Slow and Fast-Growing Chickens Use Different Antioxidant Pathways to Maintain Their Redox Balance during Postnatal Growth
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Determination of Plasma Metabolites and Redox Status of Chickens
2.3. Statistical Analysis
3. Results
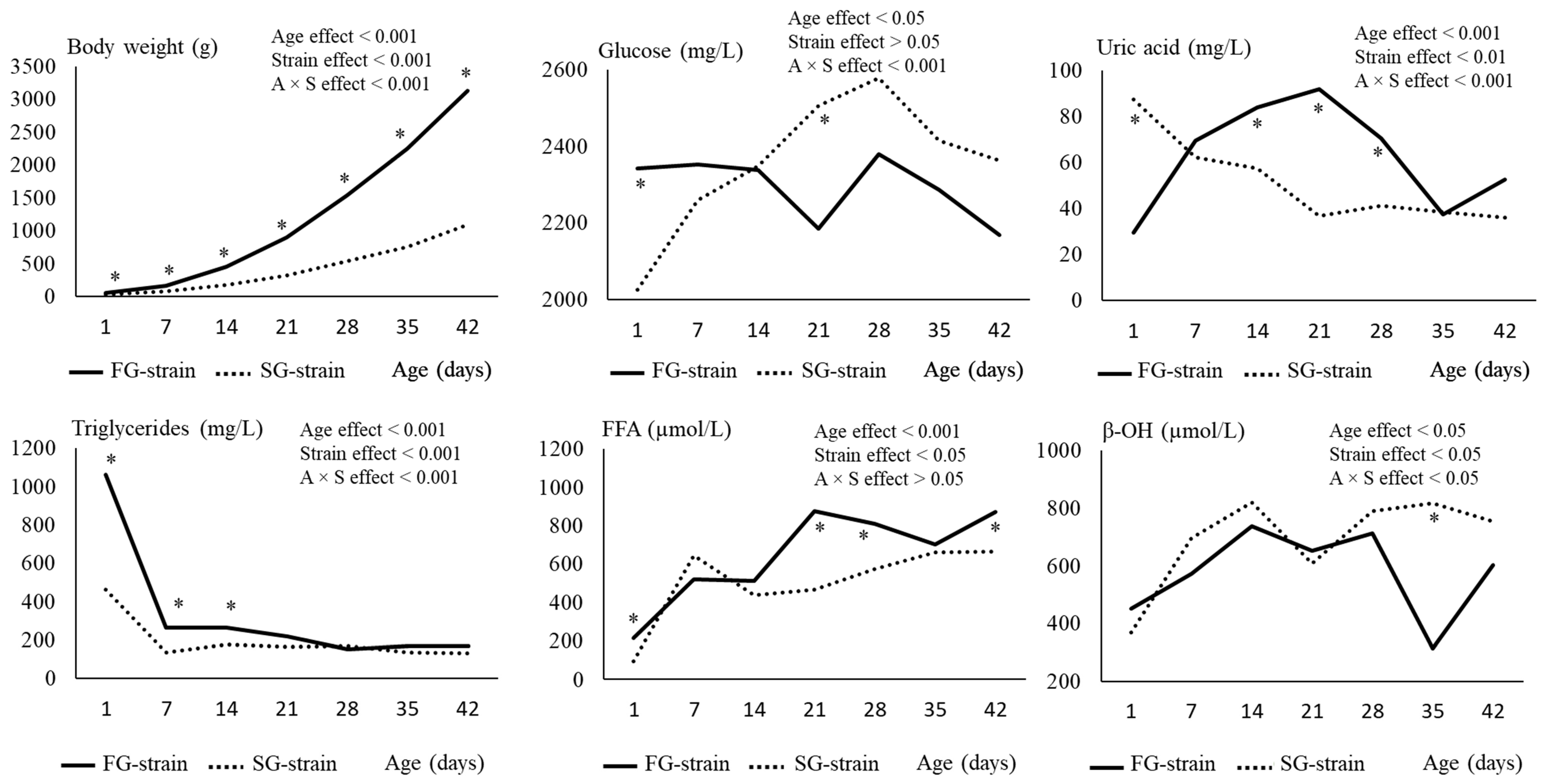
3.1. Growth Performance of Chickens
3.2. Plasma Metabolites
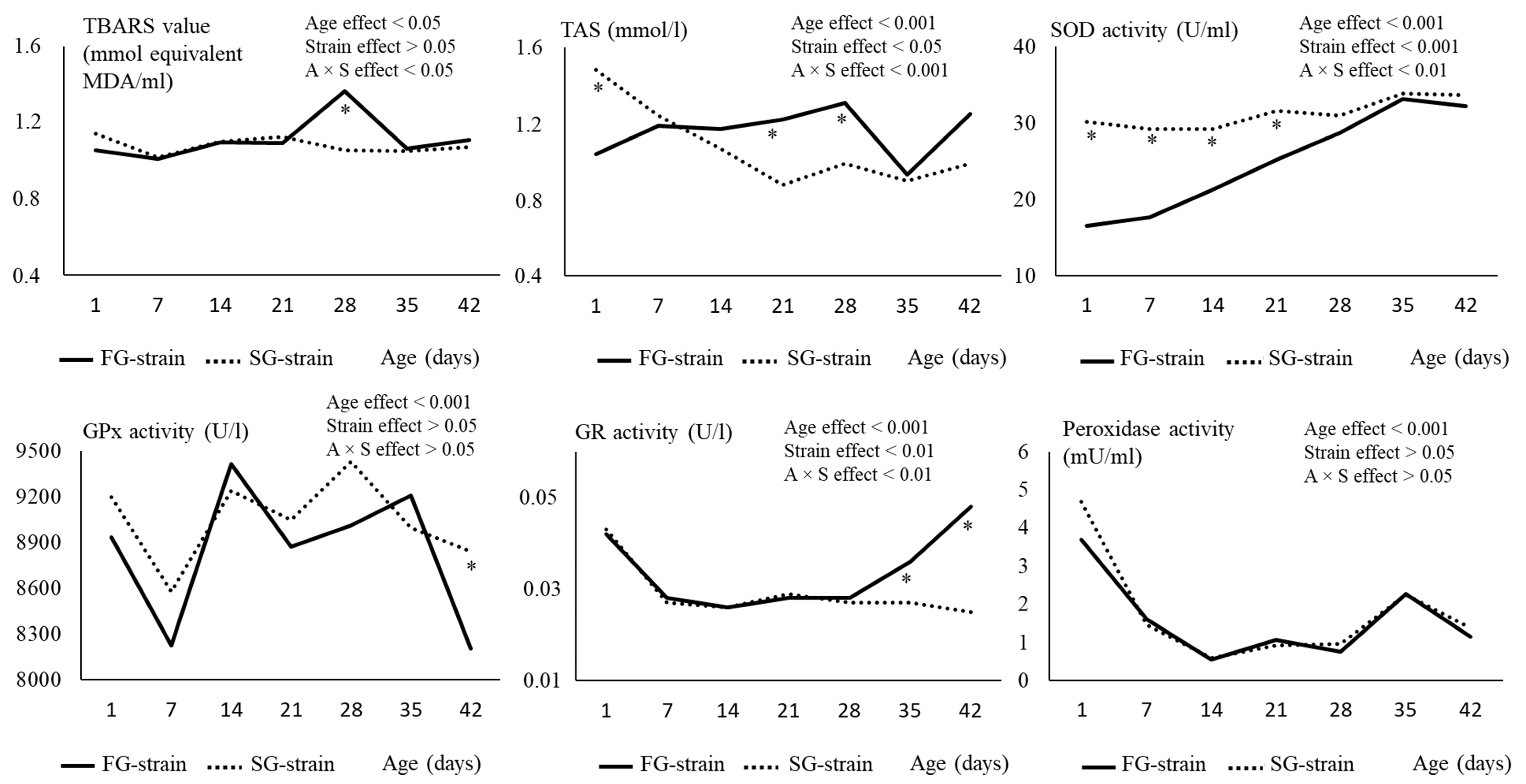
3.3. Plasma Redox Status and Enzyme Activities
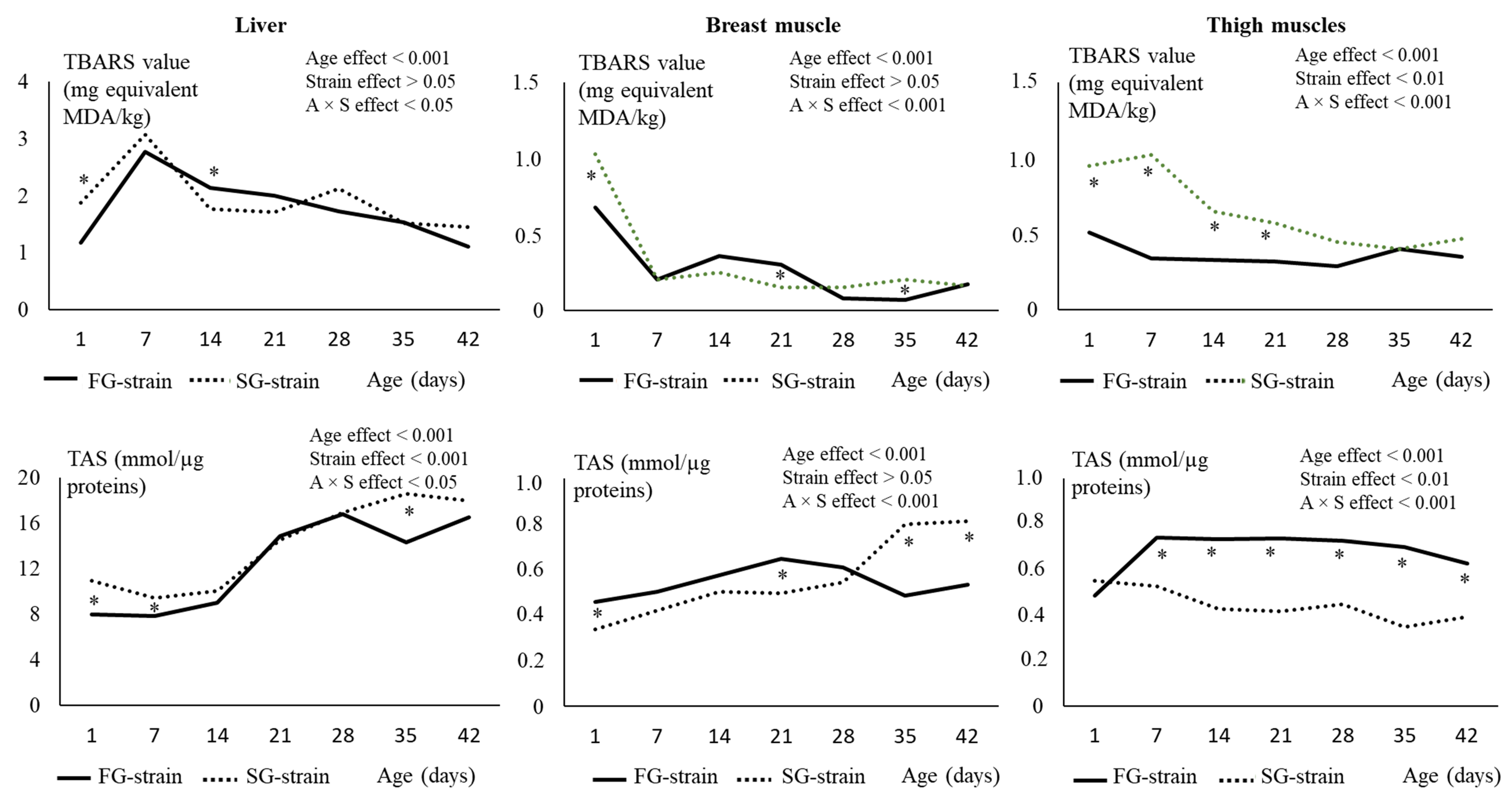
3.4. Muscles and Liver Lipid Peroxidation Status
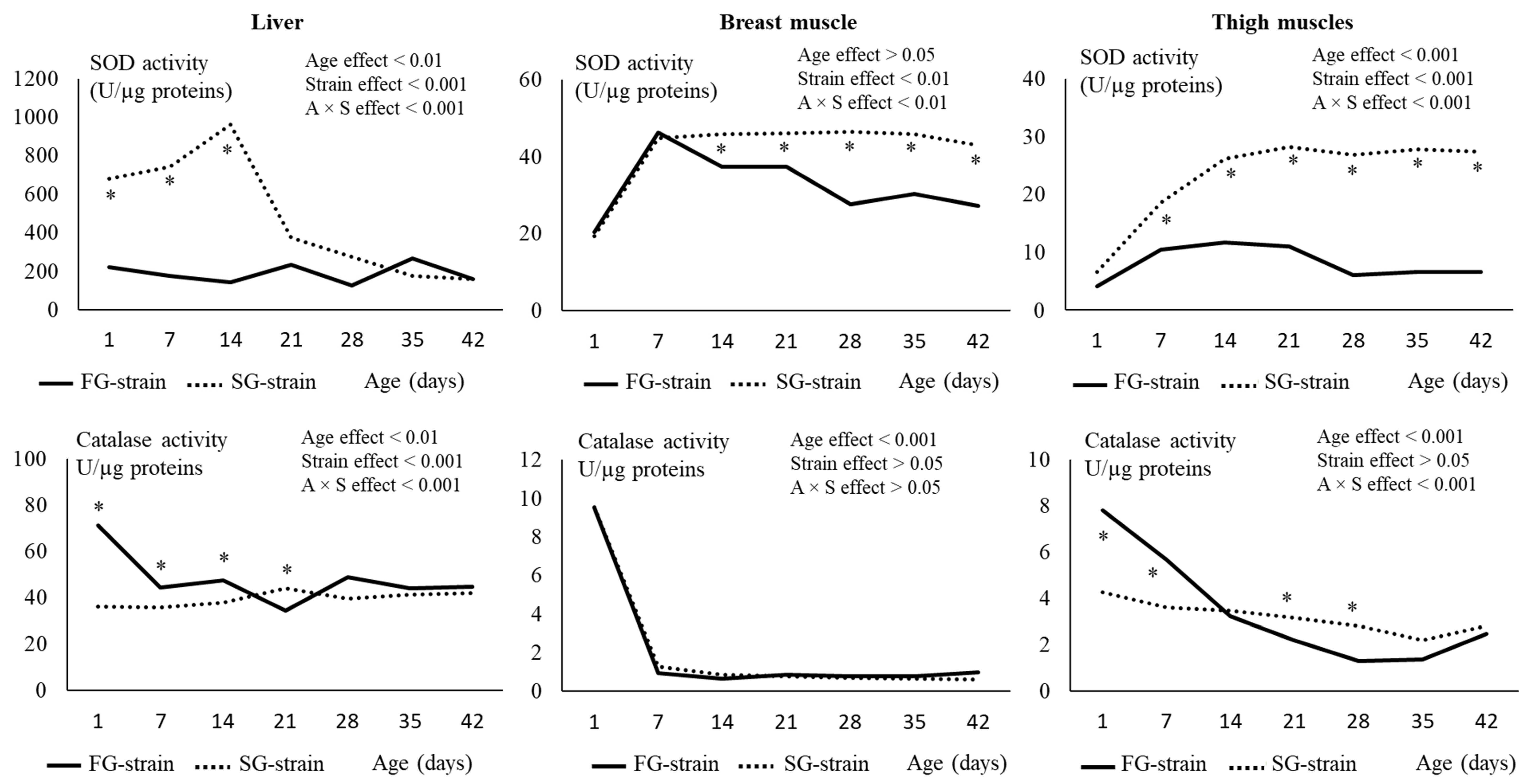
3.5. Muscles and Liver Antioxidant Status and Enzyme Activities
4. Discussion
4.1. Effect of Age on the Markers of Redox Status and Plasma Metabolites of Chickens
4.2. Effect of Strain on the Markers of Redox Status and Plasma Metabolites of Chickens
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant Defence Systems and Oxidative Stress in Poultry Biology: An Update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, A.F.; Zulkifli, I.; Omar, A.R.; Raha, A.R. Physiological responses of three chicken breeds to acute heat stress. Poult. Sci. 2011, 90, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Petracci, M.; Soglia, F.; Madruga, M.; Carvalho, L.; Ida, E.; Estevez, M. Wooden-Breast, White Striping, and Spaghetti Meat: Causes, Consequences and Consumer Perception of Emerging Broiler Meat Abnormalities. Compr. Rev. Food Sci. Food Safety 2019, 18, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Chabault, M.; Baéza, E.; Gigaud, V.; Chartrin, P.; Chapuis, H.; Boulay, M.; Arnould, C.; D’abbadie, F.; Berri, C.; Le Bihan-Duval, E. Analysis of a slow growing line reveals a large genetic control of carcass and meat quality related traits. BMC Genet. 2012, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Fanatico, A.C.; Pillai, P.B.; Hester, P.Y.; Falcone, C.; Mench, J.A.; Owens, C.M.; Emmert, J.L. Performance, liveability, and carcass yield of slow- and fast-growing chicken genotypes fed low-nutrient or standard diets and raised indoors or with outdoor access. Poult. Sci. 2008, 87, 1012–1021. [Google Scholar] [CrossRef]
- Surai, P.F. Tissue-specific changes in the activities of antioxidant enzymes during the development of the chicken embryo. Brit. Poult. Sci. 1999, 40, 397–405. [Google Scholar] [CrossRef]
- Yigit, A.A.; Panda, A.K.; Cherian, G. The avian embryo and its antioxidant defence system. World’s Poult. Sci. J. 2014, 70, 563–574. [Google Scholar] [CrossRef]
- Surai, P.F.; Fisinin, V.I.; Karadas, F. Antioxidant systems in chick embryo development. Part 1. Vitamin E, carotenoids and selenium. Anim. Nutr. 2016, 2, 1–11. [Google Scholar] [CrossRef]
- Yang, G.L.; Zhang, K.Y.; Ding, X.M.; Zheng, P.; Luo, Y.H.; Bai, S.P.; Wang, J.P.; Xuan, Y.; Su, Z.W.; Zeng, Q.F. Effects of dietary DL-2-hydroxy-4(methylthio) butanoic acid supplementation on growth performance, indices of ascites syndrome, and antioxidant capacity of broilers reared at low ambient temperature. Int. J. Biometeorol. 2016, 60, 1193–1203. [Google Scholar] [CrossRef]
