Genetic Heterogeneity and Mutated PreS Analysis of Duck Hepatitis B Virus Recently Isolated from Ducks and Geese in China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, Virus Screening, and Isolation
2.2. PCR Amplification and Sequencing
2.3. Sequence Alignment and Mutation Analysis
2.4. Evolution and Recombination Analysis
3. Results
3.1. Sample Screening and Genome Amplification
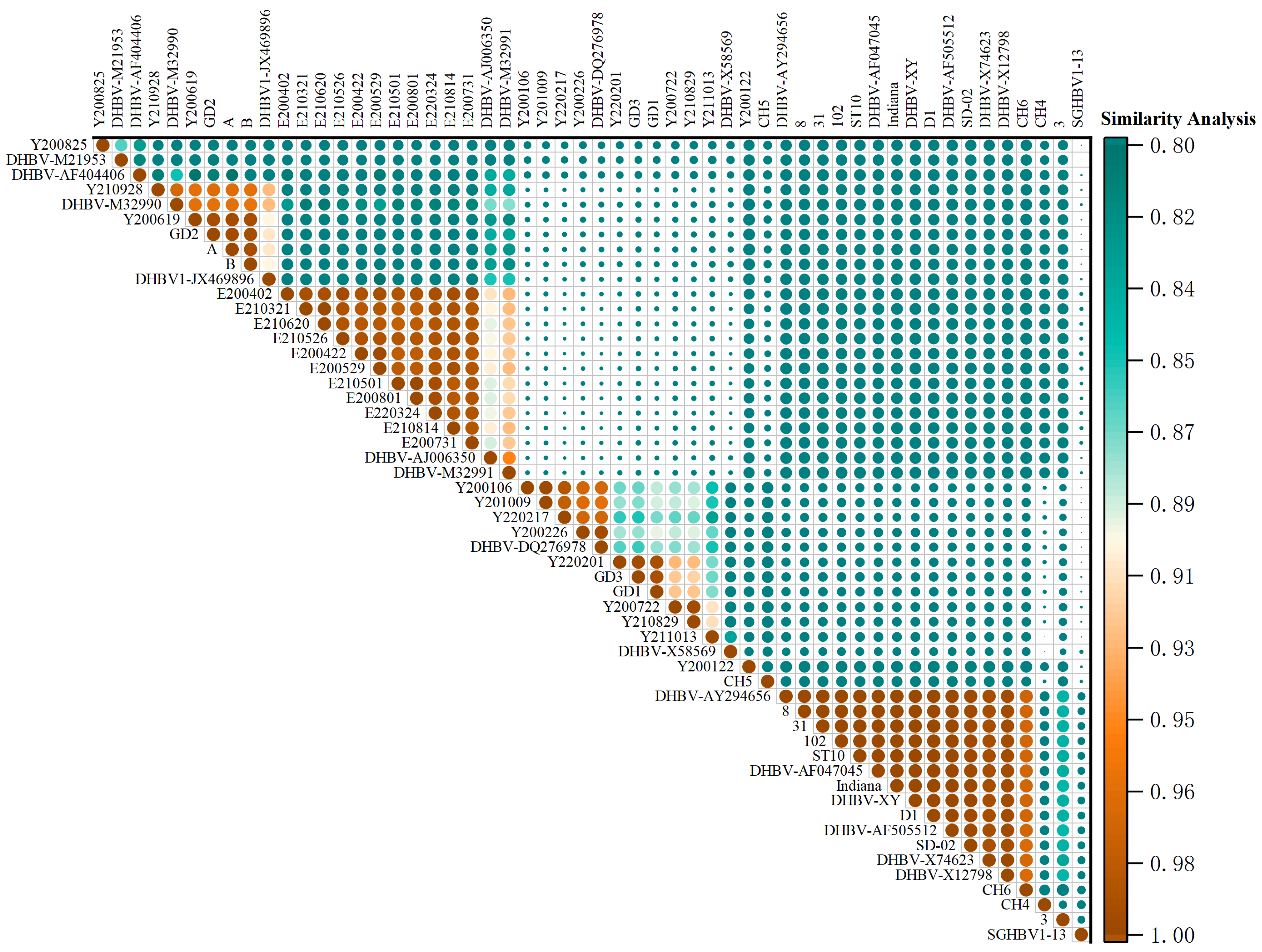
3.2. DNA Alignment and Identity Analysis
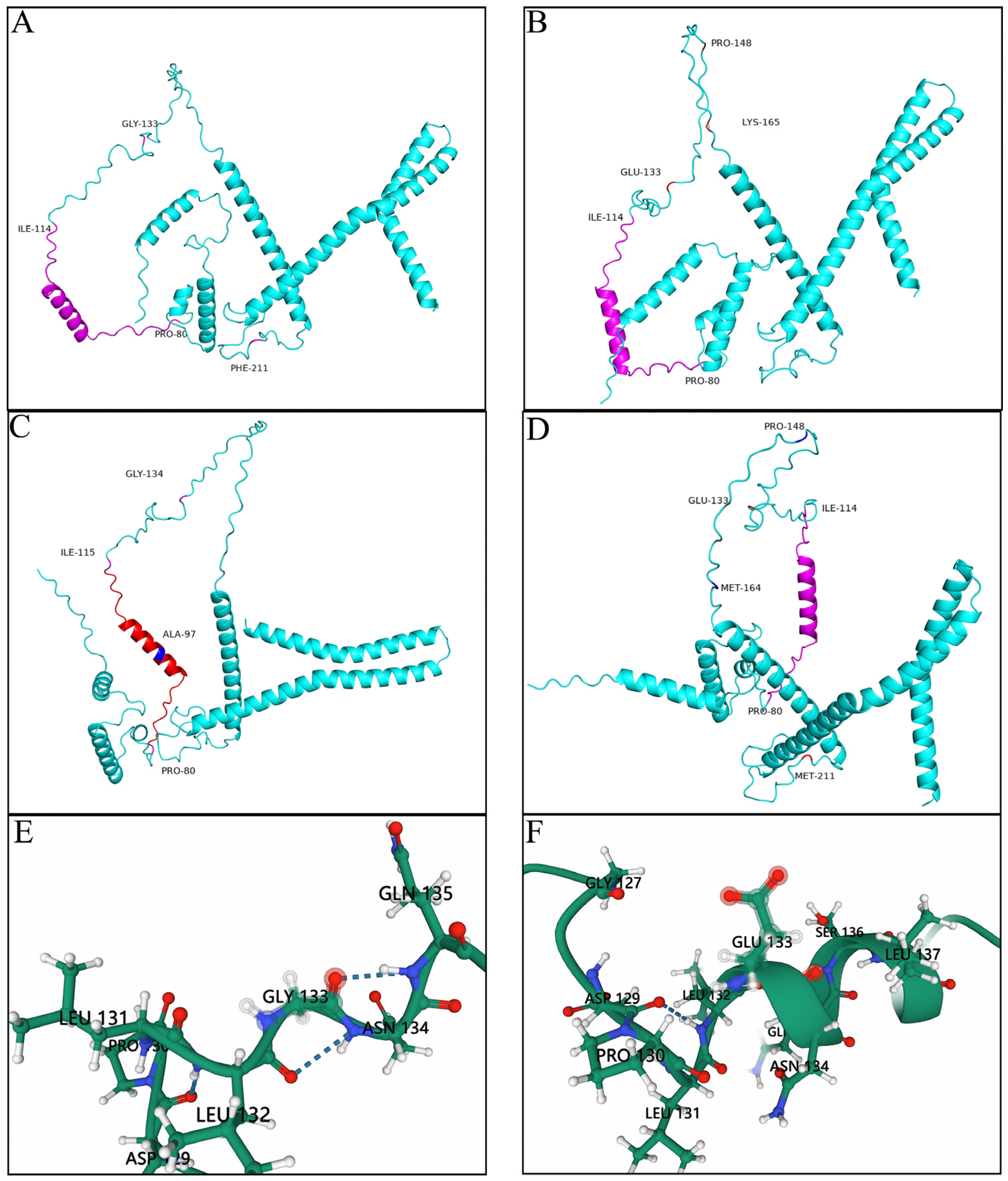
3.3. Prediction of the Tertiary Structures of the Key Mutation Loci
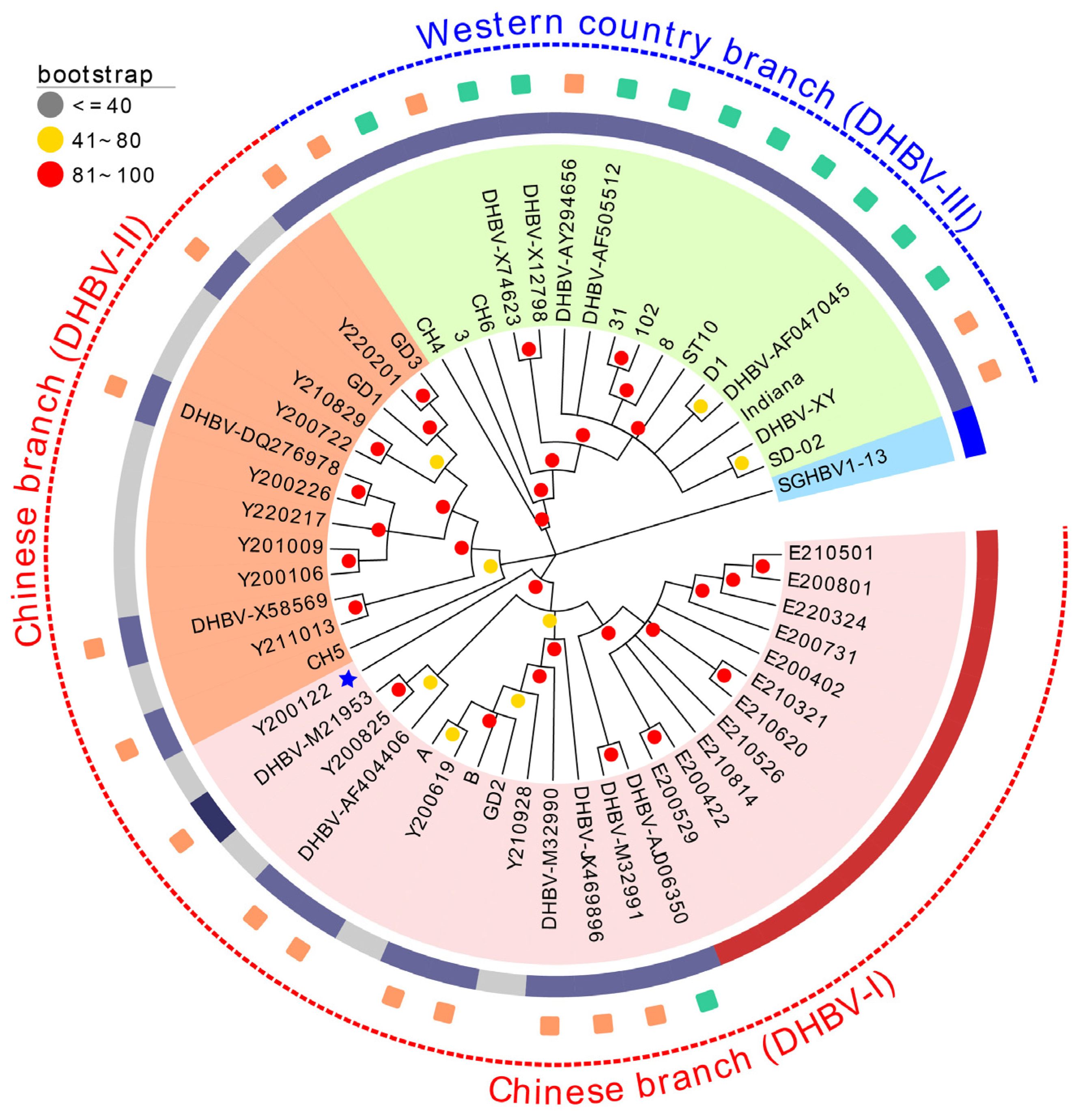
3.4. Phylogenetic Analysis and Frequency Distribution
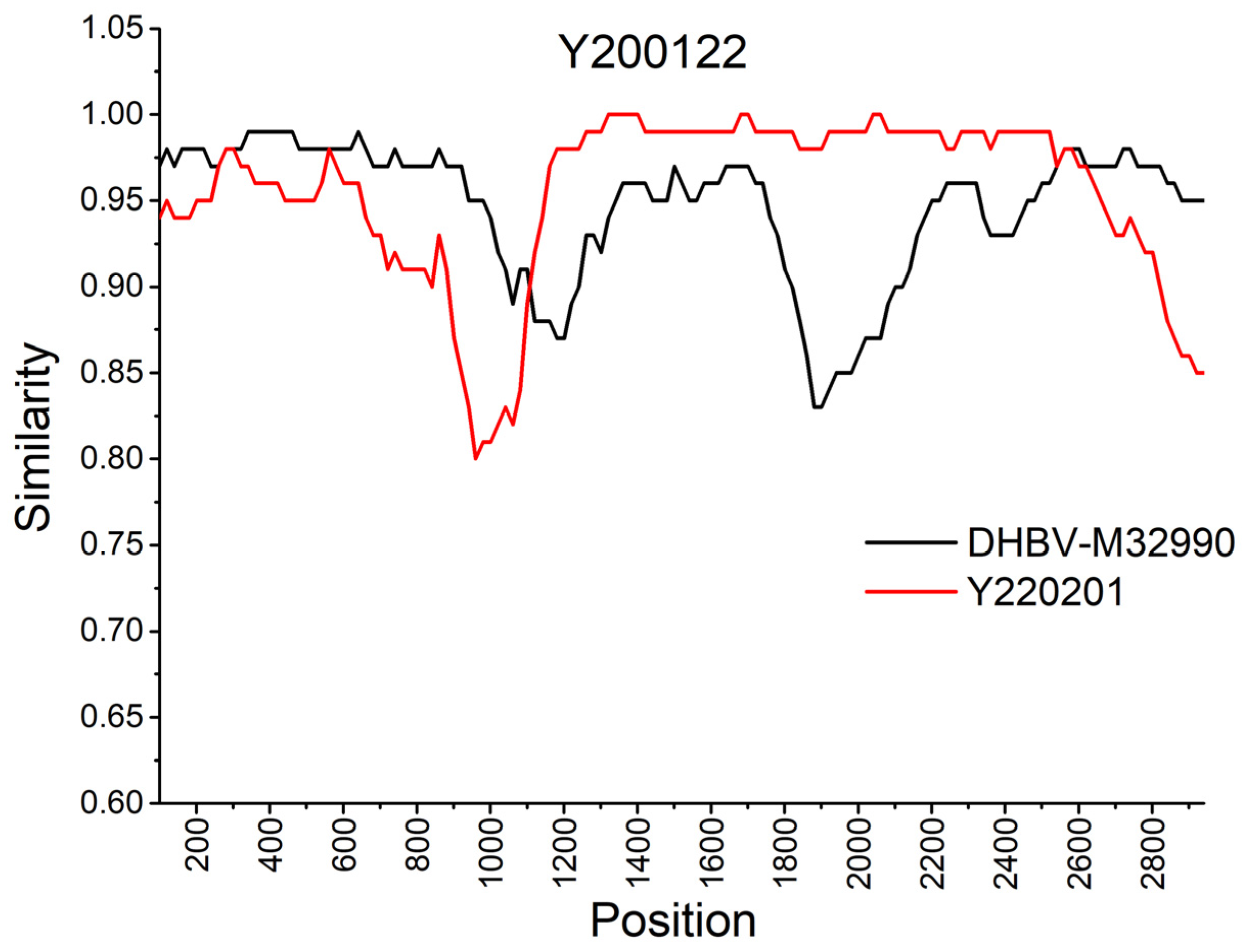
3.5. Recombination Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mason, W.S.; Seal, G.; Summers, J. Virus of Pekin ducks with structural and biological relatedness to human hepatitis B virus. J. Virol. 1980, 36, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jia, R.; Wang, M.; Huang, J.; Zhu, D.; Chen, S.; Yin, Z.; Wang, Y.; Chen, X.; Cheng, A. Cloning, expression and purification of duck hepatitis B virus (DHBV) core protein and its use in the development of an indirect ELISA for serologic detection of DHBV infection. Arch. Virol. 2014, 159, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Mangisa, N.P.; Smuts, H.E.; Kramvis, A.; Linley, C.W.; Skelton, M.; Tucker, T.J.; De La, M.; Hall, P.; Kahn, D.; Jilbert, A.R.; et al. Molecular characterization of duck hepatitis B virus isolates from South African ducks. Virus Genes 2004, 28, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Mattes, F.; Tong, S.; Teubner, K.; Blum, H.E. Complete nucleotide sequence of a German duck hepatitis B virus. Nucleic Acids Res. 1990, 18, 6140. [Google Scholar] [CrossRef]
