Activity of Patchouli and Tea Tree Essential Oils against Staphylococci Isolated from Pyoderma in Dogs and Their Synergistic Potential with Gentamicin and Enrofloxacin
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain Origin and Identification
2.2. Antimicrobial Susceptibility Testing
2.3. Antibiotics and Essential Oils Analysis
2.4. Activity of Antibiotics and Essential Oils against Staphylococci
2.4.1. Individual MIC
2.4.2. Checkerboards
2.4.3. Fractional Inhibitory Concentrations
2.5. Qualitative Analysis of the Composition of Essential Oils
3. Results
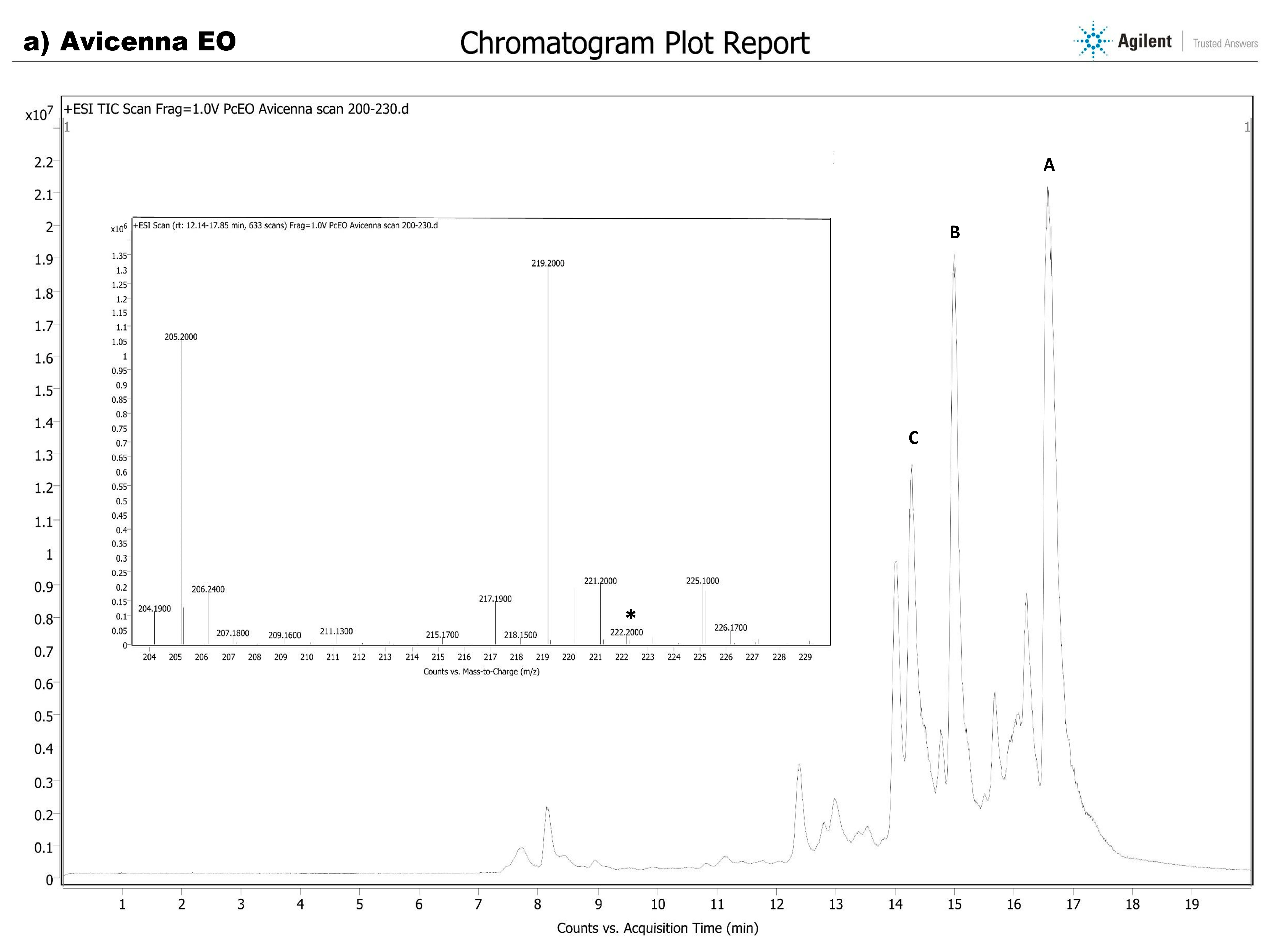
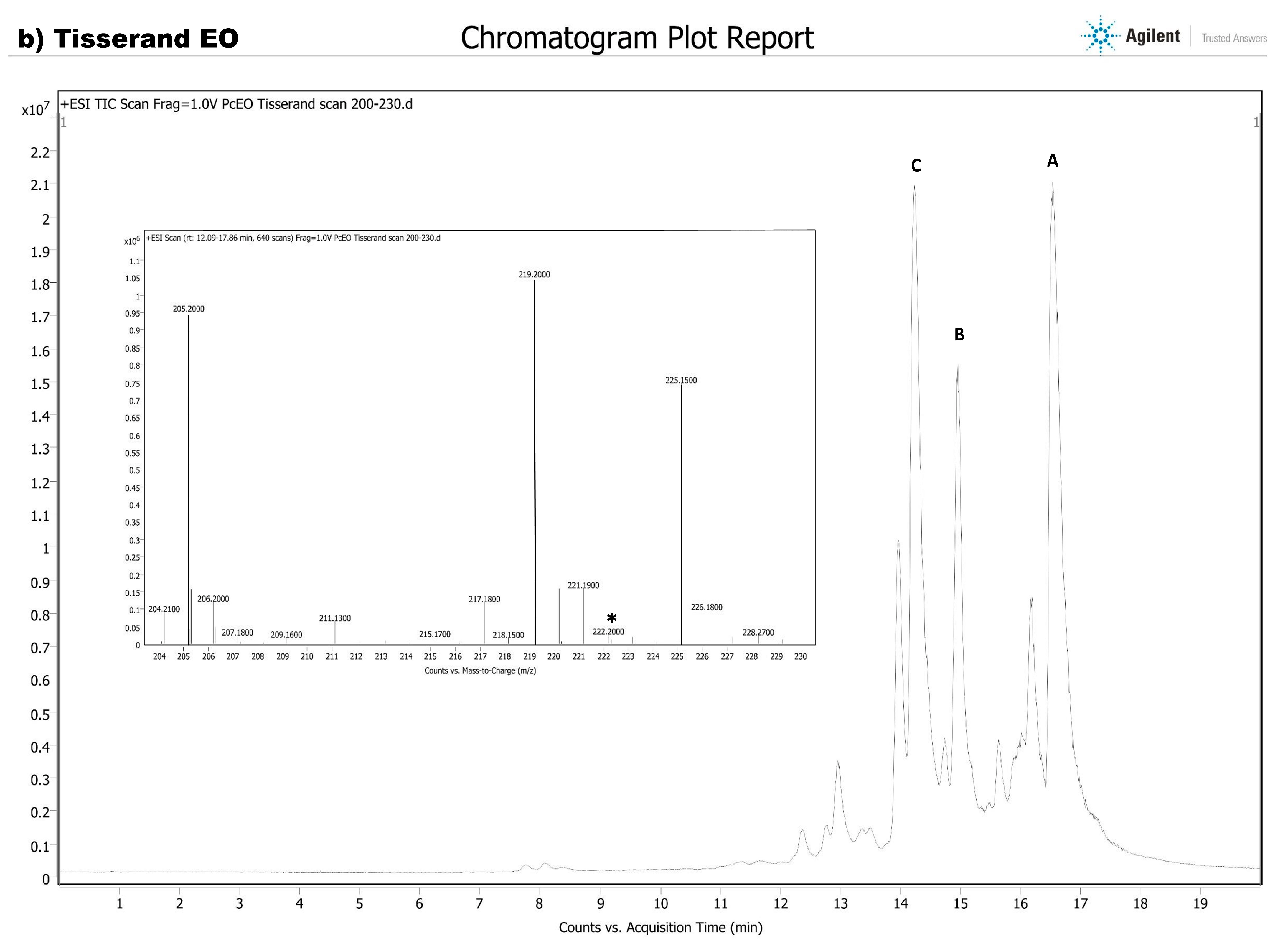
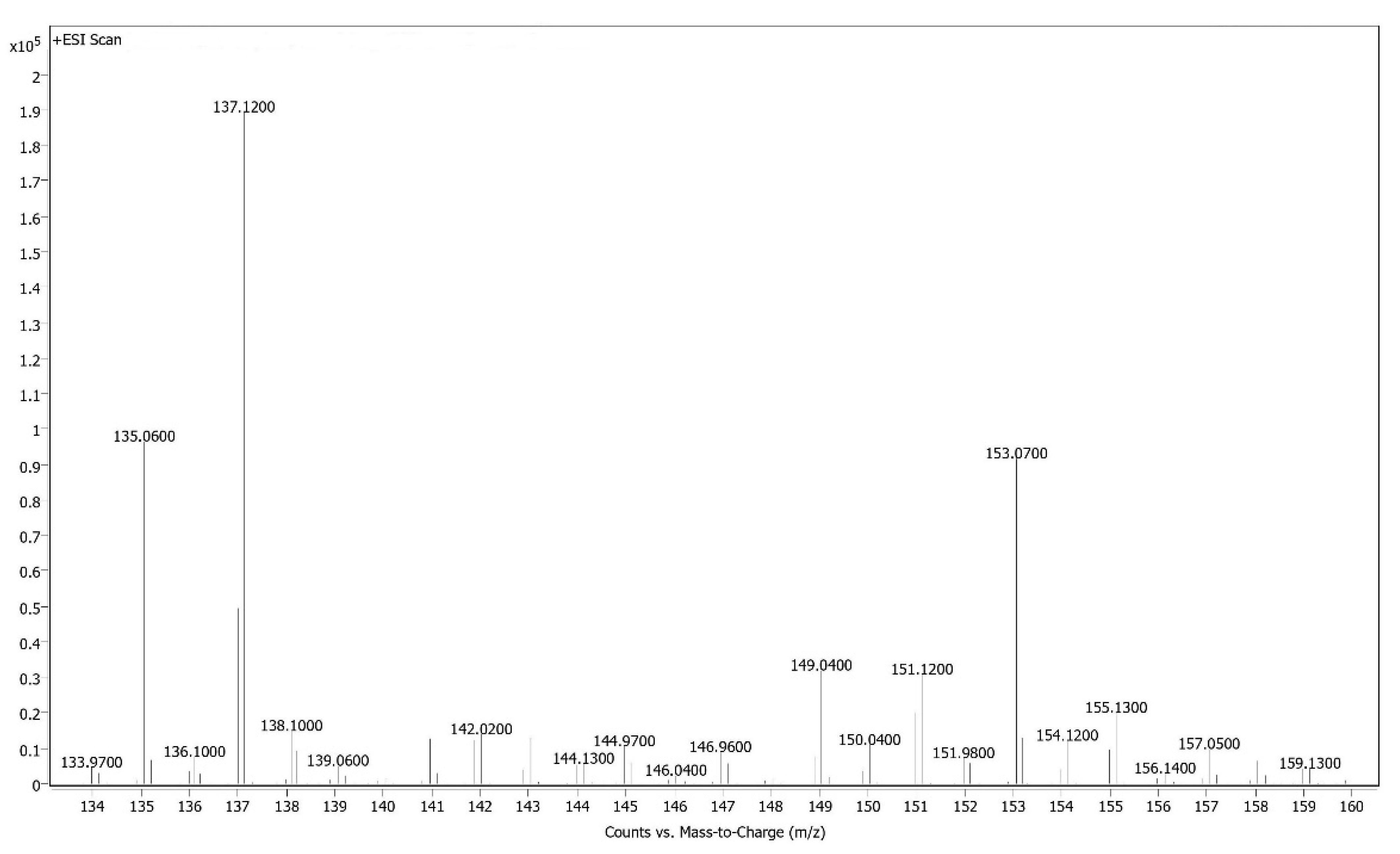
HPLC-MS Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loeffler, A.; Lloyd, D.H. What has changed in canine pyoderma? A narrative review. Vet. J. 2018, 235, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Seckerdieck, F.; Mueller, R.S. Recurrent pyoderma and its underlying primary diseases: A retrospective evaluation of 157 dogs. Vet. Rec. 2018, 182, 434. [Google Scholar] [CrossRef] [PubMed]
- Mesman, M.L.; Kirby, A.L.; Rosenkrantz, W.S.; Griffin, C.E. Residual antibacterial activity of canine hair treated with topical antimicrobial sprays against Staphylococcus pseudintermedius in vitro. Vet. Dermatol. 2016, 27, 261-e61. [Google Scholar] [CrossRef] [PubMed]
- Löwenstein, C. Pyoderma in dogs. Tierärztl. Prax. 2011, 39, 405–417. [Google Scholar]
- Szewczuk, M.A.; Zych, S.; Sablik, P. Participation and drug resistance of coagulase-positive staphylococci isolated from cases of pyoderma and otitis externa in dogs. Slov. Vet. Res. 2020, 57, 33–43. [Google Scholar]
- Beco, L.; Guaguère, E.; Lorente Méndez, C.; Noli, C.; Nuttall, T.; Vroom, M. Suggested guidelines for using systemic antimicrobials in bacterial skin infections: Part 2-antimicrobial choice, treatment regimens and compliance. Vet. Rec. 2013, 172, 156–160. [Google Scholar] [CrossRef]
- Nocera, F.P.; Mancini, S.; Najar, B.; Bertelloni, F.; Pistelli, L.; De Filippis, A.; Fiorito, F.; De Martino, L.; Fratini, F. Antimicrobial activity of some essential oils against methicillin-susceptible and methicillin-resistant Staphylococcus pseudintermedius-associated pyoderma in dogs. Animals 2020, 10, 1782. [Google Scholar] [CrossRef]
