Susceptibility of Ovine Bone Marrow-Derived Mesenchymal Stem Cell Spheroids to Scrapie Prion Infection
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bone Marrow Extraction and Ovine Mesenchymal Stem Cell Isolation and Culture
2.2. Ovine Mesenchymal Stem Cell Characterization
2.3. Formation and Culture of Ovine Mesenchymal Stem Cell Spheroids
2.4. Neurogenic Differentiation of Ovine Mesenchymal Stem Cells and Spheroids
2.4.1. Neurogenic Differentiation
2.4.2. Nissl Bodies Staining
2.4.3. Expression Analysis of Neuronal Markers
2.5. Scrapie Inocula and Infection of 2D Ovine Mesenchymal Stem Cell Cultures and Spheroids in Growth and Neurogenic Conditions
2.6. PrPSc Detection
2.6.1. ELISA (Enzyme-linked Immunosorbent Assay)
2.6.2. Immunocytochemistry of Infected Spheroids
2.7. Cell Viability Assay
3. Results
3.1. Ovine Mesenchymal Stem Cell Differentiation into Mesodermal Lineages
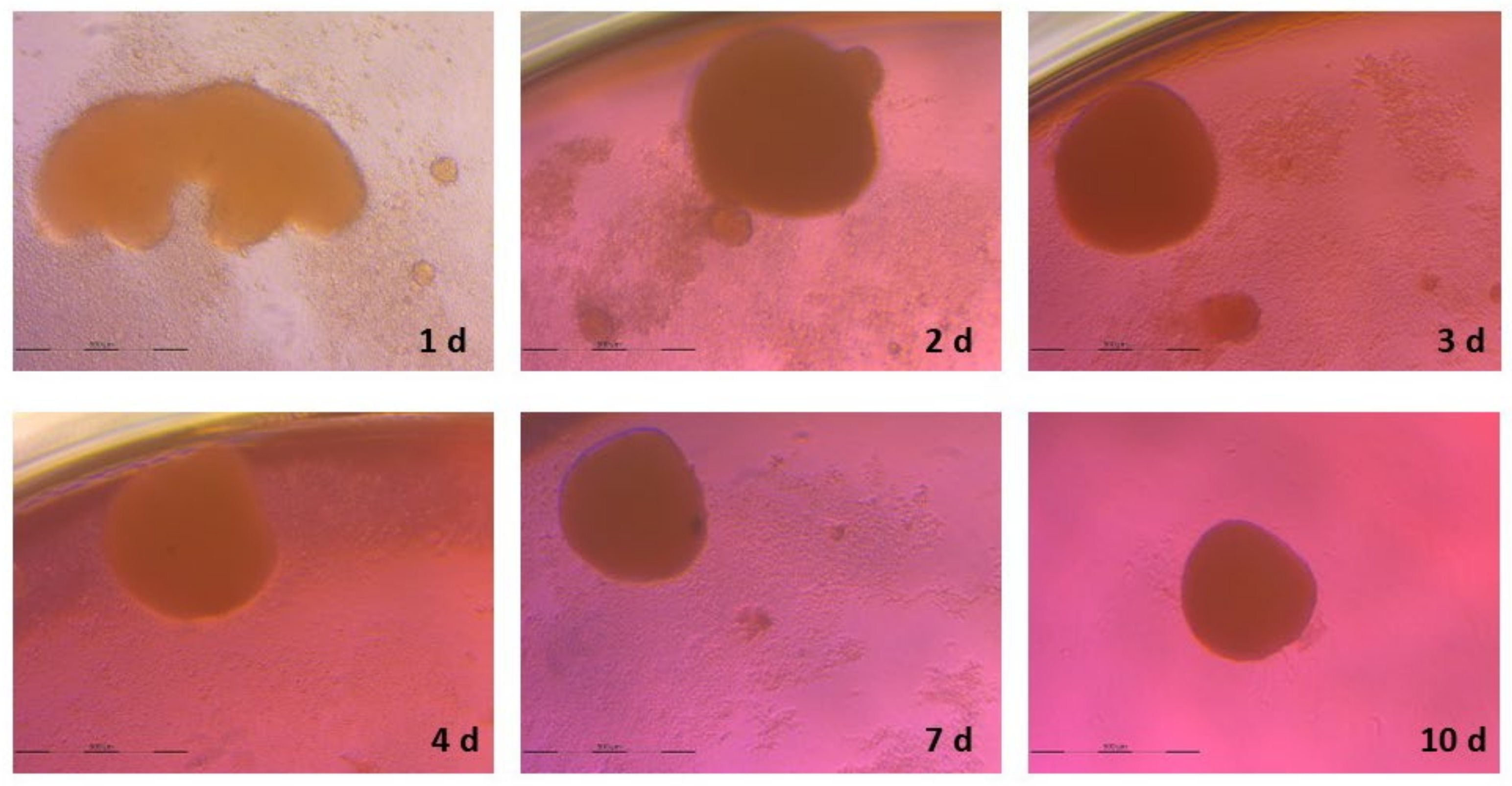
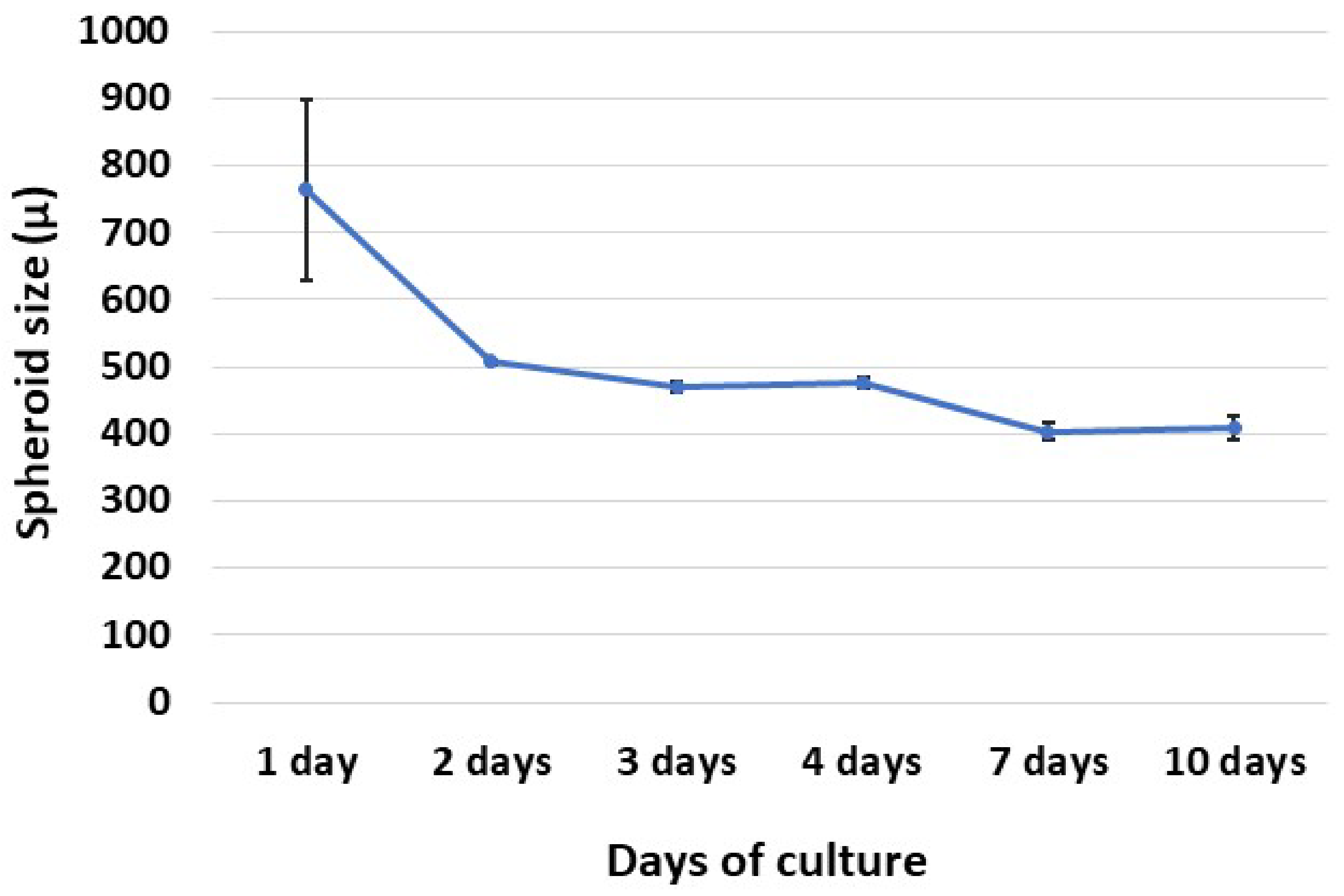
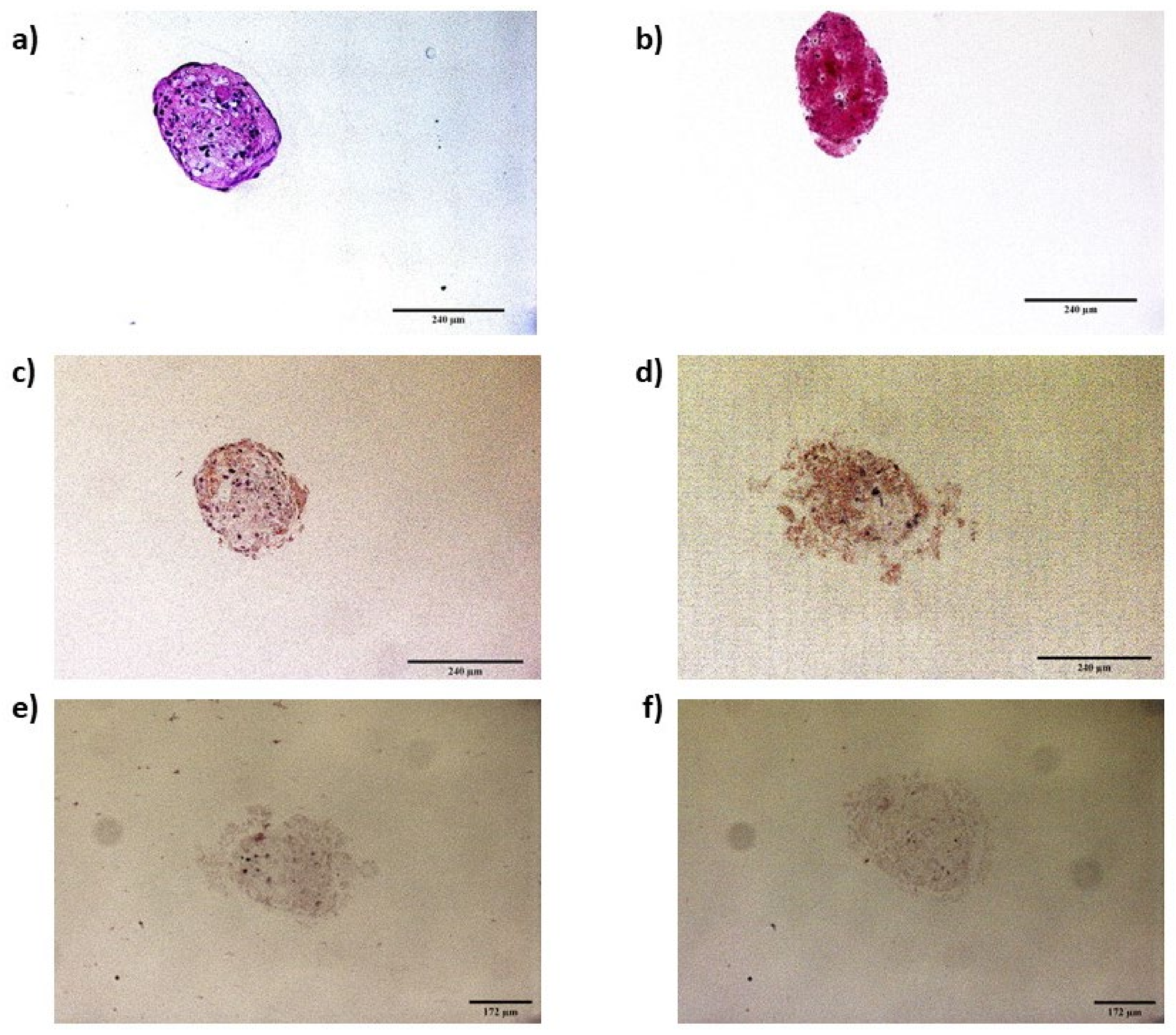
3.2. Spheroid Formation
3.3. Neurogenic Differentiation
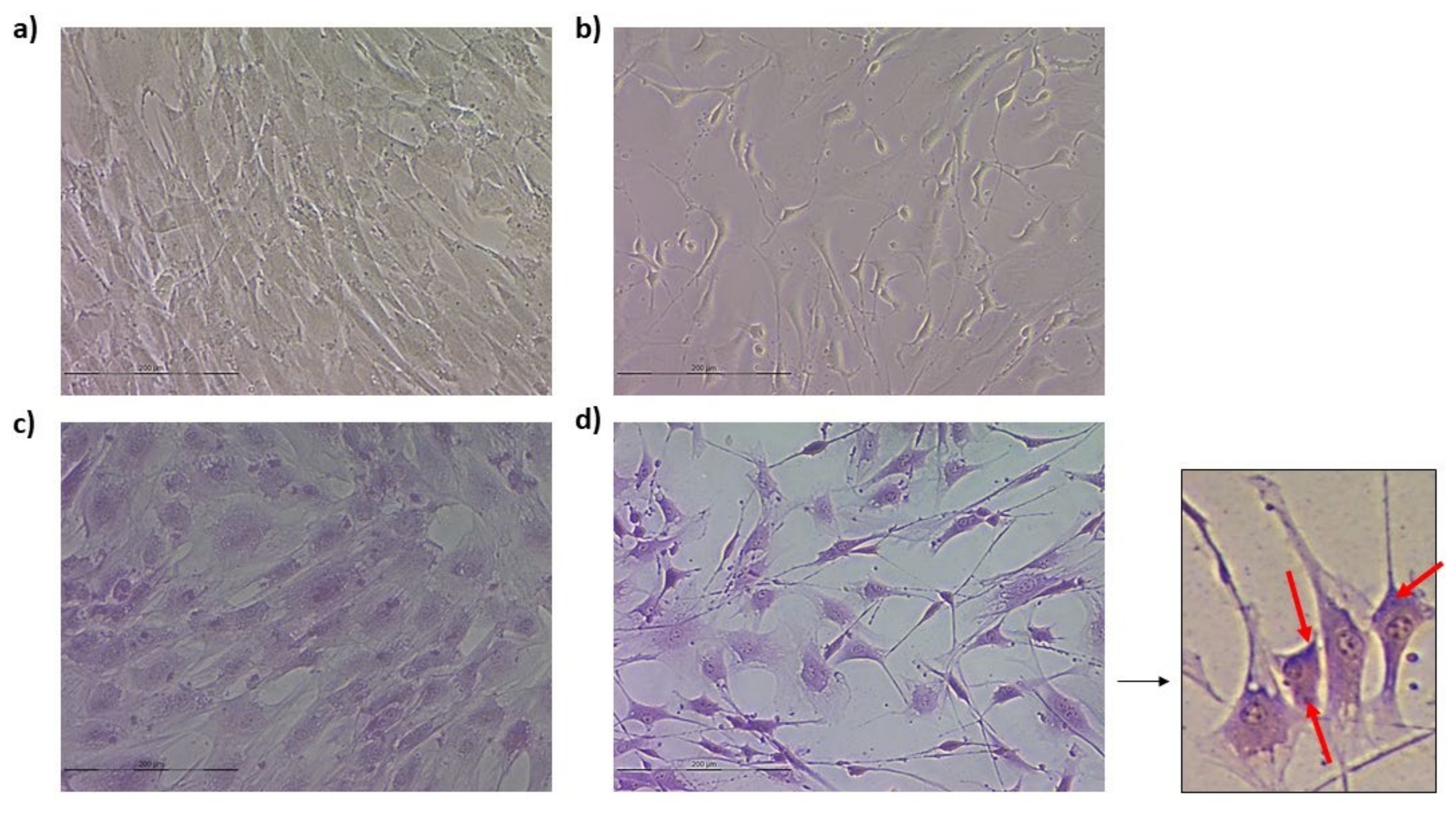
3.3.1. Ovine Mesenchymal Stem Cell 2D Cultures
3.3.2. Spheroids
3.4. Levels of PrPSc after Prion Infection
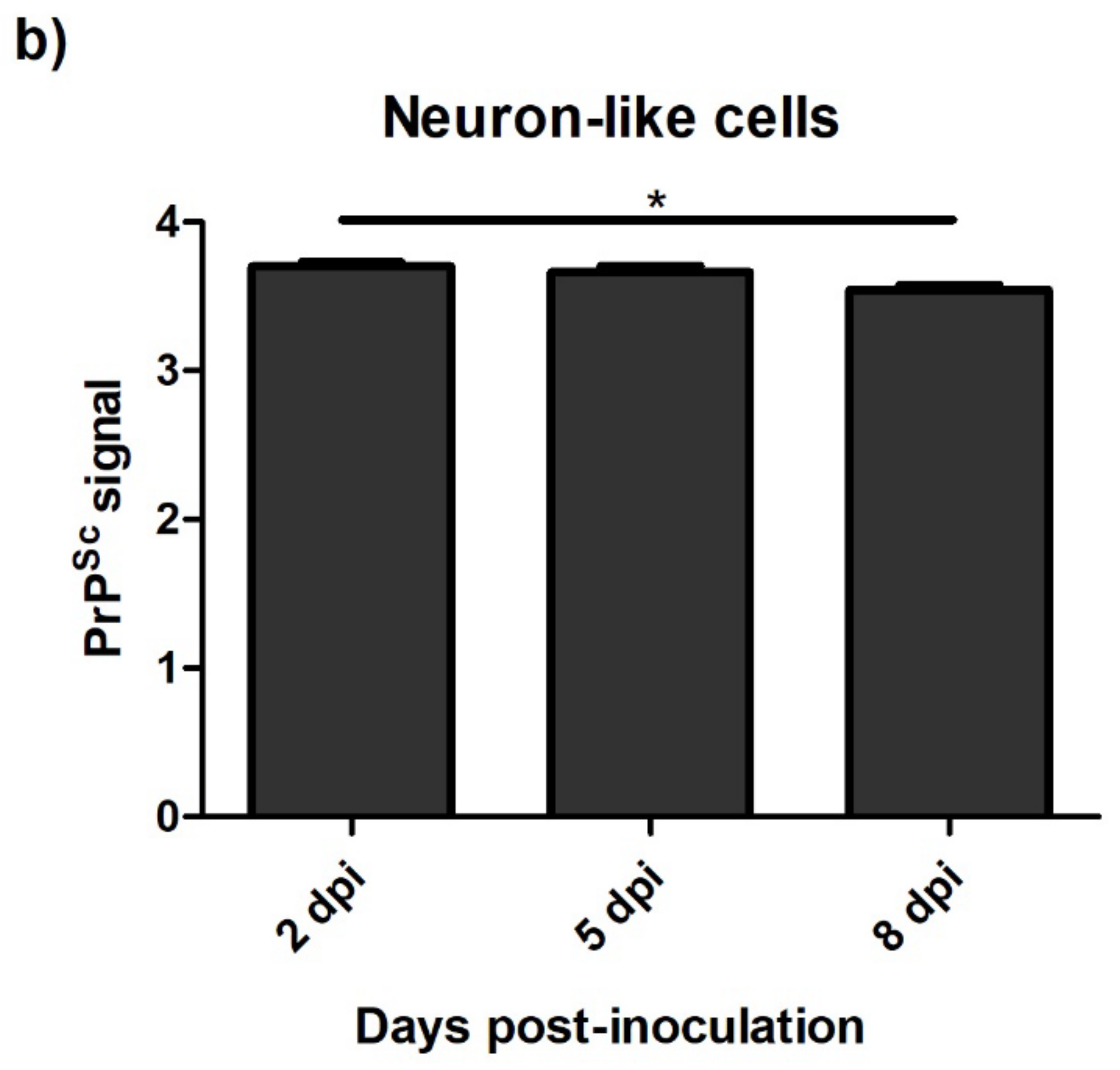
3.4.1. Ovine Mesenchymal Stem Cells and Neuron-like Cells Infected with Scrapie
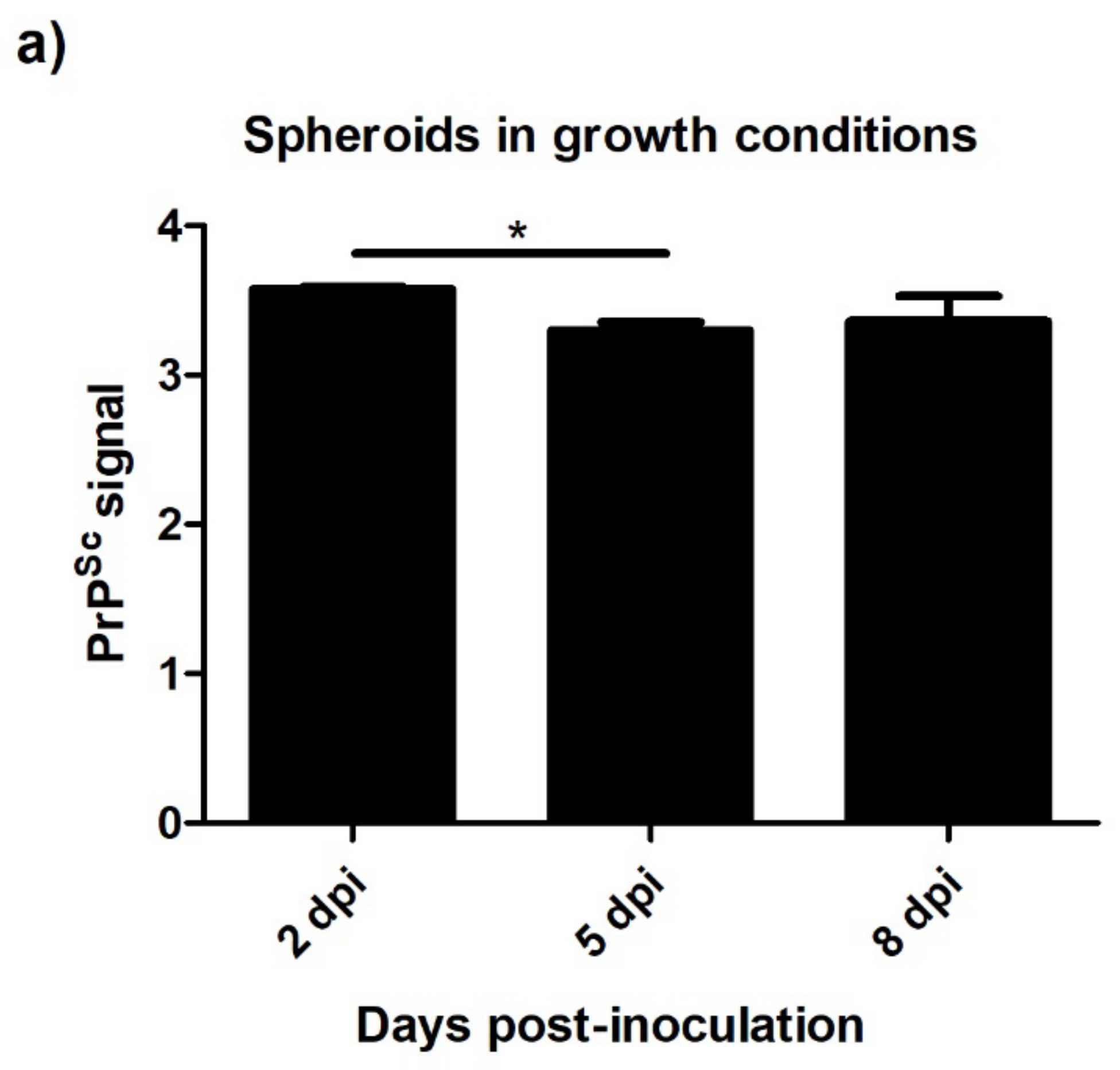
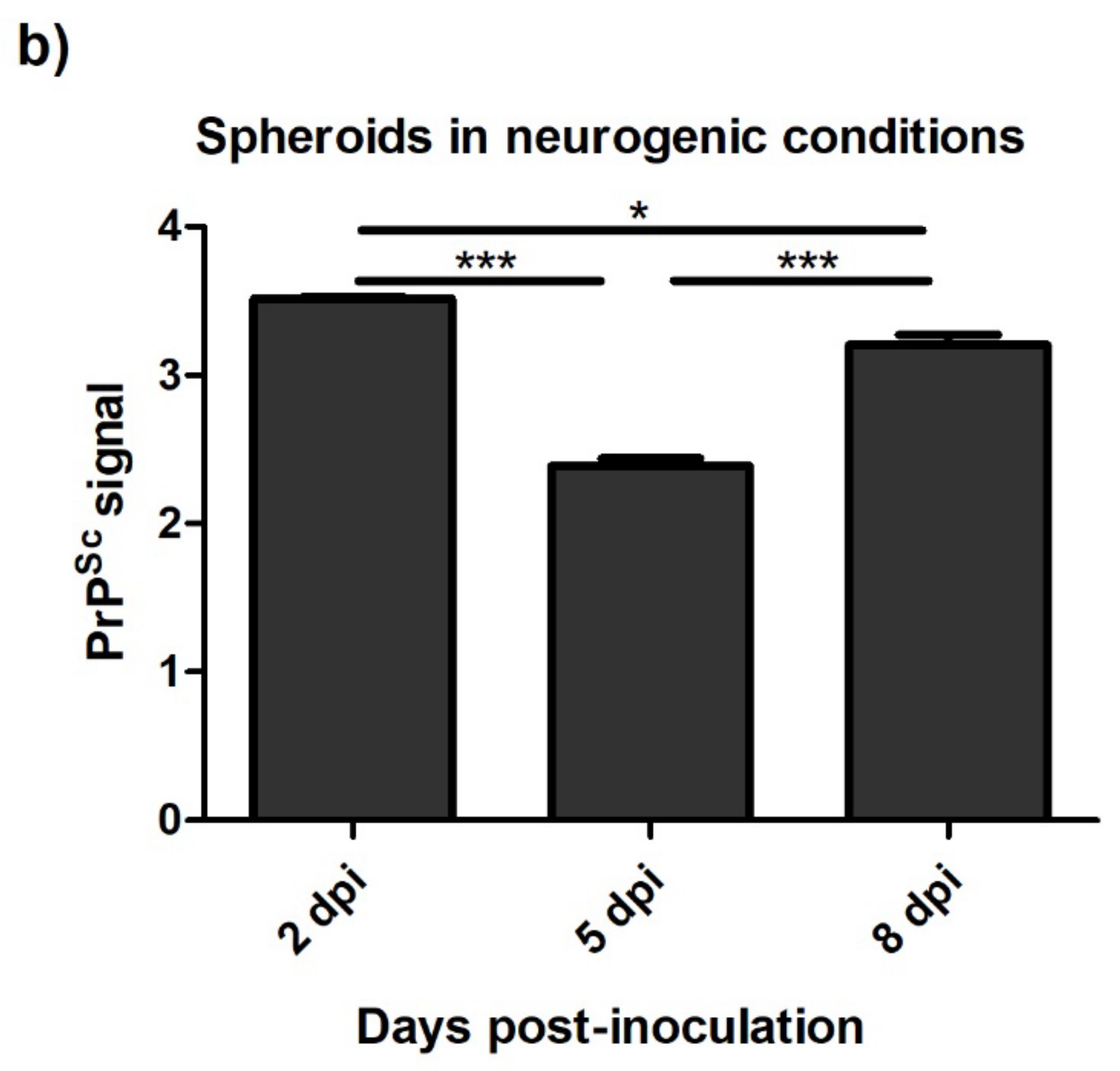
3.4.2. Spheroids in Growth and Neurogenic Conditions Infected with Scrapie
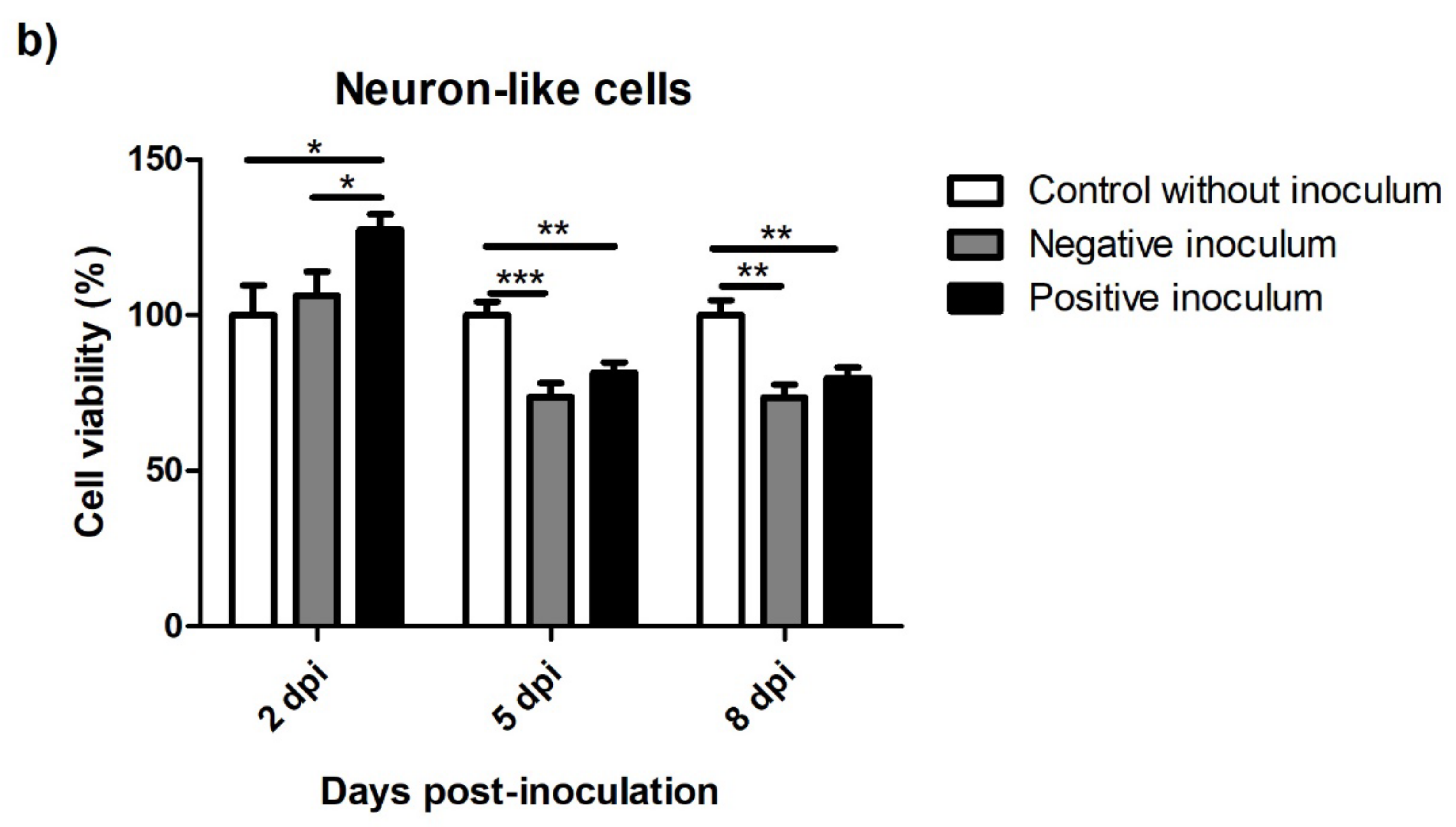
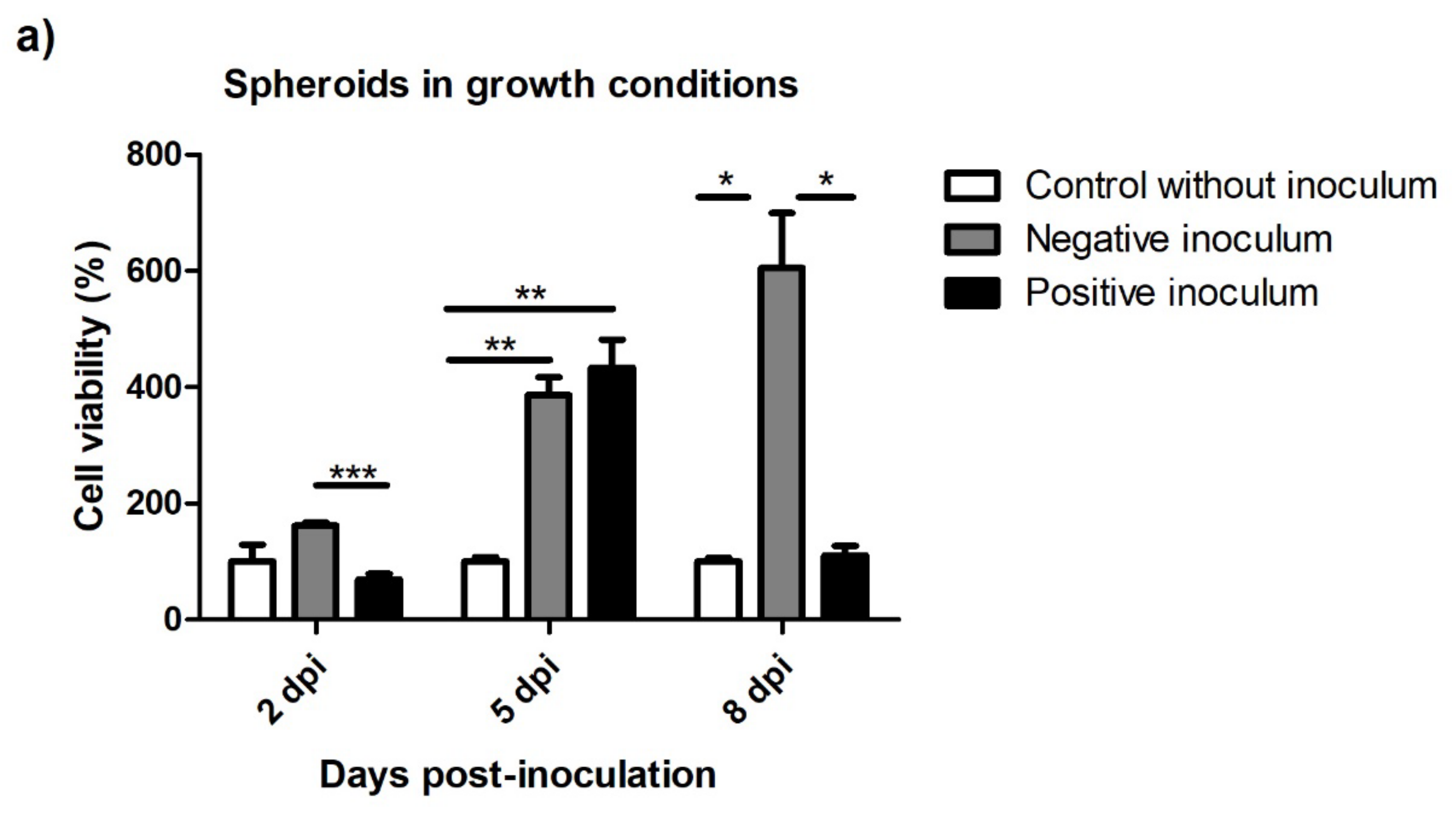
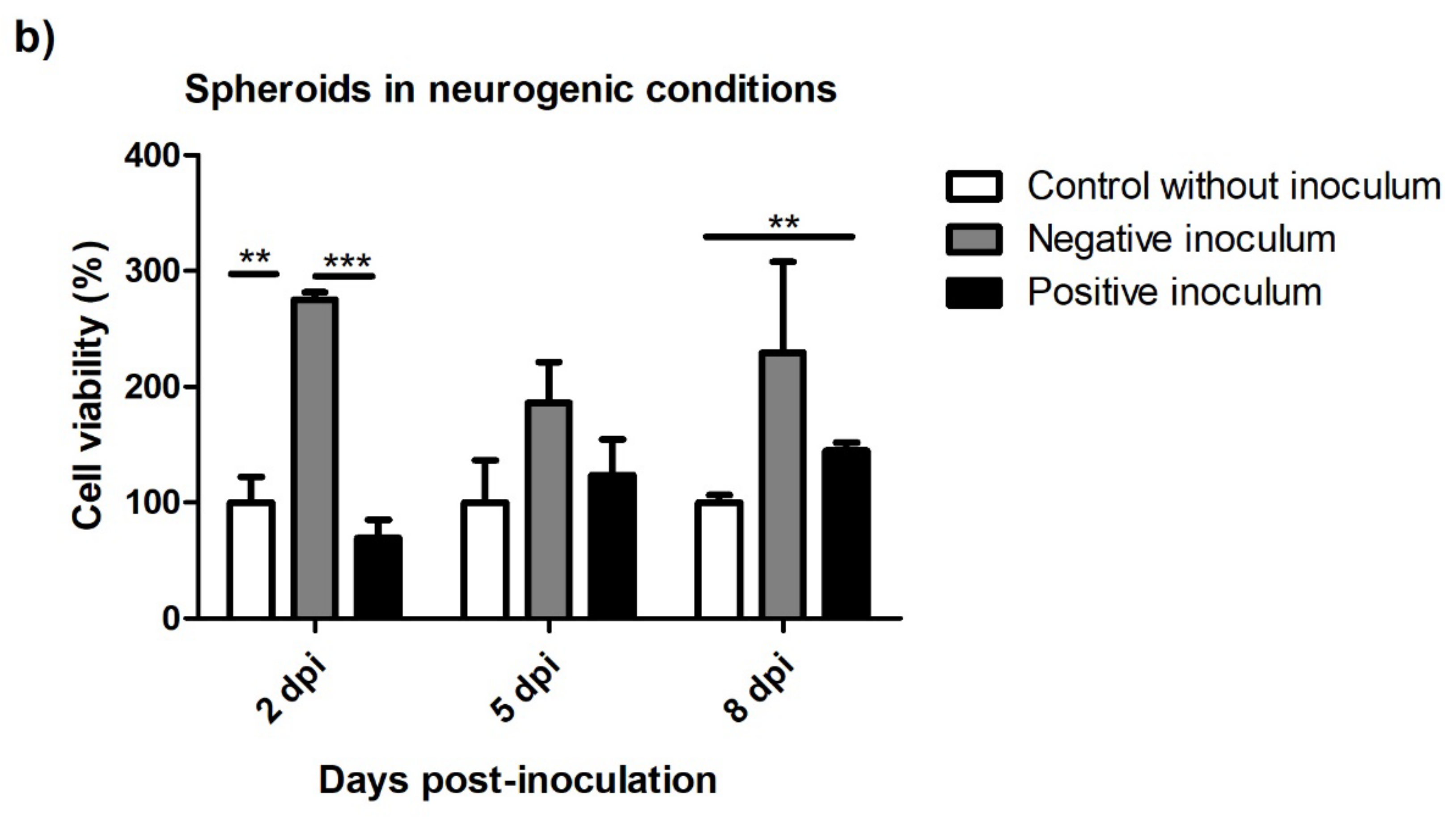
3.5. Viability after Prion Infection
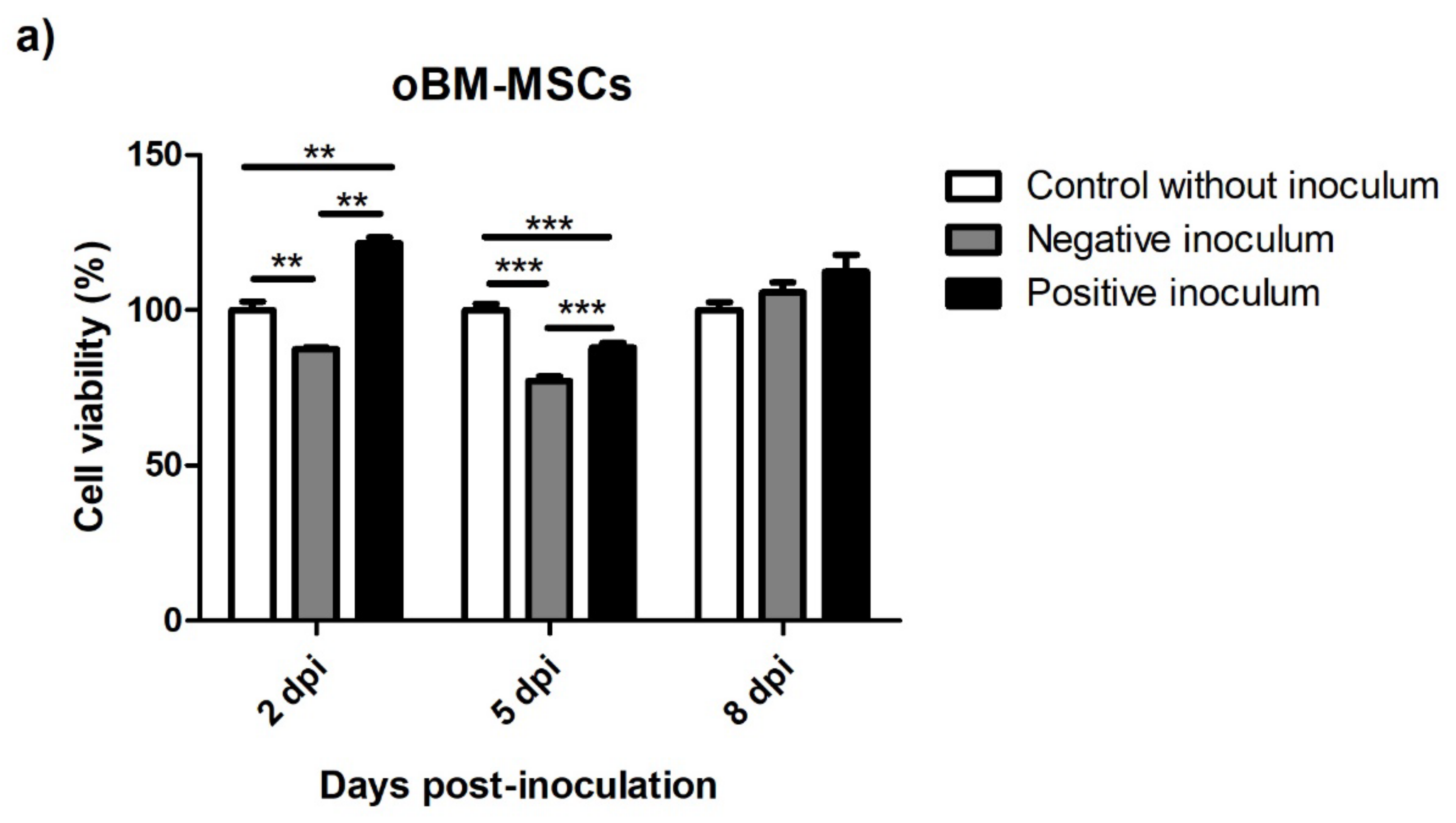
3.5.1. oBM-MSC and Neuron-like Cell 2D Cultures
3.5.2. Spheroids in Growth and Neurogenic Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-Dimensional Cell Culture Systems as an in Vitro Platform for Cancer and Stem Cell Modeling. World J. Stem Cells 2019, 11, 1065–1083. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-Dimensional Cell Culture: A Powerful Tool in Tumor Research and Drug Discovery. Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, B.; Ghajar, C.M.; Bissell, M.J. The Need for Complex 3D Culture Models to Unravel Novel Pathways and Identify Accurate Biomarkers in Breast Cancer. Adv. Drug Deliv. Rev. 2014, 69–70, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Paul Solomon, F.D. 3D Cell Culture Systems: Advantages and Applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Martinez, E.; Suazo-Sanchez, I.; Celis-Romero, M.; Carnero, A. 3D and Organoid Culture in Research: Physiology, Hereditary Genetic Diseases and Cancer. Cell Biosci. 2022, 12, 39. [Google Scholar] [CrossRef]