- Triyatni, M.; Ey, P.L.; Tran, T.; Le, M.M.; Qiao, M.; Burrell, C.J.; Jilbert, A.R. Sequence comparison of an Australian duck hepatitis B virus strain with other avian hepadnaviruses. J. Gen. Virol. 2001, 82, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jia, R.; Liu, S.; Wang, M.; Zhu, D.; Chen, S.; Liu, M.; Yin, Z.; Jing, B.; Cheng, A. Complete genome sequence of the novel duck hepatitis B virus strain SCP01 from Sichuan Cherry Valley duck. Springerplus 2016, 5, 1353. [Google Scholar] [CrossRef]
- Jilbert, A.R.; Kotlarski, I. Immune responses to duck hepatitis B virus infection. Dev. Comp. Immunol. 2000, 24, 285–302. [Google Scholar] [CrossRef]
- Lenhoff, R.J.; Luscombe, C.A.; Summers, J. Competition in vivo between a cytopathic variant and a wild-type duck hepatitis B virus. Virology 1998, 251, 85–95. [Google Scholar] [CrossRef]
- Lenhoff, R.J.; Luscombe, C.A.; Summers, J. Acute liver injury following infection with a cytopathic strain of duck hepatitis B virus. Hepatology 1999, 29, 563–571. [Google Scholar] [CrossRef]
- Marion, P.L.; Knight, S.S.; Ho, B.K.; Guo, Y.Y.; Robinson, W.S.; Popper, H. Liver disease associated with duck hepatitis B virus infection of domestic ducks. Proc. Natl. Acad. Sci. USA 1984, 81, 898–902. [Google Scholar] [CrossRef]
- Funk, A.; Mhamdi, M.; Will, H.; Sirma, H. Avian hepatitis B viruses: Molecular and cellular biology, phylogenesis, and host tropism. World J. Gastroenterol. 2007, 13, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Mayerat, C.; Mantegani, A.; Frei, P.C. Does hepatitis B virus (HBV) genotype influence the clinical outcome of HBV infection. J. Viral Hepat. 1999, 6, 299–304. [Google Scholar] [CrossRef]
