Ten Years of Animal Tuberculosis Monitoring in Free-Living European Bison (Bison bonasus) in Poland
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Samples
2.2. Mycobacterial Isolation
2.3. DNA Extraction and Multiplex PCR
2.4. Strain Species Identification
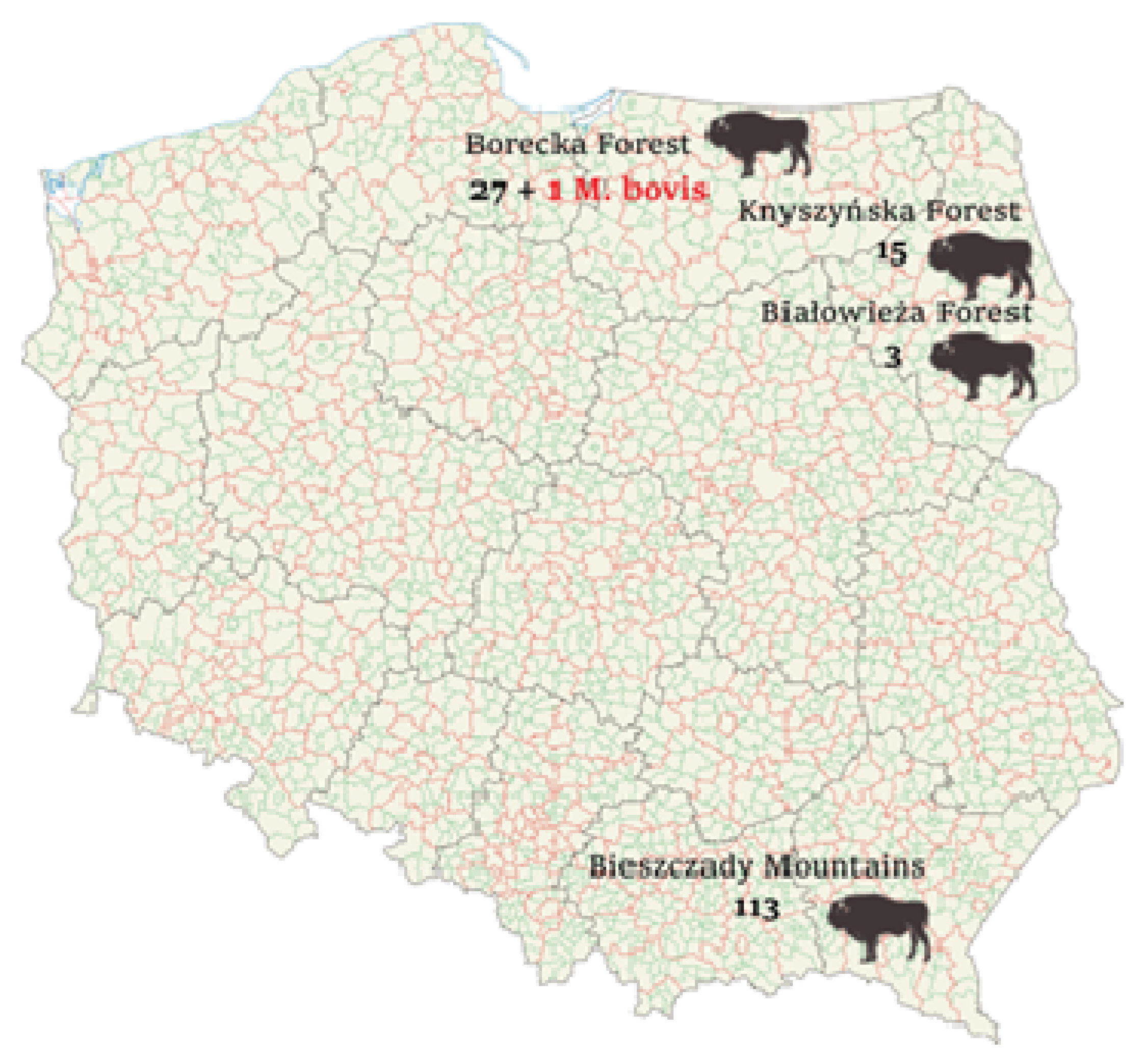
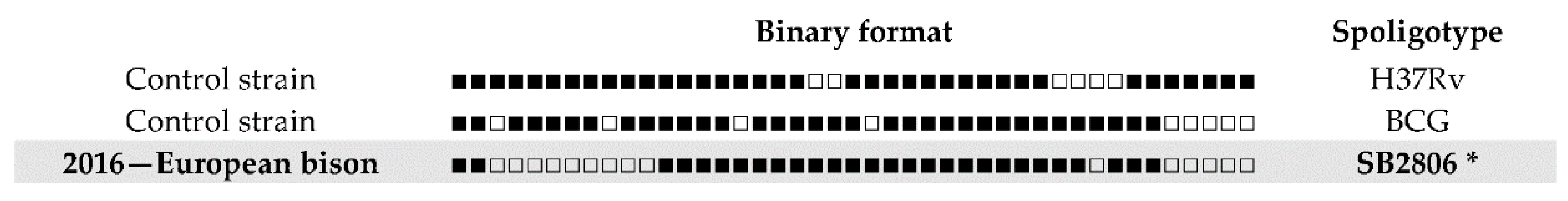
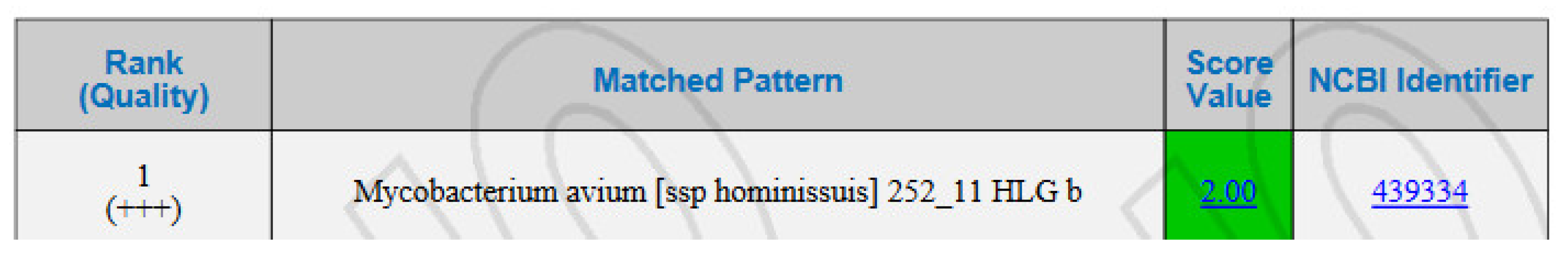
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duszewska, A.M.; Baraniewicz-Kołek, M.; Wojdan, J.; Barłowska, K.; Bielecki, W.; Gręda, P.; Niżański, W.; Olech, W. Establishment of a Wisent (Bison bonasus) Germplasm Bank. Animals 2022, 12, 1239. [Google Scholar] [CrossRef] [PubMed]
- Raczyński, J.; Bołbot, M. European bison Pedigree Book 2019; Białowieża Natlional Park: Białowieża, Poland, 2020; pp. 1–83. [Google Scholar]
- Raczyński, J.; Bołbot, M. European bison Pedigree Book 2021; Białowieża Natlional Park: Białowieża, Poland, 2022; pp. 1–83. [Google Scholar]
- Olech, W. The Genetic Variability within Bison bonasus species 90 years after bottleneck. In Restoration of Endangered and Extinct Animals; Słomski, R., Ed.; Poznan University of Life Sciences: Poznan, Poland, 2010; pp. 48–57. [Google Scholar]
- Krajewska, M.; Zabost, A.; Welz, M.; Lipiec, M.; Orłowska, B.; Anusz, K.; Brewczyński, P.; Augustynowicz-Kopeć, E.; Szulowski, K.; Bielecki, W.; et al. Transmission of Mycobacterium caprae in a herd of European bison in the Bieszczady Mountains, Southern Poland. Eur. J Wild. Res. 2015, 61, 429–433. [Google Scholar] [CrossRef]
- Kędrak-Jabłońska, A.; Budniak, S.; Szczawińska, A.; Reksa, M.; Krupa, M.; Krzysiak, M.; Szulowski, K. Isolation and identification of Pasteurella multocida from European bison in Poland. Post. Mikrobiol. 2017, 56, 69–70. [Google Scholar]
