Simple Summary
One-third of shark and ray species are threatened due to overfishing, but a lack of information on each species makes conservation decisions difficult. To address this issue, we conducted a study to identify the different species of sharks and rays in Malaysian waters using DNA barcoding of the CO1 gene, which is akin to DNA fingerprinting for species. We collected 175 individuals between June 2015 and June 2022, randomly selecting up to six specimens from each species. We successfully generated DNA barcodes for 67 species, belonging to 44 genera, 20 families, and 11 orders. Accurate species identification will improve species-specific catch landing data and accelerate the identification of use and illegal trade in Malaysia.
Abstract
The data provided in this article are partial fragments of the Cytochrome c oxidase subunit 1 mitochondrial gene (CO1) sequences of 175 tissues sampled from sharks and batoids collected from Malaysian waters, from June 2015 to June 2022. The barcoding was done randomly for six specimens from each species, so as to authenticate the code. We generated barcodes for 67 different species in 20 families and 11 orders. DNA was extracted from the tissue samples following the Chelex protocols and amplified by polymerase chain reaction (PCR) using the barcoding universal primers FishF2 and FishR2. A total of 654 base pairs (bp) of barcode CO1 gene from 175 samples were sequenced and analysed. The genetic sequences were blasted into the NCBI GenBank and Barcode of Life Data System (BOLD). A review of the blast search confirmed that there were 68 valid species of sharks and batoids that occurred in Malaysian waters. We provided the data of the COI gene mid-point rooting phylogenetic relation trees and analysed the genetic distances among infra-class and order, intra-species, inter-specific, inter-genus, inter-familiar, and inter-order. We confirmed the addition of Squalus edmundsi, Carcharhinus amboinensis, Alopias superciliosus, and Myliobatis hamlyni as new records for Malaysia. The establishment of a comprehensive CO1 database for sharks and batoids will help facilitate the rapid monitoring and assessment of elasmobranch fisheries using environmental DNA methods.
1. Introduction
Elasmobranchs, a subclass of Chondrichthyans that include sharks and batoids (rays, skates, guitarfish, and sawfish), are widely distributed in the Southeast Asian (SEA) region and encompass more than 300 species from freshwater environments to deep seas [1]. Within the SEA region, Malaysia is one of the top countries with rich biodiversity of elasmobranch species. To date, there are at least 70 species (19 families) of sharks and 91 species (11 families) of batoids that have been reported to occur in Malaysian waters (Supplementary Materials Table S1) [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]. Although new species are continuously discovered, the populations of sharks and batoids have gradually decreased over the past decades [18]. The reduction in the population sizes of sharks and batoids is mainly due to high bycatch rates within commercial fisheries and recreational fishing activities [19]. Additionally, due to their K-selected life histories, some elasmobranch species are threatened with extinction [20,21]. Malaysia is the world’s ninth largest producer of shark products, especially shark fins, and the second largest importer in terms of volume, as reported by the Food and Agriculture Organisation (FAO) of the United Nations [22]. It is also one of the major countries globally with high annual elasmobranch landings [8]. These sharks and batoids are generally landed whole and are mostly fully utilized. According to the reported annual fish landings in Malaysia (http://www.dof.gov.my, accessed on 5 January 2023), the biomass of sharks and batoids reduced around 15-fold (6487 tonnes to 438 tonnes) and 9-fold (11,993 tonnes to 1372 tonnes), respectively, from 2018 to 2021 [18,23,24]. These data suggested the depletion of shark and batoid populations in Malaysian waters, parallel to the findings on global shark and batoid landings [20,25]. However, as explained by the Department of Fisheries Malaysia (DOFM) [26], sharks are not specifically targeted by fishers in Malaysia, but they are caught as bycatch along with other commercially important species by various fishing gears.
Globally, the estimated species numbers of elasmobranchs threatened with extinction have increased from approximately one-quarter [20] to more than one-third [27] due to targeted and incidental overfishing. This can also be explained by their conservative life history traits, including slow growth, long lifespan, late maturity, and low fecundity, that render elasmobranch populations difficult to recover from anthropogenic pressures [20,21,28,29]. The decrease in elasmobranch populations demonstrated the urgent need for conservation and management plans [20,21,25,30,31]. Due to the lack of species-specific information and variability of population sizes among species, the current landing records fail to provide details as to which species require protection the most. Accurate species identification is one of the most important elements to take into consideration in order to carry out suitable conservation and management programmes. The field identification of several closely related sharks (including carcharhinid, sphyrnid, and triakid sharks) and batoids (Myliobatiformes and skates) is often challenging, which might result in inaccurate species compositions and diversity in catch reports [32,33]. Moreover, only a few detailed studies have been conducted on the taxonomy and diversity of elasmobranchs in Malaysia [9,34,35]. The lack of data in this field is mainly due to large specimen sizes, ethical reasons, lack of experienced taxonomists, and high field survey costs, which, in turn, render accurate identification more challenging [36]. Thus, there is the potential that many undiscovered species remain unknown. A simple and accurate species identification tool is therefore crucial to allow the species-specific development of fishery conservation and management plans, especially those pertaining to the mislabelling and misidentification of shark and batoid products [12].
Over the past two decades, DNA barcoding has been introduced for its efficiency and accuracy in the identification of challenging species from different taxa [37,38]. The idea of this approach is conceptually straightforward—it uses a short DNA fragment of a specific gene of an approximately 650 bp region to be compared against reference sequences for accurate animal species identification [38]. The Cytochrome c oxidase subunit 1 mitochondrial gene (CO1) is suggested as a highly suitable marker for DNA barcoding, as it can discriminate between closely related species across diverse animal phyla [36], including sharks and rays [39,40]. To date, DNA barcoding has been successful in identifying elasmobranch species from dried fins, tissues, and carcasses [41,42,43,44,45,46,47]. This is highly beneficial for non-taxonomists to identify species with reasonable confidence even in the absence of whole specimens. Species can be identified by comparing their DNA barcode sequences against an online repository of barcode references, such as the NCBI GenBank (https://www.ncbi.nlm.nih.gov/genbank, accessed on 5 January 2023) and Barcode of Life Data System (BOLD) (http://www.boldsystems.org, accessed on 5 January 2023) [38,48,49]. However, DNA barcoding has limitations, such as the possibility of errors due to poor DNA quality or contamination, the potential for hybridisation or incomplete lineage sorting, and the lack of universality of CO1 primers across different taxonomic groups.
DNA barcoding—more specifically, CO1 analysis—has been used widely to aid in the species identification of elasmobranchs from various regions, such as Australia [36,42,43], China [44], the Philippines [50], Indonesia [51], Singapore [45,47], India [19,52,53], Bangladesh [54], Southern Africa [46,55], the United Kingdom [56], the Mediterranean Sea [57], the North Atlantic Ocean [58], the United States [49], and Brazil [59,60,61]. To date, limited published DNA barcoding studies in Malaysia have focused on rays but not on sharks [9,34]. From the available records in BOLD [62], a total of 783 sequences representing 120 species (primarily from the orders Myliobatiformes and Carcharhiniformes) from Malaysian waters were found. These suggested that elasmobranchs in Malaysian waters are not completely barcoded with potential for undiscovered species. Therefore, the present study used DNA barcoding to elucidate the species diversity of sharks and batoids, as well as to update the online database of DNA sequences of elasmobranchs in Malaysian waters. The results could reveal a greater elasmobranch diversity and help support the implementation of elasmobranch conservation and management programs in Malaysian waters.