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; et al. Comparison of 2D- and 3D-Culture Models as Drug-Testing Platforms in Breast Cancer. Oncol. Rep. 2015, 33, 1837–1843. [Google Scholar] [CrossRef]
- Tekin, H.; Simmons, S.; Cummings, B.; Gao, L.; Adiconis, X.; Hession, C.C.; Ghoshal, A.; Dionne, D.; Choudhury, S.R.; Yesilyurt, V.; et al. Effects of 3D Culturing Conditions on the Transcriptomic Profile of Stem-Cell-Derived Neurons. Nat. Biomed. Eng. 2018, 2, 540–554. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Arneri, A.; Bersini, S.; Shin, S.R.; Zhu, K.; Goli-Malekabadi, Z.; Aleman, J.; Colosi, C.; Busignani, F.; Dell’Erba, V.; et al. Bioprinting 3D Microfibrous Scaffolds for Engineering Endothelialized Myocardium and Heart-on-a-Chip. Biomaterials 2016, 110, 45–59. [Google Scholar] [CrossRef]
- Cesarz, Z.; Tamama, K. Spheroid Culture of Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 9176357. [Google Scholar] [CrossRef]
- Bates, R.C.; Edwards, N.S.; Yates, J.D. Spheroids and Cell Survival. Crit. Rev. Oncol. Hematol. 2000, 36, 61–74. [Google Scholar] [CrossRef]
- Cheng, N.-C.; Chen, S.-Y.; Li, J.-R.; Young, T.-H. Short-Term Spheroid Formation Enhances the Regenerative Capacity of Adipose-Derived Stem Cells by Promoting Stemness, Angiogenesis, and Chemotaxis. Stem Cells Transl. Med. 2013, 2, 584–594. [Google Scholar] [CrossRef]
- Frith, J.E.; Thomson, B.; Genever, P.G. Dynamic Three-Dimensional Culture Methods Enhance Mesenchymal Stem Cell Properties and Increase Therapeutic Potential. Tissue Eng. Part C Methods 2010, 16, 735–749. [Google Scholar] [CrossRef]
- Wang, W.; Itaka, K.; Ohba, S.; Nishiyama, N.; Chung, U.I.; Yamasaki, Y.; Kataoka, K. 3D Spheroid Culture System on Micropatterned Substrates for Improved Differentiation Efficiency of Multipotent Mesenchymal Stem Cells. Biomaterials 2009, 30, 2705–2715. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, Y.H.; Hebisch, M.; Sliwinski, C.; Lee, S.; D’Avanzo, C.; Chen, H.; Hooli, B.; Asselin, C.; Muffat, J.; et al. A Three-Dimensional Human Neural Cell Culture Model of Alzheimer’s Disease. Nature 2014, 515, 274–278. [Google Scholar] [CrossRef]
