Comparative Genetic Characterization of Pathogenic Escherichia coli Isolated from Patients and Swine Suffering from Diarrhea in Korea
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. E. coli Strains
2.2. Antimicrobial Susceptibility Test
2.3. Multilocus Sequence Typing (MLST)
2.4. Statistical Analysis
3. Results
3.1. Pathotypes and Virotypes (Combination of Colonization Factors and Toxin Genes)
3.2. Antimicrobial Susceptibility Test
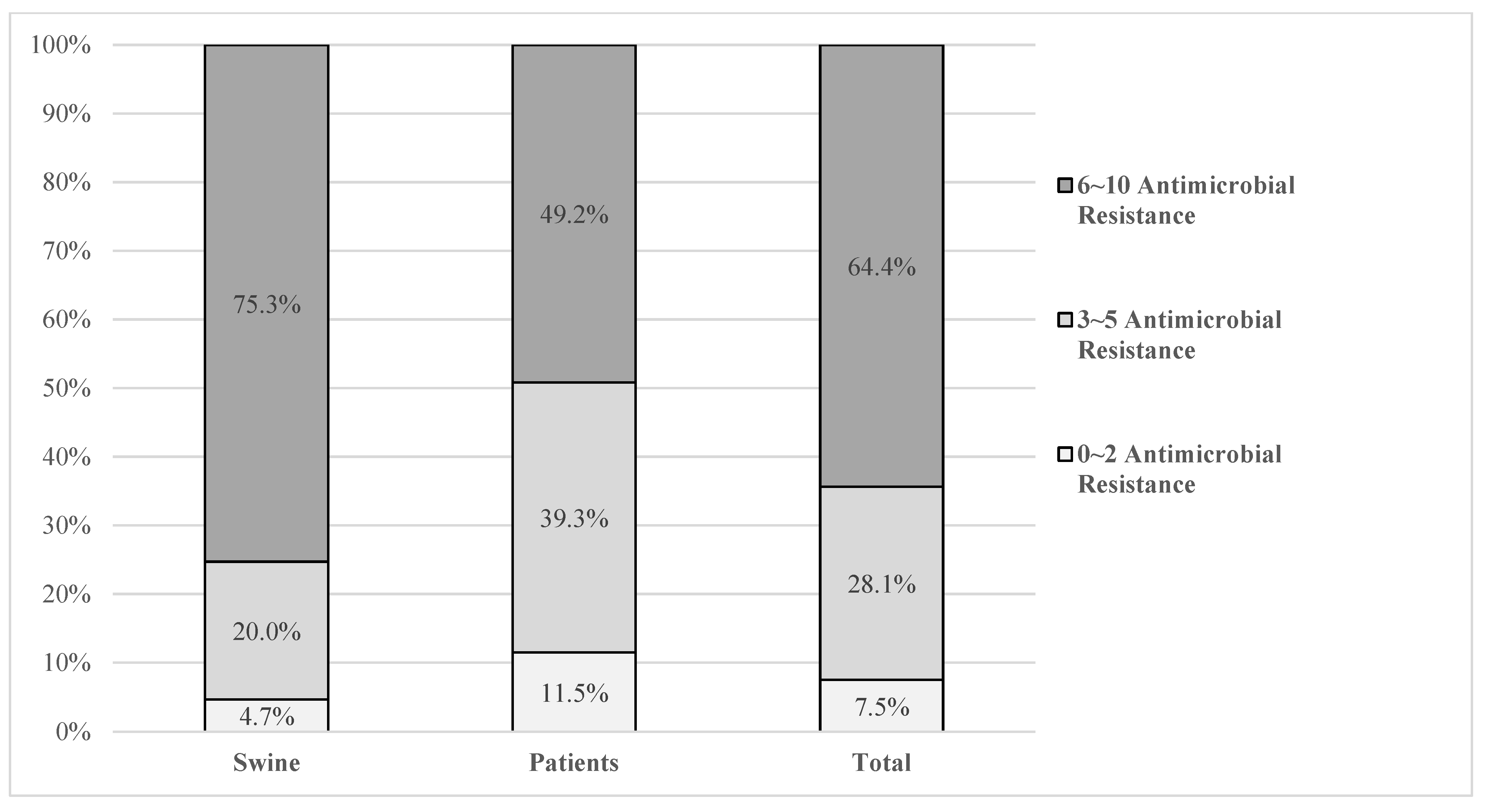
3.3. Multidrug Resistance Rates
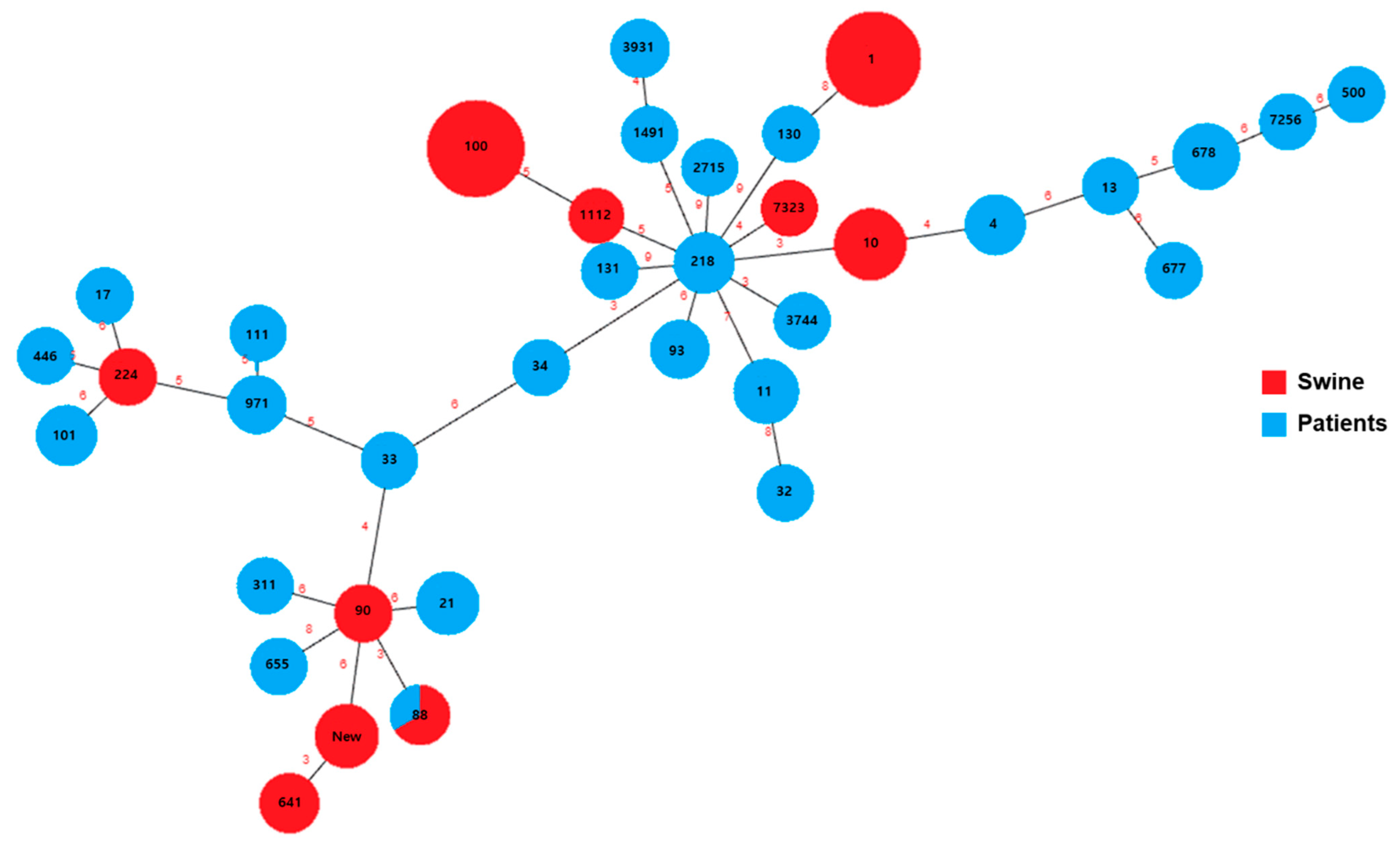
3.4. MLST
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rhouma, M.; Beaudry, F.; Thériault, W.; Bergeron, N.; Laurent-Lewandowski, S.; Fairbrother, J.M.; Letellier, A. Impacts of colistin sulfate on fecal Escherichia coli resistance and on growth. Safepork Posters 2005, 361–365. [Google Scholar]
- Do, K.-H.; Byun, J.-W.; Lee, W.-K. Antimicrobial resistance, adhesin and toxin genes of porcine pathogenic Escherichia coli following the ban on antibiotics as the growth promoters in feed. Pak. Vet. J. 2021, 41, 519–523. [Google Scholar]
- Joseph, A.; Cointe, A.; Mariani Kurkdjian, P.; Rafat, C.; Hertig, A. Shiga toxin-associated hemolytic uremic syndrome: A narrative review. Toxins 2020, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union Summary Report on antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2018/2019. EFSA J. 2021, 19, e06490. [Google Scholar]
- Animal and Plant Quarantine Agency (APQA). Antimicrobial Use and Antimicrobial Resistance Monitoring in Animals and Animal Products; Animal and Plant Quarantine Agency (APQA): Gimcheon, Republic of Korea, 2019. [Google Scholar]
- Szmolka, A.; Nagy, B. Multidrug resistant commensal Escherichia coli in animals and its impact for public health. Front. Microbiol. 2013, 4, 258. [Google Scholar] [CrossRef]
- Iguchi, A.; Iyoda, S.; Seto, K.; Morita-Ishihara, T.; Scheutz, F.; Ohnishi, M. Pathogenic, E. coli Working Group in Japan. Escherichia coli O-genotyping PCR: A comprehensive and practical platform for molecular O serogrouping. J. Clin. Microbiol. 2015, 53, 2427–2432. [Google Scholar]
- Bai, X.; Hu, B.; Xu, Y.; Sun, H.; Zhao, A.; Ba, P.; Fu, S.; Fan, R.; Jin, Y.; Wang, H.; et al. Molecular and phylogenetic characterization of non-O157 Shiga Toxin-producing Escherichia coli strains in China. Front. Cell Infect. Microbiol. 2016, 6, 143. [Google Scholar] [CrossRef]
