Prepartum Magnesium Butyrate Supplementation of Dairy Cows Improves Colostrum Yield, Calving Ease, Fertility, Early Lactation Performance and Neonatal Vitality
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animals, Housing and Experimental Design
2.3. Colostrum Quantity and Quality
2.4. Calving Ease
2.5. Calf Vitality and Health
2.6. Fertility and Health of Dairy Cows
2.7. Milk Production, Body Condition Score and Lameness Status of the Cows
2.8. Statistical Analyses
3. Results
3.1. Colostrum Quality and Quantity
3.2. Newborn Calf Vitality, Size and Health
3.3. Cow Fertility and Health
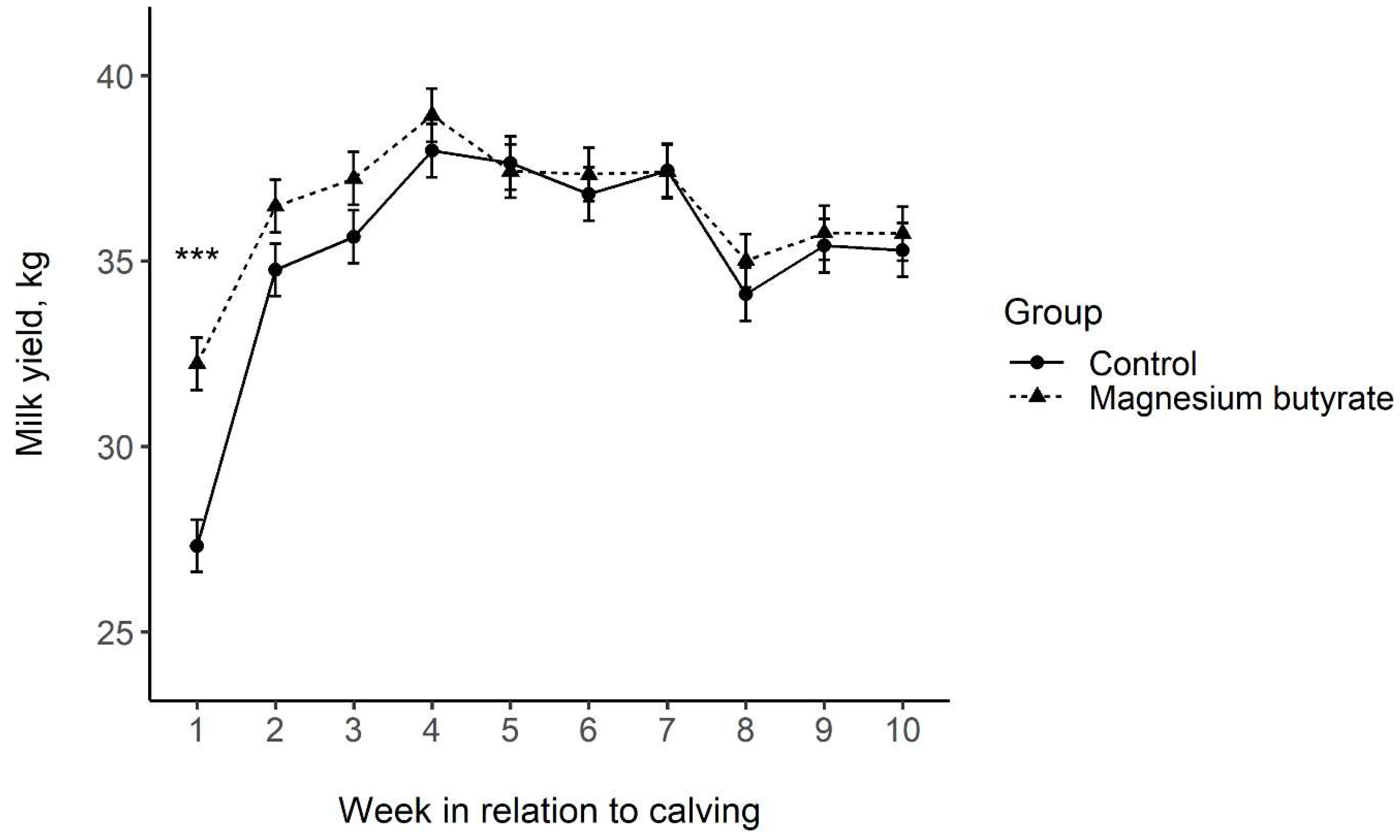
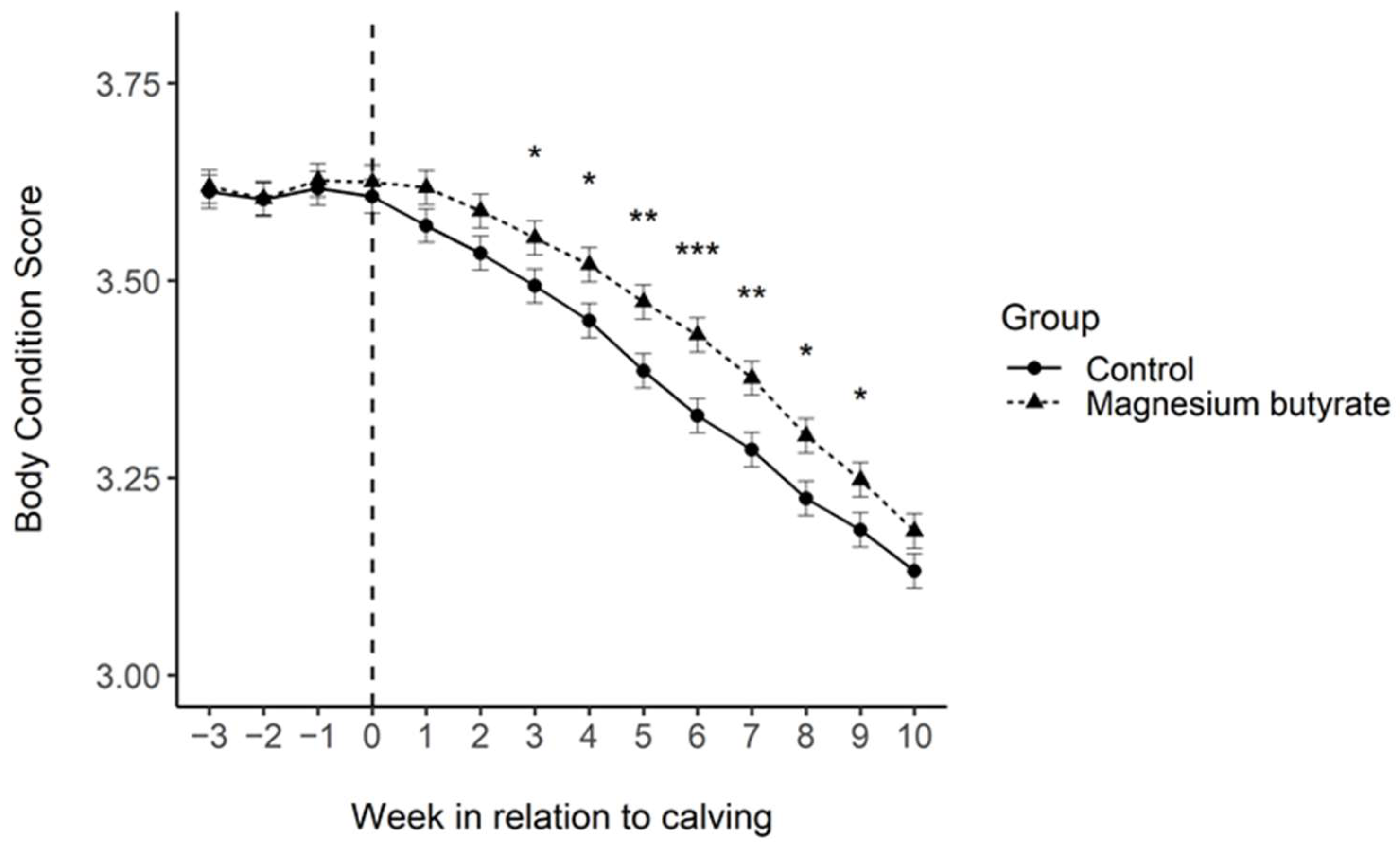
3.4. Milk Yield and BCS
4. Discussion
4.1. Colostrum Quality and Quantity
4.2. Newborn Calf Vitality and Health
4.3. Cow Fertility, Health and BCS
4.4. Milk Yield
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakata, T.; Tamate, H. Rumen epithelial cell proliferation accelerated by rapid increase in intraruminal butyrate. J. Dairy Sci. 1978, 61, 1109–1113. [Google Scholar] [CrossRef]
- Mentschel, J.; Leiser, R.; Mülling, C.; Pfarrer, C.; Claus, R. Butyric acid stimulates rumen mucosa development in the calf mainly by a reduction of apoptosis. Arch. Anim. Nutr. 2001, 55, 85–102. [Google Scholar] [CrossRef]
- Laarman, A.H.; Dionissopoulos, L.; AlZahal, O.; Greenwood, S.L.; Steele, M.A.; McBride, B.W. Butyrate and subacute ruminal acidosis affect abundance of membrane proteins involved with proton and short chain fatty acid transport in the rumen epithelium of dairy cows. Am. J. Anim. Vet. Sci. 2013, 8, 220–229. [Google Scholar] [CrossRef]
- Storm, A.C.; Hanigan, M.D.; Kristensen, N.B. Effects of ruminal ammonia and butyrate concentrations on reticuloruminal epithelial blood flow and volatile fatty acid absorption kinetics under washed reticulorumen conditions in lactating dairy cows. J. Dairy Sci. 2011, 94, 3980–3994. [Google Scholar] [CrossRef]