2. Materials and Methods
2.1. Tissue Sampling and Processing
Shark and batoid tissue samples were collected at various landing sites and markets from four major coastal areas: west coast of Peninsular Malaysia (WP), east coast of Peninsular Malaysia (EP), Sarawak (SR), and Sabah (SB) (Figure 1), from June 2015 to June 2022. These landing sites were chosen based on previous surveys conducted by the Department of Fisheries Malaysia (DOFM), which highlighted prime locations for local elasmobranch fisheries. Moreover, additional samples along EP, SR, and SB were obtained through opportunistic fisheries—independent demersal trawl surveys within the Exclusive Economic Zone (EEZ) organised by the Department of Fisheries Malaysia (July 2015 to July 2016). They were identified in the field and the laboratory by KCL and AJXL using authoritative species identification guides and taxonomic references of the region [4,6,13,14,63,64,65,66]. No live animals were collected or killed during this study. No permission to collect data from EP was needed at the time of the research, and permission to collect data from Sarawak waters was granted by the Fisheries Research Institute Sarawak, Department of Fisheries Malaysia. A Sabah sampling permit was provided by the Sabah Biodiversity Council (SaBC), Ref. No. JKM/MBS.1000-2/2 JLD.9 (22) and JKM/MBS.1000-2/3JLD.4 (18). At least one fin clip of each recorded species was collected and preserved either in absolute ethanol or gently squashed onto a Whatman FTA® Elute card before the subsequent molecular analysis.
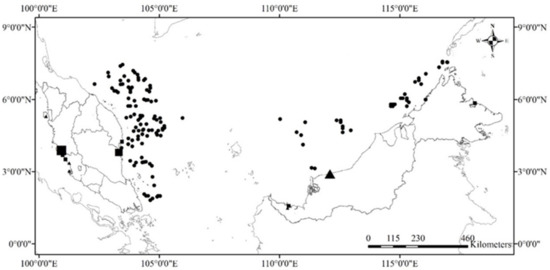
Figure 1.
Map of the selected elasmobranch sampling sites in Malaysia.
2.2. Extraction, PCR Amplification, and DNA Sequencing
One to six tissue samples for each species were selected for barcoding and molecular analysis (Table 1). Particular attention was given to specimens showing morphological ambiguities during field identification and potential cryptic species, as well as species that have not been barcoded to date; molecular identification was used to clarify species identities in these cases. These includes samples of Cephaloscyllium sarawakense (2-013 and 3-640); Carcharhinus leucas (3-129); Squalus altipinnis (2-041 and 2-047); Narcine maculata (2-874 and 4-439); Rhinobatos borneensis (KK7, 2-070, 2-678, S6, S16, and S17); Okamejei boesemani (2-057, 2-093, and 4-065); Hemitrygon parvonigra (2-059, 2-127, 2-448, and T8); Maculabatis gerrardi (TW5, 2-160, 2-162, 2-166, JHR5TC, and S27E); Urogymnus lobistoma (3-051, 3-821, and 3-876); Myliobatis hamlyni (T7, 2-027, and 2-029); and Chimaera phantasma (2-023 and 2-025). For samples stored in absolute ethanol, the total DNA was extracted using 10% Chelex resin incubated for two minutes at 60 °C, followed by 25 min at 103 °C, following a modified protocol of Hyde et al. [67]. For samples stored using Whatman FTA® Elute cards, the total DNA was extracted using the modified protocol in Rigby et al. [68].

Table 1.
Verification of the identity of barcoded shark and batoids species. Given are the sample code, sample location, NCBI accession number, field identification, best matched species from NCBI GenBank, similarity species and % from BOLD, tree-based identification, and the consensus identification. West coast of Peninsular Malaysia (WP): HM = Hutan Melintang, Pen = Penang, PP = Pasir Penambang, Sb = Sungai Besar, and Tk = Tanjung Karang; East coast of Peninsular Malaysia (EP): En = Endau, EP(D) = East coast of Peninsular Malaysia (DOF survey), Kt = Kuantan, Mer = Mersing, and Trg = Terengganu; Sarawak (SR): Btw = Bintawa, Kc = Kuching, Mk = Mukah, Sib = Sibu, SR(D) = Sarawak (DOF survey), and Srk = Sarikei; Sabah (SB): Kd = Kudat, KK = Kota Kinabalu, San = Sandakan, SB(D) = Sabah (DOF survey), and Tw = Tawau.
Amplification of the partial cytochrome c oxidase subunit I (CO1) gene (650 base pairs, nucleotide position 51-701) was done using universal primers FishF2– 5′ TCG ACT AAT CAT AAA GAT ATC GGC AC 3′ and FishR2—5′ ACT TCA GGG TGA CCG AAG AAT CAG AA 3′ [38]. PCR reactions were performed in 25 μL volumes containing 12.5 μL of 2X PCR Master Mix (1st Base), 1 μL of 10 mM of both primers, 1 μL DNA templates, and 9.5 μL sterilized distilled water. The thermal conditions consisted of the initial preheating at 94 °C for 5 min, denaturation at 94 °C for 30 s, annealing at 44–54 °C for 30 s, and extension at 72 °C for 1 min, then repeated for 36–40 cycles, followed by a final extension at 72 °C of 5 min. The PCR products were checked using 1% agarose in TAE buffer. Only PCR products that showed good PCR amplification were sent for sequencing service at Apical Scientific Sdn Bhd (Selangor, Malaysia). The sequencing results were checked for confirmation of the species using the BLAST tool of the NCBI and compared with field identification. The species identification matching was based on the query cover (%) in the BLAST tool as the query cover that describes how similar the sample sequence is to the reference sequence in GenBank and the similarity in the BOLD system.
2.3. Data Analysis
For the data analysis, the obtained sequences were edited and aligned using BioEdit version 7.2.5 [69]. Molecular species identification was achieved using two approaches: Basic Local Alignment Search Tool (BLAST®) and phylogenetic tree reconstruction. For the first approach, reviewed sequences were uploaded to BLAST® (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 5 January 2023) and BOLDSYSTEMS (http://www.boldsystems.org/index.php/IDS_OpenIdEngine, accessed on 5 January 2023) in search for local similarities with the available sequences in the National Center for Biotechnology Information (NCBI) GenBank and Barcode of Life Database (BOLD), respectively. The results from the searches were in the form of scores, query cover, and percentage identity of the 200 most similar sequences available in the databases. The list of sequences that produced significant alignments or best matches with each of our sequence was reviewed manually for validity of the suggested identity. This included their percentage similarity, query coverage, reliability of the sequence sources (from respectable authors in the field), congruency among sequences from different sources, etc. Suspicious sequences in the database were excluded in the process.
For the second approach, a phylogenetic tree was constructed using sequences from the current study and reference sequences available in the NCBI GenBank (Supplementary Materials Table S2). The selection of the reference sequences included the best matched sequences in the BLAST® search, as well as available sequences of elasmobranchs that are recorded in Malaysian waters. Reference sequences from Malaysian waters were prioritized over sequences from neighbouring waters. The list of elasmobranchs (70 sharks and 91 batoid species) and chimaera species that had been recorded in Malaysia (include some unverified records) is shown in Supplementary Materials Table S1. The number of reference sequences used for each species was limited to one or two sequences, except for the species complex of Maculabatis gerardi and M. macrura.
A midpoint rooted tree maximum likelihood (ML) based on Kimura-2-Parameter (K2P) distances was created using MEGA X [70] with 1000 bootstrap replicates. The general time-reversible model plus gamma distribution rate plus evolutionarily invariable model (GTR + G + I) was selected by MEGA X as the best-fitting substitution model based on the Akaike Information Criterion (AIC). Sequences of closely related cartilaginous fish (Chimaera phantasma) were used as the outgroup. In this approach, morphological identification was verified based on the clustering of the samples in the phylogenetic tree in comparison to the reference sequences. Furthermore, the genetic distance was calculated to evaluate the usefulness of COI across taxonomic rank. We also examined genetic distances (p-distance) within and between species and subspecies. The interindividual distances calculated in MEGA X were sorted into eight inter-rank categories (intraspecific, interspecific, inter-genus, inter-subfamily, inter-family, inter-order, inter-infraclass, and inter-class) based on the species classification in the Eschmeyer’s Catalog of Fishes (CAS) [71] and the International Union for Conservation of Nature’s (IUCN) [72] Red List of Threatened Species. The mean and ranges of p-distance for these categories were calculated and boxplot was generated using Statistica 7 [73]. In addition, the analysis of variance (ANOVA) supplemented with Tukey’s HSD pairwise comparison was performed to determine the variations among inter-rank categories, between infraclass, and among orders of elasmobranchs.