- Yuen, M.F.; Chen, D.S.; Dusheiko, G.M.; Janssen, H.L.A.; Lau, D.T.Y.; Locarnini, S.A.; Peters, M.G.; Lai, C.L. Hepatitis B virus infection. Nat. Rev. Dis. Prim. 2018, 4, 18035. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, J.; Liu, J.; Xie, Y. Identification of natural recombination in duck hepatitis B virus. Virus Res. 2010, 149, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.J.; Teng, W.; Chen, C.L.; Sun, C.P.; Teng, R.D.; Huang, Y.H.; Liang, K.H.; Chen, Y.W.; Lin, C.C.; Su, C.W.; et al. Clinical Implications of HBV PreS/S Mutations and the Effects of PreS2 Deletion on Mitochondria, Liver Fibrosis, and Cancer Development. Hepatology 2021, 74, 641–655. [Google Scholar] [CrossRef]
- Li, F.; Li, X.; Yan, T.; Liu, Y.; Cheng, Y.; Xu, Z.; Shao, Q.; Liao, H.; Huang, P.; Li, J.; et al. The preS deletion of hepatitis B virus (HBV) is associated with liver fibrosis progression in patients with chronic HBV infection. Hepatol. Int. 2018, 12, 107–117. [Google Scholar] [CrossRef]
- Kim, M.H.; Kang, S.Y.; Lee, W.I. Occult HBV among Anti-HBc Alone: Mutation Analysis of an HBV Surface Gene and Pre-S Gene. Yonsei Med. J. 2017, 58, 557–563. [Google Scholar] [CrossRef]
- Li, J.S.; Cova, L.; Buckland, R.; Lambert, V.; Deléage, G.; Trépo, C. Duck hepatitis B virus can tolerate insertion, deletion, and partial frameshift mutation in the distal pre-S region. J. Virol. 1989, 63, 4965–4968. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, M.; Robinson, W.S.; Marion, P.L. Duck hepatitis B virus replicates in the yolk sac of developing embryos. J. Virol. 1987, 61, 2273–2279. [Google Scholar] [CrossRef]
- O’Connell, A.P.; Urban, M.K.; London, W.T. Naturally occurring infection of Pekin duck embryos by duck hepatitis B virus. Proc. Natl. Acad. Sci. USA 1983, 80, 1703–1706. [Google Scholar] [CrossRef]