- Gorka, P.; Kowalski, Z.M.; Pietrzak, P.; Kotunia, A.; Jagusiak, W.; Holst, J.J.; Guilloteau, P.; Zabielski, R. Effect of method of delivery of sodium butyrate on rumen development in newborn calves. J. Dairy Sci. 2011, 94, 5578–5588. [Google Scholar] [CrossRef]
- Kowalski, Z.M.; Górka, P.; Flaga, J.; Barteczko, A.; Burakowska, K.; Oprządek, J.; Zabielski, R. Effect of microencapsulated sodium butyrate in the close-up diet on performance of dairy cows in the early lactation period. J. Dairy Sci. 2015, 98, 3284–3291. [Google Scholar] [CrossRef]
- Liebich, H.G.; Dirksen, G.; Arbel, A.; Dori, S.; Mayer, E. Fütterungsabhängige Veränderungen der Pansenschleimhaut von Hochleistungskühen im Zeitraum von der Trockenstellung bis acht Wochen postpartum. J. Vet. Med. Ser. A 1987, 34, 661–672. [Google Scholar] [CrossRef]
- Engelking, L.E.; Ambrose, D.J.; Oba, M. Effects of dietary butyrate supplementation and oral nonsteroidal anti-inflammatory drug administration on serum inflammatory markers and productivity of dairy cows during the calving transition. J. Dairy Sci. 2022, 105, 4144–4155. [Google Scholar] [CrossRef]
- Martens, H.; Rabbani, I.; Shen, Z.; Stumpff, F.; Deiner, C. Changes in rumen absorption processes during transition. Anim. Feed Sci. Technol. 2012, 172, 95–102. [Google Scholar] [CrossRef]
- Schonewille, J.T. Magnesium in dairy cow nutrition: An overview. Plant Soil 2013, 368, 167–178. [Google Scholar] [CrossRef]
- van de Braak, A.E.; Van’t Klooster, A.T.; Malestein, A. Influence of a deficient supply of magnesium during the dry period on the rate of calcium mobilisation by dairy cows at parturition. Res. Vet. Sci. 1987, 42, 101–108. [Google Scholar] [CrossRef]
- Morek-Kopeć, M.; Zarnecki, A.; Ptak, E.; Otwinowska-Mindur, A. Effect of calving difficulties and calf mortality on functional longevity in polish Holstein-Friesian cows. Animals 2021, 11, 2792. [Google Scholar] [CrossRef]
- Dallago, G.M.; Cue, R.I.; Wade, K.M.; Lacroix, R.; Vasseur, E. Birth conditions affect the longevity of Holstein offspring. J. Dairy Sci. 2022, 105, 1255–1264. [Google Scholar] [CrossRef]