- Schooneere, N.; Istasse, L. Le sélénium dans la reproduction des oiseaux. Ann. Méd. Vét. 2006, 150, 203–211. [Google Scholar]
- Surai, P.F.; Ionov, I.A.; Kuchmistova, E.F.; Noble, R.C.; Speake, B.K. The relationship between the levels of a-tocopherol and carotenoids in the maternal feed, yolk and neonatal tissues: Comparison between the chicken, turkey, duck and goose. J. Sci. Food Agric. 1998, 76, 593–598. [Google Scholar] [CrossRef]
- Del Vesco, A.P.; Khatlab, A.S.; Goes, E.S.R.; Utsunomiya, K.S.; Vieira, J.S.; Oliveira Neto, A.R.; Gasparino, E. Age-related oxidative stress and antioxidant capacity in heat-stressed broilers. Animal 2017, 11, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, K.Z.; Edens, F.W. Influence of selenium sources on age-related and mild heat stress-related changes of blood and liver glutathione redox cycle in broiler chickens (Gallus domesticus). Comp. Biochem. Physiol. Part B 2003, 136, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y. Changing in superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase activities and thiobarbituric acid-reactive products levels in early stages of development in dystrophic chickens. Experiment. Neurol. 1984, 84, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.M.; Frei, B. Mechanisms of copper- and iron-dependent oxidative modifications of human low-density lipoprotein. J. Lipid Res. 1993, 34, 1745–1753. [Google Scholar] [CrossRef]