- Do, K.H.; Byun, J.W.; Lee, W.K. Prevalence of O-serogroups, virulence genes, and F18 antigenic variants in Escherichia coli isolated from weaned piglets with diarrhea in Korea during 2008–2016. J. Vet. Sci. 2019, 20, 43–50. [Google Scholar] [CrossRef]
- Jaureguy, F.; Landraud, L.; Passet, V.; Diancourt, L.; Frapy, E.; Guigon, G.; Carbonnelle, E.; Laortholary, O.; Clermont, O.; Denamur, E.; et al. Phylogenetic and genomic diversity of human bacteremic Escherichia coli strains. BMC Genom. 2008, 9, 560. [Google Scholar] [CrossRef]
- CLSI. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement; M100-S24; CLSI: Wayne, PA, USA, 2020. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Magiorakos, A.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2011, 18, 268–281. [Google Scholar] [CrossRef]
- Paul, N. Review Virulence nature of Escherichia coli in neonatal swine. Online J. Anim. Feed Res. 2015, 5, 169–174. [Google Scholar]
- Sinwat, N.; Angkittitrakul, S.; Coulson, K.F.; Pilapil, F.M.I.R.; Meunsene, D.; Chuanchuen, R. High prevalence and molecular characteristics of multidrug-resistant Salmonella in pigs, pork and humans in Thailand and Laos provinces. J. Med. Microbiol. 2016, 65, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Boerlin, P.; McEwen, S.A.; Boerlin-Petzold, F.; Wilson, J.B.; Johnson, R.P.; Gyles, C.L. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 1999, 37, 497–503. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, H.T.; Kim, Y.H.; Kang, H.J.; Cha, I.H. Isolation of enteropathogenic Escherichia coli, thermophilic Campylobacter and Salmonelleae from scouring piglets. Korean J. Vet. Res. 1988, 28, 67–73. [Google Scholar]
- Do, K.-H.; Byun, J.-W.; Lee, W.-K. Serogroups, virulence genes and antimicrobial resistance of F4+ and F18+ Escherichia coli isolated from weaned piglets. Pak. Vet. J. 2019, 39, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Nandre, R.; Zhou, M.; Zhu, G. Type I fimbriae mediate in vitro adherence of porcine F18ac+ enterotoxigenic Escherichia coli (ETEC). Ann. Microbiol. 2017, 67, 793–799. [Google Scholar] [CrossRef]
