Effects of Low-Phosphorus Diets Supplemented with Phytase on the Production Performance, Phosphorus-Calcium Metabolism, and Bone Metabolism of Aged Hy-Line Brown Laying Hens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Feeding Management
2.2. Production Performance and Egg Quality
2.3. Serum Parameters
2.4. Determination of Levels of Copper, Iron, Manganese, and Zinc in Serum
2.5. Determination of Serum Calcium and Phosphorus Metabolism-Related Hormones
2.6. Tibia Quality
2.7. Determination of Serum Bone Turnover Markers
2.8. Real-Time Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.9. Statistical Analyses
3. Results
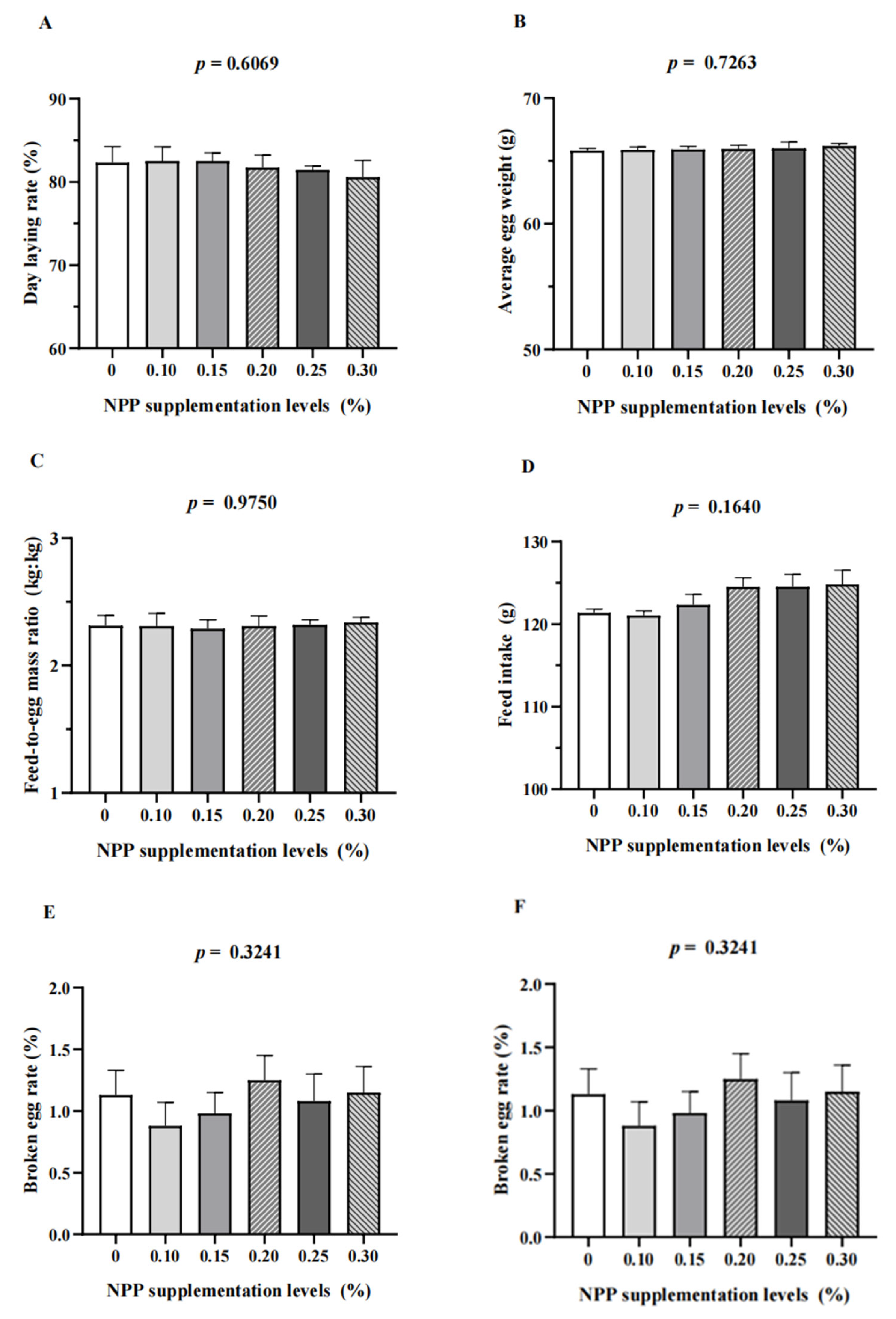
3.1. Production Performance and Egg Quality
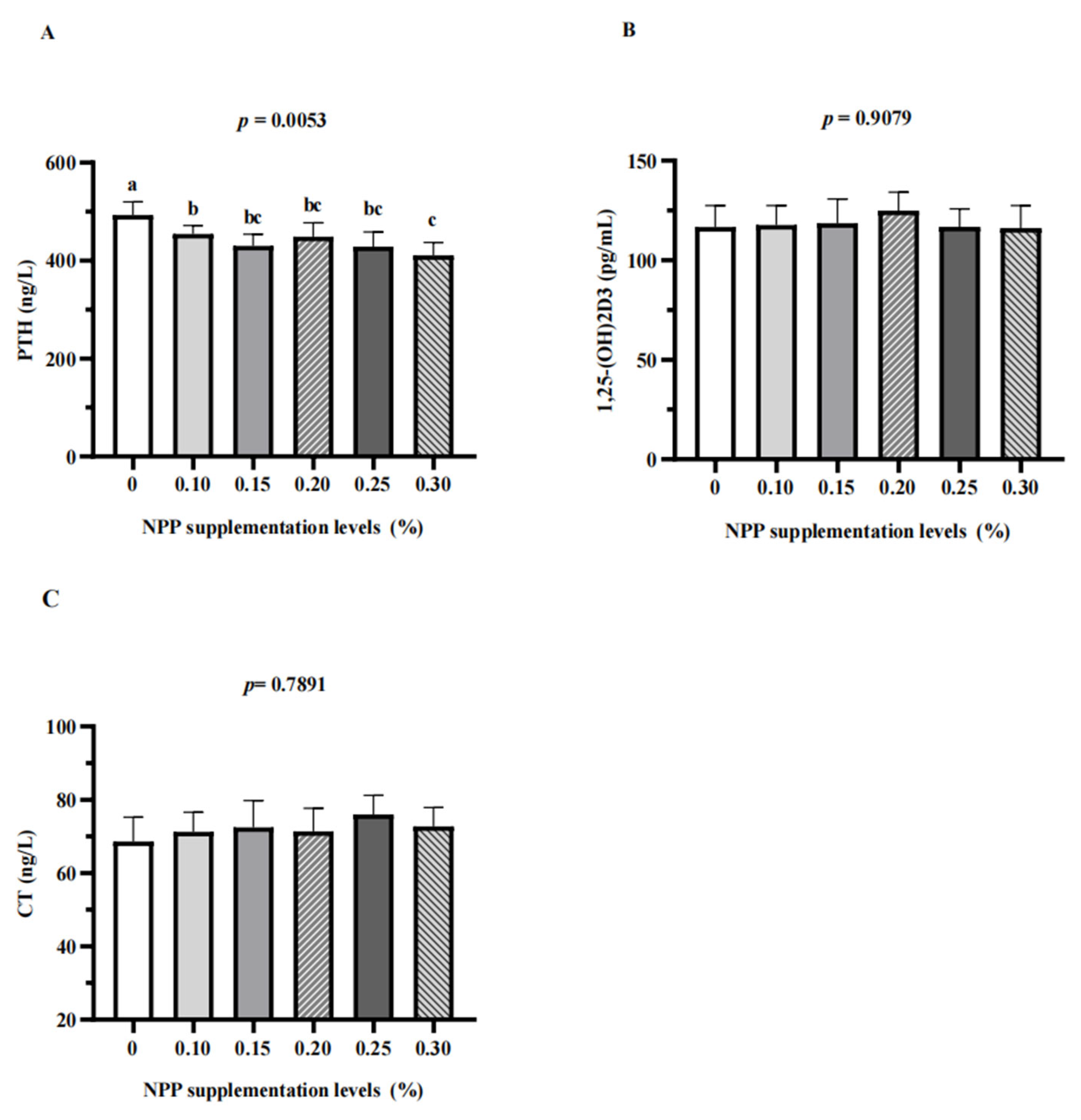
