Aggregative Behaviour of Spiny Butterfly Rays (Gymnura altavela, Linnaeus, 1758) in the Shallow Coastal Zones of Gran Canaria in the Eastern Central Atlantic
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
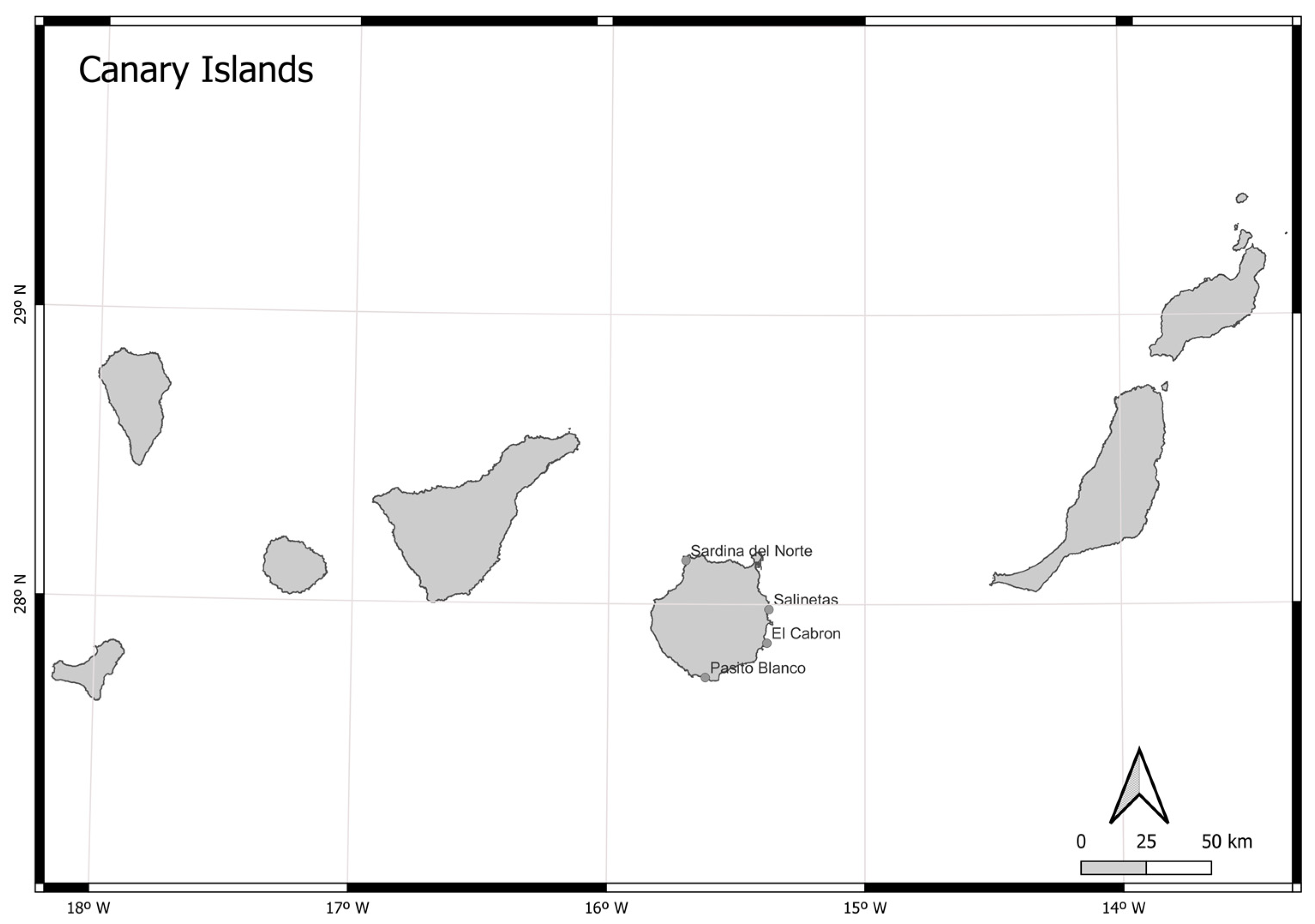
2.1. Data Collection
2.2. Data Analysis
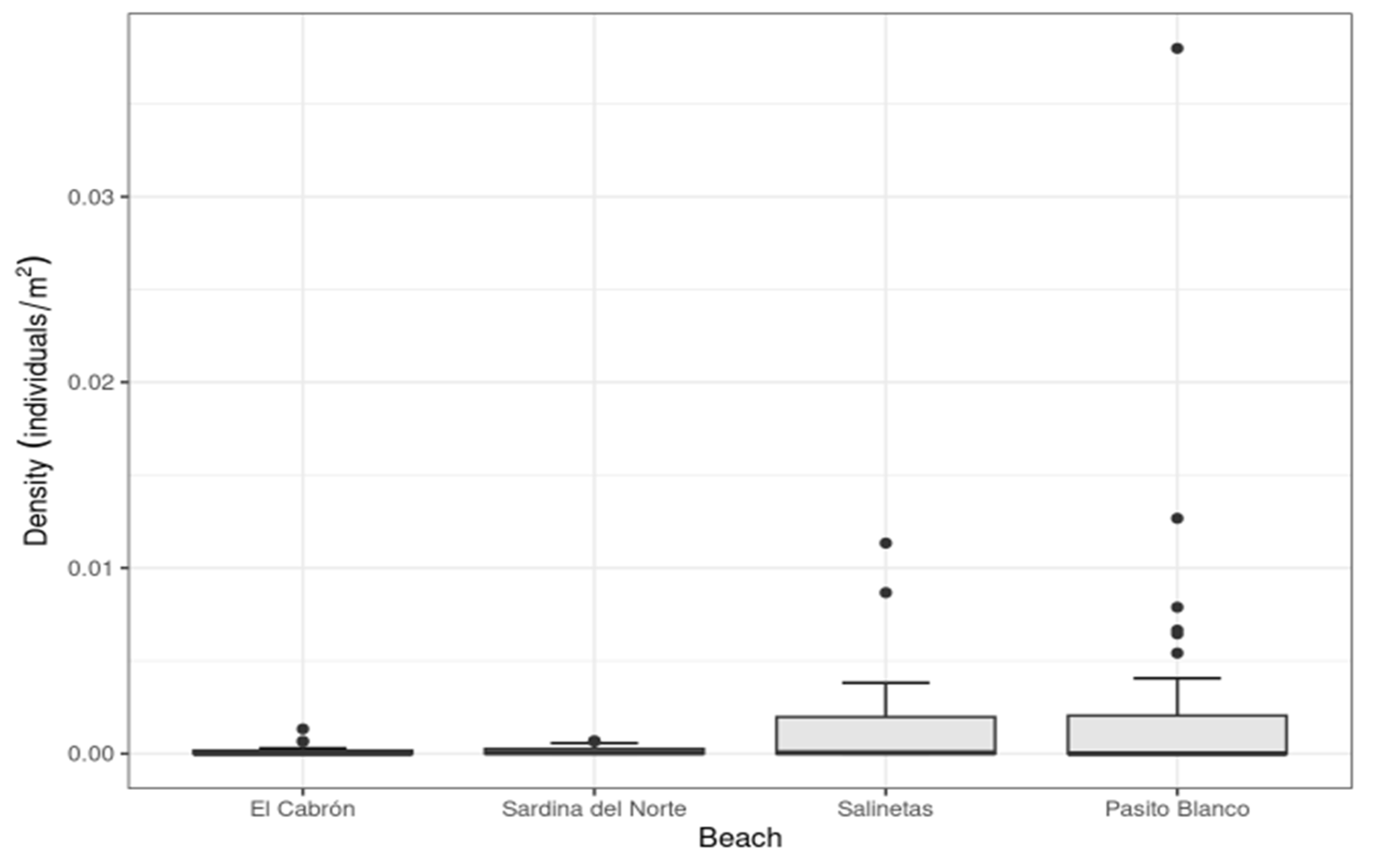
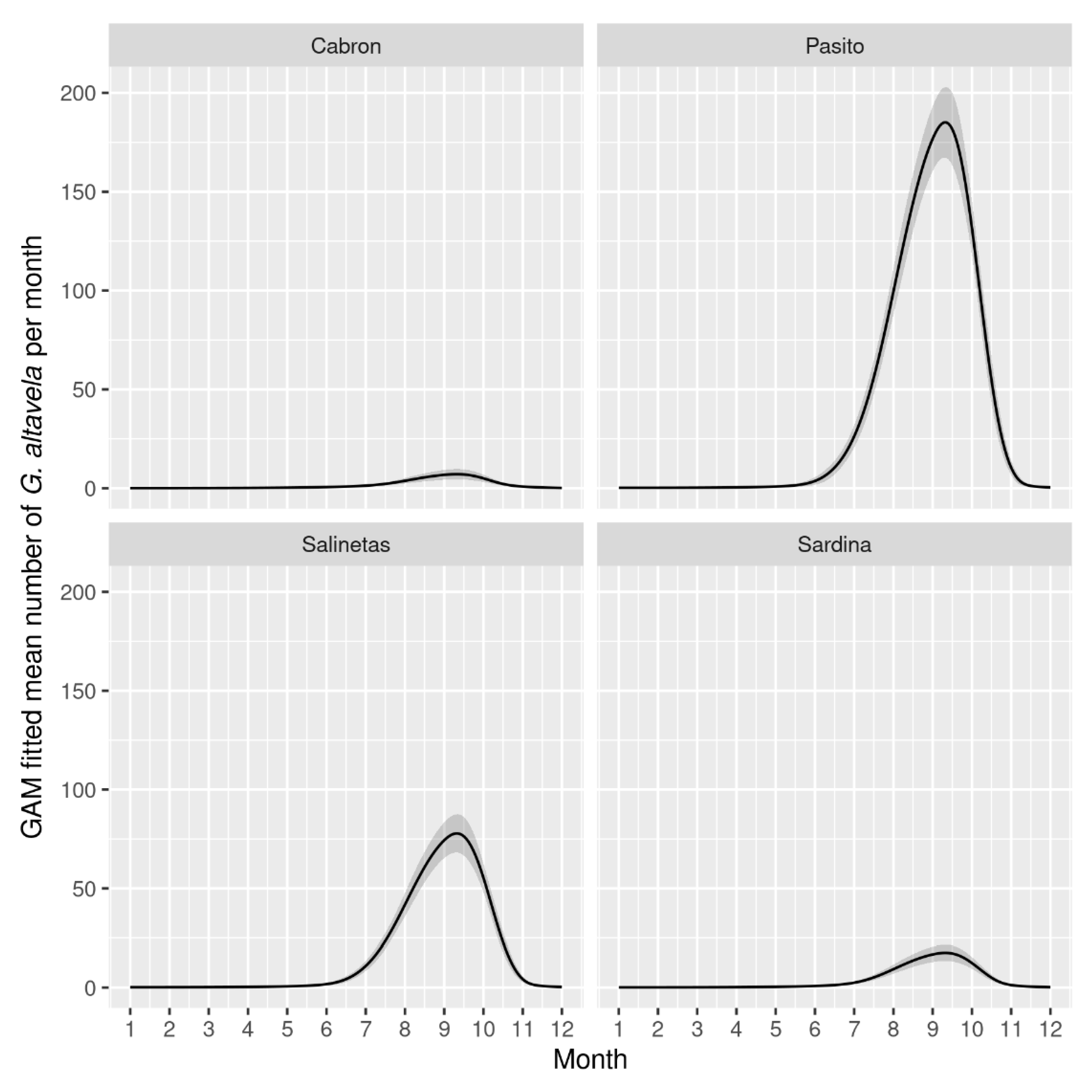
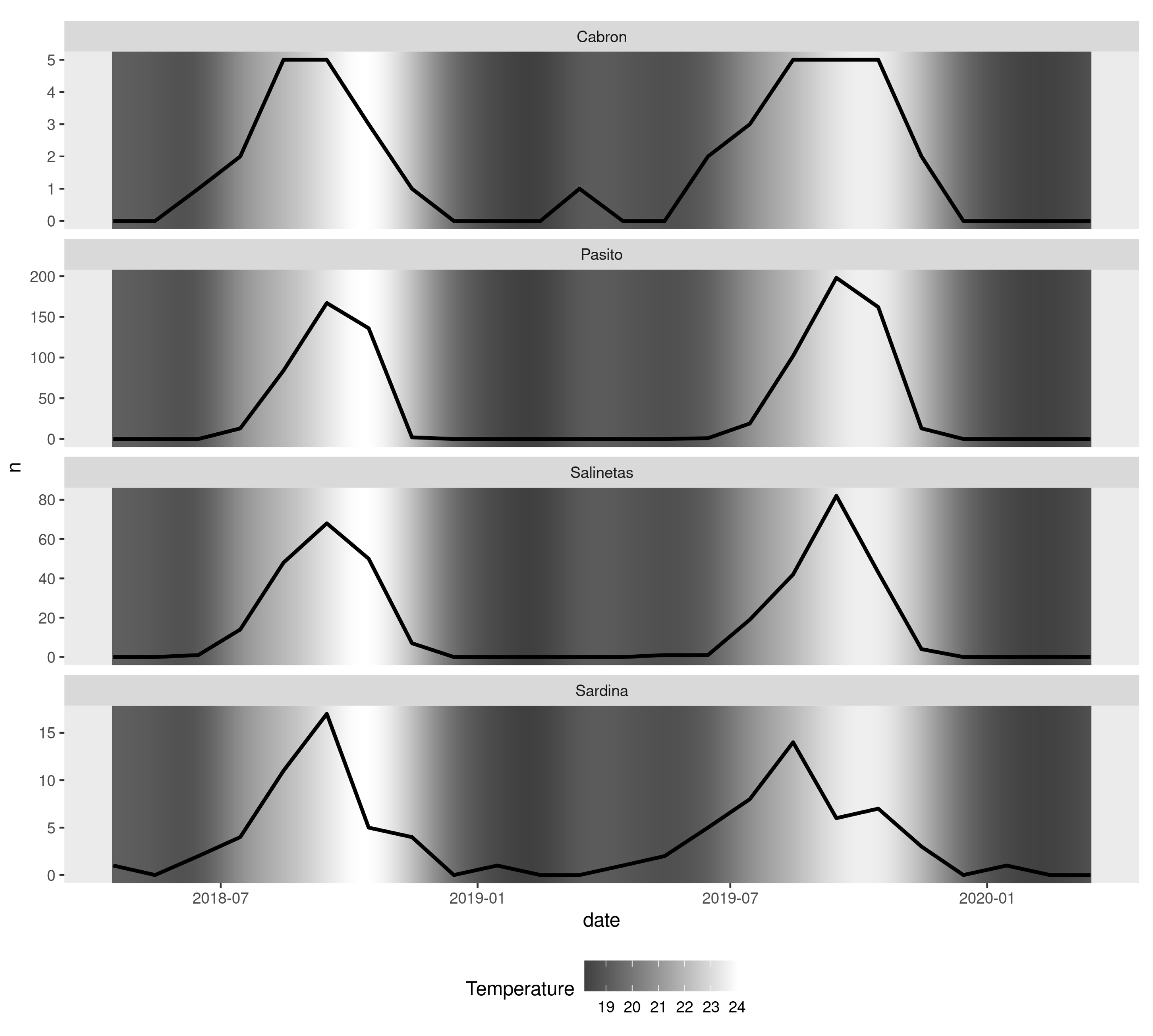
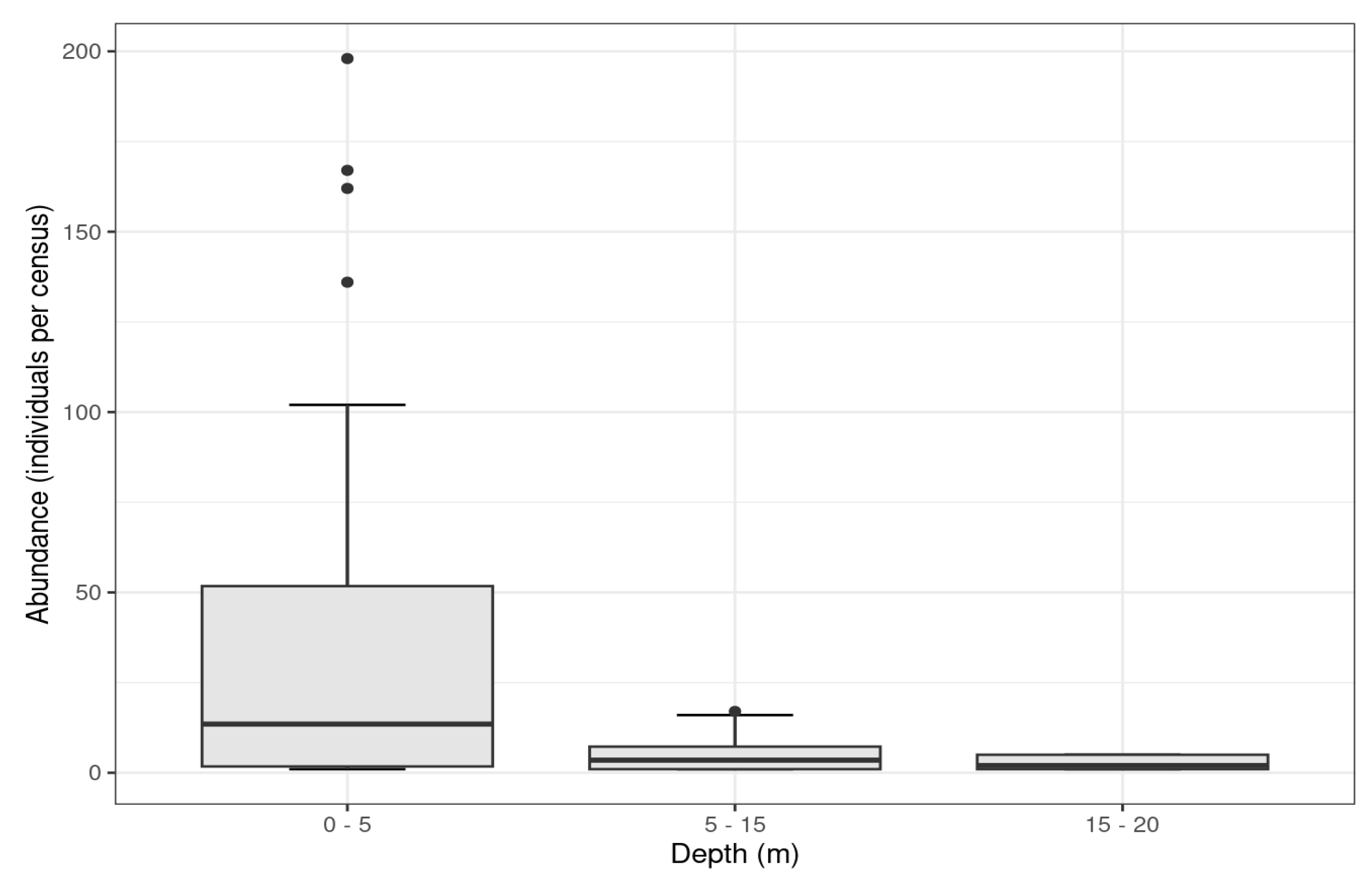
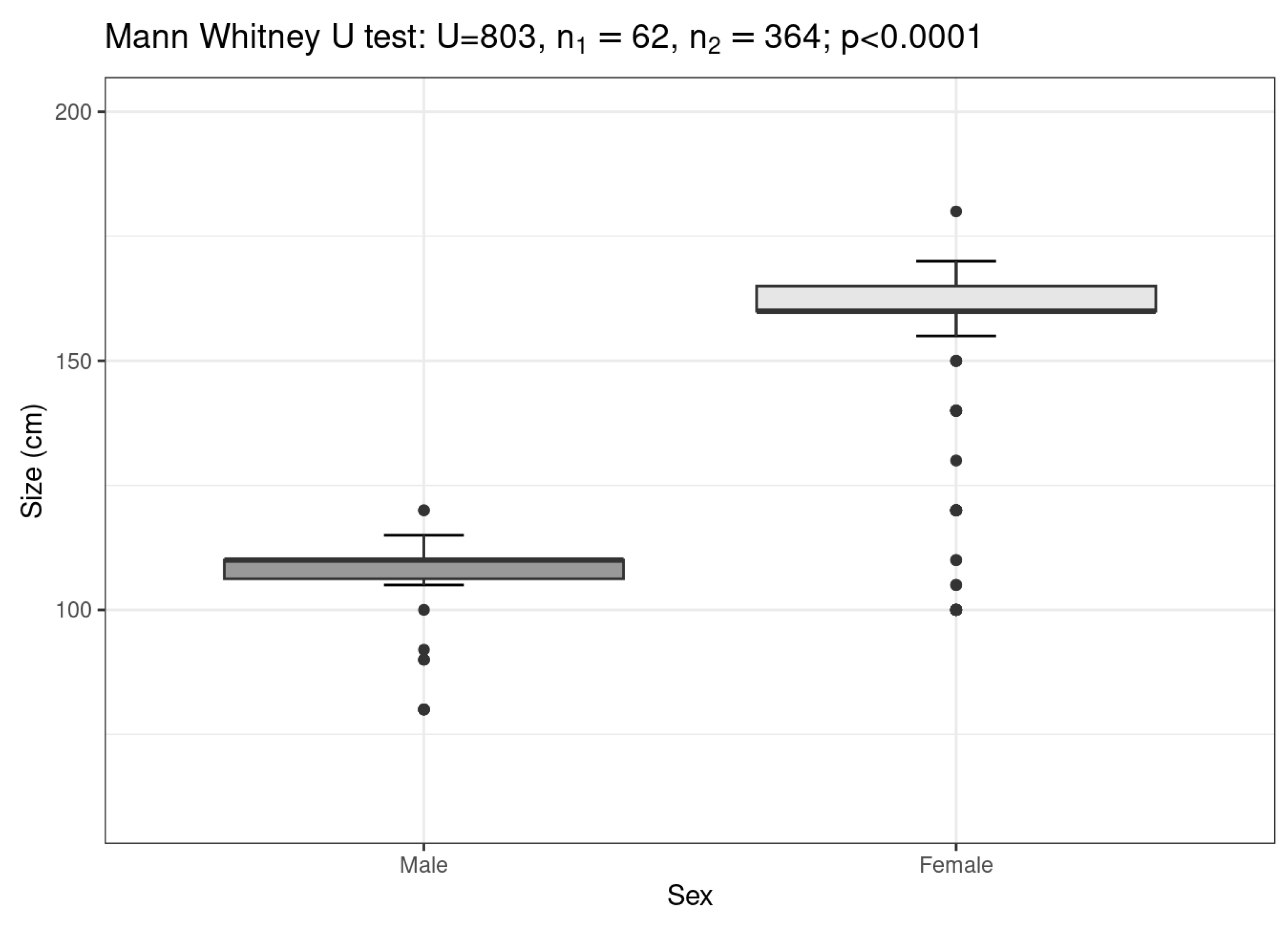
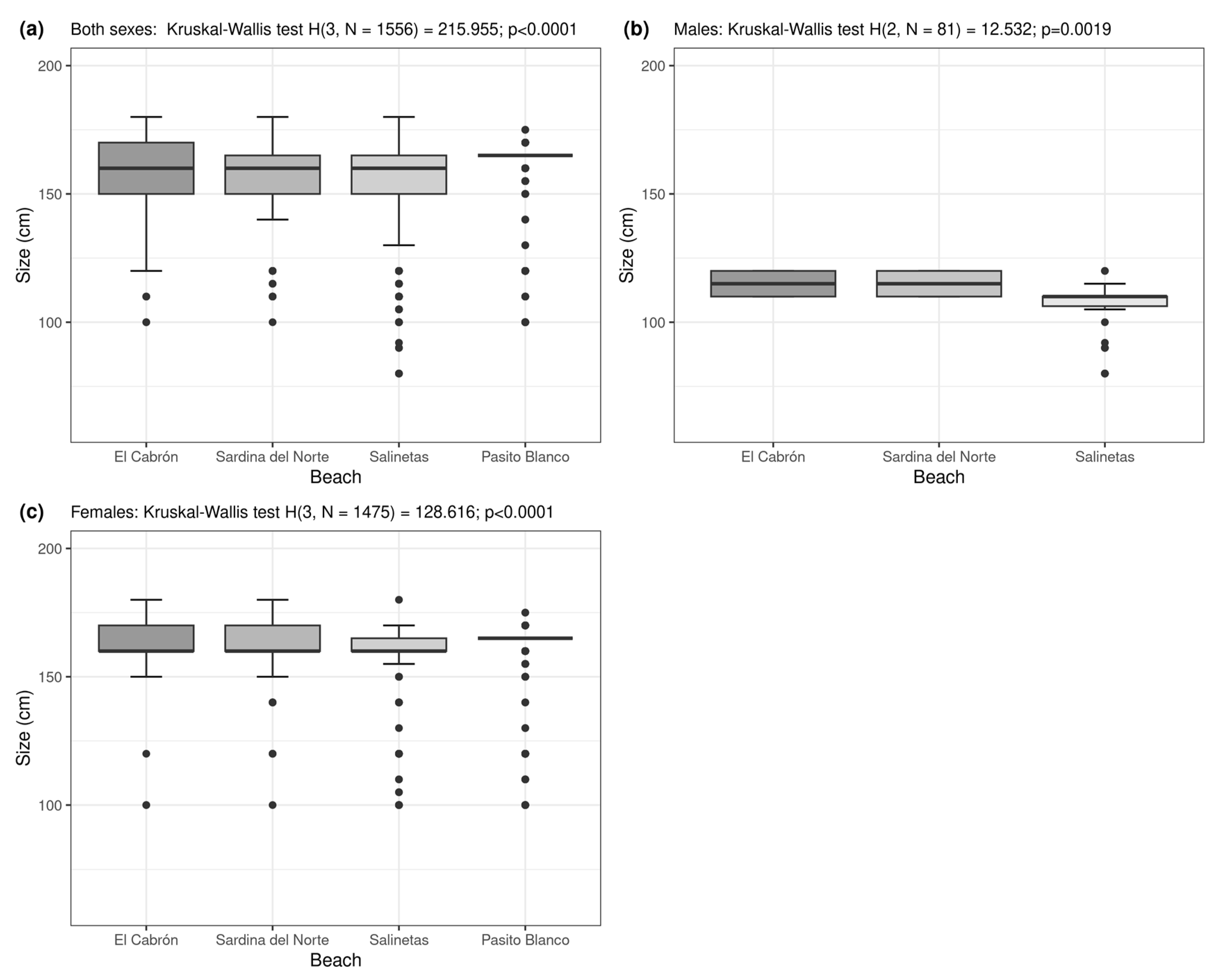
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dulvy, K.N.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; Carlson, J.K.; Davidson, L.N.K.; Fordham, S.V.; Francis, M.P.; et al. Extinction risk and conservation of the world’s sharks and rays. eLife 2014, 3, e00590. [Google Scholar] [CrossRef]