- Cherian, G. Nutrition and metabolism in poultry: Role of lipids in early diet. J. Anim. Sci. Biotechnol. 2015, 6, 28. [Google Scholar] [CrossRef]
- Hicks, J.A.; Porter, T.E.; Sunny, N.E.; Hsiao-Ching Liu, H.C. Delayed Feeding Alters Transcriptional and Post-Transcriptional Regulation of Hepatic Metabolic Pathways in Peri-Hatch Broiler Chicks. Genes 2019, 10, 272. [Google Scholar] [CrossRef]
- Moran, E.T. Nutrition of the developing embryo and hatchling. Poult. Sci. 2007, 86, 1043–1049. [Google Scholar] [CrossRef]
- Uni, Z.; Ferket, P.R. Methods for early nutrition and their potential. World’s Poult. Sci. J. 2004, 60, 101–111. [Google Scholar] [CrossRef]
- Khan, A.A.; Rahmani, A.H.; Aldebasi, Y.H.; Aly, S.M. Biochemical and Pathological Studies on Peroxidases-An Updated Review. Glob. J. Health Sci. 2014, 6, 87–98. [Google Scholar] [CrossRef]
- Renerre, M.; Dumont, F.; Gatellier, P. Antioxidative enzyme activities in relation to oxidation of lipid and myoglobin. Meat Sci. 1996, 43, 111–121. [Google Scholar] [CrossRef]
- Lombardi-Boccia, G.; Martinez-Dominguez, B.; Aguzzi, A. Optimization of heme iron analysis in raw and cooked red meat. Food Chem. 2002, 78, 505–510. [Google Scholar] [CrossRef]
- Castellini, C.; Mugnai, C.; Dal Bosco, A. Effect of Organic Production System on Broiler Carcass and Meat. Meat Sci. 2002, 60, 219–225. [Google Scholar] [CrossRef]
- Lin, Y.F.; Tsai, H.L.; Lee, Y.C.; Chang, S.J. Maternal vitamin E supplementation affects the antioxidant capability and oxidative status of hatching chicks. J. Nutr. 2005, 135, 2457–2461. [Google Scholar] [CrossRef] [PubMed]