- Papadimitriou, C.; Celikkaya, H.; Cosacak, M.I.; Mashkaryan, V.; Bray, L.; Bhattarai, P.; Brandt, K.; Hollak, H.; Chen, X.; He, S.; et al. 3D Culture Method for Alzheimer’s Disease Modeling Reveals Interleukin-4 Rescues Aβ42-Induced Loss of Human Neural Stem Cell Plasticity. Dev. Cell 2018, 46, 85–101.e8. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, S.H.; D’Avanzo, C.; Hebisch, M.; Sliwinski, C.; Bylykbashi, E.; Washicosky, K.J.; Klee, J.B.; Brüstle, O.; Tanzi, R.E.; et al. A 3D Human Neural Cell Culture System for Modeling Alzheimer’s Disease. Nat. Protoc. 2015, 10, 985–1006. [Google Scholar] [CrossRef]
- Kwak, S.S.; Washicosky, K.J.; Brand, E.; von Maydell, D.; Aronson, J.; Kim, S.; Capen, D.E.; Cetinbas, M.; Sadreyev, R.; Ning, S.; et al. Amyloid-Β42/40 Ratio Drives Tau Pathology in 3D Human Neural Cell Culture Models of Alzheimer’s Disease. Nat. Commun. 2020, 11, 1377. [Google Scholar] [CrossRef]
- Ranjan, V.D.; Qiu, L.; Lee, J.W.L.; Chen, X.; Jang, S.E.; Chai, C.; Lim, K.L.; Tan, E.K.; Zhang, Y.; Huang, W.M.; et al. A Microfiber Scaffold-Based 3D in Vitro Human Neuronal Culture Model of Alzheimer’s Disease. Biomater. Sci. 2020, 8, 4861–4874. [Google Scholar] [CrossRef]
- Park, J.; Wetzel, I.; Marriott, I.; Dréau, D.; D’Avanzo, C.; Kim, D.Y.; Tanzi, R.E.; Cho, H. A 3D Human Triculture System Modeling Neurodegeneration and Neuroinflammation in Alzheimer’s Disease. Nat. Neurosci. 2018, 21, 941–951. [Google Scholar] [CrossRef]
- Pomeshchik, Y.; Klementieva, O.; Gil, J.; Martinsson, I.; Hansen, M.G.; de Vries, T.; Sancho-Balsells, A.; Russ, K.; Savchenko, E.; Collin, A.; et al. Human IPSC-Derived Hippocampal Spheroids: An Innovative Tool for Stratifying Alzheimer Disease Patient-Specific Cellular Phenotypes and Developing Therapies. Stem Cell Rep. 2020, 15, 256–273. [Google Scholar] [CrossRef]
- Chen, X.; Sun, G.; Tian, E.; Zhang, M.; Davtyan, H.; Beach, T.G.; Reiman, E.M.; Blurton-Jones, M.; Holtzman, D.M.; Shi, Y. Modeling Sporadic Alzheimer’s Disease in Human Brain Organoids under Serum Exposure. Adv. Sci. 2021, 8, 2101462. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Jang, S.Y.; Lee, D.; Lee, J.; Kang, U.; Chang, H.; Kim, H.J.; Han, S.H.; Seo, J.; Choi, M.; et al. A Logical Network-Based Drug-Screening Platform for Alzheimer’s Disease Representing Pathological Features of Human Brain Organoids. Nat. Commun. 2021, 12, 280. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Fu, Y.; Yamazaki, Y.; Ren, Y.; Davis, M.D.; Liu, C.C.; Lu, W.; Wang, X.; Chen, K.; Cherukuri, Y.; et al. APOE4 Exacerbates Synapse Loss and Neurodegeneration in Alzheimer’s Disease Patient IPSC-Derived Cerebral Organoids. Nat. Commun. 2020, 11, 5540. [Google Scholar] [CrossRef] [PubMed]