3. Results
A total of 175 individuals belonging to 29 shark and 38 batoid species based on field identification were barcoded in this study. In addition, two individuals of chimaera Chimaera phantasma (closely related cartilaginous fishes) were also barcoded. All sequences were uploaded to GenBank, accession numbers: OQ384975–OQ385149 (n = 175). Another 231 sequences from NCBI GenBank were retrieved for phylogenetic tree reconstruction (Supplementary Materials Table S2). These sequences covered 82% of the total elasmobranch species recorded in Malaysia, i.e., there are 29 remaining elasmobranch species in Malaysian waters that have not been barcoded to date.
3.1. Matches with BLAST
Table 1 provides, for each barcoded individual, the percent matches to the best matched species in the NCBI GenBank and the similarity matched in the BOLD database. The results showed that 137 of the 175 samples strongly matched (>99%) with sequences of the same identity only (average 99.86%). The remaining individuals with uncertainty were noted as being matched with different species (13 individuals), low similarity (88.01–98.93%) with the best matched species (15 individuals), matched with multiple species at average genetic distance of 99.7% (17 individuals), or matched with unknown species (1 individual) (see Table 1).
3.2. Tree Matches
The ML tree (Figure 2a–e) matched most of the present sequences to reference sequences forming distinct clades by species (Supplementary Materials Table S3). Some of the exceptions in shark species were listed as follows. Field-identified S. altipinnis (samples 2-041 and 2-047) was found to be paraphyletic based on the ML tree; sample 2-041 formed a potential unique clade on its own (1.3–5.2% genetic difference with other highly similar Squalus species), while sample 2-047 clustered with the reference sequence of S. edmundsi (0.2% genetic distance). Sphyrna lewini (samples 2-226, 2-604, 3-225, and T3) formed two distinct clades, with sample 2-604 and T3 branching out from the other sequences, forming a unique clade (3.7–3.8% genetic distance). Cephaloscyllium sarawakense (samples 2-013 and 3-640) formed a clade with a reference sequence named C. umbratile, with genetic distance ranges from 0.6 to 1.3%. Carcharhinus leucas showed two separate clades: samples S1, 2-246, and 2-530 formed a clade with a reference sequence of C. leucas, while sample 3-129 formed a clade with a reference sequence of C. amboinensis. Sequences of Carcharhinus amblyrhynchoides and C. limbatus from the present study and the reference sequences used formed two separate clades; however, the genetic distance between the clades was low (0.6–0.8%).
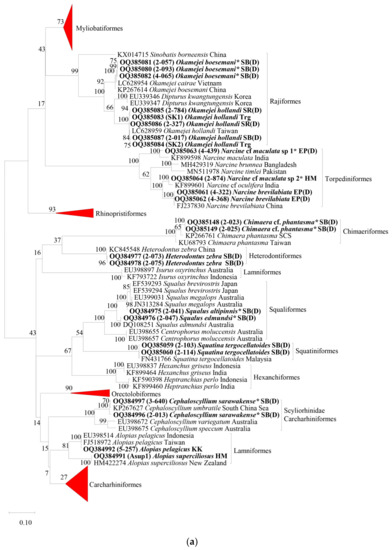

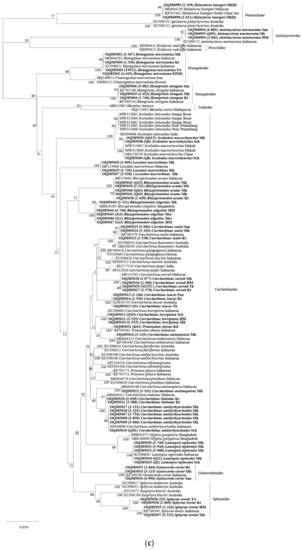
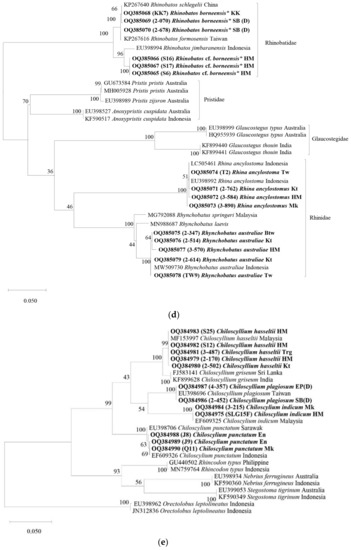
Figure 2.
Maximum likelihood (ML) mid-point rooting tree based on Kimura-2-Parameter (K2P) distances of COI gene of (a) sharks and batoids. (b) Myliobatiformes species. (c) Carcharhiniformes species. (d) Rhinopristiformes species. (e) Orectolobiformes species. The bootstrap values (ML) are shown at branches. Sequence names in bold are from the present study. * Indicate specimens of concern. See Table 1 for the abbreviation of locations for sequences from the present study.
The exceptions among batoids were listed as follows. Hemitrygon parvonigra (samples 2-059, 2-127, 2-448, and T8) was separated from the reference sequence of the species at 5.2% inter-clade genetic distance. The species identity of the Neotrygon kuhlii reference sequences found in Malaysian waters were updated to N. malaccensis, N. orientalis, and N. varidens, according to the assigned species names in Borsa et al. [74]. After renaming the references sequences, the Neotrygon sequences from the present study (samples S10, S11, Q12, 3-459, 3-619, 4-421, and 4-457) were found to cluster with the reference sequence of their respective species. Urogymnus lobistoma recorded in the present study showed a unique clade on its own as separate from other Urogymnus reference sequences (genetic distances ranged from 12.1 to 13.7%).
Sequences of M. gerrardi and M. macrura were paraphyletic showing multiple overlapping clades. The inter-individual genetic distances ranged from 0 to 5.1%. The sequence of O. boesemani formed a clade with reference sequences of O. boesemani from China (0.9–1.1% genetic distance with present sequences) and O. cairae from Vietnam (0.5–0.6% genetic distance with present sequences). Two N. maculata barcoded in this study were found to be different genetically and did not form clades with any of the best matched species; the genetic distance between samples 4-439 and N. maculata from India was 4.3% while that between samples 2-874 and N. cf. oculifera was 1.6%. Two or possibly three separate clades of field identified Rhinobatos borneensis were found with samples from Sabah (KK7, 2-070, and 2-678) forming clades with reference sequences named R. formosensis and R. schlegelii (0 to 0.3% within clade genetic distance), while samples from WP (S6, S16, and S17) were likely a unique clade separated from the aforementioned clade at 3.2 to 4.0% genetic distance and from R. jimbaranensis at 2.2% genetic distance. Myliobatis hamlyni (samples 2-027, 2-029, and T7) formed a clade with the reference sequence of the species (species name of sequence EU398924 was revised to M. hamlyni by White et al. [62]), as well as with M. tobijei sequence from South China Sea (0–0.3% genetic distance).
Although the sequences of C. phantasma were not the focus of this study, it was worth noting that the sequences obtained were separated from the reference sequences of C. phantasma at a genetic distance of 2.7 to 3.8%.