- Kwon, D.; Choi, C.; Jung, T.; Chung, H.K.; Kim, J.P.; Bae, S.S.; Cho, W.S.; Kim, J.; Chae, C. Genotypic prevalence of the fimbrial adhesins (F4, F5, F6, F41 and F18) and toxins (LT, STa, STb and Sbc2e) in Escherichia coli isolated from postweaning pigs with diarrhoea or oedema disease in Korea. Vet. Rec. 2002, 150, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Rayamahji, N.; Lee, W.J.; Cha, S.B.; Shin, M.K.; Roh, Y.M.; Yoo, H.S. Genotypes, antibiogram, and pulsed-field gel electrophoresis profiles of Escherichia coli strains from piglets in Korea. J. Vet. Diagn. Investig. 2009, 21, 510–516. [Google Scholar] [CrossRef]
- Do, K.H.; Park, H.E.; Byun, J.W.; Lee, W.K. Virulence and antimicrobial resistance profiles of Escherichia coli encoding mcr gene from diarrhoeic weaned piglets in Korea during 2007–2016. J. Glob. Antimicrob. Resist. 2020, 20, 324–327. [Google Scholar] [CrossRef]
- Jung, J.; Kim, H.; Jo, A.; Kim, J.; Lee, W.; Byun, J. Enrichment media for Stx2e production in Shiga toxin-producing Escherichia coli. J. Biomed. Transl. Res. 2017, 18, 103–107. [Google Scholar] [CrossRef]
- Lee, J.B.; Han, D.; Lee, H.T.; Wi, S.M.; Park, J.H.; Jo, J.W.; Cho, Y.J.; Hahn, T.W.; Lee, S.; Kang, B.; et al. Pathogenic and phylogenetic characteristics of non-O157 Shiga toxin-producing Escherichia coli isolates from retail meats in South Korea. J. Vet. Sci. 2018, 19, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Waddell, T.E.; Lingwood, C.A.; Gyles, C.L. Interaction of verotoxin 2e with pig intestine. Infect. Immun. 1966, 64, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Neut, C. Carriage of multidrug-resistant bacteria in healthy people: Recognition of several risk groups. Antibiotics 2021, 10, 1163. [Google Scholar] [CrossRef]
- Luppi, A. Swine enteric colibacillosis: Diagnosis, therapy and antimicrobial resistance. Porc. Health Manag. 2017, 3, 16. [Google Scholar] [CrossRef]
- Niewerth, U.; Frey, A.; Voss, T.; Le Bouguénec, C.; Baljer, G.; Franke, S.; Schmidt, M.A. The AIDA autotransporter System Is Associated with F18 and Stx2e in Escherichia coli Isolates from Pigs Diagnosed with edema Disease and Postweaning diarrhea. Clin. Diagn. Lab. Immunol. 2001, 8, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, M.; Ruesch, L.; Omot, A.; Francis, D. Prevalence of virulence genes in Escherichia coli strains recently isolated from young pigs with diarrhea in the US. Vet. Microbiol. 2007, 123, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, X.; Xu, X.; Song, G.; Liu, X. Analysis of the AIDA-I gene sequence and prevalence in Escherichia coli isolates from pigs with post-weaning diarrhoea and oedema disease. Vet. J. 2009, 180, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Xia, P.; Nandre, R.; Zhang, W.; Zhu, G. Review of newly identified functions associated with the heat-labile toxin of enterotoxigenic Escherichia coli. Front. Cell Infect. Microbiol. 2019, 9, 292. [Google Scholar] [CrossRef]