- Fiore, N.J.; Tamer-Mahoney, J.D.; Beheshti, A.; Nieland, T.J.F.; Kaplan, D.L. 3D Biocomposite Culture Enhances Differentiation of Dopamine-like Neurons from SH-SY5Y Cells: A Model for Studying Parkinson’s Disease Phenotypes. Biomaterials 2022, 290, 121858. [Google Scholar] [CrossRef] [PubMed]
- Gilmozzi, V.; Gentile, G.; Riekschnitz, D.A.; Von Troyer, M.; Lavdas, A.A.; Kerschbamer, E.; Weichenberger, C.X.; Rosato-Siri, M.D.; Casarosa, S.; Conti, L.; et al. Generation of HiPSC-Derived Functional Dopaminergic Neurons in Alginate-Based 3D Culture. Front. Cell Dev. Biol. 2021, 9, 708389. [Google Scholar] [CrossRef]
- Li, Z.F.; Cui, L.; Jin, M.M.; Hu, D.Y.; Hou, X.G.; Liu, S.S.; Zhang, X.; Zhu, J.H. A Matrigel-Based 3D Construct of SH-SY5Y Cells Models the α-Synuclein Pathologies of Parkinson’s Disease. Dis. Model. Mech. 2022, 15, dmm049125. [Google Scholar] [CrossRef]
- Taylor-Whiteley, T.R.; Le Maitre, C.L.; Duce, J.A.; Dalton, C.F.; Smith, D.P. Recapitulating Parkinson’s Disease Pathology in a Three-Dimensional Human Neural Cell Culture Model. Dis. Model. Mech. 2019, 12, dmm038042. [Google Scholar] [CrossRef]
- Kim, H.; Park, H.J.; Choi, H.; Chang, Y.; Park, H.; Shin, J.; Kim, J.; Lengner, C.J.; Lee, Y.K.; Kim, J. Modeling G2019S-LRRK2 Sporadic Parkinson’s Disease in 3D Midbrain Organoids. Stem Cell Rep. 2019, 12, 518–531. [Google Scholar] [CrossRef]
- Ma, J.; Wang, F. Prion Disease and the “Protein-Only Hypothesis”. Essays Biochem. 2014, 56, 181–191. [Google Scholar] [CrossRef]
- Pattison, I.H.; Jones, K.M. The Astrocytic Reaction in Experimental Scrapie in the Rat. Res. Vet. Sci. 1967, 8, 160–165. [Google Scholar] [CrossRef]
- Prusiner, S.B. Novel Proteinaceous Infectious Particles Cause Scrapie. Science 1982, 216, 136–144. [Google Scholar] [CrossRef]
- Hope, J. The Biology and Molecular Biology of Scrapie-like Diseases. Arch. Virol. Suppl. 1993, 7, 201–214. [Google Scholar] [CrossRef]
- Groveman, B.R.; Foliaki, S.T.; Orru, C.D.; Zanusso, G.; Carroll, J.A.; Race, B.; Haigh, C.L. Sporadic Creutzfeldt-Jakob Disease Prion Infection of Human Cerebral Organoids. Acta Neuropathol. Commun. 2019, 7, 90. [Google Scholar] [CrossRef]