3.3. Final Taxonomic Identification
Considering the results of the BLAST matches and the ML tree, taxonomic identities of 121 individuals that showed consensus using both approaches were clearly verified. Seven samples of Neotrygon species were also confirmed after updated species identity of the reference sequences according to Borsa et al. [74]. Six samples of C. amblyrhynchoides (3-754, 3-850, 3-960, 3-131, 3-133, and Q3E) and two samples of C. limbatus (3-038 and 2-588) were cautiously treated as correctly identified due to the presence of misidentified sequences in NCBI GenBank. This suggests the need to review the identity of all submitted sequences in NCBI GenBank to prevent future confusion. Four samples of S. lewini (2-226, 2-604, 3-225, and T3) were also cautiously treated as correctly identified. However, the presence of two distinct clades suggested cryptic species of S. lewini that need further taxonomic evaluation. Three samples of M. hamlyni (T7, 2-027, and 2-029) were treated as correctly identified, backed by the reference sequence (EU398924) and specimens that were used in M. hamlyni redescription [66]. Two individuals, i.e., samples 2-047 and 3-129, were determined to be field misidentifications and were assigned as S. edmundsi and C. amboinensis, respectively, based on the phylogenetic tree information.
The identities of the remaining 30 individuals remained inconclusive based on the two molecular approaches. Field-identified S. altipinnis (2-041) and U. lobistoma (3-051, 3-821, and 3-876) were treated as the final species assignment as sequences of these species were not available in NCBI GenBank. The species identity of the two C. sarawakense samples was retained, as the morphological characteristics of the specimens did not match those of C. umbratile, suggesting a possible error in the submitted C. umbratile sequences in NCBI GenBank. Similarly, the identity of the U. cf. aurantiacus sample was retained, as the morphological characteristics of the sample were found to be different from the best matched species of U. expansus. Species identities of the sequences of three R. borneensis (from Sabah), three O. boesemani, and six M. gerrardi were also retained, as they belong to species complexes that need taxonomic clarification. The three other sequences of R. borneensis from WP were treated as R. cf. borneensis considering the low similarity with the available reference sequences of the genus. Eight sequences that matched at low similarity levels were each treated as N. cf. maculata sp1 (sample 4-439); N. cf. maculata sp2 (sample 2-874); H. cf. parvonigra (sample 2-059, 2-127, 2-448, and T8); and C. cf. phantasma (2-023 and 2-025).
3.4. Genetic Distance
Pairwise genetic distances were progressively increased as the inter-rank categories increased (Figure 3). These increases were significant (p < 0.001)-based overall and pairwise comparisons in the ANOVA and Tukey’s HSD tests. However, the range for among inter-rank genetic distances were considered large and overlapped especially between the neighbouring categories.
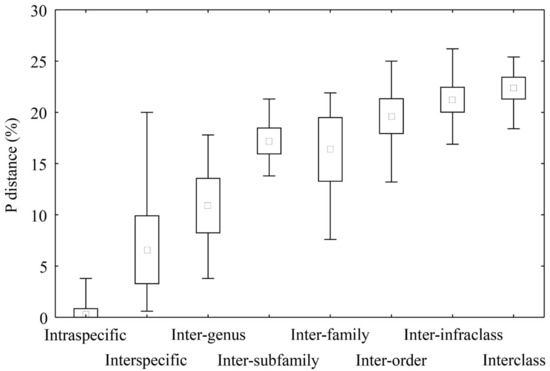
Figure 3.
Genetic distance ranges among inter-rank categories. The middle point, box, and whiskers represent the mean, mean ± standard deviation, and total range, respectively.
Comparison across class/infraclass for each of the inter-rank categories is shown in Figure 4. Within intraspecific categories, the genetic distance of Holocephali was significantly higher than the elasmobranchs (p < 0.001). The genetic distance within batoids, on the other hand, was significantly higher than sharks for the interspecific, inter-genus, inter-family, and inter-order categories (p < 0.001). Comparisons across orders provide further details for the inter-individual distances (Figure 4). Within the intraspecific categories, it showed that most of the genetic distances were in the range from 0 to 1%, except in Hexanchiformes and Chimaeriformes. For interspecific distance, the range for most batoids was found above 8%, except Rhinopristiformes, while below 8% in most of the sharks, except Lamniformes and part of the Orectolobiformes. For the inter-genus category, the genetic distance within Myliobatiformes was significantly higher than the other comparable orders (p < 0.001). For inter-family, the genetic distances within Myliobatiformes and Squaliformes were each highest and lowest among the comparable orders (p < 0.001). Other significant comparisons can be found between Orectolobiformes and Lamniformes, Orectolobiformes and Rhinopristiformes, and Carcharhiniformes with the others, except Orectolobiformes (p < 0.05 or p < 0.001).
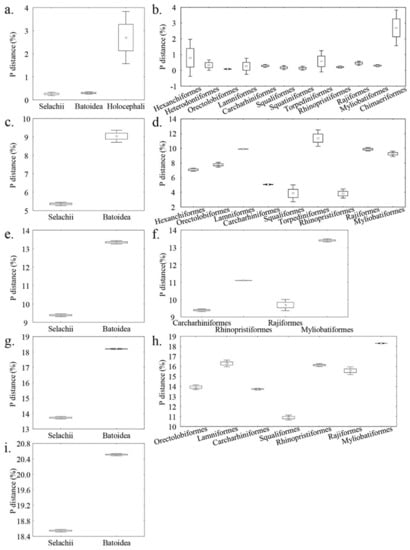
Figure 4.
Genetic distances among infraclass (or class) (a,c,e,g,i) and order (b,d,f,h). (a,b) Intraspecific; (c,d) interspecific; (e,f) inter-genus; (g,h) inter-family; and (i) inter-order. The middle point, box, and whiskers represent the mean, mean ± standard error, and mean ± 1.96 standard error, respectively.
4. Discussion
A comprehensive DNA barcode reference library is one of the fundamental building blocks allowing application of the increasingly popular environmental DNA (eDNA) metabarcoding approach for the assessment and environmental monitoring of biodiversity [75]. While gaps remain in the current elasmobranch biodiversity reference library, collective efforts, including ours, have advanced barcoding data for both common and rarely occurring freshwater and marine species of sharks, batoids, and skate in Malaysia. Additionally, the field identification and molecular verification efforts added four new records for Malaysia, namely Squalus edmundsi, Carcharhinus amboinensis, Alopias superciliosus, and Myliobatis hamlyni. With the exception of A. superciliosus that was sampled from WP, the other three species were sampled from Borneo (Sabah and Sarawak). These findings further affirm that the waters of Malaysian Borneo remain understudied in terms of the full biodiversity characterization of local elasmobranchs.
From the collation of available studies, there are 70 species of sharks (19 families) and 91 species of batoids (11 families) recorded in Malaysian waters (Supplementary Materials Table S1). The combined records from various studies had excluded species that were unaccepted in World Register of Marine Species (WORMS) (Cephaloscyllium circulopullum and Narcine indica) and species that had been verified as absent from Malaysian waters (Brevitrygon walga and B. imbricata in Last et al. [13]). These records have been improved in terms of classification within the family Dasyatidae [13]; incorporation of the revision of Telatrygon species in Malaysian waters [14]; delimitation of cryptic species in Neotrygon kuhlii complexes [15,74,76]; new recorded species in Malaysian water, including Carcharhinus tjutjot [10], Fluvitrygon kittipongi, and F. oxyrhyncha [16,17]); and Scoliodon laticaudus [35] (previously revised as S. macrorhynchos in Last et al. [6]).
Among the new species records, there are two species that are field-misidentified based on their morphological characteristics, namely S. edmundsi and C. amboinensis. These misidentifications are unsurprising, given how uncommon these two species are worldwide; the former was only described in 2007 in specimens from Australia [77], while the latter is likely often misidentified as highly similar-looking bull shark C. leucas, resulting in poor distribution records [78]. The usefulness of the molecular approach is particularly evident in separating these two morphologically similar carcharhinid species that are genetically distinctive. The discovery of spurdog S. edmundsi was possible due to the Department of Fisheries Malaysia trawl survey within the deeper EEZ waters off the coast of Borneo. This relatively newly described species is currently known to occur only off the continental waters of Australia and Indonesia [79].