- Pungpian, C.; Sinwat, N.; Angkititrakul, S.; Prathan, R.; Chuanchuen, R. Presence and Transfer of antimicrobial Resistance Determinants in Escherichia coli in Pigs, Pork, and Humans in Thailand and Lao PDR Border Provinces. Microb. Drug Resist. 2021, 27, 571–584. [Google Scholar] [CrossRef]
- Brilhante, M.; Perreten, V.; Donà, V. Multidrug resistance and multivirulence plasmids in enterotoxigenic and hybrid Shiga toxin-producing/enterotoxigenic Escherichia coli isolated from diarrheic pigs in Switzerland. Vet. J. 2019, 244, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Do, K.H.; Byun, J.W.; Lee, W.K. Virulence genes and antimicrobial resistance of pathogenic Escherichia coli isolated from diarrheic weaned piglets in Korea. J. Anim. Sci. Technol. 2020, 62, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.M.; Zhao, C.; DebRoy, C.; Torcolini, J.; Zhao, S.; White, D.G.; Wagner, D.D.; McDermott, P.F.; Walker, R.D.; Meng, J. Antimicrobial resistance of Escherichia coli O157 isolated from humans, cattle, swine, and food. Appl. Environ. Microbiol. 2002, 68, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.; Wales, A. Antimicrobial resistance on farms: A review including biosecurity and the potential role of disinfectants in resistance selection. Compr. Rev. Food Sci. Food Saf. 2019, 18, 753–774. [Google Scholar] [CrossRef]
- FAO. Joint FAO/WHO/OIE expert meeting on critically important antimicrobials. In Proceedings of the FAO/WHO/OIE Expert Meeting FAO Headquarters, Rome, Italy, 26–30 November 2007. [Google Scholar]
- Hu, Y.S.; Shin, S.; Park, Y.H.; Park, K.T. Prevalence and mechanism of fluoroquinolone resistance in Escherichia coli isolated from swine feces in Korea. J. Food Prot. 2017, 80, 1145–1151. [Google Scholar] [CrossRef]
- Van Den Bogaard, A.E.J.M.; London, N.; Stobberingh, E.E. Antimicrobial resistance in pig faecal samples from the Netherlands (five abattoirs) and Sweden. J. Antimicrob. Chemother. 2000, 45, 663–671. [Google Scholar] [CrossRef]