- Pacoureau, N.; Rigby, C.L.; Kyne, P.M.; Sherley, R.B.; Winker, H.; Carlson, J.K.; Dulvy, N.K. Half a century of global decline in oceanic sharks and rays. Nature 2021, 589, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.D.; Bonfil, R.; Dulvy, N.K.; Walker, P.A. The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci. 2000, 57, 476–494. [Google Scholar] [CrossRef]
- Bornatowski, H.; Navia, A.F.; Braga, R.R.; Abilhoa, V.; Corrêa, M.F.M. Ecological importance of sharks and rays in a structural foodweb analysis in southern Brazil. ICES J. Mar. Sci. 2014, 71, 1586–1592. [Google Scholar] [CrossRef]
- Hammerschlag, N.; Williams, L.; Fallows, M.; Fallows, C. Disappearance of white sharks leads to the novel emergence of an allopatric apex predator, the seven gill shark. Sci. Rep. 2019, 9, 1908. [Google Scholar] [CrossRef]
- Myers, R.A.; Baum, J.K.; Shepherd, T.D.; Powers, S.P.; Peterson, C.H. Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science 2007, 315, 1846–1850. Available online: https://www.science.org/doi/10.1126/science.1138657 (accessed on 28 February 2023). [CrossRef] [PubMed]
- Ferretti, F.; Worm, B.; Britten, G.L.; Heithaus, M.R.; Lotze, H.K. Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 2010, 13, 1055–1071. [Google Scholar] [CrossRef]
- Couce-Montero, L.; Christensen, V.; Castro, J.J. Simulating trophic impacts of fishing scenarios on two oceanic islands using Ecopath with Ecosim. Mar. Environ. Res. 2021, 169, 105341. [Google Scholar] [CrossRef]