- Barrier, A.C.; Haskell, M.J.; Birch, S.; Bagnall, A.; Bell, J.; Dickinson, D.J.; Macrae, A.I.; Dwyer, C.M. The impact of dystocia on dairy calf health, welfare, performance and survival. Vet. J. 2013, 195, 86–90. [Google Scholar] [CrossRef]
- Kovács, L.; Kézér, F.L.; Bodó, S.; Ruff, F.; Palme, R.; Szenci, O. Salivary cortisol as a non-invasive approach to assess stress in dystocic dairy calves. Sci. Rep. 2021, 11, 6200. [Google Scholar] [CrossRef]
- Marnila, P.; Korhonen, H. Milk proteins. Immunglobulins. In Encyclopedia of Dairy Sciences, 1st ed.; Roginski, H., Fuquay, J.W., Fox, P.F., Eds.; Academic Press: London, UK, 2002; pp. 1950–1956. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Wang, J.Q.; Yang, Y.X.; Bu, D.P.; Li, S.S.; Zhou, L.Y. Comparative proteomic analysis of changes in the bovine whey proteome during the transition from colostrum to milk. Asian-Australas. J. Anim. Sci. 2011, 24, 272–278. [Google Scholar] [CrossRef]
- Jang, Y.D.; Lindemann, M.D.; Monegue, H.J.; Monegue, J.S. The effect of coated sodium butyrate supplementation in sow and nursery diets on lactation performance and nursery pig growth performance. Livest. Sci. 2017, 195, 13–20. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Q.; Li, Y.; Tang, Z.; Sun, W.; Zhang, X.; Sun, J.; Sun, Z. Comparative effects of dietary supplementations with sodium butyrate, medium-chain fatty acids, and n-3 polyunsaturated fatty acids in late pregnancy and lactation on the reproductive performance of sows and growth performance of suckling piglets. J. Anim. Sci. 2019, 97, 4256–4267. [Google Scholar] [CrossRef]
- Hiltz, R.L.; Laarman, A.H. Effect of butyrate on passive transfer of immunity in dairy calves. J. Dairy Sci. 2019, 102, 4190–4197. [Google Scholar] [CrossRef] [PubMed]
- Sprecher, D.J.; Hostetler, D.E.; Kanneene, J.B. A lameness scoring system that uses posture and gait to predict dairy cattle reproductive performance. Theriogenology 1997, 47, 1179–1187. [Google Scholar] [CrossRef]