- Börjesson, S.; Gómez-Sanz, E.; Ekström, K.; Torres, C.; Grönlund, U. Staphylococcus pseudintermedius can be misdiagnosed as Staphylococcus aureus in humans with dog bite wounds. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 839–844. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Kaloustian, J.; Chevalier, J.; Mikail, C.; Martino, M.; Abou, L.; Vergnes, M.F. Étude de six huiles essentielles composition chimique et activité antibactérienne. Phytothérapie 2008, 6, 160–164. [Google Scholar] [CrossRef]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella Typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Stefanakis, M.K.; Touloupakis, E.; Anastasopoulos, E.; Ghanotakis, D.; Katerinopoulos, H.E.; Makridis, P. Antibacterial activity of essential oils from plants of the genus Origanum. Food Control 2013, 34, 539–546. [Google Scholar] [CrossRef]
- Van Vuuren, S.; Viljoen, A. Plant-based antimicrobial studies—Methods and approaches to study the interaction between natural products. Planta Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef]
- Sasaki, T.; Tsubakishita, S.; Tanaka, Y.; Sakusabe, A.; Ohtsuka, M.; Hirotaki, S.; Kawakami, T.; Fukata, T.; Hiramatsu, K. Multiplex-PCR method for species identification of coagulase-positive staphylococci. J. Clin. Microbiol. 2010, 48, 765–769. [Google Scholar] [CrossRef]
- Ruzauskas, M.; Couto, N.; Pavilonis, A.; Klimiene, I.; Siugzdiniene, R.; Virgailis, M.; Vaskeviciute, L.; Anskiene, L.; Pomba, C. Characterization of Staphylococcus pseudintermedius isolated from diseased dogs in Lithuania. Pol. J. Vet. Sci. 2016, 19, 7–14. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, M100, 31st ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, VET01S, 5th ed.; CLSI Supplement: Wayne, PA, USA, 2020. [Google Scholar]
- Mi, H.; Wang, D.; Xue, Y.; Zhang, Z.; Niu, J.; Hong, Y.; Drlica, K.; Zhao, X. Dimethyl sulfoxide protects Escherichia coli from rapid antimicrobial-mediated killing. Antimicrob. Agents Chemother. 2016, 60, 5054–5058. [Google Scholar] [CrossRef]