- Hammerschlag, N.; Fallows, C.; Meÿer, M.; Seakamela, S.M.; Orndorff, S.; Kirkman, S.; Kotze, D.; Creel, S. Loss of an apex predator in the wild induces physiological and behavioural changes in prey. Biol. Lett. 2022, 18, 20210476. [Google Scholar] [CrossRef]
- Castro, J. The origins and rise of shark biology in the 20th century. Mar. Fish. Rev. 2016, 78, 14–33. [Google Scholar]
- Dent, F.; Clarke, S.; State of the Global Market for Shark Products. FAO Fisheries and Aquaculture Technical Paper; I, III, IV, VII, VIII, 1–159, 161–167, 169–179, 181–185, 187. 2015. Available online: https://www.proquest.com/scholarly-journals/state-global-market-shark-products/docview/1708482071/se-2 (accessed on 25 February 2021).
- McCully, S.R.; Scott, F.; Ellis, J.R. Lengths at maturity and conversion factors for skates (Rajidae) around the British Isles, with an analysis of data in the literature. ICES J. Mar. Sci. 2012, 69, 1812–1822. [Google Scholar] [CrossRef]
- Bräutigam, A.; Callow, M.; Campbell, I.R.; Camhi, M.D.; Cornish, A.S.; Dulvy, N.K.; Fordham, S.V.; Fowler, S.L.; Hood, A.R.; McClennen, C.; et al. Global Priorities for Conserving Sharks and Rays: A 2015–2025 Strategy. Global Sharks and Rays Initiative, IUCN Species Survival Commission (SSC), Shark Specialist Group, The Shark Trust, UK, TRAFFIC International, Wildlife Conservation Society (WCS), US, WWF. 2015. Available online: https://portals.iucn.org/library/sites/library/files/documents/2016-007.pdf (accessed on 28 February 2023).
