A Descriptive Methodology for Studying the Ontogeny of Object Play and Breed Differences in Dogs (Canis lupus familiaris)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Stimuli
2.3. Procedure
2.4. Behavioral Analysis
| Behavior | Definitions |
|---|---|
| Agonistic Behavior | Behaviors seen: snapping; biting; piloerection; agonistic pucker; growling; or rushing another puppy (see Table 2 for more detail). |
| Solitary Behaviors (SOL) | |
| Bite (SOL) | Closing jaw with mouth or teeth (pups can exhibit this behavior before they have teeth) on an object and then quickly releasing. |
| Carry (SOL) | Picking up and holding an object with teeth and moving greater than 2 steps before depositing the object; the object does not touch the substrate in transit (compared to drag). |
| Chew (SOL) | Repeatedly manipulating an object with mouth or teeth. Using teeth may include biting (usually with incisors and canine teeth) or gnawing (using the molar teeth) the object; may involve ingesting pieces of the object. |
| Dig (SOL) | Rapid extension and flexion of alternating forelimbs on an object or pen substrate adjacent to the object. |
| Drag (SOL) | Picking up an object with mouth and moving greater than 2 steps away before depositing the object; portion of the object remains in contact with substrate in transit. |
| Grab (SOL) | Closing mouth or teeth on an object for at least 2 s while remaining stationary or moving no more than 1 full step. |
| Hold Object (SOL) | Possessing an object either in mouth or between forepaws while stationary. |
| Lie on Object (SOL) | Placing body over an object; usually pup rolls on top of the object. |
| Lick (SOL) | Extruding tongue from mouth and passing over an object. |
| Nose (SOL) | Touching an object with nose. Modifiers: Nudge: moving an object with nose. Touch/sniff: touching nose to an object without resulting in movement of the object (may include sniffing the object). |
| Pickup and Drop (SOL) | Repeatedly picking up and immediately dropping object. |
| Tear (SOL) | Grabbing an object with teeth and pulling it with force; usually the object is held down by forepaws as teeth and head pulls up. |
| Toss (SOL) | Flexing neck down and then rapidly extending neck while releasing the object held in mouth. |
| Tug-pull (SOL) | Grabbing with teeth and pulling back on a non-movable object or another puppy that does not tug in return; often accompanied by growling. The other puppy may let go immediately or hold onto the object for a few seconds before releasing it but does not tug back. (Previously called pull-tug [27].) |
| Behaviors occurring in both contexts (BOTH) | |
| Approach (BOTH) | Moving from one area in the enclosure towards an object or puppy in another area intentionally, i.e., the pup is clearly headed toward the object or pup and does not merely run into the object or pup. |
| Approach-retreat (BOTH) | Repeatedly stepping or rocking toward and then away from the object or puppy with an object. |
| Avoid (BOTH) | Moving body or head away from object or another puppy; may be as subtle as looking away or turning the head away, or may include turning body and crawling, walking, or trotting away from the object and/or pup. |
| Exaggerated Approach (BOTH) | Moving toward an object, puppy with an object, or approaching another puppy while carrying an object with a bouncy gait at a speed greater than a walk, often with side-to-side motion movement of head and shoulders that is exaggerated from normal approach. |
| Grab-headshake (BOTH) | Grabbing an object with mouth or teeth, followed by rapid side-to-side movement of head with an object in mouth. This often includes growling. Pups less than 5 weeks display uncoordinated head and body shake. At approximately 5 weeks, the movement becomes more limited to the head and varies in speed and intensity, often seen during tug-of-war. Other names used for this behavior in adult wolves include bite shake [50] and headshake [51], and it is labelled bite-shake in our previous dog paper [27]. |
| Paw (BOTH) | Extending forepaw toward an object with or without making contact with the object. Modifiers: No-contact: extending paw toward the object without touching it. Smack: extending and pressing down on the object with paw. Touch: extending paw and lightly touching the object with paw. Bring-in: Extending and flexing paw on the object to rake the object toward body. |
| Play Bow (BOTH) | Dropping elbows to substrate with hind limbs remaining in an upright position; may include side-to-side movement. |
| Pounce (BOTH) | Rapidly jumping towards another pup or an object with only front limbs leaving the ground. |
| Stand Over (BOTH) | Standing over an object or another puppy lying down with an object. |