- Ermaya, D.; Sari, S.P.; Patria, A.; Hidayat, F.; Razi, F. Identification of patchouli oil chemical components as the results on distillation using GC-MS. IOP Conf. Ser. Earth Environ. Sci. 2019, 365, 012039. [Google Scholar] [CrossRef]
- Santos, L.L.; Brandão, B.L.; Martins, L.R.; Rabelo, M.E.; Rodrigues, L.A.B.; Araújo, C.V.C.M.; Sobral, F.T.; Galardo, R.A.K.; Ameida, M.S.S.S. Evaluation of the larvicidal potential of the essential oil Pogostemon cablin (Blanco) Benth in the control of Aedes aegypti. Pharmaceuticals 2019, 12, 53. [Google Scholar] [CrossRef]
- Souhoka, F.A.; Al Aziz, A.Z.; Nazudin, N. Patchouli oil isolation and identification of chemical components using GC-MS. Indo. J. Chem. Res. 2020, 8, 108–113. [Google Scholar] [CrossRef]
- Xie, B.; Wu, X.F.; Luo, H.T.; Huang, X.L.; Huang, F.; Zhang, Q.Y.; Zhou, X.; Wu, H.Q. Chemical profiling and quality evaluation of Pogostemon cablin Benth by liquid chromatography tandem mass spectrometry combined with multivariate statistical analysis. J. Pharm. Biomed. Anal. 2022, 209, 114526. [Google Scholar] [CrossRef] [PubMed]
- Deguerry, F.; Pastore, L.; Wu, S.; Clark, A.; Chappell, J.; Schalk, M. The diverse sesquiterpene profile of patchouli, Pogostemon cablin, is correlated with a limited number of sesquiterpene synthases. Arch. Biochem. Biophys. 2006, 454, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, L.; Tang, K.; Xu, M.; Miao, Z. Matching is the key factor to improve the production of patchoulol in the plant chassis of Marchantia paleacea. ACS Omega 2020, 51, 33028–33038. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, T.A.; Joulain, D. The essential oil of patchouli, Pogostemon cablin: A review. Flavour Fragr. J. 2018, 33, 6–51. [Google Scholar] [CrossRef]
- De Groot, A.C.; Schmidt, E. Tea tree oil: Contact allergy and chemical composition. Contact Dermat. 2016, 75, 129–143. [Google Scholar] [CrossRef]
- ISO 4730:2017-02; Essential Oil of Melaleuca Terpinen-4-ol Type (Tea Tree Oil). International Organisation for Standardisation: Geneva, Switzerland, 2017.
