Size-Mediated Trophic Interactions in Two Syntopic Forest Salamanders
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Species
2.3. Salamanders’ Diet Sampling
2.4. Data Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hardin, G. The Competitive Exclusion Principle: An idea that took a century to be born has implications in ecology, economics, and genetics. Science 1960, 131, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Schoener, T.W. The newest synthesis: Understanding the interplay of evolutionary and ecological dynamics. Science 2011, 331, 426–429. [Google Scholar] [CrossRef]
- Amarasekare, P. Interference competition and species coexistence. Proc. R. Soc. Lond. 2002, 269, 2541–2550. [Google Scholar] [CrossRef]
- Zhang, L.; Andersen, K.H.; Dieckmann, U.; Brännström, Å. Four types of interference competition and their impacts on the ecology and evolution of size-structured populations and communities. J. Theor. Biol. 2015, 380, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Tannerfeldt, M.; Elmhagen, B.; Angerbjörn, A. Exclusion by interference competition? The relationship between red and arctic foxes. Oecologia 2002, 132, 213–220. [Google Scholar] [CrossRef]
- Stephens, D.W.; Brown, J.S.; Ydenberg, R.C. Foraging: Behavior and Ecology; University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Harding, A.M.; Welcker, J.; Steen, H.; Hamer, K.C.; Kitaysky, A.S.; Fort, J.; Grémillet, D. Adverse foraging conditions may impact body mass and survival of a high Arctic seabird. Oecologia 2011, 167, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Beguin, M.; Requier, F.; Rollin, O.; Odoux, J.F.; Aupinel, P.; Decourtye, A. A common pesticide decreases foraging success and survival in honey bees. Science 2012, 336, 348–350. [Google Scholar] [CrossRef]
- Godin, J.G.J.; Smith, S.A. A fitness cost of foraging in the guppy. Nature 1988, 333, 69–71. [Google Scholar] [CrossRef]
- Ritchie, M.E. Optimal foraging and fitness in Columbian ground squirrels. Oecologia 1990, 82, 56–67. [Google Scholar] [CrossRef]
- Pfennig, D.W.; Rice, A.M.; Martin, R.A. Ecological opportunity and phenotypic plasticity interact to promote character displacement and species coexistence. Ecology 2006, 87, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Hairston, N.G.; Nishikawa, K.C.; Stenhouse, S.L. The evolution of competing species of terrestrial salamanders: Niche partitioning or interference? Evol. Ecol. 1987, 1, 247–262. [Google Scholar] [CrossRef]
- Walls, S.C. Differences in foraging behaviour explain interspecific growth inhibition in competing salamanders. Anim. Behav. 1996, 52, 1157–1162. [Google Scholar] [CrossRef]
- Jaeger, R.G.; Gollmann, B.; Anthony, C.D.; Gabor, C.R.; Kohn, N.R. Behavioral Ecology of the Eastern Red-Backed Salamander: 50 Years of Research; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Salvidio, S.; Oneto, F.; Ottonello, D.; Costa, A.; Romano, A. Trophic specialization at the individual level in a terrestrial generalist salamander. Can. J. Zool. 2015, 93, 79–83. [Google Scholar] [CrossRef]