- Ji, J.; Xu, X.; Wu, Q.; Wang, X.; Li, W.; Yao, L.; Kan, Y.; Yuan, L.; Bi, Y.; Xie, Q. Simple and visible detection of duck hepatitis B virus in ducks and geese using loop-mediated isothermal amplification. Poult. Sci. 2020, 99, 791–796. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, J.; Qin, Y.; Liang, B.; Gu, Y.; Liang, L.; Liu, L.; Liu, Y.; Su, H. Glucose Homeostasis Is Dysregulated in Ducks Infected with Duck Hepatitis B Virus. Intervirology 2021, 64, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, B.; Gao, S.; Lercher, M.J.; Hu, S.; Chen, W.H. Evolview v3: A webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 2019, 47, W270–W275. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015, 1, vev003. [Google Scholar] [CrossRef]
- Ji, J.; Xu, S.; Li, W.; Xu, X.; Kan, Y.; Yao, L.; Bi, Y.; Xie, Q. Genome analysis and recombination characterization of duck hepatitis B virus isolated from ducks and geese in central China, 2017 to 2019. Poult. Sci. 2023, 102, 102641. [Google Scholar] [CrossRef]
- Robertson, D.L.; Sharp, P.M.; McCutchan, F.E.; Hahn, B.H. Recombination in HIV-1. Nature 1995, 374, 124–126. [Google Scholar] [CrossRef]
- Kalinina, O.; Norder, H.; Magnius, L.O. Full-length open reading frame of a recombinant hepatitis C virus strain from St Petersburg: Proposed mechanism for its formation. J. Gen. Virol. 2004, 85, 1853–1857. [Google Scholar] [CrossRef]
- Simmonds, P.; Midgley, S. Recombination in the genesis and evolution of hepatitis B virus genotypes. J. Virol. 2005, 79, 15467–15476. [Google Scholar] [CrossRef]
- Yang, J.; Xing, K.; Deng, R.; Wang, J.; Wang, X. Identification of Hepatitis B virus putative intergenotype recombinants by using fragment typing. J. Gen. Virol. 2006, 87, 2203–2215. [Google Scholar] [CrossRef]