| Social Behaviors (SOC) | |
| Chase (SOC) | Moving faster than walk in pursuit of another puppy that is in possession of an object. |
| Guard (SOC) | Standing over an object when approached by another puppy; may be accompanied by lunging at the other puppy, piloerection, or other agonistic behaviours. |
| Keep Away (SOC) | Carrying an object and turning head or body away from an approaching pup; gait is usually faster than a walk and is followed by chase from the other pup. |
| Leap (SOC) | Jumping with all four feet off the ground towards another puppy with an object. |
| Paw Face (SOC) | To extend or wave the paw to touch another puppy in the face that has an object. Modifier: Paw no-contact/paw touch/paw smack (see above) |
| Tackle (SOC) | Jumping on or running into another pup with an object, usually knocking other pup over; may occur during a keep-away/chase episode. |
| Tug-of-war (SOC) | Two or more pups grasping an object in mouth and pulling back in opposite directions; weight is shifted to back limbs; may growl, grab-headshake, or paw at the other pup; bout ends with at least one pup dropping the object. |
| Behavior | Definition |
|---|---|
| |
| Bark | Short, loud, and relatively high-pitched vocalization with abrupt onset; frequency modulation often has both tonality and noise and is subject to rapid repetition. |
| Growl | A throaty, low-frequency rumbling. |
| Whimper | Emitting repeated (approximately 2–3 times per second) high-pitched monotone vocalization on exhalation. |
| Whine | Emitting repeated (usually longer than 0.5 s) vocalization falling pitch. |
| |
| Agonistic Pucker | Vertically retracting the lips with either the corners of the mouth forward, showing canines and incisors only (offensive) or corners of mouth drawn back exposing pre-molars (defensive). |
| Growl | A throaty rumbling vocalization that is usually low pitched and can be used defensively or offensively in aggressive interactions. |
| Hackles | Piloerection of the fur along the spine. Hackles can be scruff/withers (H1), back (H2), rump (H3), and tail (H4). Piloerection increased with arousal usually in sequential order ranging from H1 to all 4 hackles (H1234) depending on arousal level. Nonsequential combination of hackles also occurred such as H13. |
| Lunge | A direct rapid approach at another puppy that is close to the object, usually with an agonistic pucker, snap, and sometimes a bite. The movement may take the form of a jump or a few running steps. |
| Snap | A rapid bite. When the jaws come together, the teeth make an audible sound. This is often an air snap where the pup does not make contact with the other pup but could include contact with the other pup. |
| Rush | A short run directed at another puppy. |
| Yelp | A quick sharp shrill bark or cry. |
2.5. Ethogram Development
2.6. Object Play Contexts
Statistical Analysis
3. Results
3.1. Behavioral Repertoire
3.1.1. Basic Ethogram
3.1.2. Behavior Observations
3.1.3. Behaviors Excluded or Combined for Statistical Analysis
Agonistic Behavior
3.1.4. Ontogenetic Changes in Play Behaviors
3.2. Play Context
3.2.1. Overall Interaction
3.2.2. Behavior Development by Breed
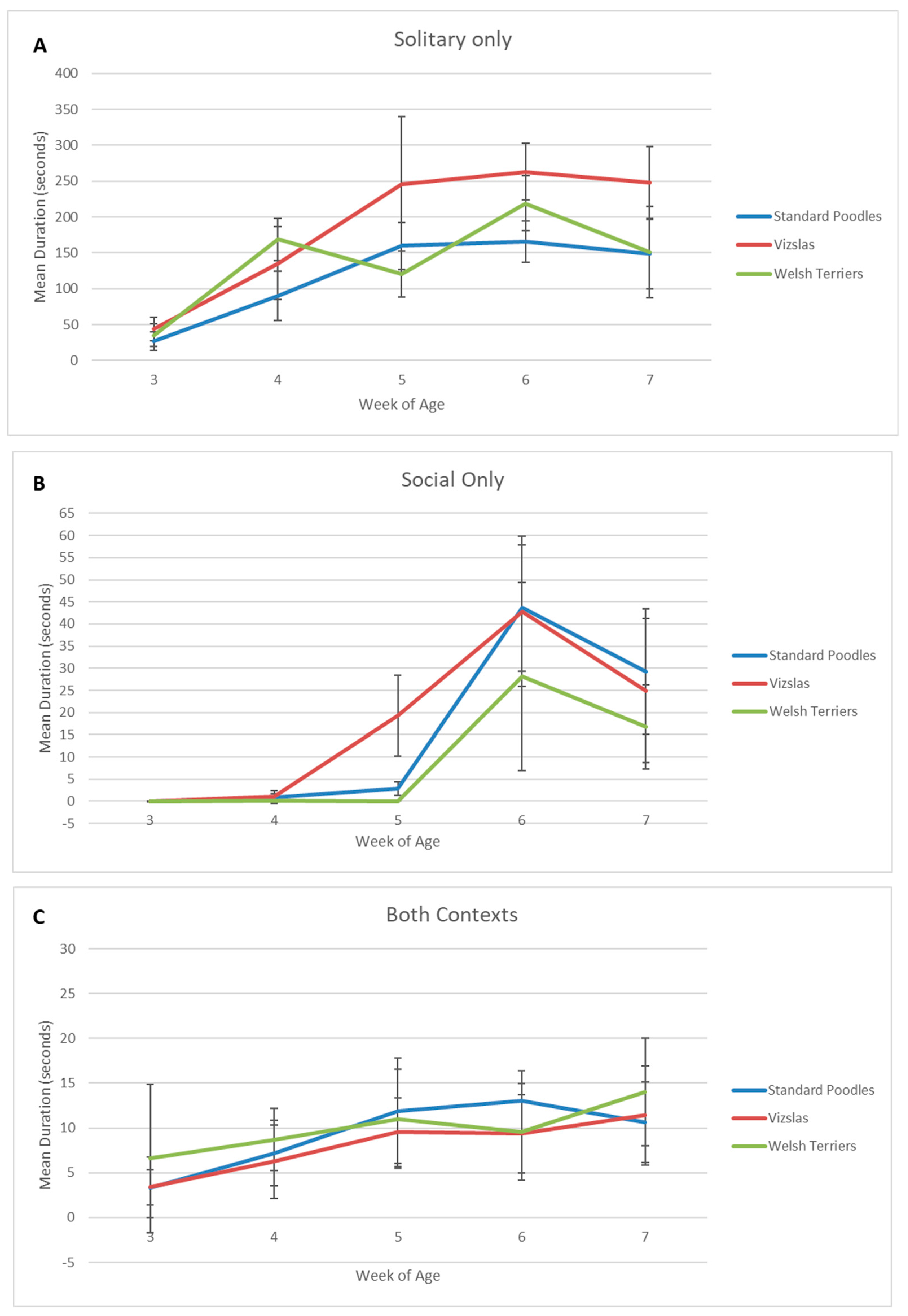
Solitary
Social
Both
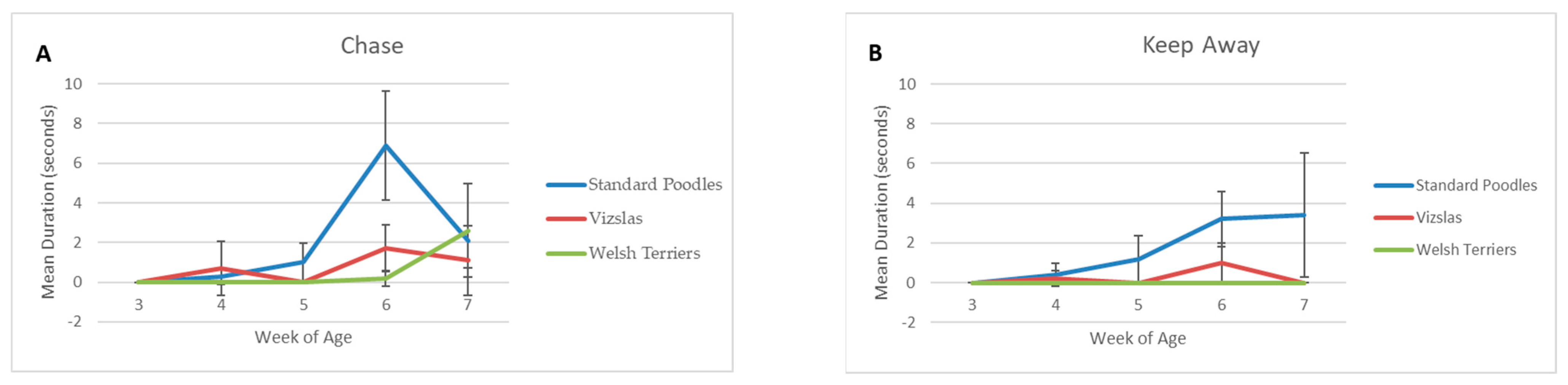
3.2.3. Individual Behaviors within Each Group
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Observer Pairs | Behaviors | Duration | Duration Sequence |
|---|---|---|---|
| GSS/AMS | 0.75 | 0.94 | 0.96 |
| CW/GSS | 0.82 | 0.96 | 0.97 |
| AMS/TA | 0.83 | 0.94 | 0.96 |
| TA/TV | 0.76 | 0.94 | 0.96 |
| TA/GSS | 0.72 | 0.95 | 0.96 |
| CW/TV | 0.83 | 0.92 | 0.95 |
| TV/GSS | 0.74 | 0.95 | 0.97 |
| AMS/TV | 0.91 | 0.96 | 0.96 |
| CW/TA | 0.83 | 0.95 | 0.97 |
| Overall Kappa | 0.80 | 0.94 | 0.96 |
| SOL Behaviors | Age (in Weeks) | Standard Poodles | Vizslas | Welsh Terriers | |||
|---|---|---|---|---|---|---|---|
| Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation | ||
| Pickup and Drop | 3 | 0 | 0 | 0.2 | 0.7 | 0 | 0 |
| 4 | 0.4 | 1.3 | 0 | 0 | 0 | 0 | |
| 5 | 0.7 | 1.2 | 0 | 0 | 0 | 0 | |
| 6 | 1.8 | 4.1 | 0.6 | 1.1 | 0.2 | 0.6 | |
| 7 | 0.2 | 0.6 | 0.7 | 1.3 | 0.1 | 0.5 | |
| Bite | 3 | 0 | 0 | 0.8 | 0.9 | 2.5 | 4.8 |
| 4 | 5.1 | 5 | 0.8 | 1.5 | 5 | 6 | |
| 5 | 5.3 | 4.1 | 4.2 | 6.2 | 2.8 | 2.4 | |
| 6 | 6.3 | 5.8 | 9.7 | 10.8 | 5 | 4.9 | |
| 7 | 5 | 7.1 | 1.6 | 3 | 14.2 | 22.5 | |
| Carry | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0.9 | 2.8 | 3 | 4.5 | 0.2 | 0.5 | |
| 5 | 2.7 | 4.2 | 7.6 | 8.8 | 0.5 | 0.9 | |
| 6 | 7.9 | 7.2 | 5.6 | 6 | 0.8 | 0.9 | |
| 7 | 6.9 | 10.7 | 7.2 | 15 | 1.2 | 2.2 | |
| Chew | 3 | 4.9 | 7.1 | 5.3 | 6.2 | 4.5 | 8.6 |
| 4 | 48.1 | 59.8 | 63.2 | 57.7 | 71.1 | 57.6 | |