- Kovács, L.; Kézér, F.L.; Szenci, O. Effect of calving process on the outcomes of delivery and postpartum health of dairy cows with unassisted and assisted calvings. J. Dairy Sci. 2016, 99, 7568–7573. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 17th ed.; AOAC International: Arlington, VA, USA, 2003. [Google Scholar]
- Mee, J.F.; Berry, D.P.; Cromie, A.R. Risk factors for calving assistance and dystocia in pasture-based Holstein–Friesian heifers and cows in Ireland. Vet. J. 2011, 187, 189–194. [Google Scholar] [CrossRef]
- Szenci, O. Correlations between muscle tone and acid-base balance in newborn calves: Experimental substantiation of a simple new score system proposed for neonatal status diagnosis. Acta Vet. Acad. Sci. Hung. 1982, 30, 79–84. [Google Scholar] [PubMed]
- Love, W.J.; Lehenbauer, T.W.; Kass, P.H.; Van Eenennaam, A.L.; Aly, S.S. Development of a novel clinical scoring system for on-farm diagnosis of bovine respiratory disease in pre-weaned dairy calves. PeerJ 2014, 2, e238. [Google Scholar] [CrossRef] [PubMed]
- Calf Health Scoring Criteria. University of Wisconsin-Madison. Available online: https://www.vetmed.wisc.edu/fapm/svm-dairy-apps/calf-health-scorer-chs/ (accessed on 13 February 2022).
- Bourne, N.; Laven, R.; Wathes, D.C.; Martinez, T.; McGowan, M. A meta-analysis of the effects of Vitamin E supplementation on the incidence of retained foetal membranes in dairy cows. Theriogenology 2006, 67, 494–501. [Google Scholar] [CrossRef]
- Beagley, J.C.; Whitman, K.J.; Baptiste, K.E.; Scherzer, J. Physiology and treatment of retained fetal membranes in cattle. J. Vet. Intern. Med. 2010, 24, 261–268. [Google Scholar] [CrossRef]
- Ferguson, J.D.; Galligan, D.T.; Thomsen, N. Principal descriptors of body condition score in Holstein cows. J. Dairy Sci. 1994, 77, 2695–2703. [Google Scholar] [CrossRef]
- Whay, H. Locomotion scoring and lameness detection in dairy cattle. Practice 2002, 24, 444–449. [Google Scholar] [CrossRef]
- Mee, J.F. Managing the dairy cow at calving time. Vet. Clin. N. Am. Food Anim. Pract. 2004, 20, 521–546. [Google Scholar] [CrossRef]
- R Core Team. R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 24 February 2023).