- ICES. NEAFC and OSPAR joint request on the status and distribution of deep-water elasmobranchs. In ICES Special Request Advice. Ecoregion in the Northeast Atlantic and Adjacent Seas; ICES: Copenhagen, Denmark, 2020. [Google Scholar]
- Chatzispyrou, A.; Koutsikopoulos, C. Tracing Patterns and Biodiversity Aspects of the Overlooked Skates and Rays (Subclass Elasmobranchii, Superorder Batoidea) in Greece. Diversity 2023, 15, 55. [Google Scholar] [CrossRef]
- Liu, K.-M.; Huang, Y.-W.; Hsu, H.-H. Management Implications for Skates and Rays Based on Analysis of Life History Parameters. Front. Mar. Sci. 2021, 8, 664611. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; 243p. [Google Scholar]
- Davidson, L.N.K.; Krawchuk, M.A.; Duley, N.K. Why have global shark and ray landings declined: Improved management or overfishing. Fish Fish. 2016, 17, 438–458. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Reynolds, J.D.; Metcalfe, J.D.; Glanville, J. Fisheries stability, local extinctions and shifts in community structure in skates. Conserv. Biol. 2000, 14, 283–293. [Google Scholar] [CrossRef]
- King, J.R.; McFarlane, G.A. Marine fish life history strategies: Applications to fishery management. Fish. Manag. Ecol. 2003, 10, 249–264. [Google Scholar] [CrossRef]
- Scientific, Technical and Economic Committee for Fisheries (STECF). Long-Term Management of Skates and Rays (STECF-17-21); JRC109366; Publications Office of the European Union: Luxembourg, 2017; ISBN 978-92-79-67493-8. [Google Scholar] [CrossRef]
- Taylan, B.; Bayhan, B.; Saglam, C.; Kara, A. First observation of the embryos of spiny butterfly rayspiny butterfly rayspiny butterfly ray, Gymnura altavela (Linnaeus, 1758) (Chondrichthyes: Gymnuridae) from Eastern Mediterranean, a species critically endangered. Fresenius Environ. Bull. 2019, 28, 3147–3152. [Google Scholar]
- Brito, A.; Pascual, P.J.; Falcón, J.M.; Sancho, A.; González, G. Peces de las Islas Canarias. Catálogo Comentado e Ilustrado; Francisco Lemus Editor: San Cristóbal de La Laguna, Spain, 2002. [Google Scholar]
- Moreno, J.; Solleleit-Ferreira, S.E.; Riera Elena, R. Distribution and Abundance of Coastal Elasmobranchs in Tenerife (Canary Islands, NE Atlantic Ocean) with Emphasis on the Bull Ray, Aetomylaeus bovinus. Thalass. Int. J. Mar. Sci. 2021, 38, 723–731. [Google Scholar] [CrossRef]
- Walls, R.; Vacchi, M.; Notarbartolo di Sciara, G.; Serena, F.; Dulvy, N. Gymnura altavela (Europe Assessment). The IUCN Red List of Threatened Species 2015: e.T63153A48931613. 2015. Available online: https://www.iucnredlist.org/ (accessed on 28 February 2023).
- Walls, R.H.L.; Vacchi, M.; Notarbartolo di Sciara, G.; Serena, F.; Dulvy, N.K. Gymnura altavela (Mediterranean Assessment). The IUCN Red List of Threatened Species 2016: e.T63153A16527909. 2016. Available online: https://www.iucnredlist.org/ (accessed on 28 February 2023).
- Dulvy, N.K.; Charvet, P.; Carlson, J.; Badji, L.; Blanco-Parra, M.P.; Chartrain, E.; De Bruyne, G.; Derrick, D.; Dia, M.; Doherty, P.; et al. Gymnura altavela. The IUCN Red List of Threatened Species 2021: e.T63153A3123409. 2021. Available online: https://dx.doi.org/10.2305/IUCN.UK.2021-1.RLTS.T63153A3123409.en (accessed on 28 February 2023).
- McEachran, J.D.; Capape, C. Rhinobatidae. In Fishes of the North-Eastern Atlantic and the Mediterranean; Unesco: Paris, France, 1984; Volume 1, pp. 156–158. [Google Scholar]
- McEachran, J.D.; De Carvalho, M.R.; Carpenter, K.E. Batoid fishes. In The Living Marine Resources of the Western Central Atlantic; FAO: Rome, Italy, 2002; Volume 1, pp. 507–589. [Google Scholar]
- Menni, R.C.; Lucifora, L.O. Chondrichthyes from Argentina and Uruguay; ProBiota, Serie Técnica-Didáctica; UNLP: La Plata, Argentina, 2007; Volume 11, pp. 1–15. Available online: http://hdl.handle.net/1834/19532 (accessed on 28 February 2023).
- Ray, C.; Robins, C.R. A Field Guide to Atlantic Coast Fishes: North America; Houghton Mifflin Harcourt: Boston, MA, USA, 2016. [Google Scholar]
- Stehmann, M. Gymnuridae. In FAO Species Identification Sheets for Fishery Purposes; Fischer, W., Bianchi, G., Scott, W.B., Eds.; Eastern Central Atlantic (Fishing Areas 34, 47 (In Part)); FAO: Rome, Italy, 1981; Volume 5. [Google Scholar]
- International Game Fish Association. World Record Game Fishes; International Game Fish Association: Dania Beach, FL, USA, 1991. [Google Scholar]
- Dulçic, J.; Jardas, I.; Onofri, V.; Bolotin, J. The roughtail stingray Dasyatis centroura (Pisces: Dasyatidae) and spiny butterfly ray Gymnura altavela (Pisces: Gymnuridae) from the southern Adriatic. Marine Biological Association of the United Kingdom. J. Mar. Biol. Assoc. UK 2003, 83, 871–872. [Google Scholar] [CrossRef]
- Meyers, E.K.M.; Tuya, F.; Barker, J.; Jiménez-Alvarado, D.; Castro-Hernández, J.J.; Haroun, R.; Rödder, D. Population structure, distribution and habitat use of the critically endangered angelshark, Squatina squatina, in the Canary Islands. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 1133–1144. [Google Scholar] [CrossRef]
- Tuya, F.; Asensio, M.; Navarro, A. “Urbanite” rays and sharks: Presence, habitat use and population structure in an urban semi-enclosed lagoon. Reg. Stud. Mar. Sci. 2020, 37, 101342. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2021-1. 2021. Available online: www.iucnredlist.org (accessed on 19 May 2022).