- Groveman, B.R.; Ferreira, N.C.; Foliaki, S.T.; Walters, R.O.; Winkler, C.W.; Race, B.; Hughson, A.G.; Zanusso, G.; Haigh, C.L. Human Cerebral Organoids as a Therapeutic Drug Screening Model for Creutzfeldt-Jakob Disease. Sci. Rep. 2021, 11, 5165. [Google Scholar] [CrossRef]
- García-Mendívil, L.; Mediano, D.R.; Hernaiz, A.; Sanz-Rubio, D.; Vázquez, F.J.; Marín, B.; López-Pérez, Ó.; Otero, A.; Badiola, J.J.; Zaragoza, P.; et al. Effect of Scrapie Prion Infection in Ovine Bone Marrow-Derived Mesenchymal Stem Cells and Ovine Mesenchymal Stem Cell-Derived Neurons. Animals 2021, 11, 1137. [Google Scholar] [CrossRef]
- Mediano, D.R.; Sanz-Rubio, D.; Bolea, R.; Marín, B.; Vázquez, F.J.; Remacha, A.R.; López-Pérez, Ó.; Fernández-Borges, N.; Castilla, J.; Zaragoza, P.; et al. Characterization of Mesenchymal Stem Cells in Sheep Naturally Infected with Scrapie. J. Gen. Virol. 2015, 96, 3715–3726. [Google Scholar] [CrossRef]
- Lyahyai, J.; Mediano, D.R.; Ranera, B.; Sanz, A.; Remacha, A.R.; Bolea, R.; Zaragoza, P.; Rodellar, C.; Martín-Burriel, I. Isolation and Characterization of Ovine Mesenchymal Stem Cells Derived from Peripheral Blood. BMC Vet. Res. 2012, 8, 169. [Google Scholar] [CrossRef]
- Ranera, B.; Ordovás, L.; Lyahyai, J.; Bernal, M.L.; Fernandes, F.; Remacha, A.R.; Romero, A.; Vázquez, F.J.; Osta, R.; Cons, C.; et al. Comparative Study of Equine Bone Marrow and Adipose Tissue-Derived Mesenchymal Stromal Cells. Equine Vet. J. 2012, 44, 33–42. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Jäger, M.; Bachmann, R.; Scharfstädt, A.; Krauspe, R. Ovine Cord Blood Accommodates Multipotent Mesenchymal Progenitor Cells. In Vivo 2006, 20, 205–214. [Google Scholar]
- Palay, S.L.; Palade, G.E. The Fine Structure of Neurons. J. Biophys. Biochem. Cytol. 1955, 1, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bolea, R.; Monleón, E.; Schiller, I.; Raeber, A.J.; Acín, C.; Monzón, M.; Martín-Burriel, I.; Struckmeyer, T.; Oesch, B.; Badiola, J.J. Comparison of Immunohistochemistry and Two Rapid Tests for Detection of Abnormal Prion Protein in Different Brain Regions of Sheep with Typical Scrapie. J. Vet. Diagn. Investig. 2005, 17, 467–469. [Google Scholar] [CrossRef]
- Bresciani, G.; Hofland, L.J.; Dogan, F.; Giamas, G.; Gagliano, T.; Zatelli, M.C. Evaluation of Spheroid 3D Culture Methods to Study a Pancreatic Neuroendocrine Neoplasm Cell Line. Front. Endocrinol. 2019, 10, 682. [Google Scholar] [CrossRef] [PubMed]