The new finding of the bigeye thresher A. superciliosus in a major fisheries landing site in Hutan Melintang, along the central-west coast of Peninsula Malaysia, was rather surprising. This thresher species is thought to occur in deeper waters and generally less catchable than the tropical congener A. pelagicus [80], which had only been reported off the open waters of Sabah. The trawl fisheries in the landing site where A. supercilious was found typically operates in the relatively shallow Strait of Malacca. Further investigations into this finding will help shed light on the ecology of the thresher.
For the eagle ray M. hamlyni, existing molecular evidence suggested a best match with M. tobijei from South China Sea (0% genetic difference) and M. hamlyni from Indonesia (0.3% genetic distance). The latest distributional assessment on both species by White et al. [66] showed that M. tobijei is restricted to the western North Pacific, while M. hamlyni occurs in neighbouring Indonesia and the Philippines. Moreover, the morphological characteristic of the present specimen best matches the species description of M. hamlyni in White et al. [66], especially the purplish brown dorsal coloration (compared to yellowish brown in M. tobijei), as well as the slightly convex cranial fontanelle that is visible in the dorsal view (nearly straight and broader in M. tobijei). More importantly, the M. hamlyni specimen corresponded to the reference sequence used in the present analysis (EU398924), i.e., one of the type of specimens in White et al. [66]. This suggests that the reference sequence for M. tobijei KP267630 (published in December 2015) was likely an outdated species identity. Prior to the White et al. [66] redescription study, M. tobijei was thought to be distributed from Japan down south to Indonesia and the Philippines [66]. The field-identified U. cf. aurantiacus showed the highest genetic similarity to U. expansus found only in Southwestern Australia, but the known distribution of U. aurantiacus in the Philippines makes the latter species identity more feasible. The U. cf. aurantiacus specimen may therefore be an undescribed species, and the significance of this finding is that no urolophids are known to occur within Malaysia to date.
One of the most fundamental requirements in conservation and fishery management of sharks and batoids is correct species identification. While some advances have been made from our study, improvement in the records is still needed, including verification of the 40 recorded species of concern (recorded by single/multiple reference/s by the same research team or absent in Malaysia according to the IUCN assessment). The use of available genetic sequences without careful morphological examination and corroboration from existing distributional records would result in erroneous species assignment. The BOLD species assignment of Narcine cf. oculifera and Cephaloscyllium umbratile are inconclusive, as the percentage match with the present sequences (except sample 3-640) was less than 99%, and they had not been previously recorded in Malaysian waters. Despite the use of multiple approaches, our work highlights the need to focus on select taxonomic groups that may be species complexes or cryptic species. Sphyrna lewini (which was also pointed out by Naylor et al. [81]), the R. borneensis-schlegelli group, and the M. gerrardi-macrura group are among those requiring further investigations that are feasible due to the relatively common occurrence locally from our past surveys. On the other hand, taxonomic work to resolve unclear identities of uncommon species of S. altipinnis (2-041); C. sarawakense (2-013 and 3-064); N. maculata (4-439 and 2-874); and H. parvonigra (2-059, 2-448, 2-127, and T8) may be hampered without extensive field samplings.
The use of barcoding approaches is also fundamental in describing species that are still unknown to the scientific communities. This is important to prevent the loss of species before even being discovered, as seen in Carcharhinus obsolerus [82]. However, to advance the use of barcoding for accurate biodiversity monitoring, there remains the urgent need for the scientific names in the NCBI database to be updated regularly. One example is the revision of the family Dasyatidae that has resulted in the renaming of Himantura walga to Brevitrygon heterura (the revised B. walga is found to be confined to the Indian Ocean) [13]. Another example is the identity of reference sequence EU398924 as M. tobijei in NCBI GenBank, which likely should be M. hamlyni after the updated study of White et al. [66]. It is imperative to review the identity of all submitted sequences in NCBI GenBank to prevent future confusion, preferably through collaboration with reputable taxonomist with molecular knowledge to further enhance the validity of the submitted sequences. Moving forward, it is also essential to increase the amount of localized data on individual species, and the correct identification of species through traditional morphology and the uploading of correct sequences for the right species are highly essential to allow full functionality of barcoding technology for species identification.
5. Conclusions
The barcoding of animal tissues has proven to be an important technique to identify species from animal remains that cannot be identified by conventional morphological means and therefore cannot be adequately traced. Accurate species identification will improve species-specific catch landing data and ease and accelerate the identification of their illegal trade and use in Malaysia. Overall, this will provide baseline and monitoring data for conservation and fisheries management. The sharks and batoids CO1 database establishment and improvement will also help facilitate the rapid monitoring and assessment of elasmobranch fish through environmental DNA methods. In the future, rapid and large-scale surveys can be conducted to obtain the distribution points of cartilaginous fishes and predict their potential habitat areas, thereby providing a scientific basis and basis for the identification of elasmobranch conservation priority areas.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani13061002/s1: Table S1: Compilation of elasmobranch records in Malaysia waters, latest IUCN status, and COI sequence availability in NCBI GenBank. Summary represent inferences based on available records. Table S2: The 231 references sequences from NCBI GenBank were retrieved for phylogenetic tree reconstruction. Table S3: Data for the estimates of evolutionary divergence between sequences.
Author Contributions
Designed the study: K.-H.L., K.-C.L., A.Y.-H.T., S.A. and J.D. Performed the field work: K.-H.L., K.-C.L., A.J.-X.L., J.M. and S.A. Conducted the statistical analysis of the data: K.-H.L., K.-C.L. and A.J.-X.L. Conducted the molecular analyses: K.-H.L., K.-C.L. and A.J.-X.L. Captured morphological and meristic data: A.J.-X.L., K.-C.L. and S.A. Wrote the manuscript: K.-H.L., A.Y.-H.T. and K.-C.L. Have checked and revised the manuscript: W.H. and A.S. Funding acquisition: K.-H.L., J.D., C.W.B., A.W. and S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Third Institute of Oceanography, Ministry of Natural Resources, China, grant number IF004-2022. Financial support for the lead author K.-H.L. was provided by the WWF-Malaysia, grant numbers PV049-2019 and PV023-2022; funding for renting the facility instruments for the molecular analyses was provided. The APC was funded by the China-ASEAN Maritime Cooperation fund projects “Marine Protected Areas Network in China-ASEAN Countries” and “Monitoring and Conservation of the coastal ecosystem in the South China Sea”; National Natural Science foundation of China (No: 41961144022). Field sampling was supported by Universiti Malaya grants BK018-2015, RP018C-16SUSC, and IF030B-2019.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All sequence (n = 175) results are uploaded to GenBank, accession numbers: OQ384975–OQ385149.