- Bas, C.; Castro, J.J.; Hernandez-Garcia, V.; Lorenzo, J.M.; Moreno, T.; Pajuelo, J.M.; Gonzalez Ramos, A.J. La Pesca en Canarias y Areas de Influencia; Cabildo Insular de Gran Canaria: Las Palmas de Gran Canaria, Spain, 1995. [Google Scholar]
- Falcón, J.M.; Bortone, S.A.; Brito, A.; Bundrick, C.M. Structure of and relationships within and between the littoral, rock-substrate fish communities off four islands in the Canarian Archipelago. Mar. Biol. 1996, 125, 215–231. [Google Scholar] [CrossRef]
- Tuya, F.; Boyra, A.; Sanchez-Jerez, P.; Barbera, C.; Haroun, R.J. Relationships among fishes, the long-spined sea urchin Diadema antillarum and algae throughout the Canarian Archipelago. Mar. Ecol. Prog. Ser. 2004, 278, 157–169. [Google Scholar] [CrossRef]
- Martínez Saavedra, J. Analysis of the State of Fishery Resources from Gran Canaria Study of the Historical Catch Series. Master’s Thesis, University of Las Palmas of Gran Canaria, Las Palmas de Gran Canaria, Spain, 2011. [Google Scholar]
- Noviello, N.; McGonigle, C.; Jacoby, D.M.P.; Meyers, E.K.M.; Jiménez-Alvarado, D.; Barker, J. Modelling Critically Endangered marine species: Bias-corrected citizen science data inform habitat suitability for the angelshark (Squatina squatina). Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 3451–3465. [Google Scholar] [CrossRef]
- Wetz, J.J.; Ajemian, M.J.; Shipley, B.; Stunz, G.W. An assessment of two visual survey methods for documenting fish community structure on artificial platform reefs in the Gulf of Mexico. Fish. Res. 2020, 225, 105492. [Google Scholar] [CrossRef]
- Jacoby, D.M.P.; Croft, D.P.; Sis, D.W. Social behaviour in sharks and rays: Analysis, patterns and implications for conservation. Fish Fish. 2011, 13, 399–417. [Google Scholar] [CrossRef]
- Mendonça, S.A.; Macena, B.C.L.; Afonso, A.S.; Hazin, H.V. Seasonal aggregation and diel activity by the sicklefin devil ray Mobula tarapacana off a small, equatorial outcrop of the Mid-Atlantic Ridge. J. Fish Biol. 2018, 93, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Labrosse, P.; Kulbicki, M.; Ferraris, J. Underwater Visual Fish Census Surveys: Proper Use and Implementation; Secretariat of Pacific Community: Noumea, New Caledonia, 2002; Volume 61. [Google Scholar]
- Mildenberger, T.K.; Taylor, M.H.; Wolff, M. TropFishR: An R package for fisheries analysis with length-frequency data. Methods Ecol. Evol. 2017, 8, 1520–1527. [Google Scholar] [CrossRef]
- Wetherall, J.A.; Polovina, J.J.; Ralston, S. Estimating growth and mortality in steady-state fish stocks from length-frequency data. ICLARM Conf. Proc. 1987, 13, 53–74. [Google Scholar]
- Aguiar, A.A.; Valentin, J.L.; Rosa, R.S. Habitat use by Dasyatis americana in a south-western Atlantic oceanic island. J. Mar. Biol. Assoc. UK 2009, 89, 1147–1152. [Google Scholar] [CrossRef]
- Sims, D.W. Differences in Habitat Selection and Reproductive Strategies of Male and Female Sharks; Cambridge University Press: Cambridge, UK, 2005; pp. 127–147. [Google Scholar]
- Powter, D.M.; Gladstone, W. Habitat-mediated use of space by juvenile and mating adult port Jackson sharks, Heterodontus portusjacksoni, in Eastern Australia. Pac. Sci. 2009, 63, 1–14. [Google Scholar] [CrossRef]
- Schlaff, A.; Heupel, M.R.; Simpfendorfer, C.A. Influence of environmental factors on the shark and ray movement, behaviour and habitat use: A review. Rev. Fish Biol. Fish. 2014, 24, 1089–1103. Available online: https://link.springer.com/content/pdf/10.1007/s11160-014-9364-8.pdf (accessed on 28 February 2023). [CrossRef]
- Jaine, F.R.A.; Rohner, C.A.; Weeks, S.J.; Couturier, L.I.E.; Bennett, M.B.; Townsend, K.A.; Richardson, A.J. Movements and habitat use of reef manta rays off eastern Australia: Offshore excursions, deep diving and eddy affinity revealed by satellite telemetry. Mar. Ecol. Prog. Ser. 2014, 510, 73–86. [Google Scholar] [CrossRef]
- Gaspar, C.; Chateau, O.; Galzin, R. Feeding sites frequentation by the pink whipray Himantura fai in Moorea (French Polynesia) as determined by acoustic telemetry. Cybium 2008, 32, 153–164. [Google Scholar]
- Davy, L.E.; Simpfendorfer, C.A.; Heupel, M.R. Movement patterns and habitat use of juvenile mangrove whiprays (Himantura granulata). Mar. Freshw. Res. 2015, 66, 481–492. [Google Scholar] [CrossRef]
- Martins, A.P.B.; Heupel, M.R.; Chin, A.; Simpfendorfer, C.A. Batoid nurseries: Definition, use and importance. Mar. Ecol. Prog. Ser. 2018, 595, 253–267. [Google Scholar] [CrossRef]
- Mull, C.G.; Lowe, C.G.; Young, K.A. Photoperiod and water temperature regulation of seasonal reproduction in male round stingrays (Urobatis halleri). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 151, 717–725. [Google Scholar] [CrossRef]
- Hammerschlag, N.; Skubel, R.A.; Clich, H.; Nelson, E.R.; Shiffman, D.S.; Wester, J.; Macdonald, C.C.; Cain, S.; Jennings, L.; Enchelmaier, A.; et al. Nocturnal and crepuscular behavior in elasmobranchs: A review of movement, habitat use, foraging, and reproduction in the dark. Bull. Mar. Sci. 2017, 93, 355–374. [Google Scholar] [CrossRef]
- Nordell, S.E. Observations of the mating behavior and dentition of the stingray, Urolophus helleri. Environ. Biol. Fishes 1994, 39, 219–229. [Google Scholar] [CrossRef]
- Kiszka, J.J.; Mourier, J.; Gastrich, K.; Heithaus, M.R. Using unmanned aerial vehicles (UAVs) to investigate shark and ray densities in a shallow coral lagoon. Mar. Ecol. Prog. Ser. 2016, 560, 237–242. [Google Scholar] [CrossRef]