- Khawaja, G.; Buronfosse, T.; Jamard, C.; Guerret, S.; Zoulim, F.; Luxembourg, A.; Hannaman, D.; Evans, C.; Hartmann, D.; Cova, L. Enhanced magnitude and breadth of neutralizing humoral response to a DNA vaccine targeting the DHBV envelope protein delivered by in vivo electroporation. Virology 2012, 425, 61–69. [Google Scholar] [CrossRef] [PubMed]
| (A) | |||||||||||||||||||||||||||||||||
| Strains Name | Substitution of amino acid residues in PreS | ||||||||||||||||||||||||||||||||
| 67 | 78 | 79 | 80 | 81 | 82 | 83 | 84 | 85 | 87 | 88 | 89 | 90 | 91 | 92 | 93 | 94 | 96 I a | 99 | 100 | 101 | 102 | 103 | 104 | 105 | 106 | 107 | 108 | 110 | 111 | 113 | 114 | 133 | |
| 31 | A | P | T | P | Q | E | I | P | Q | Q | W | T | P | E | E | D | Q | E | A | F | R | R | Y | Q | E | E | R | P | E | T | T | G | |
| Y200825 | - b | - | A | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | E | |
| Y200106 | E | L | L | L | K | R | S | L | S | S | G | L | R | K | K | I | R | L | L | S | V | V | T | R | K | K | D | H | E | T | P | P | - |
| Y201009 | E | L | L | L | K | R | S | L | S | S | G | L | R | K | K | I | R | L | L | S | D | V | T | R | K | K | D | H | E | T | P | P | - |
| Y210928 | - | - | - | - | H | - | T | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | E | |
| Y220201 | E | - | A | - | - | - | - | - | - | K | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Y211013 | E | - | A | - | - | - | - | - | - | K | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Y220217 | E | L | L | L | K | R | S | L | S | N | G | L | R | K | K | I | R | L | L | S | V | V | T | R | K | K | D | P | E | T | P | P | - |
| Y200122 | - | - | - | - | H | - | T | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Y200226 | E | - | A | - | - | - | - | - | - | K | - | - | - | - | A | - | - | K | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Y200619 | - | - | - | - | - | - | T | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | E | |