- 32019R0006; Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on Veterinary Medicinal Products and Repealing Directive 2001/82/EC. Official Journal of the European Union: Luxembourg, 2018.
- Lima, C.O.; Barreto, H.M.; de Oliveira Lima, E.; de Souza, E.L.; de Siqueira, J.P., Jr. Antimicrobial effect of the essential oil from Rosmarinus officinalis L. against Staphylococcus pseudintermedius isolated from dogs. Rev. Bras. Biocienc. 2013, 11, 280–283. [Google Scholar]
- Ebani, V.V.; Bertelloni, F.; Najar, B.; Nardoni, S.; Pistelli, L.; Mancianti, F. Antimicrobial activity of essential oils against Staphylococcus and Malassezia strains isolated from canine dermatitis. Microorganisms 2020, 8, 252. [Google Scholar] [CrossRef]
- Junren, C.; Xiaofang, X.; Mengting, L.; Qiuyun, X.; Gangmin, L.; Huiqiong, Z.; Guanru, C.; Xin, X.; Yanpeng, Y.; Fu, P.; et al. Pharmacological activities and mechanisms of action of Pogostemon cablin Benth: A review. Chin. Med. 2021, 16, 5. [Google Scholar] [CrossRef]
- Bilcu, M.; Grumezescu, A.M.; Oprea, A.E.; Popescu, R.C.; Mogoșanu, G.D.; Hristu, R.; Stanciu, G.A.; Mihailescu, D.F.; Lazar, V.; Bezirtzoglou, E.; et al. Efficiency of vanilla, patchouli and ylang essential oils stabilized by iron oxide@C14 nanostructures against bacterial adherence and biofilms formed by Staphylococcus aureus and Klebsiella pneumoniae clinical strains. Molecules 2014, 19, 17943–17956. [Google Scholar] [CrossRef]
- Karimi, A. Characterization and antimicrobial activity of patchouli essential oil extracted from Pogostemon cablin [Blanco] Benth. [lamiaceae]. Adv. Environ. Biol. 2014, 8, 2301–2309. [Google Scholar]
- Szweda, P.; Zalewska, M.; Pilch, J.; Kot, B.; Milewski, S. Essential oils as potential anti-staphylococcal agents. Acta Vet. Beogr. 2018, 68, 95–107. [Google Scholar]
- Yang, X.; Zhang, X.; Yang, S.P.; Liu, W.Q. Evaluation of the antibacterial activity of patchouli oil. Iran J. Pharm. Res. 2013, 12, 307–316. [Google Scholar] [PubMed]
- Tadtong, S.; Puengseangdee, C.; Prasertthanawut, S.; Hongratanaworakit, T. Antimicrobial constituents and effects of blended eucalyptus, rosemary, patchouli, pine, and cajuput essential oils. Nat. Prod. Commun. 2016, 11, 267–270. [Google Scholar] [CrossRef] [PubMed]
- May, J.; Chan, C.H.; King, A.; Williams, L.; French, G.L. Time-kill studies of tea tree oils on clinical isolates. J. Antimicrob. Chemother. 2000, 45, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Mee, B.J.; Riley, T.V. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob. Agents Chemother. 2002, 46, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.R.S. In vitro activities of five plant essential oils against methicillin–resistant Staphylococcus aureus and vancomycin—Resistant Enterococcus faecium. J. Antimicrob. Chemother. 1997, 40, 305–306. [Google Scholar] [CrossRef]
- Harkenthal, M.; Reichling, J.; Geiss, H.K.; Saller, R. Comparative study on the in vitro antibacterial activity of Australian tea tree oil, niaouli oil, manuka oil, kanuka oil, and eucalyptus oil. Pharmazie 1999, 54, 460–463. [Google Scholar]
- Oliva, A.; Costantini, S.; De Angelis, M.; Garzoli, S.; Božović, M.; Mascellino, M.T.; Vullo, V.; Ragno, R. High potency of Melaleuca alternifolia essential oil against multi-drug resistant Gram-negative bacteria and methicillin-resistant Staphylococcus aureus. Molecules 2018, 23, 2584. [Google Scholar] [CrossRef]
- De Martini, M.C.A.; Suthovski, G.; Catarina, A.S.; Fagundes, K.R.; Giotto, C.C.; Schmitz, E.P.S.; Gallina, A.L.; Azevedo, M.G.B.; Starikoff, K.R.; Champion, T.; et al. Antimicrobial activity of essential oils against positive coagulase Staphylococcus isolated from external canine otitis cases. Rev. Agric. Acad. 2021, 4, 43–52. [Google Scholar] [CrossRef]
- Mann, M.C.; Markham, L.J. A new method for determining the minimum inhibitory concentration of essential oils. J. Appl. Microbiol. 1998, 84, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Benjamin, J.C.; Lawrence, R. Antibacterial activity of tea tree (Melaleuca alternifolia) oil against methicillin resistant Staphylococcus aureus. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 1116–1123. [Google Scholar]