- Foudah, D.; Monfrini, M.; Donzelli, E.; Niada, S.; Brini, A.T.; Orciani, M.; Tredici, G.; Miloso, M. Expression of Neural Markers by Undifferentiated Mesenchymal-like Stem Cells from Different Sources. J. Immunol. Res. 2014, 2014, 987678. [Google Scholar] [CrossRef]
- Wu, S.H.; Liao, Y.T.; Huang, C.H.; Chen, Y.C.; Chiang, E.R.; Wang, J.P. Comparison of the Confluence-Initiated Neurogenic Differentiation Tendency of Adipose-Derived and Bone Marrow-Derived Mesenchymal Stem Cells. Biomedicines 2021, 9, 1503. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Avci, H.X.; Ochalek, A.; Rösingh, L.N.; Molnár, K.; László, L.; Bellák, T.; Téglási, A.; Pesti, K.; Mike, A.; et al. Comparison of 2D and 3D Neural Induction Methods for the Generation of Neural Progenitor Cells from Human Induced Pluripotent Stem Cells. Stem Cell Res. 2017, 25, 139–151. [Google Scholar] [CrossRef]
- Akimov, S.; Vasilyeva, I.; Yakovleva, O.; McKenzie, C.; Cervenakova, L. Murine Bone Marrow Stromal Cell Culture with Features of Mesenchymal Stem Cells Susceptible to Mouse-Adapted Human TSE Agent, Fukuoka-1. Folia Neuropathol. 2009, 47, 205–214. [Google Scholar]
- Cervenakova, L.; Akimov, S.; Vasilyeva, I.; Yakovleva, O.; McKenzie, C.; Cervenak, J.; Piccardo, P.; Asher, D.M. Fukuoka-1 Strain of Transmissible Spongiform Encephalopathy Agent Infects Murine Bone Marrow-Derived Cells with Features of Mesenchymal Stem Cells. Transfusion 2011, 51, 1755–1768. [Google Scholar] [CrossRef]
- Akimov, S.; Yakovleva, O.; Vasilyeva, I.; McKenzie, C.; Cervenakova, L. Persistent Propagation of Variant Creutzfeldt-Jakob Disease Agent in Murine Spleen Stromal Cell Culture with Features of Mesenchymal Stem Cells. J. Virol. 2008, 82, 10959–10962. [Google Scholar] [CrossRef]
- Jauković, A.; Abadjieva, D.; Trivanović, D.; Stoyanova, E.; Kostadinova, M.; Pashova, S.; Kestendjieva, S.; Kukolj, T.; Jeseta, M.; Kistanova, E.; et al. Specificity of 3D MSC Spheroids Microenvironment: Impact on MSC Behavior and Properties. Stem Cell Rev. Rep. 2020, 16, 853–875. [Google Scholar] [CrossRef]
- Sart, S.; Tsai, A.C.; Li, Y.; Ma, T. Three-Dimensional Aggregates of Mesenchymal Stem Cells: Cellular Mechanisms, Biological Properties, and Applications. Tissue Eng. Part B Rev. 2013, 20, 365–380. [Google Scholar] [CrossRef]
- Liu, Y.; Muñoz, N.; Tsai, A.C.; Logan, T.M.; Ma, T. Metabolic Reconfiguration Supports Reacquisition of Primitive Phenotype in Human Mesenchymal Stem Cell Aggregates. Stem Cells 2017, 35, 398–410. [Google Scholar] [CrossRef]