- Demiaszkiewicz, A.W.; Moskwa, B.; Gralak, A.; Laskowski, Z.; Myczka, A.W.; Kołodziej-Sobocińska, M.; Kaczor, S.; Plis-Kuprianowicz, E.; Krzysiak, M.; Filip-Hutsch, K. The Nematodes Thelazia gulosa Railiet and Henry, 1910 and Thelazia skrjabini Erschov, 1928 as a Cause of Blindness in European Bison (Bison bonasus) in Poland. Acta Parasit. 2020, 65, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Bruczyńska, M.; Didkowska, A.; Dzikowski, A.; Rudy, M.; Orłowska, B.; Welz, M.; Krajewska-Wędzina, M.; Olech, W.; Anusz, K. Legal Obstacles in the Eradication of Bovine Tuberculosis in European bison (Bison bonasus)—A Threat to an Effective Reintroduction Strategy. Divercity 2022, 14, 710. [Google Scholar] [CrossRef]
- Krzysiak, M.K.; Jabłoński, A.; Iwaniak, W.; Krajewska, M.; Kęsik-Maliszewska, J.; Larska, M. Seroprevalence and risk factors for selected respiratory and reproductive tract pathogen exposure in European bison (Bison bonasus) in Poland. Vet. Microbiol. 2018, 215, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Larska, M.; Krzysiak, M.; Smreczak, M.; Polak, M.P.; Żmudziński, J.F. First detection of Schmallenberg virus in elk (Alces alces) indicating infection of wildlife in Białowieża National Park in Poland. Vet. J. 2013, 198, 279–281. [Google Scholar] [CrossRef]
- Larska, M.; Krzysiak, M.K. Infectious diseases monitoring as an element of Bison bonasus species protection. In Compendium of the European Bison (Bison bonasus) Health Protection, 2nd ed.; Larska, M., Krzysiak, M.K., Eds.; National Veterinary Research Institute, Polish Academy of Sciences and Białowieża National Park: Puławy, Poland, 2022; pp. 71–96. [Google Scholar]