- Glantzounis, G.K.; Tsimoyiannis, E.C.; Kappas, E.M.; Galaris, D.A. Uric acid and oxidative stress. Curr. Pharm. Des. 2005, 11–32, 4145–4151. [Google Scholar] [CrossRef] [PubMed]
- Machin, M.; Simoyi, M.F.; Blemings, K.P.; Klandorf, H. Increased dietary protein elevates plasma uric acid and is associated with decreased oxidative stress in rapidly-growing broilers. Comp. Biochem. Physiol. Part B 2004, 137, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Hubert, S.; Athrey, G. Transcriptomic signals of mitochondrial dysfunction and OXPHOS dynamics in fast-growth chicken. PeerJ 2022, 10, e13364. [Google Scholar] [CrossRef]


| Standard Diets | Label Rouge Diets | |||||
|---|---|---|---|---|---|---|
| 1–14 d | 15–28 d | 29–42 d | 1–14 d | 15–28 d | 29–42 d | |
| Wheat | 350.00 | 420.04 | 456.56 | 333.60 | 350.00 | 500.00 |
| Soybean meal | 342.61 | 100.00 | 100.00 | 334.83 | 100.00 | 100.00 |
| Corn | 237.32 | 163.11 | 200.00 | 268.24 | 343.95 | 200.00 |
| Rapeseed meal | 78.64 | 80.00 | 40.75 | 80.00 | ||
| Dehulled sunflower meal | 65.00 | 14.59 | 65.00 | 18.95 | ||
| Corn gluten | 70.00 | 40.00 | 16.27 | |||
| Tradigriller 1 | 30.00 | 50.00 | 30.00 | 50.00 | ||
| Soya oil | 18.11 | 16.90 | 3.70 | 15.47 | 5.25 | |
| Rapeseed oil | 10.00 | 20.00 | 20.00 | 10.00 | 20.00 | 20.00 |
| Dicalcium phosphate | 19.36 | 14.18 | 13.57 | 18.88 | 14.20 | 9.05 |
| Calcium carbonate | 11.53 | 9.32 | 10.27 | 9.76 | 7.52 | 6.07 |