| Y200722 | E | - | A | - | - | - | - | - | - | K | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | E | |
| Y210829 | E | - | A | - | - | - | - | - | - | K | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | E | |
| E200402 | - | - | A | - | - | - | T | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | E | |
| E200422 | - | - | A | - | - | - | T | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | T | - | - | - | E | |
| E200731 | - | - | A | - | - | - | T | - | - | - | - | - | - | - | - | - | - | D | - | - | - | - | - | - | - | - | - | - | - | - | - | E | |
| E210501 | T | - | A | - | H | - | T | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | E | |
| E210321 | - | - | A | - | - | - | T | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | E | |
| E210526 | - | - | A | - | - | - | T | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | E | |
| E210620 | - | - | A | - | - | - | T | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | E | |
| E210814 | - | - | - | - | - | - | T | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | E | |
| E220324 | - | - | A | - | - | - | T | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | E | |
| E200529 | - | - | A | - | - | - | T | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | E | |
| E200801 | I | - | A | - | H | - | T | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | E | |
| (B) | |||||||||||||||||||||||||||||||||
| Strains Name | Substitution of amino acid residues in PreS | ||||||||||||||||||||||||||||||||
| 148 I a | 164 I a | 169 | 185 | 211 | 224 | 229 | 231 | 267 | 269 | 277 | 301 | 304 | 305 | 332 | 333 | 334 | |||||||||||||||||
| 31 | - b | - | S | S | K | I | V | S | G | T | A | T | L | S | Y | K | S | ||||||||||||||||