- Kęsik-Maliszewska, J.; Krzysiak, M.K.; Grochowska, M.; Lechowski, L.; Chase, C.; Larska, M. Epidemiology of Schmallenberg virus in European bison (Bison bonasus) in Poland. J. Wildl. Dis. 2018, 54, 272–282. [Google Scholar] [CrossRef]
- Krzysiak, M.K.; Anusz, K.; Konieczny, A.; Rola, J.; Salat, J.; Strakova, P.; Olech, W.; Larska, M. The European bison (Bison bonasus) as an indicatory species for the circulation of tick-borne encephalitis virus (TBEV) in natural foci in Poland. Ticks Tick Borne Dis. 2021, 12, 101799. [Google Scholar] [CrossRef]
- Krzysiak, M.K.; Puchalska, M.; Olech, W.; Anusz, K. A Freedom of Coxiella burnetii Infection Survey in European Bison (Bison bonasus) in Poland. Animals 2021, 11, 651. [Google Scholar] [CrossRef]
- Larska, M.; Krzysiak, M.K.; Jabłoński, A.; Kęsik, J.; Bednarski, M.; Rola, J. Hepatitis E virus antibody prevalence in wildlife in Poland. Zoonoses Public Health 2015, 62, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Radulski, Ł.; Krajewska-Wędzina, M.; Lipiec, M.; Szulowski, K. Infection of a Free-Living Wild Boar (Sus scrofa) with a Bacterium from the Mycobacterium kansasii Complex. Animals 2022, 12, 964. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, M. Characterization of Mycobacterium bovis Strains Isolated from Animals in Poland. Ph.D. Thesis, National Veterinary Research Institute, Pulawy, Poland, 2015. [Google Scholar]
- Marmiroli, N.; Maestri, W. Polymerase Chain Reaction (PCR) in Food Toxicants Analysis, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2007; pp. 147–187. [Google Scholar]
- Hain Lifescience. Geno Type MTBC—Manufacturer Instruction for the Test no. 301-11. Available online: https://www.hain-lifescience.de/en/instructions-for-use.html (accessed on 4 March 2022).