- Sayah, R.S.; Kaneene, J.B.; Johnson, Y.; Miller, R. Patterns of antimicrobial resistance observed in Escherichia coli isolates obtained from domestic- and wild-animal fecal samples, human Septage, and surface water. Appl. Environ. Microbiol. 2005, 71, 1394–1404. [Google Scholar] [CrossRef]
- Barton, M.D. Impact of antibiotic use in the swine industry. Curr. Opin. Microbiol. 2014, 19, 9–15. [Google Scholar] [CrossRef]
- Zarrilli, R.; Tripodi, M.F.; Di Popolo, A.; Fortunato, R.; Bagattini, M.; Crispino, M.; Florio, A.; Triassi, M.; Utili, R. Molecular epidemiology of high-level aminoglycoside-resistant enterococci isolated from patients in a university hospital in southern Italy. J. Antimicrob. Chemother. 2005, 56, 827–835. [Google Scholar] [CrossRef]
- van Breda, L.K.; Dhungyel, O.P.; Ward, M.P. Antibiotic resistant Escherichia coli in southeastern Australian pig herds and implications for surveillance. Zoonoses Publ. Health 2018, 65, e1–e7. [Google Scholar] [CrossRef]
- Jackson, C.R.; Fedorka-Cray, P.J.; Barrett, J.B.; Ladely, S.R. High-level aminoglycoside resistant enterococci isolated from swine. Epidemiol. Infect. 2005, 133, 367–371. [Google Scholar] [CrossRef]
- Aarestrup, F.M.; Oliver Duran, C.; Burch, D.G.S. Antimicrobial resistance in swine production. Anim. Health Res. Rev. 2008, 9, 135–148. [Google Scholar] [PubMed]
- Pan, Y.; Hu, B.; Bai, X.; Yang, X.; Cao, L.; Liu, Q.; Sun, H.; Li, J.; Zhang, J.; Dong, J.; et al. Antimicrobial resistance of non-O157 Shiga toxin-producing Escherichia coli isolated from humans and domestic animals. Antibiotics 2021, 10, 74. [Google Scholar] [CrossRef]
- Johnson, T.J.; Logue, C.M.; Johnson, J.R.; Kuskowski, M.A.; Sherwood, J.S.; Barnes, H.J.; DebRoy, C.; Wannemuehler, Y.M.; Obata-Yasuoka, M.; Spanjaard, L.; et al. Associations between multidrug resistance, plasmid content, and virulence potential among extraintestinal pathogenic and commensal Escherichia coli from humans and poultry. Foodborne Pathog. Dis. 2012, 9, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Korea Institute for Health and Social Affairs. Drug Use Evaluation; Korea Institute for Health and Social Affairs: Seoul, Republic of Korea, 2000. [Google Scholar]
- Organization for Economic Co-operation and Development. Antimicrobial Resistance. OECD. Available online: http://www.oecd.org/els/health-systems/antimicrobial-resistance.htm (accessed on 19 October 2022).