- Salvidio, S.; Costa, A.; Crovetto, F. Individual trophic specialisation in the Alpine newt increases with increasing resource diversity. Ann. Zool. Fenn. 2019, 56, 17–24. [Google Scholar] [CrossRef]
- Anthony, C.D.; Venesky, M.D.; Hickerson, C.A.M. Ecological separation in a polymorphic terrestrial salamander. J. Anim. Ecol. 2008, 77, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, A.J.; Lowe, W.H.; Marra, P.P. Using stable isotopes to test for trophic niche partitioning: A case study with stream salamanders and fish. Freshw. Biol. 2012, 57, 1399–1409. [Google Scholar] [CrossRef]
- Reed, D.T.; Tosh, C.R. Diversity loss is predicted to increase extinction risk of specialist animals by constraining their ability to expand niche. J. Theor. Biol. 2019, 476, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Pardi, M.I.; DeSantis, L.R. Dietary plasticity of North American herbivores: A synthesis of stable isotope data over the past 7 million years. Proc. R. Soc. Lond. 2021, 288, 20210121. [Google Scholar]
- Covich, A.P.; Palmer, M.A.; Crowl, T.A. The role of benthic invertebrate species in freshwater ecosystems: Zoobenthic species influence energy flows and nutrient cycling. BioScience 1999, 49, 119–127. [Google Scholar] [CrossRef]
- Fortin, D.; Beyer, H.L.; Boyce, M.S.; Smith, D.W.; Duchesne, T.; Mao, J.S. Wolves influence elk movements: Behavior shapes a trophic cascade in Yellowstone National Park. Ecology 2005, 86, 1320–1330. [Google Scholar] [CrossRef]
- Hilderbrand, G.V.; Schwartz, C.C.; Robbins, C.T.; Jacoby, M.E.; Hanley, T.A.; Arthur, S.M.; Servheen, C. The importance of meat, particularly salmon, to body size, population productivity, and conservation of North American brown bears. Can. J. Zool. 1999, 77, 132–138. [Google Scholar] [CrossRef]
- Ramakrishnan, U.; Coss, R.G.; Pelkey, N.W. Tiger decline caused by the reduction of large ungulate prey: Evidence from a study of leopard diets in southern India. Biol. Conserv. 1999, 89, 113–120. [Google Scholar] [CrossRef]
- Galetti, M.; Bovendorp, R.S.; Guevara, R. Defaunation of large mammals leads to an increase in seed predation in the Atlantic forests. Glob. Ecol. Conserv. 2015, 3, 824–830. [Google Scholar] [CrossRef]
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.; Fischman, D.L.; Waller, R.W. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.A. Amphibians as models for studying environmental change. ILAR J. 2007, 48, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Salvidio, S.; Costa, A.; Oneto, F.; Pastorino, M.V. Variability of a subterranean prey-predator community in space and time. Diversity 2020, 12, 17. [Google Scholar] [CrossRef]
- Costa, A.; Romano, A.; Rosa, G.; Salvidio, S. Weighted individual-resource networks in prey–predator systems: The role of prey availability on the emergence of modular structures. Int. Zool. 2022, 17, 115–127. [Google Scholar] [CrossRef]