- Hain Lifescience. Geno Type CM—Manufacturer Instruction for the Test no. 299A-02. Available online: https://www.hain-lifescience.de/en/instructions-for-use.html (accessed on 4 January 2023).
- Smith, N.H.; Upton, P. Naming spoligotype patterns for the RD9-deleted lineage of the Mycobacterium tuberculosis complex. Infect. Genet. Evol. 2012, 12, 873–876. [Google Scholar] [CrossRef]
- Didkowska, A.; Orłowska, B.; Krajewska-Wędzina, M.; Krzysiak, M.; Bruczyńska, M.; Wiśniewski, J.; Klich, D.; Olech, W.; Anusz, K. Intra-Palpebral Tuberculin Skin Test and Interferon Gamma Release Assay in Diagnosing Tuberculosis Due to Mycobacterium caprae in European Bison (Bison bonasus). Pathogens 2022, 11, 260. [Google Scholar] [CrossRef]
- Didkowska, A.; Krajewska-Wędzina, M.; Bielecki, W.; Brzezińska, S.; Augustynowicz-Kopeć, E.; Olech, W.; Anusz, K.; Sridhara, A.A.; Johnathan-Lee, A.; Elahi, R.; et al. Antibody responses in European bison (Bison bonasus) naturally infected with Mycobacterium caprae. Vet. Microbiol. 2021, 253, 108952. [Google Scholar] [CrossRef] [PubMed]
- Gortazar, C.; Vicente, J.; Samper, S.; Garrido, J.M.; Fernández-de-Mera, I.G.; Gavin, P.; Juste, R.A.; Martín, C.; Acevedo, P.; De La Puente, M.; et al. Molecular characterization of Mycobacterium tuberculosis complex isolates from wild ungulates in south-central Spain. Vet. Res. 2005, 36, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Hermoso de Mendoza, L.; Parra, A.; Tato, A.; Alonso, J.M.; Rey, J.M.; Pena, J.; García-Sánchez, A.; Larrasa, J.; Teixidó, J.; Manzano, G.; et al. Bovine tuberculosis in wild boar (Sus scrofa), red deer (Cervus elaphus) and cattle (Bos taurus) in a Mediterranean ecosystem (1992-2004). Prev. Vet. Med. 2006, 74, 239–247. [Google Scholar] [CrossRef]
- Maas, M.; Michel, A.L.; Rutten, V.P.M.G. Facts and dilemmas in diagnosis of tuberculosis in wildlife. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 269–285. [Google Scholar] [CrossRef]
- Anusz, K.; Orłowska, B.; Krajewska-Wędzina, M.; Augustynowicz-Kopeć, E.; Krzysiak, M.; Bielecki, W.; Witkowski, L.; Welz, M.; Kita, J. Ante-mortem and post-mortem tuberculosis diagnostics in three European Bison (Bison bonasus caucasicus) from the enclosure in Bukowiec in the Bieszczady National Park in Poland. Med. Weter. 2017, 73, 642–646. [Google Scholar] [CrossRef]
- Naranjo, V.; Gortazar, C.; Vicente, J.; de la Fuente, J. Evidence of the role of European wild boar as a reservoir of Mycobacterium tuberculosis complex. Vet. Microbiol. 2008, 127, 1–9. [Google Scholar] [CrossRef]
- Fredriksson-Ahomaa, M. Wild Boar: A Reservoir of Foodborne Zoonoses. Foodborne Pathog. Dis. 2019, 16, 153–165. [Google Scholar] [CrossRef]
- Miller, R.S.; Sweeney, S.J. Mycobacterium bovis (bovine tuberculosis) infection in North American wildlife: Current status and opportunities for mitigation of risks of further infection in wildlife populations. Epidemiol. Infect. 2013, 141, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Briones, V.; de Juan, L.; Sánchez, C.; Vela, A.I.; Galka, M.; Montero, N.; Goyache, J.; Aranaz, A.; Mateos, A.; Domínguez, L. Bovine Tuberculosis and the Endangered Iberian Lynx. Emerg. Infect. Dis. 2000, 6, 189–191. [Google Scholar] [CrossRef]