- Chattaway, M.A.; Day, M.; Mtwale, J.; White, E.; Rogers, J.; Day, M.; Powell, D.; Ahmad, M.; Harris, R.; Talukder, K.A.; et al. Clonality, virulence and antimicrobial resistance of enteroaggregative Escherichia coli from Mirzapur, Bangladesh. J. Med. Microbiol. 2017, 66, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Chen, X.; Zheng, S.; Han, D.; Wang, Y.; Wang, R.; Wang, B.; Chen, Y. Prevalence and genetic diversity of human diarrheagenic Escherichia coli isolates by multilocus sequence typing. Int. J. Infect. Dis. 2018, 67, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Savin, M.; Bierbaum, G.; Kreyenschmidt, J.; Schmithausen, R.M.; Sib, E.; Schmoger, S.; Kasbohrer, A.; Hammerl, J.A. Clinically relevant Escherichia coli isolates from process waters and wastewater of poultry and pig slaughterhouses in Germany. Microorganisms 2021, 9, 698. [Google Scholar] [CrossRef]
- Crémet, L.; Caroff, N.; Giraudeau, C.; Dauvergne, S.; Lepelletier, D.; Reynaud, A.; Corvec, S. Occurrence of ST23 complex phylogroup a Escherichia coli isolates producing extended-spectrum AmpC β-lactamase in a French hospital. Antimicrob. Agents Chemother. 2010, 54, 2216–2218. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Chanoine, M.H.; Blanco, J.; Leflon-Guibout, V.; Demarty, R.; Alonso, M.P.; Caniça, M.M.; Park, Y.J.; Lavigne, J.P.; Pitout, J.; Johnson, J.R. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 2008, 61, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shao, W.; Wei, L.; Chen, L.; Zhu, A.; Pan, Z. Subtyping Salmonella isolated from pet dogs with multilocus sequence typing (MLST) and clustered regularly interspaced short palindromic repeats (CRISPRs). AMB Express 2021, 11, 60. [Google Scholar] [CrossRef]
| Pathotype /Virotype | No. (%) of Pathogenic E. coli Isolates | Chi-Square Value | Degrees of Freedom | p-Value | ||
|---|---|---|---|---|---|---|
| Swine (n = 85) | Patients (n = 61) | Total (n = 146) | ||||
| ETEC * | 40 (47.1%) | 14 (23.0%) | 54 (37.0%) | 12.695 | 1 | 0.000 |
| F4:LT:STb:EAST1 * | 30 (75.0%) | - | 30 (55.6%) | 120.000 | 1 | 0.000 |
| ST * | - | 5 (35.7%) | 5 (9.3%) | 43.902 | 1 | 0.000 |
| F4:paa:LT:STb:EAST1 * | 1 (2.5%) | 3 (21.4%) | 4 (7.4%) | 15.341 | 1 | 0.000 |
| LT * | - | 3 (21.4%) | 3 (5.6%) | 23.464 | 1 | 0.000 |
| LT:ST * | - | 3 (21.4%) | 3 (5.6%) | 23.464 | 1 | 0.000 |
| F18:AIDA * | 2 (5.0%) | - | 2 (3.7%) | 5.128 | 1 | 0.024 |
| F4:F18:LT:STa:STb:EAST1 * | 2 (5.0%) | - | 2 (3.7%) | 5.128 | 1 | 0.024 |