| 5 | 84.5 | 52.4 | 176.2 | 144.4 | 70.5 | 43.8 | |
| 6 | 92.8 | 55.1 | 182.4 | 91.7 | 163.6 | 59.3 | |
| 7 | 96.1 | 85.6 | 202.4 | 87.4 | 91.5 | 102 | |
| Drag | 3 | 0 | 0.2 | 0 | 0 | 0 | 0 |
| 4 | 2.2 | 4.1 | 0.5 | 1.1 | 4 | 8.3 | |
| 5 | 4.8 | 6.1 | 1 | 2.5 | 2.5 | 3.5 | |
| 6 | 7.9 | 6 | 1.9 | 3.6 | 3.5 | 3.9 | |
| 7 | 0.9 | 1.7 | 2.4 | 3.1 | 4.6 | 6.9 | |
| Grab | 3 | 0 | 0 | 0.2 | 0.9 | 0.5 | 1.5 |
| 4 | 1 | 1.9 | 2.4 | 3.6 | 3.7 | 5.3 | |
| 5 | 4.2 | 4.9 | 4.2 | 5.3 | 3.1 | 2.4 | |
| 6 | 12 | 7.4 | 12.6 | 9 | 3.9 | 2.7 | |
| 7 | 2.4 | 3.5 | 4 | 4 | 6.5 | 7.1 | |
| Hold Object | 3 | 0 | 0 | 0.2 | 0.7 | 0.9 | 2.6 |
| 4 | 0.7 | 1.3 | 2 | 4.4 | 5.4 | 9.2 | |
| 5 | 6.4 | 6.6 | 2 | 6.1 | 6.4 | 4.9 | |
| 6 | 7.3 | 8.7 | 16.5 | 16.1 | 5.4 | 11 | |
| 7 | 1.6 | 3.5 | 8.4 | 11.1 | 5.7 | 6.5 | |
| Lick | 3 | 2.1 | 4.4 | 2 | 5.4 | 6.9 | 12.6 |
| 4 | 2 | 1.7 | 0.8 | 2 | 4.5 | 6.8 | |
| 5 | 1.7 | 2.8 | 0 | 0 | 4.8 | 7.7 | |
| 6 | 1 | 1.8 | 0.8 | 1.1 | 1.2 | 1.9 | |
| 7 | 0.4 | 1.1 | 1.2 | 1.9 | 0.8 | 2.2 | |
| Nose | 3 | 20.3 | 23.6 | 34.9 | 23.6 | 19.7 | 10.4 |
| 4 | 27.6 | 18.1 | 61.2 | 49 | 67.7 | 21.1 | |
| 5 | 39.7 | 60.5 | 32 | 35.9 | 21.1 | 19.6 | |
| 6 | 19.3 | 10.4 | 17.3 | 8.7 | 23.2 | 9.7 | |
| 7 | 5.2 | 5.1 | 10.3 | 7.5 | 16.5 | 12.7 | |
| Pounce | 3 | 0 | 0 | 0.2 | 0.3 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0.1 | 0.3 | |
| 5 | 0.5 | 1.6 | 0.3 | 0.6 | 0.1 | 0.2 | |
| 6 | 2.5 | 8.2 | 0.1 | 0.2 | 0 | 0.1 | |
| 7 | 0.1 | 0.5 | 0.1 | 0.3 | 0 | 0 | |
| Tear | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 1.9 | 4.7 | 1.2 | 2.5 | 6.7 | 8.8 | |
| 5 | 3.8 | 8.7 | 18.3 | 45.1 | 8.4 | 10.1 | |
| 6 | 2.2 | 3.7 | 11.8 | 13.5 | 7.4 | 17.4 | |
| 7 | 1.6 | 4.7 | 4.9 | 8.7 | 5.4 | 15.2 | |
| Tug-pull | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0.1 | 0.6 | 0.5 | 1.7 | 0.2 | 0.7 | |
| 5 | 5.4 | 6.9 | 0 | 0 | 0.1 | 0.4 | |
| 6 | 4.5 | 4.4 | 3.9 | 5.4 | 3.2 | 6.6 | |
| 7 | 1.9 | 2.7 | 4.5 | 7.4 | 2.2 | 4 | |
| SOC Behaviors | Age (in Weeks) | Standard Poodles | Vizslas | Welsh Terriers | |||
|---|---|---|---|---|---|---|---|
| Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation | ||
| Chase | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0.3 | 1 | 0.7 | 2.7 | 0 | 0 | |
| 5 | 1 | 2 | 0 | 0.2 | 0 | 0 | |
| 6 | 6.9 | 5.6 | 1.7 | 2.1 | 0.2 | 0.5 | |
| 7 | 2.1 | 3 | 1.1 | 3.3 | 2.6 | 4 | |
| Keep Away | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0.4 | 1.1 | 0.2 | 0.8 | 0 | 0 | |
| 5 | 1.2 | 2.4 | 0 | 0 | 0 | 0 | |
| 6 | 3.2 | 2.9 | 1 | 2 | 0 | 0 | |
| 7 | 3.4 | 6.5 | 0 | 0 | 0 | 0 | |
| Leap | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 1.2 | 0.4 | |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 6 | 0 | 0 | 0 | 0 | 1.9 | 1.0 | |
| 7 | 0 | 0 | 0 | 0 | 0.9 | 0.4 | |
| Paw Face | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0.1 | 0.3 | |
| 5 | 0 | 0 | 0 | 0.1 | 0 | 0 | |
| 6 | 0.3 | 1.1 | 0.7 | 1.6 | 0 | 0 | |
| 7 | 0 | 0 | 1.5 | 2.6 | 0 | 0 | |
| Tug-of-war | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0.2 | 0.7 | 0.1 | 0.4 | 0 | 0 | |
| 5 | 0.7 | 1.4 | 18.9 | 17.8 | 0 | 0 | |
| 6 | 33.2 | 28.4 | 39.4 | 32.3 | 27.7 | 35.5 | |
| 7 | 18.6 | 21.9 | 22.4 | 28.8 | 14 | 15 | |
| BOTH Behaviors | Age (in Weeks) | Standard Poodles | Vizslas | Welsh Terriers | |||
|---|---|---|---|---|---|---|---|
| Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation | ||
| Approach | 3 | 2.4 | 6.7 | 1.1 | 1.6 | 0.3 | 1 |
| 4 | 0.2 | 0.4 | 1 | 2.7 | 1 | 2 | |
| 5 | 1.3 | 1.6 | 0.9 | 1.2 | 0.2 | 0.3 | |
| 6 | 0.9 | 0.6 | 1.1 | 1.2 | 0.4 | 1.1 | |
| 7 | 0.9 | 1.7 | 0.4 | 0.5 | 2.9 | 5.1 | |
| Approach-exaggerated | 3 | 0 | 0 | 0.3 | 0.7 | 0.7 | 2.2 |
| 4 | 0.2 | 0.5 | 0 | 0 | 0 | 0 | |
| 5 | 0.9 | 2.2 | 0.1 | 0.2 | 0.8 | 2.2 | |
| 6 | 0.7 | 0.9 | 0.9 | 3.4 | 0 | 0.1 | |
| 7 | 0.9 | 2.2 | 0 | 0 | 0.4 | 1.3 | |
| Grab-headshake | 3 | 0 | 0 | 0.2 | 0.8 | 0 | 0 |
| 4 | 1.2 | 2.6 | 3.7 | 7.3 | 2.5 | 4.3 | |
| 5 | 1.2 | 1.9 | 4.3 | 5.9 | 4.3 | 4 | |
| 6 | 4.1 | 5 | 4.2 | 3.2 | 5.5 | 5.3 | |
| 7 | 4.2 | 6.1 | 3.5 | 5.3 | 6.3 | 7 | |
| Paw | 3 | 1 | 2.5 | 1.6 | 2 | 5.6 | 14.3 |
| 4 | 5.5 | 5.5 | 1.4 | 2 | 4.7 | 4.2 | |
| 5 | 8.3 | 10.4 | 3.8 | 2.8 | 5.5 | 5.6 | |
| 6 | 6.8 | 4.7 | 3.1 | 4.6 | 3.7 | 5.6 | |
| 7 | 2.4 | 4.7 | 7.5 | 8.2 | 4.4 | 7.3 | |
| Play bow | 3 | 0 | 0 | 0.2 | 0.3 | 0 | 0 |
| 4 | 0.1 | 0.4 | 0.1 | 0.4 | 0.5 | 1.6 | |
| 5 | 0.2 | 0.5 | 0.5 | 1.6 | 0.2 | 0.4 | |
| 6 | 0.3 | 0.8 | 0.1 | 0.3 | 0 | 0 | |
| 7 | 0.2 | 1 | 0.1 | 0.2 | 0 | 0 | |
| Behavior | Age in Weeks (Pup N = 42 Weeks 3–6.5 and N = 41 Week 7) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3.0 | 3.5 | 4.0 | 4.5 | 5.0 | 5.5 | 6.0 | 6.5 | 7.0 | ||