- Orłowska, B.; Krajewska-Wędzina, M.; Augustynowicz-Kopeć, E.; Kozińska, M.; Brzezińska, S.; Zabost, A.; Didkowska, A.; Welz, M.; Kaczor, S.; Żmuda, P.; et al. Epidemiological characterization of Mycobacterium caprae strains isolated from wildlife in the Bieszczady Mountains, on the border of Southeast Poland. BMC Vet. Res. 2020, 16, 362. [Google Scholar] [CrossRef]
- Casalinuovo, F.; Ciambrone, L.; Grillone, R. Tuberculosis in wild boar and the risk of human infection by Mycobacterium bovis results of a study conducted in Southern Italy. J. Food Microbiol. 2017, 1, 22–26. [Google Scholar]
- Amato, B.; Mignacca, S.A.; Pacciarini, M.L.; Vitale, M.; Antoci, S.; Cucinotta, S.; Puleio, R.; Biasibetti, E.; Fiasconaro, M.; Capucchio, M.T.; et al. An outbreak of bovine tuberculosis in a fallow deer herd (Dama dama) in Sicily. Res. Vet. Sci. 2016, 106, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarkowska, A.; Didkowska, A.; Kwiecień, E.; Stefańska, I.; Rzewuska, M.; Anusz, K. The Mycobacterium avium complex—An underestimated threat to humans and animals. Ann. Agric. Environ. Med. 2022, 29, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Klotz, D.; Barth, S.A.; Baumgartner, W.; Hewicker-Trautwein, M. Mycobacterium avium subsp. hominissuis infection in a domestic rabbit. Emerg. Infect. Dis. 2018, 24, 596–598. [Google Scholar] [CrossRef]
- Álvarez, J.; Castellanos, E.; Romero, B.; Aranaz, A.; Bezos, J.; Rodríguez, S.; Mateos, A.; Domínguez, L.; de Juan, L. Epidemiological investigation of a Mycobacterium avium subsp. hominissuis outbreak in swine. Epidemiol. Infect. 2011, 139, 143–148. [Google Scholar] [CrossRef]
- Radulski, Ł.; Kalicki, M.; Krajewska-Wędzina, M.; Lipiec, M.; Szulowski, K. Pulmonary mycobacteriosis of sitatunga antelope caused by M. avium ssp. hominissuis. Ann. Agric. Environ. Med. 2022, 29, 220–223. [Google Scholar] [CrossRef]
- Prevots, D.R.; Marras, T.K. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: A review. Clin. Chest Med. 2015, 36, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.M. NTM working group at Queensland TB Control Centre and Queensland Mycobacterial Reference Laboratory. Changing epidemiology of pulmonary nontuberculous mycobacteria infections. Emerg. Infect. Dis. 2010, 16, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.urpl.gov.pl/pl/produkty-biob%C3%B3jcze/wykaz-produkt%C3%B3w-biob%C3%B3jczych (accessed on 10 March 2023). (In Polish)
- The European Parliament and the Council of the European Union. Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on Transmissible Animal Diseases and Amending and Repealing Certain Acts in the Area of Animal Health (‘Animal Health Law’). EU OJ L 084 of 31 March 2016. p. 1. Available online: http://data.europa.eu/eli/reg/2016/429/oj (accessed on 4 March 2023).