Acknowledgments
The authors acknowledged Universiti Malaya (UM) and the Institute of Ocean and Earth Sciences (IOES), UM, for providing the necessary facilities. We are grateful to the Department of Fisheries Malaysia and Fisheries Research Institute (FRI) Bintawa, Sarawak, for the opportunity to participate in the fisheries-independent demersal trawl survey within the Malaysian Exclusive Economic Zone (EEZ). A Sabah sampling permit was provided by the Sabah Biodiversity Council (SaBC), Ref. No. JKM/MBS.1000-2/2 JLD.9 (22) and JKM/MBS. 1000-2/3 JLD. 4(18). We thank the anonymous reviewers for their comments that have significantly improved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahmad, A.; Lim, P.K.; Fahmi; Dharmadi; Krajangdara, T. Identification Guide to Sharks, Rays and Skates of the Southeast Asian Region; SEAFDEC/MFRDMD/SP/31; SEAFDEC/MFRDMD Institutional Repository: Kuala Terengganu, Malaysia, 2017; 33p. [Google Scholar]
- Canton, T. Catalogue of Malaysian fishes. J. R. Asiat. Soc. Bengal 1894, 18, 983–991. [Google Scholar]
- Mohsin, A.K.M.; Ambak, M.A. Marine Fishes and Fisheries of Malaysia and Neighbouring Countries; University Pertanian Malaysia Press: Serdang, Selangor, Malaysia, 1996; 744p. [Google Scholar]
- Yano, K.; Ahmad, A.; Gambang, A.C.; Idris, A.H.; Solahuddin, A.R.; Aznan, Z. Sharks and Rays of Malaysia and Brunei Darussalam; SEAFDEC-MFRDMD/SP/12; SEAFDEC/MFRDMD Institutional Repository: Kuala Terengganu, Malaysia, 2005. [Google Scholar]
- Ahmad, A.; Lim, A.P.K.; Abdul Rahman, M.; Raja Bindin, R.H.; Gambang, A.C.; Nor Azman, Z. Panduan mengenali spesies ikan Yu di Malaysia, Brunei Darussalam, Indonesia dan Thailand. Kuala Terengganu: DPPSPM/DOF; SEAFDEC/MFRDMD/SP/25; SEAFDEC/MFRDMD Institutional Repository: Kuala Terengganu, Malaysia, 2008; 294p. [Google Scholar]
- Last, P.R.; White, W.T.; Caira, J.N.; Jensen, K.; Lim, A.P.; Manjaji-Matsumoto, B.M.; Naylor, G.J.; Pogonoski, J.J.; Stevens, J.D.; Yearsley, G.K. Sharks and Rays of Borneo; CSIRO Publishing: Clayton, VIC, Australia, 2010; 298p. [Google Scholar]
- Ahmad, A.; Lim, A.P.K. Field Guide to Sharks of the Southeast Asian Region; SEAFDEC/MFRDMD/SP/18; SEAFDEC/MFRDMD Institutional Repository: Kuala Terengganu, Malaysia, 2012; 210p. [Google Scholar]
- Ahmad, A.; Lim, A.P.; Krajangdara, T. Field Guide to Rays, Sketes and Chimaeras of the Southeast Asian Region; SEAFDEC/MFRDMD/SP/25 (84 sp); SEAFDEC/MFRDMD Institutional Repository: Kuala Terengganu, Malaysia, 2014; 289p. [Google Scholar]
- Lim, K.C.; Lim, P.E.; Chong, V.C.; Loh, K.H. Molecular and morphological analyses reveal phylogenetic relationships of stingrays focusing on the family Dasyatidae (Myliobatiformes). PLoS ONE 2015, 10, e0120518. [Google Scholar]
- Arai, T.; Azri, A. Diversity, occurrence and conservation of sharks in the southern South China Sea. PLoS ONE 2019, 14, e0213864. [Google Scholar] [CrossRef] [PubMed]
- Booth, H.; Chaya, F.; Ng, S.; Tan, V.; Rao, M.; Teepol, B.; Matthews, E.; Lim, A.; Gumal, M. Elasmobranch fishing and trade in Sarawak, Malaysia, with implications for management. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 3056–3071. [Google Scholar] [CrossRef]
- White, W.; Last, P. A redescription of Carcharhinus dussumieri and C sealei, with resurrection of C. coatesi and C. tjutjot as valid species (Chondrichthyes: Carcharhinidae). Zootaxa 2012, 3241, 1–34. [Google Scholar] [CrossRef]
- Last, P.R.; Naylor, G.J.; Manjaji-Matsumoto, B.M. A revised classification of the family Dasyatidae (Chondrichthyes: Myliobatiformes) based on new morphological and molecular insights. Zootaxa 2016, 4139, 345–368. [Google Scholar] [CrossRef]
- Last, P.R.; White, W.T.; Naylor, G. Three new stingrays (Myliobatiformes: Dasyatidae) from the Indo-West Pacific. Zootaxa 2016, 4147, 377–402. [Google Scholar] [CrossRef]
- Borsa, P.; Arlyza, I.S.; Hoareau, T.B.; Shen, K.N. Diagnostic description and geographic distribution of four new cryptic species of the blue-spotted maskray species complex (Myliobatoidei: Dasyatidae; Neotrygon spp.) based on DNA sequences. J. Ocean. Limnol 2018, 36, 827–841. [Google Scholar] [CrossRef]
- Hasan, V.; Gausmann, P.; Nafisyah, A.L.; Isroni, W.; Widodo, M.S.; Islam, I.; Chaidir, R.R.A. First record of longnose marbled whipray Fluvitrygon oxyrhyncha (Sauvage, 1878) (Myliobatiformes: Dasyatidae) in Malaysian waters. Ecol. Montenegrina 2021, 40, 75–79. [Google Scholar] [CrossRef]
- Lim, K.C.; Then, A.Y. Environmental DNA approach complements social media reports to detect an endangered freshwater stingray species in the wild. Endang. Species Res. 2022, 48, 43–50. [Google Scholar] [CrossRef]
- Du, J.; Ding, L.; Su, S.; Hu, W.; Wang, Y.; Loh, K.H.; Yang, S.; Chen, M.; Roeroe, K.A.; Songploy, S.; et al. Setting conservation priorities for marine sharks in China and the Association of Southeast Asian Nations (ASEAN) seas: What are the benefits of 30% conservation target? Front. Mar. Sci. Mar. Ecosyst. Ecol. 2022, 9, 933291. [Google Scholar] [CrossRef]
- Bineesh, K.K.; Gopalakrishnan, A.; Akhilesh, K.V.; Sajeela, K.A.; Abdussamad, E.M.; Pillai, N.G.K.; Basheer, V.S.; Jena, J.K.; Ward, R.D. DNA barcoding reveals species composition of sharks and rays in the Indian commercial fishery. Mitochondrial DNA Part A 2017, 28, 458–472. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; Carlson, J.K.; Davidson, L.N.; Fordham, S.V.; Francis, M.P.; et al. Extinction risk and conservation of the world sharks and rays. eLife 2014, 3, e00590. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.; Bonfi, R.; Dulvy, N.; Walker, P. The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci. 2000, 57, 476–494. [Google Scholar] [CrossRef]
- Dent, F.; Clarke, S. State of the Global Market for Shark Products; FAO Fisheries and Aquaculture Technical Paper, No. 590; FAO: Rome, Italy, 2015; 187p. [Google Scholar]
- Department of Fisheries Malaysia (DOFM). 2018. Available online: https://www.dof.gov.my/en/resources/i-extension-en/annual-statistics/ (accessed on 1 January 2023).
- Department of Fisheries Malaysia (DOFM). 2021. Available online: https://www.dof.gov.my/en/resources/i-extension-en/annual-statistics/ (accessed on 5 January 2023).