- Davic, R.D.; Welsh, H.H., Jr. On the ecological roles of salamanders. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 405–434. [Google Scholar] [CrossRef]
- Burton, T.M.; Likens, G.E. Salamander populations and biomass in the Hubbard Brook experimental forest, New Hampshire. Copeia 1975, 1975, 541–546. [Google Scholar] [CrossRef]
- Best, M.L.; Welsh, H.H., Jr. The trophic role of a forest salamander: Impacts on invertebrates, leaf litter retention, and the humification process. Ecosphere 2014, 5, 1–19. [Google Scholar] [CrossRef]
- Pough, F.H. Amphibians and reptiles as low energy systems. In Behavioral Energetics: The Cost of Survival in Vertebrates; Aspeyand, W.P., Lustick, S.I., Eds.; Ohio State University Press: Columbus, OH, USA, 1983; pp. 141–188. [Google Scholar]
- Salvidio, S.; Romano, A.; Oneto, F.; Ottonello, D.; Michelon, R. Different season, different strategies: Feeding ecology of two syntopic forest-dwelling salamanders. Acta Oecol. 2012, 43, 42–50. [Google Scholar]
- Rosa, G.; Bosio, M.; Salvidio, S.; Costa, A. Foraging success is differently affected by local climate in two syntopic forest-dwelling salamanders. Ethol. Ecol. Evol. 2022, 1–10. [Google Scholar] [CrossRef]
- Agenzia Regionale per la Protezione dell’Ambiente Ligure (ARPAL). Atlante Climatico della Liguria; Grafica KC: Genova, Italy, 2013. [Google Scholar]
- Lanza, B.; Andreone, F.; Bologna, M.A.; Corti, C.; Razzetti, E. Volume XLII—Amphibia. In Fauna d’Italia; Edizioni Calderini: Bologna, Italy, 2007. [Google Scholar]
- Lunghi, E.; Manenti, R.; Ficetola, G.F. Seasonal variation in microhabitat of salamanders: Environmental variation or shift of habitat selection? PeerJ 2015, 3, e1122. [Google Scholar] [CrossRef]
- Costa, A.; Romano, A.; Salvidio, S. Reliability of multinomial N-mixture models for estimating abundance of small terrestrial vertebrates. Biodivers. Conserv. 2020, 29, 2951–2965. [Google Scholar] [CrossRef]
- Basile, M.; Romano, A.; Costa, A.; Posillico, M.; Scinti Roger, D.; Crisci, A.; Matteucci, G. Seasonality and microhabitat selection in a forest-dwelling salamander. Sci. Nat. 2017, 104, 80. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Crovetto, F.; Salvidio, S. European plethodontid salamanders on the forest floor: Local abundance is related to fine-scale environmental factors. Herp. Conserv. Biol. 2016, 11, 344–349. [Google Scholar]
- Salvidio, S. Diet and food utilization in a rock-face population of Speleomantes ambrosii (Amphibia, Caudata, Plethodontidae). Vie Milieu 1992, 42, 35–39. [Google Scholar]
- Costa, A.; Salvidio, S.; Posillico, M.; Matteucci, G.; De Cinti, B.; Romano, A. Generalisation within specialization: Inter-individual diet variation in the only specialized salamander in the world. Sci. Rep. 2015, 5, 13260. [Google Scholar] [CrossRef]