| Vitamins and trace minerals 2 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| NaCl | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| HCl-lysine | 1.71 | 4.46 | 2.96 | 3.38 | 1.92 | |
| dl-methionine | 1.62 | 0.48 | 1.72 | 1.02 | 1.26 | |
| l-threonine | 0.24 | 0.41 | 0.15 | 0.28 | ||
| l-tryptophane | 0.44 | 0.22 | 0.13 | |||
| Coccidiostat 3 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Metabolizable energy MJ/kg | 11.91 | 12.33 | 12.33 | 11.91 | 12.12 | 12.33 |
| Crude protein | 220 | 205 | 185 | 216 | 170 | 166 |
| Lysine | 12.50 | 11.20 | 9.70 | 10.99 | 9.44 | 8.65 |
| Sulfur amino-acids | 8.30 | 7.50 | 7.20 | 8.38 | 7.12 | 7.20 |
| Methionine | 4.66 | 3.61 | 3.57 | 4.75 | 3.86 | 3.87 |
| Tryptophane | 2.65 | 2.50 | 2.15 | 2.60 | 1.90 | 1.89 |
| Threonine | 8.10 | 7.50 | 6.60 | 7.79 | 6.19 | 5.82 |
| Calcium | 10.80 | 9.00 | 9.10 | 10.00 | 8.00 | 6.50 |
| Available phosphorus | 4.40 | 3.80 | 3.70 | 4.30 | 3.60 | 3.00 |
| Kit Name | Suppliers | Reference | Postal Address of Suppliers |
|---|---|---|---|
| Gpx (glutathione peroxidase) | Randox | RS505 | 55 Diamond, Crumlin, County Antrim, BT29 4QY, UK |
| TAS (total antioxidant status) | Randox | NX2332 | 55 Diamond, Crumlin, County Antrim, BT29 4QY, UK |
| SOD (superoxide dismutase) | Sigma Aldrich | 19160-1KT-F | Sigma Aldrich Chimie S.a.r.l 80 rue de Luzais, L’Isle d’Abeau Chesnes St Quentin Fallavier Cedex 38297, France |
| NEFA-HR2 (Free Fatty Acids) | Fujifilm | 434-91795 | FUJIFILM Wako Chemicals Europe GmbH, Fuggerstraße 12, 41468 Neuss, Germany |
| 436-91995 | |||