| Agonistic | Count | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Duration | 0.8 | 0.0 | 0.0 | 0.0 | 9.7 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Approach | Count | 23 | 26 | 8 | 25 | 33 | 29 | 32 | 66 | 50 |
| Duration | 25.5 | 93.2 | 5.1 | 52.7 | 20.4 | 49.4 | 24.0 | 46.0 | 53.0 | |
| Approach-retreat | Count | 1 | 2 | 0 | 2 | 5 | 0 | 1 | 10 | 2 |
| Duration | 0.3 | 14.4 | 0.0 | 2.1 | 15.4 | 0.0 | 2.0 | 22.9 | 3.5 | |
| Avoid | Count | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Duration | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 3.6 | 0.0 | |
| Bite | Count | 11 | 11 | 35 | 55 | 77 | 66 | 74 | 125 | 88 |
| Duration | 18.5 | 58.6 | 134.2 | 168.7 | 166.9 | 189.2 | 222.6 | 374.8 | 264.1 | |
| Carry | Count | 0 | 0 | 2 | 19 | 45 | 37 | 59 | 80 | 60 |
| Duration | 0.0 | 0.0 | 11.1 | 109.8 | 192.4 | 137.8 | 195.6 | 248.6 | 231.9 | |
| Chase | Count | 0 | 0 | 1 | 3 | 8 | 4 | 59 | 34 | 27 |
| Duration | 0.0 | 0.0 | 7.9 | 22.7 | 26.9 | 15.4 | 193.1 | 92.9 | 78.8 | |
| Chew | Count | 21 | 49 | 119 | 303 | 444 | 457 | 560 | 584 | 530 |
| Duration | 149.8 | 265.3 | 1548.5 | 3423.1 | 4506.5 | 5393.2 | 6371.0 | 5491.9 | 5473.2 | |
| Dig | Count | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 1 | 2 |
| Duration | 0.0 | 0.0 | 6.0 | 0.0 | 7.8 | 2.1 | 0.0 | 2.2 | 8.0 | |
| Drag | Count | 1 | 0 | 4 | 26 | 50 | 19 | 61 | 43 | 32 |
| Duration | 1.3 | 0.0 | 22.2 | 154.0 | 171.6 | 81.6 | 269.1 | 132.3 | 98.7 | |
| Exaggerated Approach | Count | 0 | 3 | 0 | 2 | 12 | 4 | 8 | 5 | 12 |
| Duration | 0.0 | 7.8 | 0.0 | 3.0 | 27.2 | 7.2 | 17.4 | 8.1 | 16.3 | |
| Grab | Count | 1 | 3 | 15 | 39 | 73 | 46 | 118 | 196 | 83 |
| Duration | 1.0 | 15.8 | 50.3 | 131.9 | 160.5 | 136.3 | 346.4 | 497.8 | 168.5 | |
| Grab-headshake | Count | 0 | 4 | 17 | 46 | 89 | 53 | 67 | 95 | 71 |
| Duration | 0.0 | 5.7 | 42.3 | 158.5 | 186.1 | 108.2 | 176.9 | 201.2 | 190.9 | |
| Hold Object | Count | 0 | 6 | 3 | 37 | 51 | 50 | 97 | 114 | 61 |
| Duration | 0.0 | 26.7 | 9.6 | 189.7 | 216.4 | 210.7 | 383.2 | 446.5 | 207.6 | |
| Keep Away | Count | 0 | 0 | 0 | 5 | 3 | 7 | 27 | 10 | 18 |
| Duration | 0.0 | 0.0 | 0.0 | 21.0 | 18.0 | 31.8 | 106.4 | 29.5 | 57.6 | |
| Leap | Count | 0 | 0 | 0 | 0 | 1 | 0 | 7 | 1 | 2 |
| Duration | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 0.0 | 3.7 | 0.3 | 1.5 | |
| Lick | Count | 11 | 24 | 16 | 31 | 17 | 14 | 13 | 12 | 14 |
| Duration | 103.2 | 177.4 | 47.1 | 140.5 | 91.2 | 72.1 | 43.3 | 38.9 | 32.9 | |
| Lie on Object | Count | 0 | 1 | 0 | 7 | 1 | 0 | 0 | 0 | 1 |
| Duration | 0.0 | 6.8 | 0.0 | 33.4 | 8.5 | 0.0 | 0.0 | 0.0 | 5.0 | |
| Nose | Count | 275 | 231 | 507 | 460 | 418 | 320 | 300 | 336 | 205 |
| Duration | 1278.6 | 823.4 | 2419.0 | 1721.5 | 1254.3 | 1569.5 | 905.1 | 748.2 | 413.5 | |
| Paw | Count | 4 | 37 | 36 | 86 | 109 | 102 | 87 | 104 | 67 |
| Duration | 15.7 | 187.2 | 94.7 | 233.2 | 229.9 | 325.3 | 211.8 | 187.9 | 194.0 | |
| Paw Face | Count | 0 | 0 | 0 | 1 | 2 | 0 | 1 | 4 | 5 |
| Duration | 0.0 | 0.0 | 0.0 | 2.2 | 1.8 | 0.0 | 2.6 | 27.6 | 21.5 | |
| Pickup and Drop | Count | 1 | 1 | 0 | 4 | 7 | 4 | 2 | 18 | 8 |
| Duration | 2.6 | 3.0 | 0.0 | 15.1 | 19.8 | 7.5 | 6.5 | 75.9 | 14.4 | |
| Play Bow | Count | 0 | 4 | 3 | 5 | 9 | 7 | 3 | 3 | 2 |
| Duration | 0.0 | 4.7 | 6.5 | 11.4 | 9.6 | 11.0 | 11.0 | 2.8 | 5.0 | |
| Pounce | Count | 3 | 0 | 2 | 1 | 11 | 4 | 11 | 7 | 6 |
| Duration | 4.4 | 0.0 | 1.2 | 1.7 | 20.4 | 5.1 | 80.9 | 7.9 | 3.7 | |
| Stand Over | Count | 0 | 2 | 0 | 3 | 7 | 0 | 0 | 0 | 0 |
| Duration | 0.0 | 2.2 | 0.0 | 20.2 | 24.7 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Tackle | Count | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Duration | 0.0 | 0.0 | 1.8 | 0.0 | 0.0 | 0.0 | 2.8 | 0.0 | 0.0 | |
| Tear | Count | 0 | 0 | 6 | 24 | 35 | 29 | 18 | 47 | 23 |
| Duration | 0.0 | 0.0 | 44.5 | 201.1 | 273.8 | 342.9 | 173.7 | 394.5 | 155.8 | |
| Toss | Count | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Duration | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.0 | 0.0 | |
| Tug-of-war | Count | 0 | 0 | 0 | 3 | 18 | 43 | 167 | 167 | 98 |
| Duration | 0.0 | 0.0 | 0.0 | 8.5 | 158.3 | 337.2 | 1482.6 | 1358.3 | 783.4 | |
| Tug-pull | Count | 0 | 0 | 0 | 6 | 16 | 22 | 44 | 36 | 31 |
| Duration | 0.0 | 0.0 | 0.0 | 30.4 | 57.7 | 152.0 | 227.8 | 136.7 | 138.0 | |
References
- Burghardt, G.M. A brief glimpse at the long evolutionary history of play. Anim. Behav. Cogn. 2014, 1, 90–98. [Google Scholar] [CrossRef]
- Burghardt, G.M. Genesis of Animal Play: Testing the Limits; MIT Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Burghardt, G.M.; Pellis, S.M. New directions in studying the evolution of play. In The Cambridge Handbook of Play; Smith, P.K., Roopnarine, J.L., Eds.; Cambridge University Press: New York, NY, USA, 2018; pp. 11–29. ISBN 9781316640906. [Google Scholar]