- Bassos-Hull, K.; Wilkinson, K.A.; Hull, P.T.; Dougherty, D.A.; Omori, K.L.; Ailloud, L.E.; Morris, J.J.; Hueter, R.E. Life history and seasonal occurrence of the spotted eagle Aetobatus narinari, in the eastern Gulf of Mexico. Environ. Biol. Fishes 2014, 97, 1039–1056. [Google Scholar] [CrossRef]
- Perryman RJYVenables, S.K.; Tapilatu, R.F.; Marshall, A.D.; Brown, C.; Frank, D.W. Social preferences and network structure in a population reef manta rays. Behav. Ecol. Sociobiol. 2019, 73, 114. [Google Scholar] [CrossRef]
- Castillo-Geniz, J.L.; Sosa-Nishizaki, O.; Pérez-Jiménez, J.C. Morphological variation and sexual dimorphism in the California skate, Raja inornate Jordan and Gilbert, 1881, from the Gulf of California, Mexico. Zootaxa 2007, 1545, 1–16. [Google Scholar] [CrossRef]
- Orlov, A.M.; Cotton, C.F. Sexually dimorphic morphological characters in five north Atlantic deepwater skates (Chondrichthyes: Rajiformes). J. Mar. Biol. 2011, 2011, 842821. [Google Scholar] [CrossRef]
- Orlov, A.M.; Smirnov, A.A. New data on sexual dimorphism and reproductive biology of Alaska skate Bathyraja parmifera from the northwestern Pacific Ocean. J. Ichthyol. 2011, 51, 590–603. [Google Scholar] [CrossRef]
- Orlov, A.M.; Cotton, C.F.; Shevernitsky, D.A. Sexual dimorphism of external morphological characters in some deepwater skates (Rajidae, Rajifoems, Chondrichthyes) of the North Atlantic. Mosc. Univ. Biol. Sci. Bull. 2010, 65, 40–44. [Google Scholar] [CrossRef]
- Parsons, K.T.; Maisano, J.; Gregg, J.; Cotton, C.F.; Latour, R.J. Age and growth assessment of western North Atlantic spiny butterfly ray Gymnura altavela (L. 1758) using computed tomography of vertebral centra. Environ. Biol. Fishes 2018, 101, 137–151. [Google Scholar] [CrossRef]
- Coelho, R.; Erzini, K. Age and growth of the undulate ray, Raja undulata, in the Algarve (southern Portugal). J. Mar. Biol. Assoc. United Kingd. 2002, 82, 987–990. [Google Scholar] [CrossRef]
- Neer, J.A. Aspects of the Life History, Ecophysiology, Bioenergetics, and Population Dynamics of the Cownose Ray, Rhinoptera bonasus, in the Northern Gulf of Mexico; UMI Number: 3329085; Louisiana State University and Agricultural & Mechanical College: Baton Rouge, LA, USA, 2005. [Google Scholar]
- Ainsley, S.A.; Ebert, D.A.; Cailliet, G.M. Age, growth, and maturity of the whitebrow skate, Bathyraja minispinosa, from the eastern Bearing Sea. ICES J. Mar. Sci. 2011, 68, 1426–1434. [Google Scholar] [CrossRef]
- Fisher, R.A.; Call, G.C.; Grubbs, R.D. Age, growth, and reproductive biology of cownose rays in Chesapeake Bay. Mar. Coast. Fish. 2013, 5, 224–235. [Google Scholar] [CrossRef]

| Information |
|---|
| Diving centre or person who reported the sighting |
| Ability to distinguish the species |
| Date and location of sighting |
| Date or season of observation |
| Approximate length |
| Sex |
| Presence of offspring |
| Number of individuals observed |
| Aggregated or solitary status |
| Behaviour (e.g., resting, hunting or mating) |
| Body marks or injuries (e.g., by hook or spear gun) |
| Ability to reidentify the reported individuals due to those body marks |
| Correlation | Var1 | Var2 | Corr. | p | Conf. Low | Conf. High |
|---|---|---|---|---|---|---|
| Pearson | Temperature | N | 0.86 | 0.0000000988 | 0.6906081 | 0.9359712 |
| Spearman | Temperature | N | 0.81 | 0.0000017800 |
| Parameter | ELEFAN S.A. | ELEFAN G.A. |
|---|---|---|
| Asymptotic length (cm) | 206.07 | 199.28 |
| Growth coefficient k (yr−1) | 0.22 | 0.23 |
| Summer point oscillation (ts) | 0.22 | 0.39 |
| Amplitude of growth oscillation (C) | 0.35 | 0.79 |
| Growth performance index (Φ’) | 3.96 | 3.95 |
| t_anchor | 0.31 | 0.60 |
| Age_max | 14 | 14 |
| Goodness of fit (Rn_max) score | 0.58 | 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espino-Ruano, A.; Castro, J.J.; Guerra-Marrero, A.; Couce-Montero, L.; Meyers, E.K.M.; Santana-del-Pino, A.; Jimenez-Alvarado, D. Aggregative Behaviour of Spiny Butterfly Rays (Gymnura altavela, Linnaeus, 1758) in the Shallow Coastal Zones of Gran Canaria in the Eastern Central Atlantic. Animals 2023, 13, 1455. https://doi.org/10.3390/ani13091455
Espino-Ruano A, Castro JJ, Guerra-Marrero A, Couce-Montero L, Meyers EKM, Santana-del-Pino A, Jimenez-Alvarado D. Aggregative Behaviour of Spiny Butterfly Rays (Gymnura altavela, Linnaeus, 1758) in the Shallow Coastal Zones of Gran Canaria in the Eastern Central Atlantic. Animals. 2023; 13(9):1455. https://doi.org/10.3390/ani13091455
Chicago/Turabian StyleEspino-Ruano, Ana, Jose J. Castro, Airam Guerra-Marrero, Lorena Couce-Montero, Eva K. M. Meyers, Angelo Santana-del-Pino, and David Jimenez-Alvarado. 2023. "Aggregative Behaviour of Spiny Butterfly Rays (Gymnura altavela, Linnaeus, 1758) in the Shallow Coastal Zones of Gran Canaria in the Eastern Central Atlantic" Animals 13, no. 9: 1455. https://doi.org/10.3390/ani13091455
APA StyleEspino-Ruano, A., Castro, J. J., Guerra-Marrero, A., Couce-Montero, L., Meyers, E. K. M., Santana-del-Pino, A., & Jimenez-Alvarado, D. (2023). Aggregative Behaviour of Spiny Butterfly Rays (Gymnura altavela, Linnaeus, 1758) in the Shallow Coastal Zones of Gran Canaria in the Eastern Central Atlantic. Animals, 13(9), 1455. https://doi.org/10.3390/ani13091455