- He, B.; Wang, M.; Guo, H.; Jia, Y.; Yang, X.; Zhao, R. Effects of sodium butyrate supplementation on reproductive performance and colostrum composition in gilts. Animal 2016, 10, 1722–1727. [Google Scholar] [CrossRef] [PubMed]
- Hare, K.S.; Croft, E.; Wood, K.M.; Steele, M.A. Late gestation metabolizable energy intake increases colostrum yield and alters colostrum composition in beef cattle. J. Anim. Sci. 2021, 99 (Suppl. S3), 150. [Google Scholar] [CrossRef]
- Larson, B.L.; Heary, H.L., Jr.; Devery, J.E. Immunoglobulin production and transport by the mammary gland. J. Dairy Sci. 1980, 63, 665–671. [Google Scholar] [CrossRef]
- Faber, S.N.; Faber, N.E.; Mccauley, T.C.; Ax, R.L. Case study: Effects of colostrum ingestion on lactational performance. Prof. Anim. Sci. 2005, 21, 420–425. [Google Scholar] [CrossRef]
- Gomez, D.E.; Chamorro, M.F. The importance of colostrum for dairy calves. Rev. Colomb. Cienc. Pecu. 2017, 30, 241–244. [Google Scholar]
- McGuirk, S.M.; Collins, M. Managing the production, storage, and delivery of colostrum. Vet. Clin. N. Am. Food Anim. Pract. 2004, 20, 593–603. [Google Scholar] [CrossRef]
- Devery-Pocius, E.; Larson, B.L. Age and previous lactations as factors in the amount of bovine colostral immunoglobulins. J. Dairy Sci. 1983, 66, 221–226. [Google Scholar] [CrossRef]
- Tyler, J.W.; Steevens, B.J.; Hostetler, D.E.; Holle, J.M.; Denbigh, J.L., Jr. Colostral immunoglobulin concentrations in Holstein and Guernsey cows. Am. J. Vet. Res. 1999, 60, 1136–1139. [Google Scholar] [PubMed]
- Moore, M.; Tyler, J.W.; Chigerwe, M.; Dawes, M.E.; Middleton, J.R. Effect of delayed colostrum collection on colostral IgG concentration in dairy cows. J. Am. Vet. Med. Assoc. 2005, 226, 1375–1377. [Google Scholar] [CrossRef]
- Conneely, M.; Berry, D.P.; Sayers, R.; Murphy, J.P.; Lorenz, I.; Doherty, M.L.; Kennedy, E. Factors associated with the concentration of immunoglobulin G in the colostrum of dairy cows. Animal 2013, 7, 1824–1832. [Google Scholar] [CrossRef]
- Ganz, S.; Bülte, M.; Gajewski, Z.; Wehrend, A. Inhaltsstoffe des bovinen Kolostrums-Eine Übersicht. Tierärztl. Prax. Ausg. G Grobtiere/Nutztiere 2018, 46, 178–189. [Google Scholar] [CrossRef]
- Vandeputte, S.; Detilleux, J.; Rollin, F. Investigation of colostrum quality in beef cattle by radial immunodiffusion and Brix refractometry. Vet. Rec. 2014, 175, 353. [Google Scholar] [CrossRef] [PubMed]
- Coleman, L.W.; Hickson, R.E.; Amoore, J.; Laven, R.A.; Back, P.J. Colostral immunoglobulin G as a predictor for serum immunoglobulin G concentration in dairy calves. Proc. N. Z. Soc. Anim. Prod. 2015, 75, 3–8. [Google Scholar]
- Morrill, K.M.; Robertson, K.E.; Spring, M.M.; Robinson, A.L.; Tyler, H.D. Validating a refractometer to evaluate immunoglobulin G concentration in Jersey colostrum and the effect of multiple freeze-thaw cycles on evaluating colostrum quality. J. Dairy Sci. 2015, 98, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Silva-Del-Rio, N.; Rolle, D.; García-Muñoz, A.; Rodríguez-Jiménez, S.; Valldecabres, A.; Lago, A.; Pandey, P. Colostrum immunoglobulin G concentration of multiparous Jersey cows at first and second milking is associated with parity, colostrum yield, and time of first milking, and can be estimated with Brix refractometry. J. Dairy Sci. 2017, 100, 5774–5781. [Google Scholar] [CrossRef]