- Zhang, Q.; Nguyen, A.L.; Shi, S.; Hill, C.; Wilder-Smith, P.; Krasieva, T.B.; Le, A.D. Three-Dimensional Spheroid Culture of Human Gingiva-Derived Mesenchymal Stem Cells Enhances Mitigation of Chemotherapy-Induced Oral Mucositis. Stem Cells Dev. 2012, 21, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Cronier, S.; Laude, H.; Peyrin, J.M. Prions Can Infect Primary Cultured Neurons and Astrocytes and Promote Neuronal Cell Death. Proc. Natl. Acad. Sci. USA 2004, 101, 12271–12276. [Google Scholar] [CrossRef] [PubMed]
- Hannaoui, S.; Maatouk, L.; Privat, N.; Levavasseur, E.; Faucheux, B.A.; Haïk, S. Prion Propagation and Toxicity Occur in Vitro with Two-Phase Kinetics Specific to Strain and Neuronal Type. J. Virol. 2013, 87, 2535–2548. [Google Scholar] [CrossRef]
- Shan, Z.; Hirai, Y.; Nakayama, M.; Hayashi, R.; Yamasaki, T.; Hasebe, R.; Song, C.H.; Horiuchi, M. Therapeutic Effect of Autologous Compact Bone-Derived Mesenchymal Stem Cell Transplantation on Prion Disease. J. Gen. Virol. 2017, 98, 2615–2627. [Google Scholar] [CrossRef]
- Song, C.-H.; Honmou, O.; Furuoka, H.; Horiuchi, M. Identification of Chemoattractive Factors Involved in the Migration of Bone Marrow-Derived Mesenchymal Stem Cells to Brain Lesions Caused by Prions. J. Virol. 2011, 85, 11069–11078. [Google Scholar] [CrossRef] [PubMed]
- Song, C.-H.; Honmou, O.; Ohsawa, N.; Nakamura, K.; Hamada, H.; Furuoka, H.; Hasebe, R.; Horiuchi, M. Effect of Transplantation of Bone Marrow-Derived Mesenchymal Stem Cells on Mice Infected with Prions. J. Virol. 2009, 83, 5918–5927. [Google Scholar] [CrossRef]
- Wang, Z.; Chai, C.; Wang, R.; Feng, Y.; Huang, L.; Zhang, Y.; Xiao, X.; Yang, S.; Zhang, Y.; Zhang, X. Single-Cell Transcriptome Atlas of Human Mesenchymal Stem Cells Exploring Cellular Heterogeneity. Clin. Transl. Med. 2021, 11, e650. [Google Scholar] [CrossRef]
- Yin, J.Q.; Zhu, J.; Ankrum, J.A. Manufacturing of Primed Mesenchymal Stromal Cells for Therapy. Nat. Biomed. Eng. 2019, 3, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Yan, L.; Miao, Z.; Li, E.; Wong, K.H.; Xu, R.H. Spheroidal Formation Preserves Human Stem Cells for Prolonged Time under Ambient Conditions for Facile Storage and Transportation. Biomaterials 2017, 133, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, G.; Li, L.; Chen, F.; Bao, J.; Shi, Y.J.; Bu, H. Three-Dimensional Spheroid Culture of Human Umbilical Cord Mesenchymal Stem Cells Promotes Cell Yield and Stemness Maintenance. Cell Tissue Res. 2015, 360, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Regmi, S.; Raut, P.K.; Pathak, S.; Shrestha, P.; Park, P.H.; Jeong, J.H. Enhanced Viability and Function of Mesenchymal Stromal Cell Spheroids Is Mediated via Autophagy Induction. Autophagy 2021, 17, 2991–3010. [Google Scholar] [CrossRef]