- Meroni, G.; Cardin, E.; Rendina, C.; Herrera Millar, V.R.; Soares Filipe, J.F.; Martino, P.A. In vitro efficacy of essential oils from Melaleuca alternifolia and Rosmarinus officinalis, Manuka honey-based gel, and Propolis as antibacterial agents against canine Staphylococcus pseudintermedius strains. Antibiotics 2020, 9, 344. [Google Scholar] [CrossRef]
- Valentine, B.K.; Dew, W.; Yu, A.; Weese, J.S. In vitro evaluation of topical biocide and antimicrobial susceptibility of Staphylococcus pseudintermedius from dogs. Vet. Dermatol. 2012, 23, 493-e95. [Google Scholar] [CrossRef]
- Han, J.I.; Park, S.J.; Kim, S.G.; Park, H.M. Antimicrobial effects of topical skin cream containing natural oil mixtures against Staphylococcus pseudintermedius and Malassezia pachydermatis. Vet. Med. 2015, 60, 202–207. [Google Scholar] [CrossRef]
- Loughlin, R.; Gilmore, B.F.; McCarron, P.A.; Tunney, M.M. Comparison of the cidal activity of tea tree oil and terpinen-4-ol against clinical bacterial skin isolates and human fibroblast cells. Lett. Appl. Microbiol. 2008, 46, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Effects of Melaleuca alternifolia (tea tree) essential oil and the major monoterpene component terpinen-4-ol on the development of single-and multistep antibiotic resistance and antimicrobial susceptibility. Antimicrob. Agents Chemother. 2012, 56, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, L.; Figueiredo, P.; Souza, H.; Sousa, A.; Andrade-Júnior, F.; Medeiros, D.; Nóbrega, J.; Silva, D.; Martins, E.; Barbosa-Filho, J.; et al. Terpinen-4-ol as an antibacterial and antibiofilm agent against Staphylococcus aureus. Int. J. Mol. Sci. 2020, 21, 4531. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, M.A.W.; Dokla, E.M.; Serya, R.A.; Abouzid, K.A. Penicillin binding protein 2a: An overview and a medicinal chemistry perspective. Eur. J. Med. Chem. 2020, 199, 112312. [Google Scholar] [CrossRef]
- Jentzsch, P.V.; Ramos, L.A.; Ciobota, V. Detection of essential oils adulteration: A quick overview and current challenges. Am. J. Biomed. Sci. Res. 2019, 15, 10–11. [Google Scholar] [CrossRef]
- Pierson, M.; Fernandez, X.; Antoniotti, S. Type and magnitude of non-compliance and adulteration in neroli, mandarin and bergamot essential oils purchased on-line: Potential consumer vulnerability. Sci. Rep. 2021, 11, 11096. [Google Scholar] [CrossRef] [PubMed]
- Capetti, F.; Marengo, A.; Cagliero, C.; Liberto, E.; Bicchi, C.; Rubiolo, P.; Sgorbini, B. Adulteration of essential oils: A multitask issue for quality control. Three case studies: Lavandula angustifolia Mill., Citrus limon (L.) Osbeck and Melaleuca alternifolia (Maiden & Betche) Cheel. Molecules 2021, 26, 5610. [Google Scholar] [PubMed]
| Antimicrobial Agents or Tests | Staphylococci | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sa ATCC 25923 | Sa 1 | Sa 2 | Sa 3 | Sa 4 | Sa 5 | Sa 6 | Sa 7 | Sps ED99 | Sps 1 | Sps 2 | Sps 3 | Sps 4 | Sps 5 | Sps 6 | Sps 7 | Sps 8 | Sps 9 | Sps 10 | Sps 11 | Sps 12 | |
| Penicillins (penicillin G, amoxicillin) | S | R | R | R | S | S | S | S | S | R | R | R | R | R | R | R | R | R | S | S | S |
| amoxicillin potentiated with clavulanic acid | S | R | R | R | S | S | S | S | S | R | R | R | R | R | R | R | R | S | S | S | S |
| Cephalosporins (cefalexin) | S | R | R | R | S | S | S | S | S | R | R | R | R | R | R | R | R | S | S | S | S |
| tetracyclines (doxycycline, oxytetracycline) | S | R | I | S | S | S | S | S | S | R | R | R | I | I | R | S | I | S | S | S | S |
| sulfamethoxazole potentiated with trimethoprim | S | R | I | I | S | S | S | S | S | R | R | I | R | R | R | R | I | I | S | S | S |
| Aminoglycosides (neomycin, gentamicin, amikacin) | S | S | S | R | S | S | S | S | S | R | R | R | R | R | R | R | R | R | S | S | R |
| Quinolones (enrofloxacin, marbofloxacin, ciprofloxacin) | S | R | I | R | S | S | S | S | S | R | R | R | R | R | R | R | R | S | R | S | S |