| Glucose (GOD-POD) | ThermoFisher | 981780 | ThermoFisher Scientific 2 avenue des Chaumes, 78180 Montigny le Bretonne, France |
| β-OH (β-hydroxybutyrate acid) | ThermoFisher | 984392 | ThermoFisher Scientific 2 avenue des Chaumes, 78180 Montigny le Bretonne, France |
| Triglycerides | ThermoFisher | 981786 | ThermoFisher Scientific 2 avenue des Chaumes, 78180 Montigny le Bretonne, France |
| GR (glutathione reductase) | Sigma Aldrich | GRSA-1KT | Sigma Aldrich Chimie S.a.r.l 80 rue de Luzais, L’Isle d’Abeau Chesnes St Quentin Fallavier Cédex 38297, France |
| Uric acid | ThermoFisher | 981788 | ThermoFisher Scientific 2 avenue des Chaumes, 78180 Montigny le Bretonne, France |
| Hydro-peroxides and peroxidase activity | Cell Biolabs | STA-844 | Cell Biolabs 7758 Arjons Drive San Diego, CA 92126, USA |
| Catalase activity | ThermoFisher | EIACATC | ThermoFisher Scientific 2 avenue des Chaumes, 78180 Montigny le Bretonne, France |
| FG Strain | SG Strain | Strain Effect | |
|---|---|---|---|
| BW D1 (g) | 45.18 a (n = 120) | 33.30 b (n = 120) | <0.001 |
| Feed consumption D1–D14 (g/d) | 33.74 (n = 1) | 21.29 (n = 1) | nd |
| DWG D1–D14 (g/d) | 28.05 (n = 1) | 10.53 (n = 1) | nd |
| FCR D1–D14 | 1.20 (n = 1) | 2.02 (n = 1) | nd |
| BW D14 (g) | 437.86 a (n = 89) | 180.75 b (n = 87) | <0.001 |
| Feed consumption D14–D28 (g/d) | 116.55 (n = 1) | 41.37 (n = 1) | nd |
| DWG D14–D28 (g/d) | 78.06 (n = 1) | 25.23 (n = 1) | nd |
| FCR D14–D28 | 1.49 (n = 1) | 1.64 (n = 1) | nd |