- Hall, S.L. Object play in adult animals. In Animal Play: Evolutionary, Comparative, and Ecological Perspectives; Bekoff, M., Byers, J.A., Eds.; Cambridge University Press: New York, NY, USA, 1998; pp. 45–60. [Google Scholar]
- Pellis, S.M.; Burghardt, G.M. Play and exploration. In APA Handbook of Comparative Psychology: Basic Concepts, Methods, Neural Substrate, and Behavior; Call, J., Burghardt, G.M., Pepperberg, I.M., Snowden, C.T., Zentall, T., Eds.; American Psychological Association: Washington, DC, USA, 2017; APA Handbooks in Psychology®; Volume 1, pp. 699–722. [Google Scholar]
- Berlyne, D.E. Conflict and Arousal; McGraw-Hill: Chicago, IL, USA, 1960. [Google Scholar] [CrossRef]
- Hutt, C. Exploration and play in children. In Symposia of the Zoological Society of London; Academic Press: London, UK, 1966; Volume 18, pp. 61–81. [Google Scholar]
- Baldwin, J.D.; Baldwin, J.I. The role of learning phenomena in the ontogeny of exploration and play. In Primate Bio-Social Development: Biological, Social and Ecological Determinants; Chevelier-Skolnikoff, S., Poirier, F.E., Eds.; Garland STPM Press: New York, NY, USA, 1977; pp. 343–406. [Google Scholar]
- Burghardt, G.M. On the origins of play. In Play in Animals and Humans; Smith, P.K., Ed.; Basil Blackwell: Oxford, UK, 1984; pp. 6–41. [Google Scholar]
- Burghardt, G. Play, exploration, and learning. In Encyclopedia of the Sciences of Learning; Neel, N.M., Ed.; Springer Science & Business Media: New York, NY, USA, 2012; pp. 2650–2653. [Google Scholar]
- Pisula, W. Play and exploration in animals—A comparative analysis. Pol. Psychol. Bull. 2008, 39, 104–107. [Google Scholar] [CrossRef]
- Panksepp, J. Affective Neuroscience: The Foundations of Human and Animal Emotions; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Pellis, S.M.; Pellis, V.C. The Playful Brain: Venturing to the Limits of Neuroscience; Oneworld: Oxford, UK, 2009. [Google Scholar]
- Dona, H.S.G.; Solvi, C.; Kowalewska, A.; Mäkelä, K.; MaBouDi, H.; Chittka, L. Do bumble bees play? Anim. Behav. 2022, 194, 239–251. [Google Scholar] [CrossRef]
- Coppinger, R.; Coppinger, L. Differences in the behavior of dog breeds. In Genetics and the Behavior of Domestic Animals; Serpell, J., Ed.; Academic Press: Cambridge, MA, USA, 1998; pp. 167–202. [Google Scholar]
- Coppinger, R.; Coppinger, L. Dogs: A New Understanding of Canine Origin, Behavior and Evolution; University of Chicago Press: Chicago, IL, USA, 2001. [Google Scholar]
- Coppinger, R.; Feinstein, M. How Dogs Work; University of Chicago Press: Chicago, IL, USA, 2015. [Google Scholar] [CrossRef]
- Cordoni, G. Social play in captive wolves (Canis lupus): Not only an immature affair. Behaviour 2009, 146, 1363–1385. [Google Scholar] [CrossRef]
- Bekoff, M. Social play behavior. Bioscience 1984, 34, 228–233. [Google Scholar] [CrossRef]
- Smuts, B. Social behaviour among companion dogs with an emphasis on play. In The Social Dog; Kaminski, J., Marshall-Pescini, S., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 105–130. [Google Scholar]
- Mehrkam, L.R.; Hall, N.J.; Haitz, C.; Wynne, C.D.L. The influence of breed and environmental factors on social and solitary play in dogs (Canis lupus familiaris). Learn Behav. 2017, 45, 367–377. [Google Scholar] [CrossRef]
- Bateson, P.; Mendl, M.; Feaver, J. Play in the domestic cat is enhanced by rationing of the mother during lactation. Anim. Behav. 1990, 40, 514–525. [Google Scholar] [CrossRef]
- Hall, S.L.; Bradshaw, J.W.S. The influence of hunger on object play by adult domestic cats. Appl. Anim. Behav. Sci. 1998, 58, 143–150. [Google Scholar] [CrossRef]
- Biben, M. Predation and predatory play-behavior of domestic cats. Anim. Behav. 1979, 27, 81–94. [Google Scholar] [CrossRef]
- Leyhausen, P. Cat Behaviour. In The Predatory and Social Behaviour of Domestic and Wild Cats; Garland STPM Press: New York, NY, USA, 1979. [Google Scholar]
- Pullen, A.J.; Merrill, R.J.N.; Bradshaw, J.W.S. Preferences for toy types and presentations in kennel housed dogs. App. Anim. Behav. Sci. 2010, 125, 151–156. [Google Scholar] [CrossRef]
- Burghardt, G.M.; Albright, J.D.; Davis, K.M. Motivation, development and object play: Comparative perspectives with lessons from Dogs. Behaviour 2016, 153, 767–793. [Google Scholar] [CrossRef]
- Davis, K.M.; Partin, A.M.; Springer, C.M.; Burghardt, G.M. The development of object play in wolf puppies. Int. J. Play 2023, 12, 20–39. [Google Scholar] [CrossRef]
- Pullen, A.J.; Merrill, R.J.N.; Bradshaw, J.W.S. Habituation and dishabituation during object play in kennel-housed dogs. Anim. Cogn. 2012, 15, 1143–1150. [Google Scholar] [CrossRef]