| polymyxins (polymyxin B) | R | R | R | R | R | R | R | R | S | S | S | S | S | S | S | S | S | S | S | S | S |
| cefoxitin (disk diffusion method) | S | R | R | R | S | S | S | S | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| oxacillin (disk diffusion method) | [S] | [R] | [R] | [R] | [S] | [S] | [S] | [S] | S | R | R | R | R | R | R | R | R | S | S | S | S |
| M-PCR (bp) | 359 | 359 | 359 | 359 | 359 | 359 | 359 | 359 | 926 | 926 | 926 | 926 | 926 | 926 | 926 | 926 | 926 | 926 | 926 | 926 | 926 |
| mecA | - | + | + | + | - | - | - | - | - | + | + | + | + | + | + | + | + | - | - | - | - |
| blaZ | - | + | + | + | - | - | - | - | - | + | + | + | + | + | + | + | + | + | - | - | - |
| coagulase (rabbit serum) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Latex Agglutination Test | + | + | + | + | + | + | + | + | - | ± | ± | ± | ± | ± | ± | ± | ± | - | - | - | - |
| mannitol (anaerobic) | + | + | + | + | + | + | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Strain | MIC | |||
|---|---|---|---|---|
| Gentamicin | Enrofloxacin | Patchouli | Tea Tree | |
| μg/mL | μg/mL | % v/v (mg/mL) | % v/v (mg/mL) | |
| Reference strain: Staphylococcus aureus ATCC 25923 | 0.5 ÷ 1 | ≤0.125 | 0.125 ÷ 0.25 (1.2 ÷ 2.4) | 1.25 ÷ 2.5 (11.2 ÷ 22.4) |
| Staphylococcus aureus isolate Sa 1 | 0.5 ÷ 2 | 4 ÷ 8 | 0.25 ÷ 0.5 (2.4 ÷ 4.8) | 2.5 ÷ 5 (22.4 ÷ 44.8) |
| Staphylococcus aureus isolate Sa 2 | 0.5 ÷ 1 | 2 ÷ 4 | 0.25 ÷ 0.5 (2.4 ÷ 4.8) | 5 ÷ 10 (44.8 ÷ 89.6) |
| Staphylococcus aureus isolate Sa 3 | 8 ÷ 16 | 16 ÷ 32 | 0.25 ÷ 0.5 (2.4 ÷ 4.8) | 2.5 ÷ 5 (22.4 ÷ 44.8) |
| Staphylococcus aureus isolate Sa 4 | 0.5 ÷ 1 | 0.125 ÷ 0.25 | 0.25 ÷ 0.5 (2.4 ÷ 4.8) | 1.25 ÷ 2.5 (11.2 ÷ 22.4) |
| Staphylococcus aureus isolate Sa 5 | 0.25 ÷ 0.5 | 0.5 ÷ 1 | 0.125 ÷ 0.25 (1.2 ÷ 2.4) | 1.25 ÷ 2.5 (11.2 ÷ 22.4) |
| Staphylococcus aureus isolate Sa 6 | 0.125 ÷ 0.25 | 0.125 ÷ 0.25 | 0.125 ÷ 0.25 (1.2 ÷ 2.4) | 0.625 ÷ 1.25 (5.6 ÷ 11.2) |
| Staphylococcus aureus isolate Sa 7 | 0.0625 ÷ 0.125 | 0.032 ÷ 0.125 | 0.125 ÷ 0.25 (1.2 ÷ 2.4) | 0.625 ÷ 1.25 (5.6 ÷ 11.2) |
| Type strain: Staphylococcus pseudintermedius ED99 | 0.5 ÷ 1 | 0.125 ÷ 0.25 | 0.25 ÷ 0.5 (2.4 ÷ 4.8) | 1.25 ÷ 2.5 (11.2 ÷ 22.4) |
| Staphylococcus pseudintermedius isolate Sps 1 | 32 ÷ 64 | 32 ÷ 64 | 0.25 ÷ 0.5 (2.4 ÷ 4.8) | 1.25 ÷ 5 (11.2 ÷ 44.8) |
| Staphylococcus pseudintermedius isolate Sps 2 | 32 ÷ 64 | 32 ÷ 64 | 0.25 ÷ 0.5 (2.4 ÷ 4.8) | 1.25 ÷ 2.5 (11.2 ÷ 22.4) |
| Staphylococcus pseudintermedius isolate Sps 3 | 64 ÷ 128 | 32 ÷ 64 | 0.25 ÷ 0.5 (2.4 ÷ 4.8) | 1.25 ÷ 2.5 (11.2 ÷ 22.4) |
| Staphylococcus pseudintermedius isolate Sps 4 | 32 ÷ 64 | 32 ÷ 64 | 0.25 ÷ 0.5 (2.4 ÷ 4.8) | 0.625 ÷ 1.25 (5.6 ÷ 11.2) |
| Staphylococcus pseudintermedius isolate Sps 5 | 128 ÷ 256 | 32 ÷ 64 | 0.25 ÷ 0.5 (2.4 ÷ 4.8) | 1.25 ÷ 5 (11.2 ÷ 44.8) |
| Staphylococcus pseudintermedius isolate Sps 6 | 64 ÷ 128 | 32 ÷ 64 | 0.25 ÷ 0.5 (2.4 ÷ 4.8) | 2.5 ÷ 5 (22.4 ÷ 44.8) |
| Staphylococcus pseudintermedius isolate Sps 7 | 32 ÷ 128 | 64 ÷ 128 | 0.25 ÷ 0.5 (2.4 ÷ 4.8) | 0.625 ÷ 1.25 (5.6 ÷ 11.2) |
| Staphylococcus pseudintermedius isolate Sps 8 | 64 ÷ 128 | 32 ÷ 64 | 0.25 ÷ 0.5 (2.4 ÷ 4.8) | 1.25 ÷ 2.5 (11.2 ÷ 22.4) |
| Staphylococcus pseudintermedius isolate Sps 9 | 32 ÷ 64 | ≤0.125 | 0.25 ÷ 0.5 (2.4 ÷ 4.8) | 1.25 ÷ 5 (11.2 ÷ 44.8) |
| Staphylococcus pseudintermedius isolate Sps 10 | 0.0625 ÷ 0.125 | 32 ÷ 64 | 0.25 ÷ 0.5 (2.4 ÷ 4.8) | 1.25 ÷ 2.5 (11.2 ÷ 22.4) |
| Staphylococcus pseudintermedius isolate Sps 11 | 0.125 ÷ 0.5 | ≤0.125 | 0.25 ÷ 0.5 (2.4 ÷ 4.8) | 0.625 ÷ 2.5 (5.6 ÷ 22.4) |
| Staphylococcus pseudintermedius isolate Sps 12 | 8 ÷ 32 | 0.25 ÷ 0.5 | 0.25 ÷ 0.5 (2.4 ÷ 4.8) | 1.25 ÷ 5 (11.2 ÷ 44.8) |
| Antimicrobial Agent | Patchouli Oil | Tea Tree Oil | ||||||
|---|---|---|---|---|---|---|---|---|
| MICi | MICc | FIC | ∑FIC [Interaction] | MICi | MICc | FIC | ∑FIC (Interaction) | |
| Staphylococcus aureus ATCC 25923 | ||||||||
| oil (% v/v) | 0.25 | 0.008 | 0.032 | 0.282 synergy | 2.5 | 0.08 | 0.032 | 0.282 synergy |
| gentamicin (μg/mL) | 1 | 0.25 | 0.25 | 1 | 0.25 | 0.25 | ||
| oil (% v/v) | 0.125 | 0.125 | 1 | 2.0 none | 1.25 | 1.25 | 1 | 2.0 none |