| BW D28 (g) | 1530.75 a (n = 78) | 534.03 b (n = 77) | <0.001 |
| Feed consumption D28–D42 (g/d) | 192.15 (n = 1) | 115.67 (n = 1) | nd |
| DWG D28–D42 (g/d) | 114.55 (n = 1) | 39.73 (n = 1) | nd |
| FCR D28–D42 | 1.67 (n = 1) | 2.91 (n = 1) | nd |
| BW D42 (g) | 3134.40 a (n = 57) | 1090.20 b (n = 56) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coudert, E.; Baéza, E.; Chartrin, P.; Jimenez, J.; Cailleau-Audouin, E.; Bordeau, T.; Berri, C. Slow and Fast-Growing Chickens Use Different Antioxidant Pathways to Maintain Their Redox Balance during Postnatal Growth. Animals 2023, 13, 1160. https://doi.org/10.3390/ani13071160
Coudert E, Baéza E, Chartrin P, Jimenez J, Cailleau-Audouin E, Bordeau T, Berri C. Slow and Fast-Growing Chickens Use Different Antioxidant Pathways to Maintain Their Redox Balance during Postnatal Growth. Animals. 2023; 13(7):1160. https://doi.org/10.3390/ani13071160
Chicago/Turabian StyleCoudert, Edouard, Elisabeth Baéza, Pascal Chartrin, Justine Jimenez, Estelle Cailleau-Audouin, Thierry Bordeau, and Cécile Berri. 2023. "Slow and Fast-Growing Chickens Use Different Antioxidant Pathways to Maintain Their Redox Balance during Postnatal Growth" Animals 13, no. 7: 1160. https://doi.org/10.3390/ani13071160
APA StyleCoudert, E., Baéza, E., Chartrin, P., Jimenez, J., Cailleau-Audouin, E., Bordeau, T., & Berri, C. (2023). Slow and Fast-Growing Chickens Use Different Antioxidant Pathways to Maintain Their Redox Balance during Postnatal Growth. Animals, 13(7), 1160. https://doi.org/10.3390/ani13071160