| AIDA:STb:EAST1 | 1 (2.5%) | - | 1 (1.9%) | 3.046 | 1 | 0.081 |
| F18:LT:STa:STb:EAST1 | 1 (2.5%) | - | 1 (1.9%) | 3.046 | 1 | 0.081 |
| F18:paa:AIDA:STb:EAST1 | 1 (2.5%) | - | 1 (1.9%) | 3.046 | 1 | 0.081 |
| F5:paa | 1 (2.5%) | - | 1 (1.9%) | 3.046 | 1 | 0.081 |
| STEC * | 28 (32.9%) | 31 (50.8%) | 59 (40.4%) | 6.650 | 1 | 0.010 |
| F18:AIDA:stx 2e * | 14 (50.0%) | - | 14 (23.7%) | 66.667 | 1 | 0.000 |
| stx1:stx 2 * | - | 12 (38.7%) | 12 (20.3%) | 48.447 | 1 | 0.000 |
| stx1 * | - | 11 (35.5%) | 11 (18.6%) | 43.902 | 1 | 0.000 |
| stx2 * | - | 8 (25.8%) | 8 (13.6%) | 29.885 | 1 | 0.000 |
| F18:stx2:stx2e * | 7 (25.0%) | - | 7 (11.9%) | 28.571 | 1 | 0.000 |
| F18:stx2e * | 4 (14.3%) | - | 4 (6.8%) | 15.054 | 1 | 0.000 |
| F18:AIDA:stx2:stx2e * | 3 (10.7%) | - | 3 (5.1%) | 11.640 | 1 | 0.001 |
| EAEC * | - | 3 (4.9%) | 3 (2.1%) | 5.128 | 1 | 0.024 |
| aggR * | - | 3 (100.0%) | 3 (100.0%) | 200.000 | 1 | 0.000 |
| EIEC | - | 1 (1.6%) | 1 (0.7%) | 2.020 | 1 | 0.155 |
| ipaH * | - | 1 (100.0%) | 1 (100.0%) | 200.000 | 1 | 0.000 |
| EPEC | - | 1 (1.6%) | 1 (0.7%) | 2.020 | 1 | 0.155 |
| eae * | - | 1 (100.0%) | 1 (100.0%) | 200.000 | 1 | 0.000 |
| ETEC/STEC * | 17 (20.0%) | 2 (3.3%) | 19 (13.0%) | 14.198 | 1 | 0.000 |
| F18:LT:STa:stx2e * | 5 (29.4%) | - | 5 (26.3%) | 35.294 | 1 | 0.000 |
| F18:st × 2:stx2e:EAST1 * | 5 (29.4%) | - | 5 (26.3%) | 35.294 | 1 | 0.000 |
| F18:LT:stx2e * | 3 (17.6%) | - | 3 (15.8%) | 19.780 | 1 | 0.000 |
| F18:paa:STa:stx2e * | 2 (11.8%) | - | 2 (10.5%) | 12.766 | 1 | 0.000 |
| F18:stx2e:EAST1 * | 2 (11.8%) | - | 2 (10.5%) | 12.766 | 1 | 0.000 |
| F18:paa:LT:STa:stx2:stx2e * | - | 1 (50.0%) | 1 (5.3%) | 66.667 | 1 | 0.000 |
| F18:paa:LT:STb:st × 2:stx2e:EAST1 * | - | 1 (50.0%) | 1 (5.3%) | 66.667 | 1 | 0.000 |
| STEC/EPEC * | - | 3 (4.9%) | 3 (2.1%) | 5.128 | 1 | 0.024 |
| stx1:eae * | - | 3 (100.0%) | 3 (100.0%) | 200.000 | 1 | 0.000 |
| STEC/EAEC * | - | 3 (4.9%) | 3 (2.1%) | 5.128 | 1 | 0.024 |
| stx2:aggR * | - | 3 (100.0%) | 3 (100.0%) | 200.000 | 1 | 0.000 |
| Undetected * | - | 3 (4.9%) | 3 (2.1%) | 5.128 | 1 | 0.000 |
| Antimicrobial Agent 1 | No. (%) of Resistant Isolates | Chi-Square Value | Degrees of Freedom | p-Value | |||
|---|---|---|---|---|---|---|---|
| Swine (n = 85) | Patients (n = 61) | Total (n = 146) | |||||
| Aminoglycosides | GM * | 44 (51.8%) | 11 (18.0%) | 55 (37.7%) | 25.407 | 1 | 0.000 |
| S | 59 (69.4%) | 36 (59.0%) | 95 (65.1%) | 2.170 | 1 | 0.104 | |
| N * | 44 (51.8%) | 18 (29.5%) | 62 (42.5%) | 10.004 | 1 | 0.002 | |