| Y200825 | P | - | - | - | - | - | A | - | - | - | - | M | - | L | F | - | N | ||||||||||||||||
| Y200106 | - | - | - | - | - | - | A | - | - | - | - | M | - | L | - | - | - | ||||||||||||||||
| Y201009 | - | - | - | - | - | - | A | - | - | - | - | M | - | L | - | - | - | ||||||||||||||||
| Y210928 | P | K | P | - | - | - | - | - | M | I | E | M | - | L | - | - | N | ||||||||||||||||
| Y220201 | P | - | - | - | T | - | - | - | - | - | M | - | L | - | - | - | |||||||||||||||||
| Y211013 | T | - | A | - | - | - | - | - | - | - | - | M | - | L | F | - | N | ||||||||||||||||
| Y220217 | - | - | - | - | - | T | A | - | - | - | - | M | - | L | - | - | N | ||||||||||||||||
| Y200122 | P | - | - | - | - | T | - | - | - | - | - | M | - | L | - | - | - | ||||||||||||||||
| Y200226 | T | - | - | - | - | T | - | - | - | - | - | M | - | L | - | - | - | ||||||||||||||||
| Y200619 | P | K | P | - | - | - | - | - | M | I | E | M | - | L | - | - | N | ||||||||||||||||
| Y200722 | T | - | A | - | - | T | - | - | - | - | - | M | - | L | F | - | N | ||||||||||||||||
| Y210829 | T | - | A | - | - | T | - | - | - | - | - | M | - | L | F | - | N | ||||||||||||||||
| E200402 | P | K | A | G | - | - | A | F | M | I | E | M | P | L | - | R | N | ||||||||||||||||
| E200422 | P | K | - | - | - | - | A | F | M | I | E | M | - | L | - | R | N | ||||||||||||||||
| E200731 | P | K | A | G | - | - | A | Y | M | I | E | M | - | L | - | - | N | ||||||||||||||||
| E210501 | P | K | A | G | - | - | A | F | M | I | E | M | - | L | - | - | N | ||||||||||||||||
| E210321 | P | K | A | G | E | - | A | F | M | I | E | M | - | L | - | - | N | ||||||||||||||||
| E210526 | P | K | A | G | E | - | A | F | M | I | E | M | - | L | - | R | N | ||||||||||||||||