- Mitchell, R.W.; Thompson, N.S. Projects, routines, and enticements in dog–human play. In Perspectives in Ethology; Bateson, P.P.G., Klopfer, P.H., Eds.; Plenum Press: New York, NY, USA, 1991; Volume 9, pp. 189–216. [Google Scholar]
- Rooney, N.J. Play Behaviour of the Domestic Dog Canis Familiaris, and Its Effects upon the Dog-Human Relationship. Ph.D. Thesis, University of Southampton, Southampton, UK, 1999. [Google Scholar]
- Pullen, A.J. Behavioural Indicators of Candidate Enrichments for Kennel Housed Dogs. Ph.D. Thesis, University of Bristol, Bristol, UK, 2011. Available online: http://uk.bl.ethos.535234 (accessed on 15 December 2022).
- Tinbergen, N. On aims and methods of ethology. Z Tierpsychol. 1963, 20, 410–433. [Google Scholar] [CrossRef]
- Rosati, A.G.; Wobber, V.; Hughes, K.; Santos, L.R. Comparative Developmental Psychology: How is Human Cognitive Development Unique? Evol. Psychol. 2014, 12, 448–473. [Google Scholar] [CrossRef]
- Biben, M. Object play and social treatment of prey in bush dogs and crab-eating foxes. Behaviour 1982, 79, 201–211. [Google Scholar] [CrossRef]
- Feddersen-Petersen, D. The ontogeny of social play and agonistic behaviour in selected canid species. Bonn. Zool. Beitr. 1991, 42, 97–114. [Google Scholar]
- Feddersen-Peterson, D.U. Social behaviour of dogs and related canids. In The Behavioural Biology of Dogs; Jensen, P., Ed.; CAB International: Wallingford, UK, 2007; pp. 105–119. [Google Scholar] [CrossRef]
- Lund, J.D.; Vestergaard, K.S. Development of social behaviour in four litters of dogs (Canis familiaris). Acta. Vet. Scand. 1998, 39, 183–193. [Google Scholar] [CrossRef]
- Heine, C. Verhaltensontogenese Von Welpen Der Rasse Border Collie in Den Ersten Acht Lebenswochen. Ph.D. Thesis, Christian-Albrechts Universität Kiel, Physiologische Institut Der Tierärztlichen Hochschule Hannover, Hanover, Germany, 2000. [Google Scholar]
- Käufer, M. Canine Play Behavior: The Science of Dogs at Play; Dogwise Publishing: Wenatchee, WA, USA, 2013. [Google Scholar]
- Bekoff, M. Social play and play-soliciting by infant canids. Am. Zool. 1974, 14, 323–340. [Google Scholar] [CrossRef]
- Zimen, E. Wölfe und Königspudel: Vergleichende Verhaltensbeobachtungen; Piper: München, Germany, 1971. [Google Scholar]
- Scott, J.P.; Fuller, J.L. Dog Behavior: The Genetic Basis; Chicago University Press: Chicago, IL, USA, 1965. [Google Scholar]
- Packard, J.M. Wolf behavior: Reproductive, social, and intelligent. In Wolves Behavior, Ecology and Conservation; Mech, L.D., Boitani, L., Eds.; University of Chicago Press: Chicago, IL, USA, 2003; pp. 35–65. [Google Scholar]
- Lord, K. A comparison of the sensory development of wolves (Canis lupus lupus) and dogs (Canis lupus familiaris). Ethology 2013, 119, 110–120. [Google Scholar] [CrossRef]
- Klinghammer, E.; Goodmann, P.A. Socialization and management of wolves in captivity. In Man and Wolf: Advances, Issues and Problems in Captive Wolf Research; Frank, H., Ed.; Dr. W. Junk Publishers: Chicago, IL, USA, 1987; pp. 31–61. [Google Scholar]
- Parker, H.G.; Dreger, D.L.; Rimbault, M.; Davis, B.W.; Mullen, A.B.; Carpintero-Ramirez, G.; Ostrander, E.A. Genomic analyses reveal the influence of geographic origin, migration, and hybridization on modern dog breed development. Cell Rep. 2017, 19, 697–708. [Google Scholar] [CrossRef]
- Pal, S.K. Play behaviour during early ontogeny in free-ranging dogs (Canis familiaris). App. Anim. Behav. Sci. 2010, 126, 140–153. [Google Scholar] [CrossRef]
- Altmann, J. Observational study of behavior: Sampling methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef]
- Essler, J.L.; Cafazzo, S.; Marshall-Pescini, S.; Virányi, Z.; Kotrschal, K.; Range, F. Play behavior in wolves: Using the ‘50:50′ rule to test for egalitarian play styles. PLoS ONE 2016, 11, e0154150. [Google Scholar] [CrossRef]
- Goodmann, P.A.; Klinghammer, E.; Willard, J. Wolf ethogram. In Ethology; Eckard, H., Ed.; Hess Institute of Ethology: Battle Ground, IN, USA, 2002. [Google Scholar]
- Lord, K.; Feinstein, M.; Coppinger, R. Barking and mobbing. Behav. Process. 2009, 81, 358–368. [Google Scholar] [CrossRef]
- Pellis, S.M.; Pellis, V.C.; Pelletier, A.; Leca, J.-B. Is play a behavior system, and, if so, what kind? Behav. Process. 2019, 160, 1–9. [Google Scholar] [CrossRef]
- Coppinger, R.; Glendinning, J.; Torop, E.; Matthay, C.; Sutherland, M.; Smith, C. Degree of behavioral neoteny differentiates canid polymorphs. Ethology 1987, 75, 89–108. [Google Scholar] [CrossRef]
- Caro, T.M. Predatory behaviour and social play in kittens. Behaviour 1981, 76, 1–24. [Google Scholar] [CrossRef]
- Bateson, P.; Young, M. Separation from the mother and the development of play in cats. Anim. Behav. 1981, 29, 173–180. [Google Scholar] [CrossRef]