- Costa, A.; Salvidio, S.; Posillico, M.; Altea, T.; Matteucci, G.; Romano, A. What goes in does not come out: Different non-lethal dietary methods give contradictory interpretation of prey selectivity in amphibians. Amphib. Reptil. 2014, 35, 255–262. [Google Scholar] [CrossRef]
- Moya, O.; Mansilla, P.L.; Madrazo, S.; Igual, J.M.; Rotger, A.; Romano, A.; Tavecchia, G. APHIS: A new software for photo-matching in ecological studies. Ecol. Inform. 2015, 27, 64–70. [Google Scholar] [CrossRef]
- Lunghi, E. Doubling the lifespan of European plethodontid salamanders. Ecology 2022, 103, e03581. [Google Scholar] [CrossRef] [PubMed]
- Tinker, T.M.; Guimaraes, P.R.; Novak, M.; Marquitti, F.M.D.; Bodkin, J.L.; Staedler, M.; Estes, J.A. Structure and mechanism of diet specialisation: Testing models of individual variation in resource use with sea otters. Ecol. Lett. 2012, 15, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Lunghi, E.; Manenti, R.; Cianferoni, F.; Ceccolini, F.; Veith, M.; Corti, C.; Mancinelli, G. Interspecific and interpopulation variation in individual diet specialization: Do environmental factors have a role? Ecology 2020, 101, e03088. [Google Scholar] [CrossRef]
- Lunghi, E.; Cianferoni, F.; Ceccolini, F.; Zhao, Y.; Manenti, R.; Corti, C.; Mancinelli, G. Same diet, different strategies: Variability of individual feeding habits across three populations of Ambrosi’s cave salamander (Hydromantes ambrosii). Diversity 2020, 12, 180. [Google Scholar] [CrossRef]
- Albrecht, M.; Gotelli, N.J. Spatial and temporal niche partitioning in grassland ants. Oecologia 2001, 126, 134–141. [Google Scholar] [CrossRef]
- Székely, D.; Cogălniceanu, D.; Székely, P.; Denoël, M. Adult—Juvenile interactions and temporal niche partitioning between life-stages in a tropical amphibian. PLoS ONE 2020, 15, e0238949. [Google Scholar] [CrossRef]

| Municipality | Site ID | Altitude Asl | Species | Sample Size |
|---|---|---|---|---|
| Valbrevenna (GE) | TON | 789 | Salamandrina perspicillata | 11 |
| Speleomantes strinatii | 18 | |||
| CAS | 750 | Speleomantes strinatii | 20 | |
| PAR | 920 | Salamandrina perspicillata | 18 | |
| Speleomantes strinatii | 12 | |||
| Carrega Ligure (AL) | GHI | 760 | Salamandrina perspicillata | 29 |
| Speleomantes strinatii | 14 | |||
| FOS | 820 | Speleomantes strinatii | 23 | |
| Montoggio (GE) | PEN | 580 | Speleomantes strinatii | 20 |
| Cabella Ligure (AL) | GOR | 620 | Salamandrina perspicillata | 13 |
| Mongiardino Ligure (AL) | RIA | 570 | Salamandrina perspicillata | 13 |
| Salamandrina perspicillata (n = 85) | Speleomantes strinatii (n = 106) | |||||
|---|---|---|---|---|---|---|
| Prey Taxon | Number | n of Stomachs | f | Number | n of Stomachs | f |
| Pseudoscorpiones | 13 | 12 | 0.02 | 4 | 4 | 0.01 |
| Acarina | 178 | 47 | 0.30 | 76 | 37 | 0.10 |
| Opilionida | 4 | 4 | 0.01 | 13 | 10 | 0.02 |
| Araneae | 22 | 17 | 0.04 | 51 | 37 | 0.07 |
| Diplopoda | 10 | 9 | 0.02 | 65 | 39 | 0.08 |
| Geophiloorpha | 0 | 0 | 0.00 | 1 | 1 | 0.00 |