| enrofloxacin (μg/mL) | 0.125 | 0.125 | 1 | 0.125 | 0.125 | 1 | ||
| Staphylococcus aureus Sa 1 | ||||||||
| oil (% v/v) | 0.5 | 0.125 | 0.25 | 0.75 additive | 5 | 1.25 | 0.25 | 0.5 synergy |
| gentamicin (μg/mL) | 2 | 1 | 0.5 | 0.5 | 0.125 | 0.25 | ||
| oil (% v/v) | 0.5 | 0.25 | 0.5 | 1.0 additive | 5 | 1.25 | 0.25 | 0.375 synergy |
| enrofloxacin (μg/mL) | 8 | 4 | 0.5 | 8 | 1 | 0.125 | ||
| Staphylococcus aureus Sa 2 | ||||||||
| oil (% v/v) | 0.5 | 0.125 | 0.25 | 0.75 additive | 10 | 1.25 | 0.125 | 0.25 synergy |
| gentamicin (μg/mL) | 1 | 0.5 | 0.5 | 0.5 | 0.0625 | 0.125 | ||
| oil (% v/v) | 0.25 | 0.25 | 1 | 2.0 none | 5 | 5 | 1 | 2.0 none |
| enrofloxacin (μg/mL) | 4 | 4 | 1 | 4 | 4 | 1 | ||
| Staphylococcus aureus Sa 3 | ||||||||
| oil (% v/v) | 0.5 | 0.125 | 0.25 | 0.5 synergy | 2.5 | 0.625 | 0.25 | 0.5 synergy |
| gentamicin (μg/mL) | 16 | 4 | 0.25 | 8 | 2 | 0.25 | ||
| oil (% v/v) | 0.25 | 0.25 | 1 | 2.0 none | 5 | 5 | 1 | 2.0 none |
| enrofloxacin (μg/mL) | 32 | 32 | 1 | 32 | 32 | 1 | ||
| Staphylococcus aureus Sa 4 | ||||||||
| oil (% v/v) | 0.25 | 0.125 | 0.5 | 0.625 additive | 2.5 | 0.32 | 0.128 | 0.253 synergy |
| gentamicin (μg/mL) | 0.5 | 0.0625 | 0.125 | 0.5 | 0.0625 | 0.125 | ||
| oil (% v/v) | 0.25 | 0.25 | 1 | 2.0 none | 1.25 | 1.25 | 1 | 2.0 none |
| enrofloxacin (μg/mL) | 0.25 | 0.25 | 1 | 0.125 | 0.125 | 1 | ||
| Staphylococcus aureus Sa 5 | ||||||||
| oil (% v/v) | 0.25 | 0.0625 | 0.25 | 0.75 additive | 2.5 | 0.625 | 0.25 | 0.378 synergy |
| gentamicin (μg/mL) | 0.25 | 0.125 | 0.5 | 0.25 | 0.032 | 0.128 | ||
| oil (% v/v) | 0.25 | 0.25 | 1 | 2.0 none | 1.25 | 1.25 | 1 | 2.0 none |
| enrofloxacin (μg/mL) | 1 | 1 | 1 | 0.5 | 0.5 | 1 | ||
| Staphylococcus aureus Sa 6 | ||||||||
| oil (% v/v) | 0.125 | 0.016 | 0.128 | 0.384 synergy | 1.25 | 0.16 | 0.128 | 0.256 synergy |
| gentamicin (μg/mL) | 0.125 | 0.032 | 0.256 | 0.125 | 0.016 | 0.128 | ||
| oil (% v/v) | 0.125 | 0.032 | 0.256 | 0.756 additive | 1.25 | 0.625 | 0.5 | 1.0 additive |
| enrofloxacin (μg/mL) | 0.125 | 0.0625 | 0.5 | 0.125 | 0.0625 | 0.5 | ||
| Staphylococcus aureus Sa 7 | ||||||||
| oil (% v/v) | 0.25 | 0.0625 | 0.25 | 0.506 additive | 1.25 | 0.32 | 0.256 | 0.768 additive |
| gentamicin (μg/mL) | 0.125 | 0.032 | 0.256 | 0.0625 | 0.032 | 0.512 | ||
| oil (% v/v) | 0.125 | 0.0625 | 0.5 | 1.012 additive | 0.625 | 0.625 | 1 | 2.0 none |
| enrofloxacin (μg/mL) | 0.0625 | 0.032 | 0.512 | 0.125 | 0.125 | 1 | ||
| Staphylococcus pseudintermedius ED99 | ||||||||
| oil (% v/v) | 0.25 | 0.032 | 0.128 | 0.192 synergy | 2.5 | 0.32 | 0.128 | 0.256 synergy |
| gentamicin (μg/mL) | 0.5 | 0.032 | 0.064 | 0.5 | 0.064 | 0.128 | ||
| oil (% v/v) | 0.125 | 0.008 | 0.064 | 0.564 additive | 1.25 | 1.25 | 1 | 2.0 none |
| enrofloxacin (μg/mL) | 0.25 | 0.125 | 0.5 | 0.125 | 0.125 | 1 | ||
| Staphylococcus pseudintermedius Sps 1 | ||||||||
| oil (% v/v) | 0.5 | 0.0625 | 0.125 | 0.375 synergy | 5 | 0.625 | 0.125 | 0.375 synergy |
| gentamicin (μg/mL) | 64 | 16 | 0.25 | 32 | 8 | 0.25 | ||
| oil (% v/v) | 0.25 | 0.016 | 0.064 | 0.564 additive | 1.25 | 0.08 | 0.064 | 0.314 synergy |
| enrofloxacin (μg/mL) | 32 | 16 | 0.5 | 64 | 16 | 0.25 | ||
| Staphylococcus pseudintermedius Sps 2 | ||||||||
| oil (% v/v) | 0.5 | 0.125 | 0.25 | 0.75 additive | 1.25 | 0.16 | 0.128 | 0.378 synergy |
| gentamicin (μg/mL) | 64 | 32 | 0.5 | 32 | 8 | 0.25 | ||
| oil (% v/v) | 0.25 | 0.016 | 0.064 | 0.564 additive | 1.25 | 1.25 | 1 | 2.0 none |
| enrofloxacin (μg/mL) | 64 | 32 | 0.5 | 64 | 64 | 1 | ||
| Staphylococcus pseudintermedius Sps 3 | ||||||||
| oil (% v/v) | 0.5 | 0.25 | 0.5 | 0.625 additive | 1.25 | 0.16 | 0.128 | 0.191 synergy |
| gentamicin (μg/mL) | 128 | 16 | 0.125 | 64 | 4 | 0.0625 | ||
| oil (% v/v) | 0.5 | 0.032 | 0.064 | 0.564 additive | 1.25 | 1.25 | 1 | 2.0 none |
| enrofloxacin (μg/mL) | 64 | 32 | 0.5 | 64 | 64 | 1 | ||
| Staphylococcus pseudintermedius Sps 4 | ||||||||
| oil (% v/v) | 0.5 | 0.125 | 0.25 | 0.5 synergy | 0.625 | 0.16 | 0.256 | 0.381 synergy |
| gentamicin (μg/mL) | 64 | 16 | 0.25 | 32 | 4 | 0.125 | ||