- Burghardt, G.M.; Burghardt, L.S. Notes on the behavioral development of two female black bear cubs: The first eight months. Bears Biol. Manag. 1972, 2, 207–220. [Google Scholar] [CrossRef]
- Fox, M.W.; Stelzner, D. The effects of early experience on the development of inter and intraspecies social relationships in the dog. Anim. Behav. 1967, 15, 377–386. [Google Scholar] [CrossRef]
- Bekoff, M. Play signals as punctuation: The structure of social play in canids. Behavior 1995, 132, 419–429. [Google Scholar] [CrossRef]
- Byosiere, S.-E.; Espinosa, J.; Marshall-Pescini, S.; Smuts, B.; Range, F. Investigating the function of play bows in dog and wolf puppies (Canis lupus familiaris, Canis lupus occidentalis). PLoS ONE 2016, 11, e0168570. [Google Scholar] [CrossRef]
- Cordoni, G.; Nicotra, V.; Palagi, E. Unveiling the “secret” of play in dogs (Canis lupus familiaris): Asymmetry and signals. J. Comp. Psychol. 2016, 130, 278–287. [Google Scholar] [CrossRef]
- Pellis, S.M.; Pellis, V.C. Play-fighting differs from serious fighting in both target of attack and tactics of fighting in the laboratory rat Rattus norvegicus. Aggress. Behav. 1987, 13, 227–242. [Google Scholar] [CrossRef]
- Palagi, E.; Burghardt, G.M.; Smuts, B.; Cordoni, G.; Dall’Olio, S.; Fouts, H.N.; Řeháková-Petrů, M.; Siviy, S.M.; Pellis, S.M. Rough-and-tumble play as a window on animal communication. Biol. Rev. 2016, 91, 311–327. [Google Scholar] [CrossRef]
- Rooney, N.J.; Bradshaw, J.W.S.; Robinson, I.H. A comparison of dog–dog and dog–human play behaviour. Appl. Anim. Behav. Sci. 2000, 66, 235–248. [Google Scholar] [CrossRef]
- Udell, M.A.R. When dogs look back: Inhibition of independent problem-solving behaviour in domestic dogs (Canis lupus familiaris) compared with wolves (Canis lupus). Biol. Lett. 2015, 11, 20150489. [Google Scholar] [CrossRef]
- Cordoni, G.; Palagi, E. Back to the future: A glance over wolf social behavior to understand dog–human relationship. Animals 2019, 9, 991. [Google Scholar] [CrossRef]
- Salomons, H.; Smith, K.C.M.; Callahan-Beckel, M.; Callahan, M.; Levy, K.; Kennedy, B.S.; Bray, E.E.; Gnanadesikan, G.E.; Horschler, D.J.; Gruen, M.; et al. Cooperative communication with humans evolved to emerge early in domestic dogs. Curr. Biol. 2021, 31, 3137–3144.e11. [Google Scholar] [CrossRef]
- Bradshaw, J.W.S.; Casey, R.A.; Brown, S.L. The Behaviour of the Domestic Cat, 2nd ed.; CABI: Boston, MA, USA, 2012. [Google Scholar]
- Pelletier, A.N.; Kaufmann, T.; Mohak, S.; Milan, R.; Nahallage, C.A.D.; Huffman, M.A.; Gunst, N.; Rompis, A.; Wandia, I.N.; Putra, I.G.A.A.; et al. Behavior systems approach to object play: Stone handling repertoire as a measure of propensity for complex foraging and percussive tool use in the genus macaca. Anim. Behav. Cogn. 2017, 4, 455–473. [Google Scholar] [CrossRef]


| Breed | |||||
|---|---|---|---|---|---|
| Welsh Terriers | Standard Poodles | Vizslas | Total | ||
| N = 11 | N = 17 | N = 14 | N = 42 | ||
| Agonistic | Count | 0 | 0 | 3 | 3 |
| Duration | 0.0 | 0.0 | 10.5 | 10.5 | |
| Approach | Count | 52 | 122 | 118 | 292 |
| Duration | 73.3 | 181.1 | 114.9 | 369.3 | |
| Approach-retreat | Count | 7 | 6 | 10 | 23 |
| Duration | 30.5 | 6.7 | 23.5 | 60.6 | |
| Avoid | Count | 0 | 1 | 0 | 1 |
| Duration | 0.0 | 3.6 | 0.0 | 3.6 | |
| Bite | Count | 168 | 213 | 161 | 542 |
| Duration | 491.2 | 651.8 | 454.4 | 1597.5 | |
| Carry | Count | 19 | 145 | 138 | 302 |
| Duration | 47.6 | 512.2 | 567.3 | 1127.2 | |
| Chase | Count | 11 | 97 | 28 | 136 |
| Duration | 32.0 | 316.4 | 89.3 | 437.7 | |
| Chew | Count | 850 | 1049 | 1168 | 3067 |
| Duration | 7819.1 | 9465.1 | 15,338.3 | 32,622.5 | |
| Dig | Count | 5 | 2 | 0 | 7 |
| Duration | 20.2 | 6.0 | 0.0 | 26.2 | |
| Drag | Count | 73 | 117 | 46 | 236 |
| Duration | 271.0 | 524.0 | 135.8 | 930.8 | |
| Exaggerated Approach | Count | 10 | 28 | 8 | 46 |
| Duration | 6.0 | 67.0 | 14.0 | 87.0 | |
| Grab | Count | 127 | 257 | 190 | 574 |
| Duration | 316.0 | 626.7 | 565.9 | 1508.5 | |
| Grab-headshake | Count | 127 | 118 | 197 | 442 |
| Duration | 340.5 | 293.5 | 435.8 | 1069.8 | |
| Hold Object | Count | 97 | 146 | 176 | 419 |
| Duration | 461.4 | 519.3 | 709.8 | 1690.4 | |
| Keep Away | Count | 0 | 58 | 12 | 70 |
| Duration | 0.0 | 221.2 | 43.1 | 264.3 | |
| Leap | Count | 1 | 7 | 3 | 11 |
| Duration | 0.3 | 3.7 | 2.6 | 6.6 | |
| Lick | Count | 71 | 55 | 26 | 152 |
| Duration | 391.9 | 238.7 | 116.0 | 746.6 | |
| Lie on Object | Count | 10 | 0 | 0 | 10 |
| Duration | 53.7 | 0.0 | 0.0 | 53.7 | |
| Nose | Count | 896 | 954 | 1202 | 3052 |
| Duration | 3080.0 | 3725.2 | 4328.1 | 11,133.2 | |
| Paw | Count | 182 | 262 | 188 | 632 |
| Duration | 477.4 | 777.2 | 425.2 | 1679.7 | |
| Paw Face | Count | 1 | 2 | 10 | 13 |
| Duration | 2.2 | 9.5 | 44.1 | 55.8 | |