| Litobiomorpha | 0 | 0 | 0.00 | 8 | 6 | 0.01 |
| Collembola | 239 | 48 | 0.40 | 153 | 58 | 0.20 |
| Diplura | 1 | 1 | 0.00 | 0 | 0 | 0.00 |
| Coleoptera | 30 | 24 | 0.05 | 56 | 36 | 0.07 |
| Coleoptera larvae | 1 | 1 | 0.00 | 3 | 3 | 0.00 |
| Diptera | 14 | 9 | 0.02 | 20 | 17 | 0.03 |
| Diptera larvae | 12 | 11 | 0.02 | 123 | 36 | 0.16 |
| Hemiptera | 4 | 4 | 0.01 | 10 | 8 | 0.01 |
| Heteroptera | 2 | 2 | 0.00 | 6 | 5 | 0.01 |
| Hemiptera larvae | 1 | 1 | 0.00 | 0 | 0 | 0.00 |
| Hymenoptera (winged) | 2 | 2 | 0.00 | 8 | 6 | 0.01 |
| Formicidae | 12 | 8 | 0.02 | 116 | 44 | 0.15 |
| Lepidoptera larvae | 0 | 0 | 0.00 | 3 | 2 | 0.00 |
| Neuroptera | 1 | 1 | 0.00 | 2 | 2 | 0.00 |
| Neuroptera larvae | 0 | 0 | 0.00 | 3 | 3 | 0.00 |
| Plecoptera | 2 | 1 | 0.00 | 1 | 1 | 0.00 |
| Trichoptera | 1 | 1 | 0.00 | 1 | 1 | 0.00 |
| Nematoda | 0 | 0 | 0.00 | 2 | 2 | 0.00 |
| Oligochaeta | 1 | 1 | 0.00 | 20 | 5 | 0.03 |
| Lumbricidae | 0 | 0 | 0.00 | 2 | 1 | 0.00 |
| Copepoda | 0 | 0 | 0.00 | 1 | 1 | 0.00 |
| Isopoda | 43 | 14 | 0.07 | 19 | 13 | 0.02 |
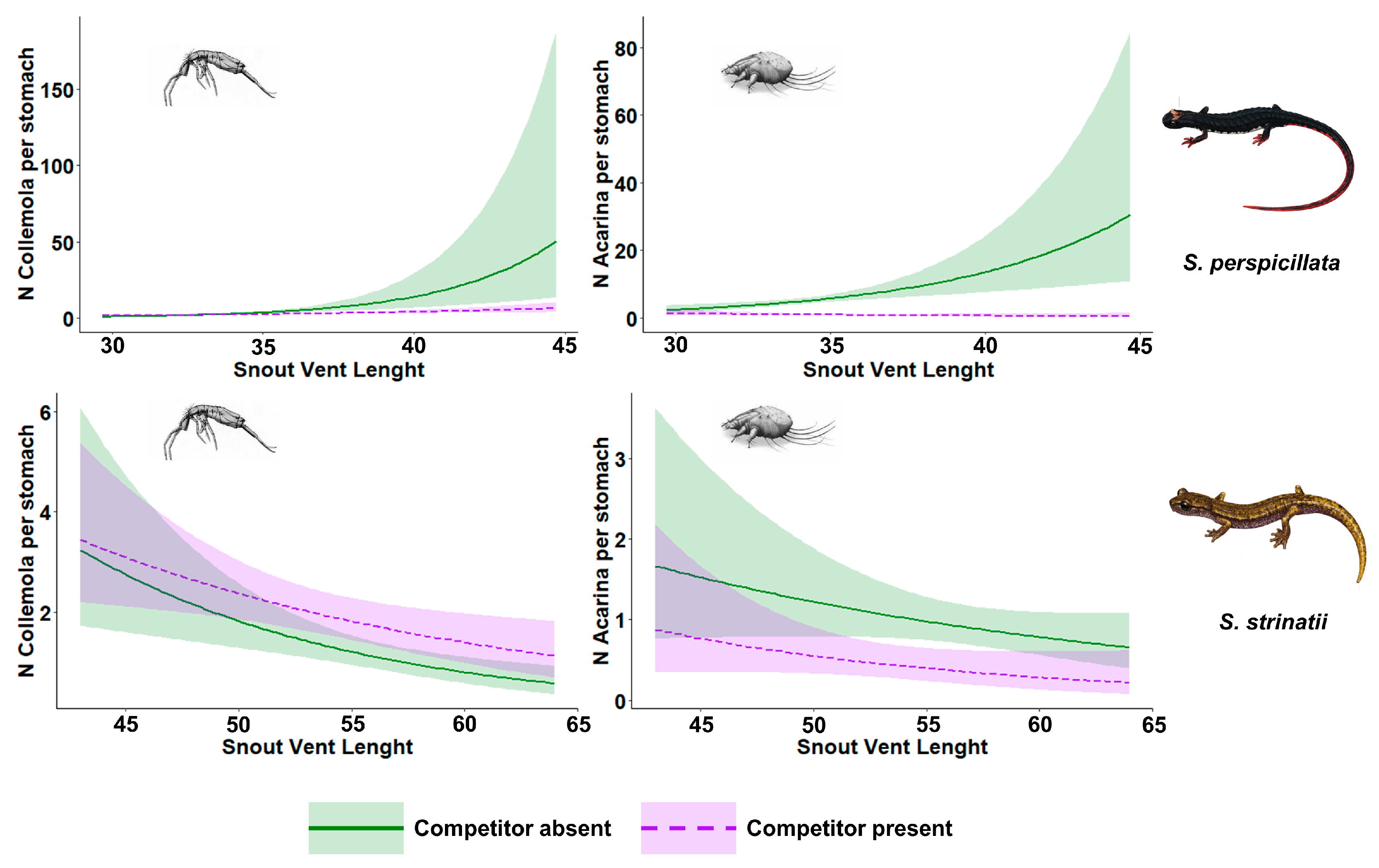
| S. perspicillata Collembola | Parameter | Estimate | Std. Error | p-Value |
| Intercept | 1.30 | 0.11 | - | |
| β–Competitor’s presence (1) | −0.36 | 0.14 | p < 0.05 * | |
| β–SVL | 0.67 | 0.16 | p < 0.001 * | |
| β–SVL * Competitor’s presence | −0.44 | 0.18 | p < 0.05 * | |
| S. perspicillata Acarina | Parameter | Estimate | Std. Error | p-value |
| Intercept | 1.75 | 0.09 | - | |
| β–Competitor’s presence (1) | −1.84 | 0.17 | p < 0.001 * | |
| β–SVL | 0.43 | 0.12 | p < 0.001 * | |
| β–SVL * Competitor’s presence | −0.61 | 0.19 | p < 0.01 * | |
| S. strinatii Collembola | Parameter | Estimate | Std. Error | p-value |
| Intercept | 4.72 | 1.32 | - | |
| β–Competitor’s presence (1) | −1.19 | 1.69 | p = 0.48 | |
| β–SVL | −0.82 | 0.23 | p < 0.01 * | |
| β–SVL * Competitor’s presence | 0.29 | 0.30 | p = 0.34 | |
| S. strinatii Acarina | Parameter | Estimate | Std. Error | p-value |
| Intercept | 2.41 | 1.57 | - | |
| β–Competitor’s presence (1) | 0.29 | 2.71 | p = 0.91 | |
| β–SVL | −0.44 | 0.28 | p = 0.11 | |
| β–SVL * Competitor’s presence | −0.22 | 0.50 | p = 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, A.; Rosa, G.; Salvidio, S. Size-Mediated Trophic Interactions in Two Syntopic Forest Salamanders. Animals 2023, 13, 1281. https://doi.org/10.3390/ani13081281
Costa A, Rosa G, Salvidio S. Size-Mediated Trophic Interactions in Two Syntopic Forest Salamanders. Animals. 2023; 13(8):1281. https://doi.org/10.3390/ani13081281
Chicago/Turabian StyleCosta, Andrea, Giacomo Rosa, and Sebastiano Salvidio. 2023. "Size-Mediated Trophic Interactions in Two Syntopic Forest Salamanders" Animals 13, no. 8: 1281. https://doi.org/10.3390/ani13081281
APA StyleCosta, A., Rosa, G., & Salvidio, S. (2023). Size-Mediated Trophic Interactions in Two Syntopic Forest Salamanders. Animals, 13(8), 1281. https://doi.org/10.3390/ani13081281