| K * | 76 (89.4%) | 25 (41.0%) | 101 (69.2%) | 50.637 | 1 | 0.000 | |
| AN * | 64 (75.3%) | 2 (3.3%) | 66 (45.2%) | 108.953 | 1 | 0.000 | |
| β-lactam/ lactamase inhibitor | AMC * | 31 (36.5%) | 44 (72.1%) | 75 (51.4%) | 24.700 | 1 | 0.000 |
| Cephalosporin 1 | CF | 35 (41.2%) | 26 (42.6%) | 61 (41.8%) | 0.082 | 1 | 0.774 |
| CZ | 29 (34.1%) | 16 (26.2%) | 45 (30.8%) | 1.524 | 1 | 0.217 | |
| Cephalosporin 2 | FOX | 25 (29.4%) | 12 (19.7%) | 37 (25.3%) | 2.667 | 1 | 0.139 |
| Cephalosporin 4 | FEP | 0 (0.0%) | 2 (3.3%) | 2 (1.4%) | 3.046 | 1 | 0.081 |
| Quinolone | NA * | 60 (70.6%) | 31 (50.8%) | 91 (62.3%) | 8.407 | 1 | 0.004 |
| Fluoroquinolone | CIP * | 35 (41.2%) | 12 (19.7%) | 47 (32.2%) | 10.402 | 1 | 0.001 |
| NOR * | 28 (32.9%) | 11 (18.0%) | 39 (26.7%) | 5.922 | 1 | 0.015 | |
| Tetracyclines | TE * | 74 (87.1%) | 31 (50.8%) | 105 (71.9%) | 30.295 | 1 | 0.000 |
| DOX * | 68 (80.0%) | 18 (29.5%) | 86 (58.9%) | 50.505 | 1 | 0.000 | |
| Aminopenicillin | AMP | 74 (87.1%) | 47 (77.0%) | 121 (82.9%) | 3.388 | 1 | 0.066 |
| Folate pathway inhibitors | SXT * | 48 (56.5%) | 21 (34.4%) | 69 (47.3%) | 10.666 | 1 | 0.001 |
| Phenicols | C * | 72 (84.7%) | 21 (34.4%) | 93 (63.7%) | 53.968 | 1 | 0.000 |
| Polymyxins | CL | 1 (1.2%) | 0 (0.0%) | 1 (0.7%) | 1.005 | 1 | 0.316 |
| Lincosamide | CC | 85 (100.0%) | 61 (100.0%) | 146 (100.0%) | Not available | Not available | Not available |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, K.-H.; Seo, K.; Jung, M.; Lee, W.-K.; Lee, W.-K. Comparative Genetic Characterization of Pathogenic Escherichia coli Isolated from Patients and Swine Suffering from Diarrhea in Korea. Animals 2023, 13, 1154. https://doi.org/10.3390/ani13071154
Do K-H, Seo K, Jung M, Lee W-K, Lee W-K. Comparative Genetic Characterization of Pathogenic Escherichia coli Isolated from Patients and Swine Suffering from Diarrhea in Korea. Animals. 2023; 13(7):1154. https://doi.org/10.3390/ani13071154
Chicago/Turabian StyleDo, Kyung-Hyo, Kwangwon Seo, Myunghwan Jung, Woo-Kon Lee, and Wan-Kyu Lee. 2023. "Comparative Genetic Characterization of Pathogenic Escherichia coli Isolated from Patients and Swine Suffering from Diarrhea in Korea" Animals 13, no. 7: 1154. https://doi.org/10.3390/ani13071154
APA StyleDo, K.-H., Seo, K., Jung, M., Lee, W.-K., & Lee, W.-K. (2023). Comparative Genetic Characterization of Pathogenic Escherichia coli Isolated from Patients and Swine Suffering from Diarrhea in Korea. Animals, 13(7), 1154. https://doi.org/10.3390/ani13071154