| E210620 | P | K | A | G | E | - | A | F | M | I | E | M | - | L | - | - | N | ||||||||||||||||
| E210814 | P | K | A | G | - | - | A | F | M | I | E | M | - | L | - | R | N | ||||||||||||||||
| E220324 | P | K | A | G | E | - | A | F | M | I | E | M | - | L | - | - | N | ||||||||||||||||
| E200529 | P | K | - | G | - | - | A | F | M | I | E | M | - | L | - | R | N | ||||||||||||||||
| E200801 | P | K | A | G | - | - | A | F | M | I | E | M | - | L | - | - | N | ||||||||||||||||
| Event | RDP | GENOCOV | BOOTSCAN | MaxChi | CHIMAERA | SISCAN | 3Seq |
|---|---|---|---|---|---|---|---|
| I | 6.90 × 10−8 | 2.08 × 10−3 | 1.40 × 10−4 | 2.28 × 10−8 | 4.92 × 10−7 | 1.12 × 10−7 | 6.13 × 10−10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Mu, X.; Xu, X.; Bi, C.; Ji, J.; Kan, Y.; Yao, L.; Bi, Y.; Xie, Q. Genetic Heterogeneity and Mutated PreS Analysis of Duck Hepatitis B Virus Recently Isolated from Ducks and Geese in China. Animals 2023, 13, 1282. https://doi.org/10.3390/ani13081282
Xu S, Mu X, Xu X, Bi C, Ji J, Kan Y, Yao L, Bi Y, Xie Q. Genetic Heterogeneity and Mutated PreS Analysis of Duck Hepatitis B Virus Recently Isolated from Ducks and Geese in China. Animals. 2023; 13(8):1282. https://doi.org/10.3390/ani13081282
Chicago/Turabian StyleXu, Shuqi, Xinhao Mu, Xin Xu, Congying Bi, Jun Ji, Yunchao Kan, Lunguang Yao, Yingzuo Bi, and Qingmei Xie. 2023. "Genetic Heterogeneity and Mutated PreS Analysis of Duck Hepatitis B Virus Recently Isolated from Ducks and Geese in China" Animals 13, no. 8: 1282. https://doi.org/10.3390/ani13081282
APA StyleXu, S., Mu, X., Xu, X., Bi, C., Ji, J., Kan, Y., Yao, L., Bi, Y., & Xie, Q. (2023). Genetic Heterogeneity and Mutated PreS Analysis of Duck Hepatitis B Virus Recently Isolated from Ducks and Geese in China. Animals, 13(8), 1282. https://doi.org/10.3390/ani13081282