- Karl, M.; Staufenbiel, R. Einflussfaktoren auf die Immunglobulinkonzentration im Erstkolostrum bei Holstein Friesian-Milchkühen und deren Beziehung zur postpartalen Kalziumkonzentration im Blut und Kolostrum. Tierärztliche Prax. Ausg. G Großtiere/Nutztiere 2017, 45, 331–341. [Google Scholar] [CrossRef]
- Besser, T.E.; Gay, C.C.; Pritchett, L. Comparison of three methods of feeding colostrum to dairy calves. J. Am. Vet. Med. Assoc. 1991, 198, 419–422. [Google Scholar]
- Davis, C.L.; Drackley, J.K. The Development, Nutrition, and Management of the Young Calf, 1st ed.; Iowa State University Press: Ames, IA, USA, 1998. [Google Scholar]
- Chigerwe, M.; Tyler, J.W.; Schultz, L.G.; Middleton, J.R.; Steevens, B.J.; Spain, J.N. Effect of colostrum administration by use of oroesophageal intubation on serum IgG concentrations in Holstein bull calves. Am. J. Vet. Res. 2008, 69, 1158–1163. [Google Scholar] [CrossRef]
- Puppel, K.; Gołębiewski, M.; Grodkowski, G.; Slósarz, J.; Kunowska-Slósarz, M.; Solarczyk, P.; Łukasiewicz, M.; Balcerak, M.; Przysucha, T. Composition and factors affecting quality of bovine colostrum: A Review. Animals 2019, 9, 1070. [Google Scholar] [CrossRef]
- Mee, J.F. Investigation of bovine abortion and stillbirth/perinatal mortality—Similar diagnostic challenges, different approaches. Ir. Vet. J. 2020, 73, 20. [Google Scholar] [CrossRef]
- Bicalho, R.C.; Galvao, K.N.; Cheong, S.H.; Gilbert, R.O.; Warnick, L.D.; Guard, C.L. Effect of stillbirths on dam survival and reproduction performance in Holstein dairy cows. J. Dairy Sci. 2007, 90, 2797–2803. [Google Scholar] [CrossRef] [PubMed]
- Buják, D.; Szelényi, Z.; Choukeir, A.; Kovács, L.; Kézér, F.L.; Boldizsár, S.Z.; Molnár, L.; Szenci, O. A Holstein-Friesian dairy farm survey of postparturient factors influencing the days to first AI and days open in Hungary. Acta Vet. Hung. 2018, 66, 613–624. [Google Scholar] [CrossRef] [PubMed]
- de Groot, B.; Edwards, J.; Schonewille, T. Magnesium butyrate is a readily available magnesium source in dairy cow nutrition. Anim. Sci. Vet. Sci. Zool. 2022; preprints. [Google Scholar] [CrossRef]
- Laven, R.A.; Peters, A.R. Bovine retained placenta: Aetiology, pathogenesis, and economic loss. Vet. Rec. 1996, 139, 465–471. [Google Scholar] [CrossRef]
- Roche, J.F. The effect of nutritional management of the dairy cow on reproductive efficiency. Anim. Reprod. Sci. 2006, 96, 282–296. [Google Scholar] [CrossRef]
- Kimura, K.; Goff, J.P.; Kehrli, M.E., Jr.; Reinhardt, T.A. Decreased neutrophil function as a cause of retained placenta in dairy cattle. J. Dairy Sci. 2002, 85, 544–550. [Google Scholar] [CrossRef]
- Drillich, M.; Mahlstedt, M.; Reichert, U.; Tenhagen, B.A.; Heuwieser, W. Strategies to improve the therapy of retained fetal membranes in dairy cows. J. Dairy Sci. 2006, 89, 627–635. [Google Scholar] [CrossRef]