- López-Pérez, Ó.; Otero, A.; Filali, H.; Sanz-Rubio, D.; Toivonen, J.M.; Zaragoza, P.; Badiola, J.J.; Bolea, R.; Martín-Burriel, I. Dysregulation of Autophagy in the Central Nervous System of Sheep Naturally Infected with Classical Scrapie. Sci. Rep. 2019, 9, 1911. [Google Scholar] [CrossRef]
- López-Pérez, Ó.; Toivonen, J.M.; Otero, A.; Solanas, L.; Zaragoza, P.; Badiola, J.J.; Osta, R.; Bolea, R.; Martín-Burriel, I. Impairment of Autophagy in Scrapie-Infected Transgenic Mice at the Clinical Stage. Lab. Investig. 2020, 100, 52–63. [Google Scholar] [CrossRef] [PubMed]



| Gene | Expression Levels (2 −∆∆Ct) in oBM-MSCs | Expression Levels (2 −∆∆Ct) in Neuron-like Cells |
|---|---|---|
| TUBB3 | 1 | 1.46 |
| NEFM | 1 | 0.50 |
| MAP2 | 1 | 321.63 |
| Gene | Expression Levels (2−∆∆Ct) in Spheroids in Basal Conditions | Expression Levels (2−∆∆Ct) in Spheroids in Neurogenic Differentiation Conditions |
|---|---|---|
| TUBB3 | 1 | 1.21 |
| NEFM | 1 | 1.13 |
| MAP2 | 1 | 2.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernaiz, A.; Cobeta, P.; Marín, B.; Vázquez, F.J.; Badiola, J.J.; Zaragoza, P.; Bolea, R.; Martín-Burriel, I. Susceptibility of Ovine Bone Marrow-Derived Mesenchymal Stem Cell Spheroids to Scrapie Prion Infection. Animals 2023, 13, 1043. https://doi.org/10.3390/ani13061043
Hernaiz A, Cobeta P, Marín B, Vázquez FJ, Badiola JJ, Zaragoza P, Bolea R, Martín-Burriel I. Susceptibility of Ovine Bone Marrow-Derived Mesenchymal Stem Cell Spheroids to Scrapie Prion Infection. Animals. 2023; 13(6):1043. https://doi.org/10.3390/ani13061043
Chicago/Turabian StyleHernaiz, Adelaida, Paula Cobeta, Belén Marín, Francisco José Vázquez, Juan José Badiola, Pilar Zaragoza, Rosa Bolea, and Inmaculada Martín-Burriel. 2023. "Susceptibility of Ovine Bone Marrow-Derived Mesenchymal Stem Cell Spheroids to Scrapie Prion Infection" Animals 13, no. 6: 1043. https://doi.org/10.3390/ani13061043
APA StyleHernaiz, A., Cobeta, P., Marín, B., Vázquez, F. J., Badiola, J. J., Zaragoza, P., Bolea, R., & Martín-Burriel, I. (2023). Susceptibility of Ovine Bone Marrow-Derived Mesenchymal Stem Cell Spheroids to Scrapie Prion Infection. Animals, 13(6), 1043. https://doi.org/10.3390/ani13061043