- The Act of March 11, 2004 on the Protection of Animal Health and Combating Infectious Animal Diseases, i.e.; Journal of Laws of o2020, Item 1421. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20200001421 (accessed on 4 March 2023). (In Polish)
| Identification | Target DNA | Primer | Sequence (5′–3′) * | Sequence Length (bp) |
|---|---|---|---|---|
| Mycobacterium genre | 16 S rRNA | Mycgen–F | AGA GTT TGA TCC TGG CTC AG | 1030 |
| Mycgen–R | TGC ACA CAG GCC ACA AGG GA | |||
| Mycobacterium tuberculosis complex | MPB70 | TB1–F | GAA CAA TCC GGA GTT GAC AA | 372 |
| TB1–R | AGC ACG CTG TCA ATC ATG TA |
| Reagent | Concentration | Volume (µL) |
|---|---|---|
| Nuclease-free water | - | 3.4 |
| Multiplex PCR Master Mix | 2x | 12.5 |
| MycF,R primers | 35 ng/µL | 1.5 |
| TB1F,R primers | 20 ng/µL | 2.5 |
| Mg2+ | 25 mM | 0.1 |
| DNA | - | 5 |
| Total volume | 25 | |
| Cycle | Temperature | Time |
|---|---|---|
| Initial denaturation | 10 min | 95 °C |
| Denaturation | 30 s | 95 °C |
| Annealing | 2 min | 65 °C |
| Elongation | 3 min | 72 °C |
| Final elongation | 10 min | 72 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajewska-Wędzina, M.; Krzysiak, M.K.; Bruczyńska, M.; Orłowska, B.; Didkowska, A.; Radulski, Ł.; Wiśniewski, J.; Olech, W.; Nowakiewicz, A.; Welz, M.; et al. Ten Years of Animal Tuberculosis Monitoring in Free-Living European Bison (Bison bonasus) in Poland. Animals 2023, 13, 1205. https://doi.org/10.3390/ani13071205
Krajewska-Wędzina M, Krzysiak MK, Bruczyńska M, Orłowska B, Didkowska A, Radulski Ł, Wiśniewski J, Olech W, Nowakiewicz A, Welz M, et al. Ten Years of Animal Tuberculosis Monitoring in Free-Living European Bison (Bison bonasus) in Poland. Animals. 2023; 13(7):1205. https://doi.org/10.3390/ani13071205
Chicago/Turabian StyleKrajewska-Wędzina, Monika, Michał K. Krzysiak, Małgorzata Bruczyńska, Blanka Orłowska, Anna Didkowska, Łukasz Radulski, Jan Wiśniewski, Wanda Olech, Aneta Nowakiewicz, Mirosław Welz, and et al. 2023. "Ten Years of Animal Tuberculosis Monitoring in Free-Living European Bison (Bison bonasus) in Poland" Animals 13, no. 7: 1205. https://doi.org/10.3390/ani13071205
APA StyleKrajewska-Wędzina, M., Krzysiak, M. K., Bruczyńska, M., Orłowska, B., Didkowska, A., Radulski, Ł., Wiśniewski, J., Olech, W., Nowakiewicz, A., Welz, M., Kaczor, S., Weiner, M., & Anusz, K. (2023). Ten Years of Animal Tuberculosis Monitoring in Free-Living European Bison (Bison bonasus) in Poland. Animals, 13(7), 1205. https://doi.org/10.3390/ani13071205