- Siatka, K.; Sawa, A.; Krężel-Czopek, S.; Piwczyński, D.; Bogucki, M. Effect of some factors on number of services per conception in dairy cows. J. Vet. Sci. Technol. 2017, 8, 465. [Google Scholar] [CrossRef]
- O’Sullivan, M.; Butler, S.T.; Pierce, K.M.; Crowe, M.A.; O’Sullivan, K.; Fitzgerald, R.; Buckley, F. Reproductive efficiency and survival of Holstein-Friesian cows of divergent Economic Breeding Index, evaluated under seasonal calving pasture-based management. J. Dairy Sci. 2020, 103, 1685–1700. [Google Scholar] [CrossRef]
- Burgers, E.E.A.; Kok, A.; Goselink, R.M.A.; Hogeveen, H.; Kemp, B.; van Knegsel, A.T.M. Fertility and milk production on commercial dairy farms with customized lactation lengths. J. Dairy Sci. 2021, 104, 443–458. [Google Scholar] [CrossRef]
- Dervishi, E.; Zhang, G.; Hailemariam, D.; Dunn, S.; Ametaj, B.N. Occurrence of retained placenta is preceded by an inflammatory state and alterations of energy metabolism in transition dairy cows. J. Anim. Sci. Biotechnol. 2016, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Herrick, K.J.; Hippen, A.R.; Kalscheur, K.F.; Schingoethe, D.J.; Casper, D.P.; Moreland, S.C.; van Eys, J.E. Single-dose infusion of sodium butyrate, but not lactose, increases plasma β-hydroxybutyrate and insulin in lactating dairy cows. J. Dairy Sci. 2017, 100, 757–768. [Google Scholar] [CrossRef] [PubMed]
| Item | Prepartum | Postpartum 2 | ||
|---|---|---|---|---|
| Control | Magnesium Butyrate 1 | |||
| Ingredient, % DM | ||||
| Corn silage | 50.5 | 49.2 | 34.7 | |
| Rye silage | - | - | 6.24 | |
| Alfalfa silage | 8.90 | 8.67 | 7.45 | |
| Grass hay | 5.96 | 5.82 | 3.06 | |
| Straw | 10.4 | 10.1 | - | |
| Brewer’s grains | 5.96 | 5.82 | 6.24 | |
| Corn flour | 5.05 | 4.93 | 22.0 | |
| Oats | 2.52 | 2.46 | 5.75 | |
| Extracted rapeseed meal | 2.52 | 2.46 | 7.45 | |
| Extracted sunflower meal | 6.31 | 6.16 | 5.08 | |
| Premix Mipro Pren 250 3 | 1.88 | 1.84 | - | |
| Premix Mipro RB 600 4 | - | - | 2.03 | |
| Rumen-Ready® 1 | - | 2.54 | - | |
| Chemical composition 5 | ||||
| Dry matter, g/kg | 437 | 441 | 426 | |
| NEl, MJ/kg of DM | 6.23 | 6.32 | 7.25 | |
| Ash, g/kg of DM | 84.0 | 86.0 | 71.0 | |
| Crude protein, g/kg of DM | 114 | 114 | 167 | |
| Ether extract, g/kg of DM | 26.0 | 29.0 | 35.0 | |
| Crude fiber, g/kg of DM | 241 | 232 | 143 | |
| NDF, g/kg of DM | 507 | 494 | 339 | |
| ADF, g/kg of DM | 303 | 280 | 190 | |
| ADL, g/kg of DM | 50.0 | 47.0 | 39.0 | |
| Starch, g/kg of DM | 154 | 158 | 283 | |
| Sugar, g/kg of DM | 26.0 | 26.0 | 29.0 | |
| Item | Control | Magnesium Butyrate | p-Values 1 | ||||
|---|---|---|---|---|---|---|---|
| Parity = 2 (n = 45) | Parity ≥ 3 (n = 67) | Parity = 2 (n = 41) | Parity ≥ 3 (n = 66) | Group | Parity | Group × Parity | |
| Colostrum yield (kg) | 7.55 ± 0.65 | 8.23 ± 0.52 | 9.68 ± 0.66 | 9.48 ± 0.55 | 0.006 | 0.686 | 0.488 |
| IgG concentration (mg/mL) | 69.2 ± 4.08 | 74.3 ± 3.31 | 71.5 ± 4.13 | 69.1 ± 3.47 | 0.547 | 0.698 | 0.324 |
| Total IgG (g) | 535 ± 53.8 | 601 ± 43.2 | 675 ± 53.1 | 679 ± 45.4 | 0.034 | 0.473 | 0.524 |
| Fat concentration (%) | 6.10 ± 0.53 | 6.67 ± 0.43 | 5.47 ± 0.53 | 6.51 ± 0.46 | 0.496 | 0.097 | 0.662 |
| Total fat (g) | 456 ± 66.4 | 544 ± 54.7 | 576 ± 67.2 | 644 ± 57.5 | 0.076 | 0.206 | 0.877 |