| Pickup and Drop | Count | 2 | 28 | 15 | 45 |
| Duration | 5.9 | 104.5 | 34.5 | 144.8 | |
| Play Bow | Count | 10 | 10 | 16 | 36 |
| Duration | 15.3 | 24.6 | 22.2 | 62.1 | |
| Pounce | Count | 14 | 18 | 13 | 45 |
| Duration | 11.8 | 99.4 | 14.1 | 125.3 | |
| Stand Over | Count | 12 | 0 | 0 | 12 |
| Duration | 47.1 | 0.0 | 0.0 | 47.1 | |
| Tackle | Count | 0 | 2 | 0 | 2 |
| Duration | 0.0 | 4.6 | 0.0 | 4.6 | |
| Tear | Count | 69 | 35 | 78 | 182 |
| Duration | 553.0 | 298.7 | 734.5 | 1586.2 | |
| Toss | Count | 0 | 1 | 0 | 1 |
| Duration | 0.0 | 2.0 | 0.0 | 2.0 | |
| Tug-of-war | Count | 74 | 204 | 218 | 496 |
| Duration | 763.7 | 1473.6 | 1891.1 | 4128.3 | |
| Tug-pull | Count | 43 | 66 | 46 | 155 |
| Duration | 169.1 | 371.4 | 202.2 | 742.6 | |
| Age in Weeks (Pup N = 42 Weeks 3–6.5 and N = 41 Week 7) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Behaviour | 3.0 | 3.5 | 4.0 | 4.5 | 5.0 | 5.5 | 6.0 | 6.5 | 7.0 |
| Agonistic | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Approach | 8 | 10 | 7 | 16 | 14 | 16 | 19 | 30 | 24 |
| Approach-retreat | 1 | 1 | 0 | 2 | 3 | 0 | 1 | 5 | 2 |
| Avoid | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Bite | 7 | 5 | 15 | 19 | 31 | 27 | 24 | 34 | 30 |
| Carry | 0 | 0 | 2 | 11 | 15 | 12 | 22 | 25 | 18 |
| Chase | 0 | 0 | 1 | 2 | 7 | 3 | 21 | 22 | 15 |
| Chew | 9 | 13 | 24 | 34 | 42 | 42 | 42 | 42 | 41 |
| Digging | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 2 |
| Drag | 1 | 0 | 2 | 13 | 21 | 12 | 26 | 22 | 19 |
| Exaggerated Approach | 0 | 2 | 0 | 1 | 10 | 4 | 6 | 3 | 9 |
| Grab | 1 | 2 | 8 | 17 | 27 | 20 | 30 | 40 | 29 |
| Grab-headshake | 0 | 1 | 9 | 20 | 25 | 15 | 30 | 42 | 29 |
| Hold Object | 0 | 4 | 3 | 13 | 27 | 17 | 30 | 38 | 29 |
| Keep Away | 0 | 0 | 0 | 4 | 3 | 3 | 11 | 6 | 6 |
| Leap | 0 | 0 | 0 | 0 | 1 | 0 | 4 | 1 | 2 |
| Lick | 5 | 8 | 13 | 11 | 7 | 8 | 8 | 11 | 12 |
| Lie on Object | 0 | 3 | 3 | 5 | 5 | 0 | 6 | 6 | 5 |
| Nose | 40 | 42 | 42 | 42 | 42 | 42 | 42 | 42 | 41 |
| Paw | 3 | 17 | 26 | 42 | 42 | 42 | 38 | 42 | 39 |
| Paw Face | 0 | 0 | 0 | 1 | 2 | 0 | 1 | 4 | 5 |
| Pickup and Drop | 1 | 1 | 0 | 2 | 5 | 3 | 2 | 9 | 7 |
| Play Bow | 0 | 3 | 3 | 3 | 8 | 4 | 3 | 3 | 2 |
| Pounce | 3 | 0 | 1 | 1 | 7 | 4 | 7 | 7 | 4 |
| Stand Over | 0 | 3 | 6 | 11 | 7 | 5 | 2 | 4 | 3 |
| Tackle | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Tear | 0 | 0 | 3 | 10 | 13 | 8 | 11 | 14 | 12 |
| Toss | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Tug-of-war | 0 | 0 | 0 | 3 | 6 | 14 | 35 | 32 | 28 |
| Tug-pull | 3 | 0 | 0 | 3 | 10 | 12 | 26 | 24 | 16 |
| Play Context | Age (in Weeks) | Standard Poodles | Vizslas | Welsh Terriers | |||
|---|---|---|---|---|---|---|---|
| Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation | ||
| Solitary | 3.0 | 27.39 | 27.47 | 43.92 | 30.71 | 34.93 | 26.87 |
| 4.0 | 90.32 | 72 | 135.45 | 96.97 | 168.92 | 49.58 | |
| 5.0 | 159.72 | 69.05 | 245.81 | 178.7 | 120.96 | 54.12 | |
| 6.0 | 165.74 | 61.24 | 263.01 | 75.46 | 219.22 | 64.32 | |
| 7.0 | 148.64 | 93.08 | 247.72 | 97.24 | 151.01 | 108.35 | |
| Both | 3.0 | 3.36 | 7.12 | 3.4 | 3.71 | 6.6 | 14.05 |
| 4.0 | 7.16 | 7.67 | 6.25 | 7.84 | 8.68 | 5.89 | |
| 5.0 | 11.89 | 12.33 | 9.52 | 7.37 | 11 | 9.33 | |
| 6.0 | 13.02 | 6.96 | 9.37 | 8.32 | 9.57 | 9.06 | |
| 7.0 | 10.62 | 8.53 | 11.41 | 10.51 | 14.02 | 10.15 | |
| Social | 3.0 | 0 | 0 | 0.03 | 0.1 | 0 | 0 |
| 4.0 | 0.96 | 1.52 | 1.04 | 2.72 | 0.15 | 0.51 | |
| 5.0 | 2.94 | 3.15 | 19.29 | 17.42 | 0 | 0 | |
| 6.0 | 43.64 | 30 | 42.84 | 32.4 | 28.1 | 35.93 | |
| 7.0 | 29.21 | 27.08 | 24.96 | 31.03 | 16.79 | 15.96 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, K.M.; Partin, A.M.; Burghardt, G.M.; Springer, C.M.; Albright, J.D. A Descriptive Methodology for Studying the Ontogeny of Object Play and Breed Differences in Dogs (Canis lupus familiaris). Animals 2023, 13, 1371. https://doi.org/10.3390/ani13081371
Davis KM, Partin AM, Burghardt GM, Springer CM, Albright JD. A Descriptive Methodology for Studying the Ontogeny of Object Play and Breed Differences in Dogs (Canis lupus familiaris). Animals. 2023; 13(8):1371. https://doi.org/10.3390/ani13081371
Chicago/Turabian StyleDavis, Karen M., Adam M. Partin, Gordon M. Burghardt, Cary M. Springer, and Julia D. Albright. 2023. "A Descriptive Methodology for Studying the Ontogeny of Object Play and Breed Differences in Dogs (Canis lupus familiaris)" Animals 13, no. 8: 1371. https://doi.org/10.3390/ani13081371
APA StyleDavis, K. M., Partin, A. M., Burghardt, G. M., Springer, C. M., & Albright, J. D. (2023). A Descriptive Methodology for Studying the Ontogeny of Object Play and Breed Differences in Dogs (Canis lupus familiaris). Animals, 13(8), 1371. https://doi.org/10.3390/ani13081371