| oil (% v/v) | 0.5 | 0.5 | 1 | 2.0 none | 1.25 | 1.25 | 1 | 2.0 none |
| enrofloxacin (μg/mL) | 32 | 32 | 1 | 64 | 64 | 1 | ||
| Staphylococcus pseudintermedius Sps 5 | ||||||||
| oil (% v/v) | 0.5 | 0.125 | 0.25 | 0.5 synergy | 5 | 1.25 | 0.25 | 0.281 synergy |
| gentamicin (μg/mL) | 256 | 64 | 0.25 | 128 | 4 | 0.031 | ||
| oil (% v/v) | 0.5 | 0.032 | 0.064 | 0.564 additive | 1.25 | 0.625 | 0.5 | 1.0 additive |
| enrofloxacin (μg/mL) | 64 | 32 | 0.5 | 64 | 32 | 0.5 | ||
| Staphylococcus pseudintermedius Sps 6 | ||||||||
| oil (% v/v) | 0.5 | 0.25 | 0.5 | 0.75 additive | 2.5 | 0.32 | 0.128 | 0.159 synergy |
| gentamicin (μg/mL) | 128 | 32 | 0.25 | 64 | 2 | 0.031 | ||
| oil (% v/v) | 0.5 | 0.5 | 1 | 2.0 none | 2.5 | 2.5 | 1 | 3.0 none |
| enrofloxacin (μg/mL) | 32 | 32 | 1 | 64 | 128 | 2 | ||
| Staphylococcus pseudintermedius Sps 7 | ||||||||
| oil (% v/v) | 0.5 | 0.25 | 0.5 | 1 additive | 0.625 | 0.16 | 0.256 | 0.381 synergy |
| gentamicin (μg/mL) | 128 | 64 | 0.5 | 32 | 4 | 0.125 | ||
| oil (% v/v) | 0.5 | 0.008 | 0.016 | 0.516 additive | 1.25 | 0.625 | 0.5 | 1.0 additive |
| enrofloxacin (μg/mL) | 64 | 32 | 0.5 | 128 | 64 | 0.5 | ||
| Staphylococcus pseudintermedius Sps 8 | ||||||||
| oil (% v/v) | 0.5 | 0.125 | 0.25 | 0.75 additive | 1.25 | 0.16 | 0.128 | 0.191 synergy |
| gentamicin (μg/mL) | 128 | 64 | 0.5 | 64 | 4 | 0.0625 | ||
| oil (% v/v) | 0.5 | 0.5 | 1 | 2.0 none | 1.25 | 1.25 | 1 | 2.0 none |
| enrofloxacin (μg/mL) | 64 | 64 | 1 | 64 | 64 | 1 | ||
| Staphylococcus pseudintermedius Sps 9 | ||||||||
| oil (% v/v) | 0.5 | 0.125 | 0.25 | 0.375 synergy | 5 | 1.25 | 0.25 | 0.375 synergy |
| gentamicin (μg/mL) | 64 | 8 | 0.125 | 32 | 4 | 0.125 | ||
| oil (% v/v) | 0.5 | 0.5 | 1 | 2.0 none | 1.25 | 1.25 | 1 | 2.0 none |
| enrofloxacin (μg/mL) | 0.125 | 0.125 | 1 | 0.125 | 0.125 | 1 | ||
| Staphylococcus pseudintermedius Sps 10 | ||||||||
| oil (% v/v) | 0.5 | 0.125 | 0.25 | 0.506 additive | 1.25 | 0.32 | 0.256 | 0.512 additive |
| gentamicin (μg/mL) | 0.125 | 0.032 | 0.256 | 0.0625 | 0.016 | 0.256 | ||
| oil (% v/v) | 0.5 | 0.5 | 1 | 2.0 none | 1.25 | 1.25 | 1 | 2.0 none |
| enrofloxacin (μg/mL) | 64 | 64 | 1 | 32 | 32 | 1 | ||
| Staphylococcus pseudintermedius Sps 11 | ||||||||
| oil (% v/v) | 0.5 | 0.25 | 0.5 | 0.75 additive | 2.5 | 0.625 | 0.25 | 0.378 synergy |
| gentamicin (μg/mL) | 0.5 | 0.125 | 0.25 | 0.125 | 0.016 | 0.128 | ||
| oil (% v/v) | 0.5 | 0.5 | 1 | 2.0 none | 0.625 | 0.625 | 1 | 2.0 none |
| enrofloxacin (μg/mL) | 0.125 | 0.125 | 1 | 0.125 | 0.125 | 1 | ||
| Staphylococcus pseudintermedius Sps 12 | ||||||||
| oil (% v/v) | 0.5 | 0.25 | 0.5 | 0.75 additive | 5 | 1.25 | 0.25 | 0.5 synergy |
| gentamicin (μg/mL) | 32 | 8 | 0.25 | 8 | 2 | 0.25 | ||
| oil (% v/v) | 0.5 | 0.5 | 1 | 2.0 none | 1.25 | 1.25 | 1 | 2.0 none |
| enrofloxacin (μg/mL) | 0.5 | 0.5 | 1 | 0.5 | 0.5 | 1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szewczuk, M.A.; Zych, S.; Oster, N.; Karakulska, J. Activity of Patchouli and Tea Tree Essential Oils against Staphylococci Isolated from Pyoderma in Dogs and Their Synergistic Potential with Gentamicin and Enrofloxacin. Animals 2023, 13, 1279. https://doi.org/10.3390/ani13081279
Szewczuk MA, Zych S, Oster N, Karakulska J. Activity of Patchouli and Tea Tree Essential Oils against Staphylococci Isolated from Pyoderma in Dogs and Their Synergistic Potential with Gentamicin and Enrofloxacin. Animals. 2023; 13(8):1279. https://doi.org/10.3390/ani13081279
Chicago/Turabian StyleSzewczuk, Małgorzata Anna, Sławomir Zych, Nicola Oster, and Jolanta Karakulska. 2023. "Activity of Patchouli and Tea Tree Essential Oils against Staphylococci Isolated from Pyoderma in Dogs and Their Synergistic Potential with Gentamicin and Enrofloxacin" Animals 13, no. 8: 1279. https://doi.org/10.3390/ani13081279
APA StyleSzewczuk, M. A., Zych, S., Oster, N., & Karakulska, J. (2023). Activity of Patchouli and Tea Tree Essential Oils against Staphylococci Isolated from Pyoderma in Dogs and Their Synergistic Potential with Gentamicin and Enrofloxacin. Animals, 13(8), 1279. https://doi.org/10.3390/ani13081279