| Protein concentration (%) | 17.0 ± 0.99 | 18.3 ± 0.82 | 17.8 ± 1.01 | 19.2 ± 0.86 | 0.363 | 0.160 | 0.975 |
| Total protein (g) | 1187 ± 120 | 1446 ± 99.2 | 1611 ± 122 | 1765 ± 104 | 0.001 | 0.065 | 0.641 |
| Lactose concentration (%) | 2.70 ± 0.12 | 2.50 ± 0.10 | 2.67 ± 0.12 | 2.58 ± 0.11 | 0.869 | 0.157 | 0.536 |
| Total lactose (g) | 215 ± 22.9 | 212 ± 18.9 | 267 ± 23.2 | 249 ± 19.9 | 0.041 | 0.621 | 0.735 |
| Solids non-fat concentration (%) | 24.9 ± 0.94 | 26.3 ± 0.74 | 25.1 ± 0.92 | 26.6 ± 0.78 | 0.790 | 0.083 | 0.998 |
| Total solids non-fat (g) | 1797 ± 164 | 2111 ± 135 | 2375 ± 166 | 2496 ± 142 | 0.002 | 0.152 | 0.533 |
| Item | Control (n = 116) | Magnesium Butyrate (n = 116) | p-Value 1 |
|---|---|---|---|
| Vitality score 2 | 9.20 ± 2.57 | 10.6 ± 2.64 | <0.001 |
| Birth weight (kg) | 43.6 ± 4.76 | 42.8 ± 4.89 | 0.259 |
| Hip width (cm) | 20.3 ± 1.80 | 20.0 ± 1.91 | 0.222 |
| Hip height (cm) | 77.8 ± 7.21 | 76.8 ± 7.31 | 0.327 |
| Item | Control | Magnesium Butyrate | p-Value 1 |
|---|---|---|---|
| Parity = 2 | |||
| Interval to first heat (d postpartum) | 23.0 (22; 25) | 20.5 (19; 21) | 0.01 |
| Services per conception (SPC) | 4 (2; 6) | 2 (1; 6) | <0.0001 |
| Interval to conception (d postpartum) | 109 (106; 134) | 77 (63; 99) | <0.0001 |
| Parity ≥ 3 | |||
| Interval to first heat (d postpartum) | 23 (22; 25) | 20 (20; 22) | 0.01 |
| Services per conception (SPC) | 4 (2; 6) | 3 (2; 6) | 0.0115 |
| Interval to conception (d postpartum) | 106 (94; 121) | 84 (81; 94) | <0.0001 |
| Item | Control | Magnesium Butyrate | p-Value 1 |
|---|---|---|---|
| Calving assistance rate (%) 2 | 36.9 | 21.3 | 0.012 |
| RFM rate (%) 3 | 14.4 | 3.70 | 0.008 |
| Embryonic/fetal mortality rate (%) | 32.4 | 13.9 | 0.001 |
| Prevalence of health disorders (%) | 21.6 | 21.3 | 1.000 |
| Culling rate within 70 DIM (%) | 16.2 | 13.0 | 0.568 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, L.; Pajor, F.; Bakony, M.; Fébel, H.; Edwards, J.E. Prepartum Magnesium Butyrate Supplementation of Dairy Cows Improves Colostrum Yield, Calving Ease, Fertility, Early Lactation Performance and Neonatal Vitality. Animals 2023, 13, 1319. https://doi.org/10.3390/ani13081319
Kovács L, Pajor F, Bakony M, Fébel H, Edwards JE. Prepartum Magnesium Butyrate Supplementation of Dairy Cows Improves Colostrum Yield, Calving Ease, Fertility, Early Lactation Performance and Neonatal Vitality. Animals. 2023; 13(8):1319. https://doi.org/10.3390/ani13081319
Chicago/Turabian StyleKovács, Levente, Ferenc Pajor, Mikolt Bakony, Hedvig Fébel, and Joan E. Edwards. 2023. "Prepartum Magnesium Butyrate Supplementation of Dairy Cows Improves Colostrum Yield, Calving Ease, Fertility, Early Lactation Performance and Neonatal Vitality" Animals 13, no. 8: 1319. https://doi.org/10.3390/ani13081319
APA StyleKovács, L., Pajor, F., Bakony, M., Fébel, H., & Edwards, J. E. (2023). Prepartum Magnesium Butyrate Supplementation of Dairy Cows Improves Colostrum Yield, Calving Ease, Fertility, Early Lactation Performance and Neonatal Vitality. Animals, 13(8), 1319. https://doi.org/10.3390/ani13081319