- Pacoureau, N.; Rigby, C.L.; Kyne, P.M.; Sherley, R.B.; Winker, H.; Carlson, J.K.; Fordham, S.V.; Barreto, R.; Fernando, D.; Francis, M.P.; et al. Half a century of global decline in oceanic sharks and rays. Nature 2021, 589, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Department of Fisheries Malaysia (DOFM). Malaysia National Plan of Action for the Conservation and Management of Shark (Plan 2); Ministry of Agriculture and Agrobased Industry Malaysia: Putrajaya, Malaysia, 2014; 50p. [Google Scholar]
- Dulvy, N.K.; Pacoureau, N.; Rigby, C.L.; Pollom, R.A.; Jabado, R.W.; Ebert, D.A.; Finucci, B.; Pollock, C.M.; Cheok, J.; Derrick, D.H.; et al. Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Curr. Biol. 2021, 31, 4773–4787. [Google Scholar] [CrossRef]
- Stobutzki, I.C.; Miller, M.J.; Heales, D.S.; Brewer, D.T. Sustainability of elasmobranchs caught as bycatch in a tropical prawn (shrimp) trawl fishery. Fishery Bulletin. 2002, 100, 800–821. [Google Scholar]
- Mardhiah, U.; Booth, H.; Simeon, B.M.; Muttaqin, E.; Ichsan, M.; Prasetyo, A.P.; Yulianto, I. Quantifying vulnerability of sharks and rays species in Indonesia: Is biological knowledge sufficient enough for the assessment? IOP Conf. Ser. Earth Environ. Sci. 2019, 278, 012043. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Baum, J.K.; Clarke, S.; Compagno, L.J.; Cortés, E.; Domingo, A.; Fordham, S.; Fowler, S.; Francis, M.P.; Gibson, C.; et al. You can swim but you can’t hide: The global status and conservation of oceanic pelagic sharks. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 459–482. [Google Scholar] [CrossRef]
- Jabado, R.W.; Kyne, P.M.; Pollom, R.A.; Ebert, D.A.; Simpfendorfer, C.A.; Ralph, G.M.; Dhaheri, S.S.; Akholesh, K.V.; Ali, K.; Ali, M.H.; et al. Troubled waters: Threats and extinction risk of the sharks, rays and chimaeras of the Arabian Sea and adjacent waters. Fish Fish. 2018, 19, 1043–1062. [Google Scholar] [CrossRef]
- Tillett, B.J.; Field, I.C.; Bradshaw, C.J.A.; Johnson, G.; Buckworth, R.C.; Meekan, M.G.; Ovenden, J.R. Accuracy of species identification by fisheries observers in a north Australian shark fishery. Fish. Res. 2012, 127–128, 109–115. [Google Scholar] [CrossRef]
- Verissimo, A.; Cotton, C.F.; Buch, R.H.; Guallart, J.; Burgess, G.H. Species diversity of the deep-water gulper sharks (Squaliformes: Centrophoridae: Centrophorus) in North Atlantic waters–current status and taxonomic issues. Zool. J. Linn. Soc. 2014, 172, 803–830. [Google Scholar] [CrossRef]
- Akbar John, B.; Muhamad Asrul, M.A.; Mohd Arshaad, W.; Jalal, K.C.A.; Sheikh, H.I. DNA barcoding of rays from the South China Sea. In DNA Barcoding and Molecular Phylogeny; Springer: Cham, Switzerland, 2020; pp. 121–136. ISBN 978-3-030-50074-0. [Google Scholar]
- Lim, K.C.; White, W.T.; Then, A.Y.H.; Naylor, G.J.P.; Arunrugstichai, S.; Loh, K.H. Integrated Taxonomy Revealed Genetic Differences in Morphologically Similar and Non-Sympatric Scoliodon macrorhynchos and S. laticaudus. Animals 2022, 12, 681. [Google Scholar] [CrossRef] [PubMed]
- Cerutti-Pereyra, F.; Meekan, M.G.; Wei, N.-W.V.; O’Shea, O.R.; Bradshaw, C.J.A.; Austin, C.M. Identification of rays through DNA barcoding: An application for ecologists. PLoS ONE 2012, 7, e36479. [Google Scholar] [CrossRef]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. London. Ser. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Ratnasingham, S.; deWaard, J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, S96–S99. [Google Scholar] [CrossRef] [PubMed]
- Hubert, N.; Hanner, R.; Holm, E.; Mandrak, N.; Taylor, E.; Burridge, M.; Watkinson, D.; Dumont, P.; Curry, A.; Bentzen, P.; et al. Identifying Canadian Freshwater Fishes through DNA Barcodes. PLoS ONE 2008, 3, e2490. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D.N. DNA barcoding Australia’s fish species. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2005, 360, 1847–1857. [Google Scholar] [CrossRef]
- Shivji, M.S.; Clarke, S.; Pank, M.; Natanson, L.; Kohler, N.; Stanhope, M. Genetic identification of pelagic shark body parts for conservation and trade monitoring. Conserv. Biol. 2002, 16, 1036–1047. [Google Scholar] [CrossRef]
- Ward, R.D.; Holmes, B.H.; White, W.T.; Last, P.R. DNA barcoding Australasian chondrichthyans: Results and potential uses in conservation. Mar. Freshw. Res. 2008, 59, 57–71. [Google Scholar] [CrossRef]
- Holmes, B.H.; Steinke, D.; Ward, R.D. Identification of shark and ray fins using DNA barcoding. Fish. Res. 2009, 95, 280–288. [Google Scholar] [CrossRef]
- Cardeñosa, D.; Fields, A.; Abercrombie, D.; Feldheim, K.; Shea, S.K.H.; Chapman, D.D. A multiplex PCR mini-barcode assay to identify processed shark products in the global trade. PLoS ONE 2017, 12, e0185368. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, B.J.; Ip, Y.C.; Neo, M.L.; Chang, J.J.; Gan, C.Z.; Clark-Shen, N.; Huang, D.; Rao, M. DNA barcoding of traded shark fins, meat and mobulid gill plates in Singapore uncovers numerous threatened species. Conserv. Genet. 2018, 19, 1393–1399. [Google Scholar] [CrossRef]
- Asbury, T.A.; Bennett, R.; Price, A.S.; da Silva, C.; Burgener, M.; Klein, J.D.; Maduna, S.N.; Sidat, N.; Fernando, S.; Bester-Van der Merwe, A.E. Application of DNA mini-barcoding reveals illegal trade in endangered shark products in southern Africa. Afr. J. Mar. Sci. 2021, 43, 511–520. [Google Scholar] [CrossRef]
- Choy, C.P.P.; Wainwright, B.J. What Is in Your Shark Fin Soup? Probably an endangered shark species and a bit of mercury. Animals 2022, 12, 802. [Google Scholar] [CrossRef]
- Zeng, Y.; Wu, Z.; Zhang, C.; Meng, Z.; Jiang, Z.; Zhang, J. DNA barcoding of mobulid ray gill rakers for implementing CITES on elasmobranch in China. Sci. Rep. 2016, 6, 37567. [Google Scholar] [CrossRef]
- Steinke, D.; Bernard, A.M.; Horn, R.L.; Hilton, P.; Hanner, R.; Shivji, M.S. DNA analysis of traded shark fins and mobulid gill plates reveals a high proportion of species of conservation concern. Sci. Rep. 2017, 7, 9505. [Google Scholar] [CrossRef] [PubMed]
- Asis, A.M.J.M.; Lacsamana, J.K.M.; Santos, M.D. Illegal trade of regulated and protected aquatic species in the Philippines detected by DNA barcoding. Mitochondrial DNA Part A 2016, 27, 659–666. [Google Scholar] [CrossRef]
- Sembiring, A.; Pertiwi, N.P.D.; Mahardini, A.; Wulandari, R.; Kurniasih, E.M.; Kuncoro, A.W.; Cahyani, N.K.D.; Anggoro, A.W.; Ulfa, M.; Madduppa, H.; et al. DNA barcoding reveals targeted fisheries for endangered sharks in Indonesia. Fish. Res. 2015, 164, 130–134. [Google Scholar] [CrossRef]
- Pavan-Kumar, A.; Gireesh-Babu, P.; Suresh Babu, P.P.; Jaiswar, A.K.; Prasad, K.P.; Chaudhari, A.; Raje, S.G.; Chakraborty, S.K.; Krishna, G.; Lakra, W.S. DNA barcoding of elasmobranchs from Indian Coast and its reliability in delineating geographically widespread specimens. Mitochondrial DNA 2015, 26, 92–100. [Google Scholar] [CrossRef]
- Bineesh, K.K.; Akhilesh, K.V.; Sajeela, K.A.; Abdussamad, E.M.; Gopalakrishnan, A.; Basheer, V.S.; Jena, J.K. DNA Barcoding Confirms the Occurrence Rare Elasmobranchs in the Arabian Sea of Indian EEZ. Middle East. J. Sci. Res. 2014, 19, 1266–1271. [Google Scholar] [CrossRef]
- Haque, A.B.; Das, S.A.; Biswas, A.R. DNA analysis of elasmobranch products originating from Bangladesh reveals unregulated elasmobranch fishery and trade on species of global conservation concern. PLoS ONE 2019, 14, e0222273. [Google Scholar] [CrossRef] [PubMed]
- Kuguru, G.; Maduna, S.; da Silva, C.; Gennari, E.; Rhode, C.; Bester-van der Merwe, A. DNA barcoding of chondrichthyans in South African fisheries. Fish. Res. 2018, 206, 292–295. [Google Scholar] [CrossRef]
- Hobbs, C.A.D.; Potts, R.W.A.; Walsh, M.B.; Usher, J.; Griffiths, A.M. Using DNA Barcoding to investigate patterns of species utilisation in UK shark products reveals threatened species on sale. Sci. Rep. 2019, 9, 1028. [Google Scholar] [CrossRef]
- Cariani, A.; Messinetti, S.; Ferrari, A.; Arculeo, M.; Bonello, J.J.; Bonnici, L.; Cannas, R.; Carbonara, P.; Cau, A.; Charilaou, C.; et al. Improving the conservation of Mediterranean chondrichthyans: The Elasmomed DNA barcode reference library. PLoS ONE 2017, 12, e0170244. [Google Scholar] [CrossRef] [PubMed]
- Serra-Pereira, B.; Moura, T.; Griffiths, A.M.; Gordo, L.S.; Figueiredo, I. Molecular barcoding of skates (Chondrichthyes: Rajidae) from the southern northeast Atlantic. Zool. Scr. 2011, 40, 76–84. [Google Scholar] [CrossRef]
- Almerón-Souza, F.; Sperb, C.; Castilho, C.L.; Figueiredo, P.I.; Gonçalves, L.T.; Machado, R.; Oliveira, L.R.; Valiati, V.H.; Fagundes, N.J. Molecular identification of shark meat from local markets in Southern Brazil based on DNA barcoding: Evidence for mislabeling and trade of endangered species. Front. Genet. 2018, 9, 138. [Google Scholar] [CrossRef]
- Feitosa, L.M.; Martins, A.P.; Giarrizzo, T.; Macedo, W.; Monteiro, I.L.; Gemaque, R.; Nunes, J.L.; Gomes, F.; Schneider, H.; Sampaio, I.; et al. DNA-based identification reveals illegal trade of threatened shark species in a global elasmobranch conservation hotspot. Sci. Rep. 2018, 8, 3347. [Google Scholar] [CrossRef]
- Bernardo, C.; de Lima Adachi, A.M.C.; da Cruz, V.P.; Foresti, F.; Loose, R.H.; Bornatowski, H. The label “Cação” is a shark or a ray and can be a threatened species! Elasmobranch trade in southern Brazil unveiled by DNA barcoding. Mar. Policy 2020, 116, 103920. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data System (www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Last, P.R.; White, T.W.; Carvalho, M.R.; Seret, B.; Stehmann, N.F.W.; Naylor, G.J.P. Rays of the World; CSIRO Publishing: Melbourne, Australia, 2016. [Google Scholar]
- Carpenter, K.E.; Niem, V.H. FAO species identification guide for fishery purposes. In The Living Marine Resources of the Western Central Pacific; Cephalopods, crustaceans, holothurians and sharks; FAO: Rome, Italy, 1998; Volume 2, pp. 687–1396. [Google Scholar]
- Carpenter, K.E.; Niem, V.H. FAO species identification guide for fishery purposes. In The living marine resources of the Western Central Pacific; Batoid fishes, chimaeras and bony fishes part 1 (Elopidae to Linophrynidae); FAO: Rome, Italy, 1999; Volume 3, pp. 1397–2068. [Google Scholar]
- White, W.T.; Kawauchi, J.; Corrigan, S.; Rochel, E.; Naylor, G.J. Redescription of the eagle rays Myliobatis hamlyni Ogilby, 1911 and M. tobijei Bleeker, 1854 (Myliobatiformes: Myliobatidae) from the East Indo-West Pacific. Zootaxa 2015, 3948, 521–548. [Google Scholar] [CrossRef]
- Hyde, J.; Lynn, E.; Humphreys, R., Jr.; Musyl, M.; West, A.; Vetter, R. Shipboard identification of fish eggs and larvae by multiplex PCR, and description of fertilized eggs of blue marlin, shortbill spearfish, and wahoo. Mar. Ecol. Prog. Ser. 2005, 286, 269–277. [Google Scholar] [CrossRef]
- Rigby, C.; Appleyard, S.; Chin, A.; Heupel, M.; Humber, F.; Simpfendorfer, C.; White, W.; Campbell, I. Rapid Assessment Toolkit for Sharks and Rays; WWF International and CSTFA, James Cook University: Townsville, Australia, 2019. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids. Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Fricke, R.; Eschmeyer, W.N.; Van der Laan, R. (Eds.) Eschmeyer’s Catalog of Fishes: Genera, Species, References. 2022. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 2 February 2022).
- IUCN. The IUCN Red List of Threatened Species. 2022. Version 2022-2. Available online: https://www.iucnredlist.org (accessed on 5 February 2023).
- StatSoft, Inc. STATISTICA (Data Analysis Software System). 2007. Version 7.0. Available online: https://www.statsoft.com (accessed on 5 February 2023).
- Borsa, P.; Shen, K.N.; Arlyza, I.S.; Hoareau, T.B. Multiple cryptic species in the blue-spotted maskray (Myliobatoidei: Dasyatidae: Neotrygon spp.): An update. Comptes Rendus Biol. 2016, 339, 9–10, 417–426. [Google Scholar] [CrossRef]
- Valentini, A.; Taberlet, P.; Miaud, C.; Civade, R.; Herder, J.; Thomsen, P.F.; Bellemain, E.; Besnard, A.; Coissac, E.; Boyer, F.; et al. Next-generation monitoring of aquatic biodiversity using environmental DNA metabarcoding. Mol. Ecol. 2016, 25, 929–942. [Google Scholar] [CrossRef]
- Last, P.R.; White, W.T.; Séret, B. Taxonomic status of maskrays of the Neotrygon kuhlii species complex (Myliobatidei: Dasyatidae) with the description of three new species from the Indo-West Pacific. Zootaxa 2016, 4083, 533–561. [Google Scholar] [CrossRef]
- White, W.T.; Last, P.R.; Stevens, J.D. Part 7—Two new species of Squalus of the mitsukurii group from the Indo-Pacific. CSIRO Mar. Atmos. Res. Pap. 2007, 14, 71–81. [Google Scholar]
- Spaet, J.L.; Cochran, J.E.; Berumen, M.L. First record of the pigeye shark, Carcharhinus amboinensis (Müller & Henle, 1839) (Carcharhiniformes: Carcharhinidae), in the Red Sea. Zool. Middle East 2011, 52, 118–121. [Google Scholar]
- Finucci, B.; White, W.T. Squalus edmundsi. The IUCN Red List of Threatened Species 2019: E.T158617A68644689. Available online: https://www.iucnredlist.org/species/158617/68644689 (accessed on 5 February 2023).
- Rigby, C.L.; Barreto, R.; Carlson, J.; Fernando, D.; Fordham, S.; Francis, M.P.; Herman, K.; Jabado, R.W.; Liu, K.M.; Marshall, A.; et al. Alopias superciliosus. The IUCN Red List of Threatened Species 2019: E.T161696A894216. Available online: https://www.iucnredlist.org/species/161696/894216 (accessed on 5 February 2023).
- Naylor, G.J.P.; Caira, J.N.; Jensen, K.; Rosana, K.A.M.; White, W.T.; Last, P.R. A DNA sequence–based approach to the identification of shark and ray species and its implications for global elasmobranch diversity and parasitology. Bull. Am. Mus. Nat. Hist. 2012, 367, 1–262. [Google Scholar] [CrossRef]
- White, W.T.; Kyne, P.M.; Harris, M. Lost before found: A new species of whaler shark Carcharhinus obsolerus from the Western Central Pacific known only from historic records. PLoS ONE 2019, 14, e0209387. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).