3.2. Serum Parameters, Serum Micronutrients, Serum Calcium and Phosphorus Metabolism-Related Hormones, and Tibia Quality
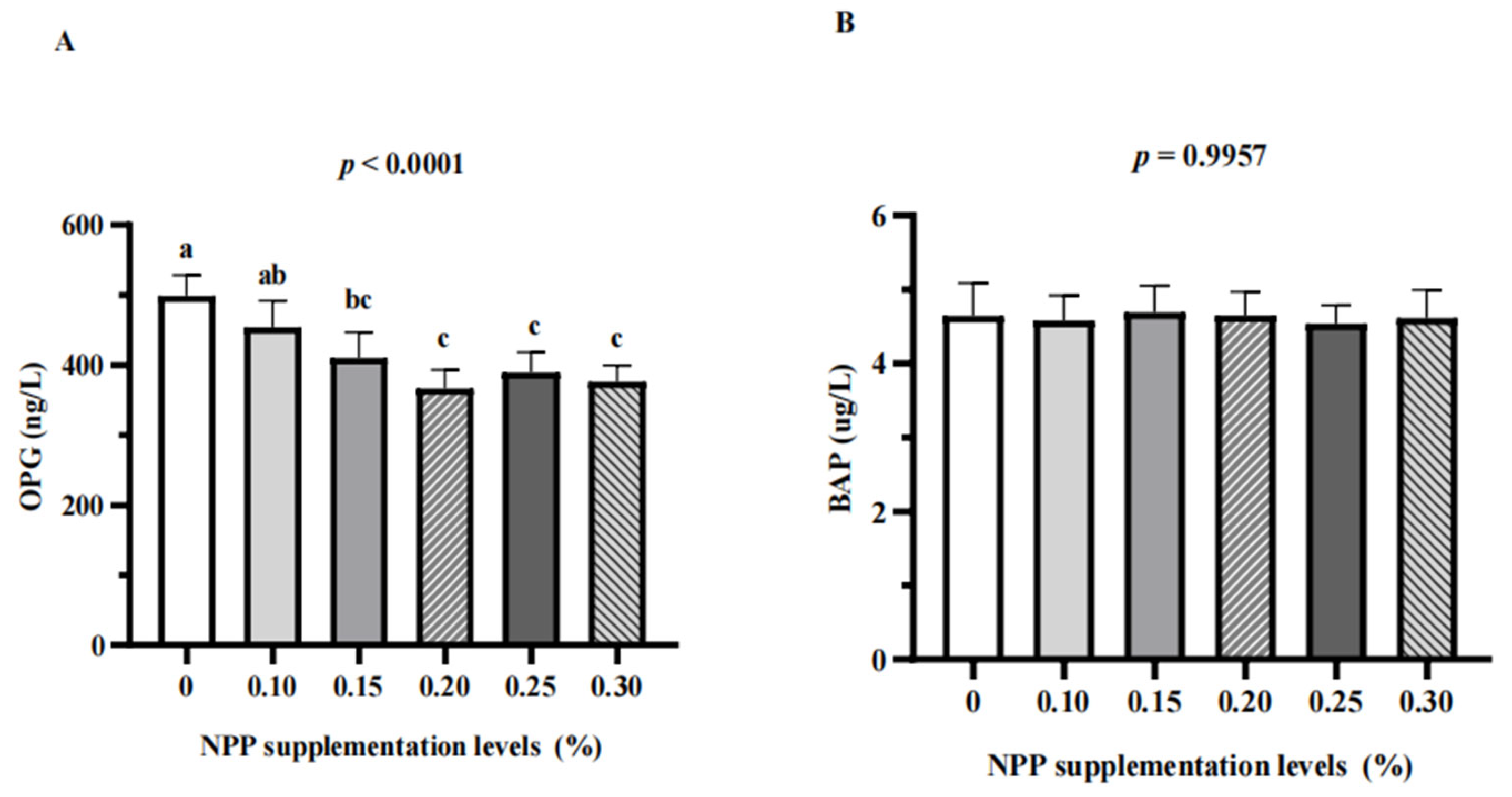
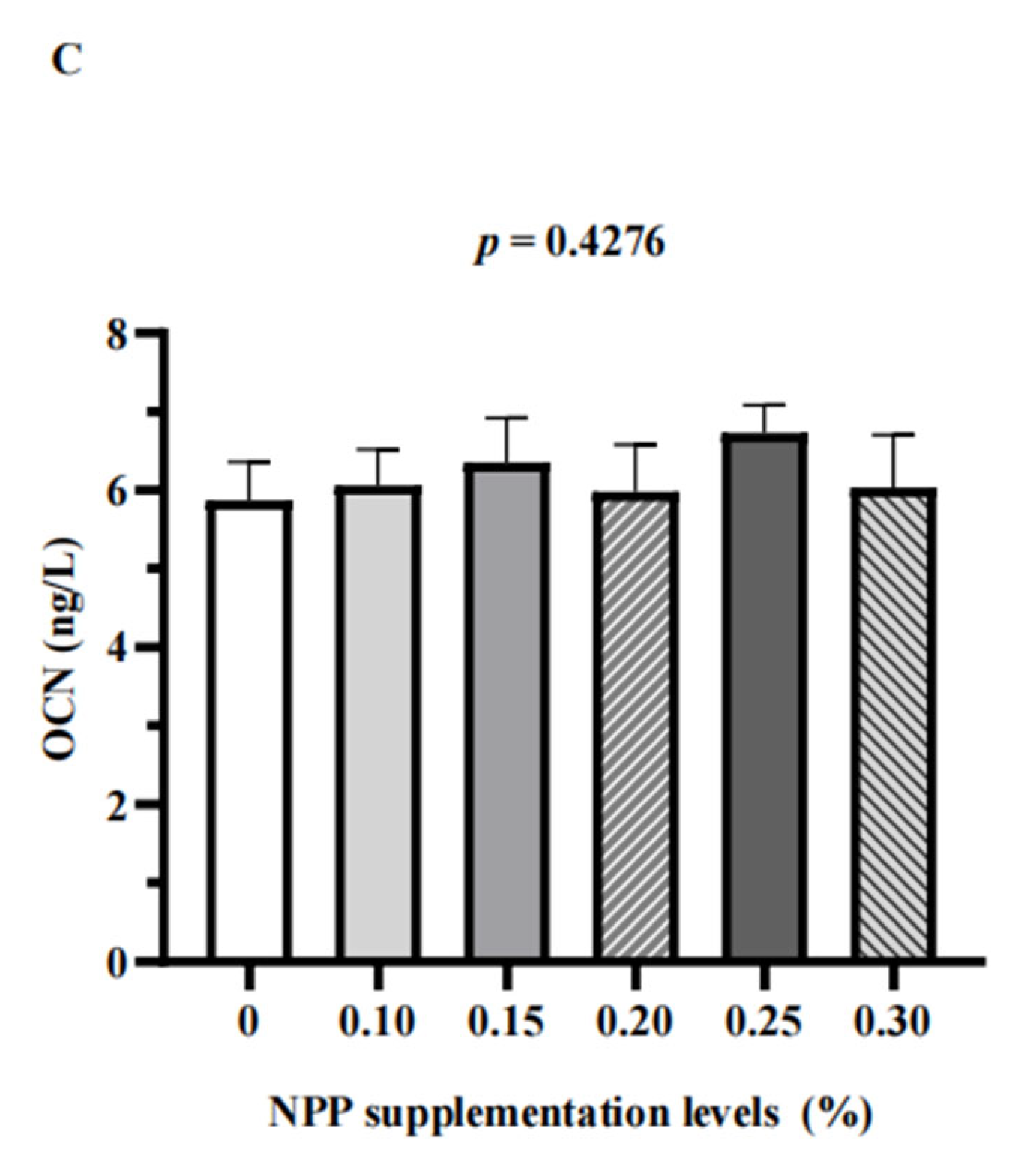
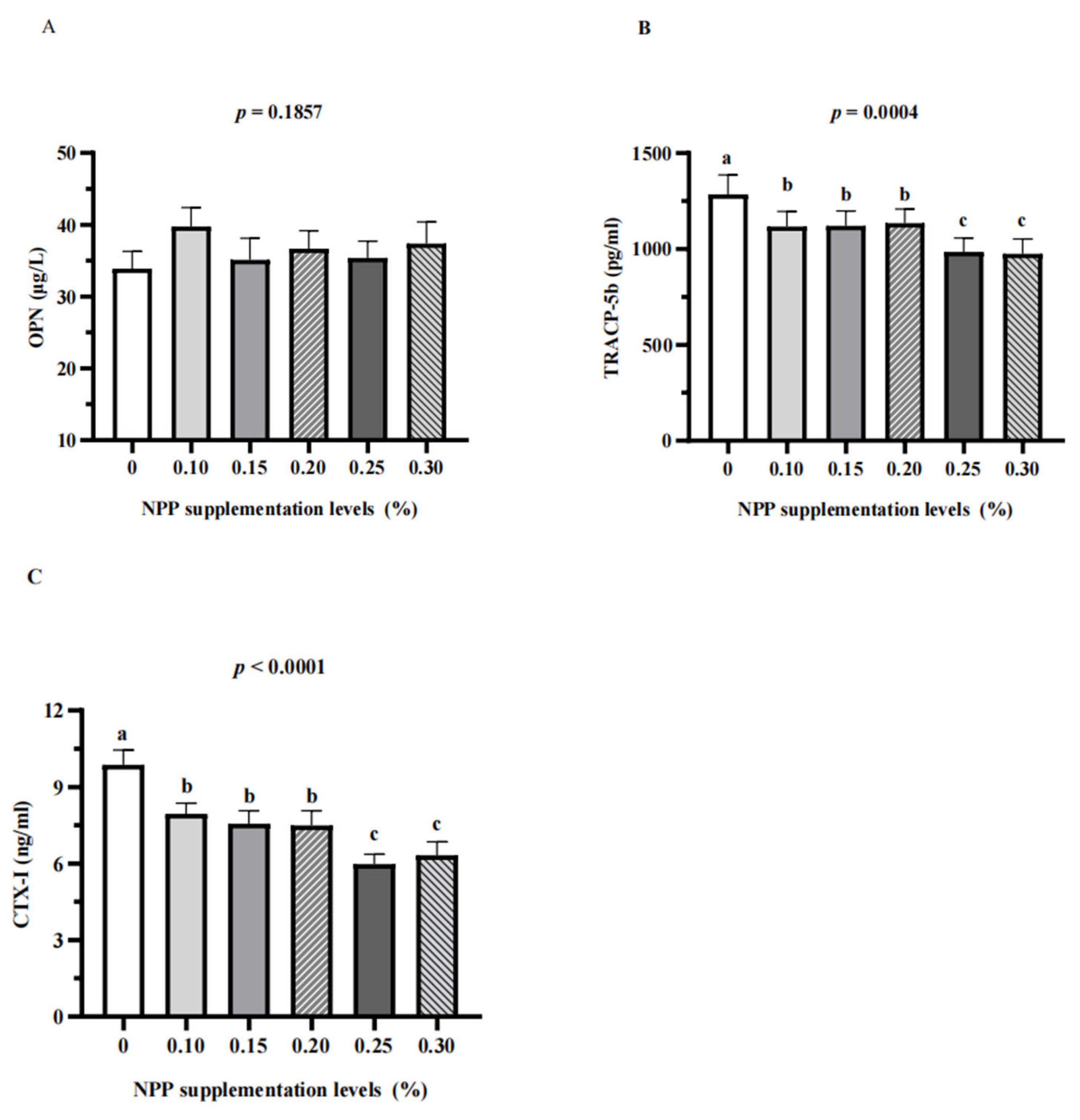
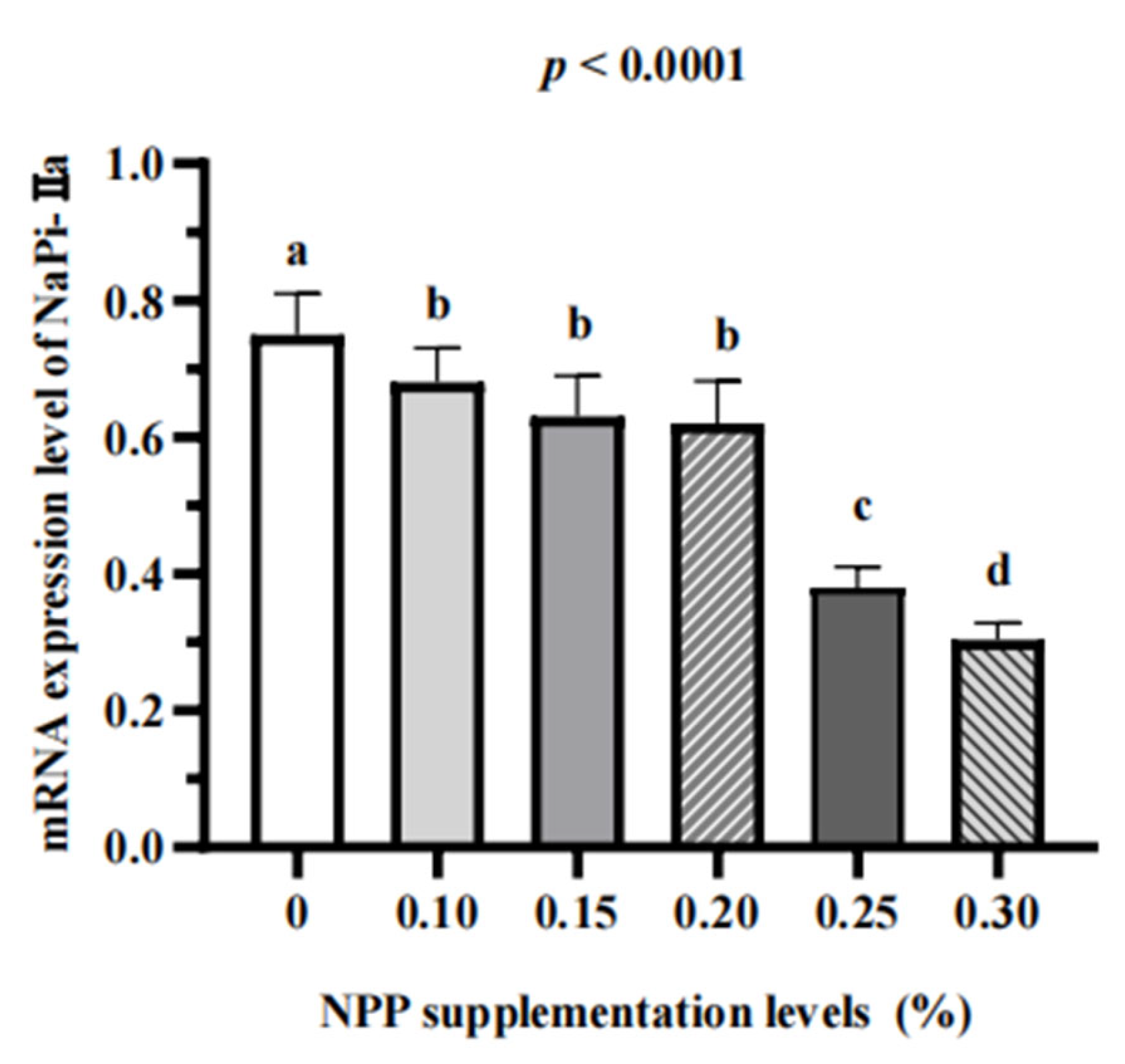
3.3. Serum Bone Turnover Markers and Renal Expression of NaPi-Ⅱa
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Selle, P.H.; Ravindran, V. Microbial phytase in poultry nutrition. Anim. Feed Sci. Technol. 2007, 135, 1–41. [Google Scholar] [CrossRef]
- Nelson, T.S. The hydrolysis of phytate phosphorus by chicks and laying hens. Poult. Sci. 1976, 55, 2262–2264. [Google Scholar] [CrossRef] [PubMed]
- NRC. National Research Council: Nutrient Requirements of Poultry; NRC: Washington, DC, USA, 1994. [Google Scholar]
- Goyette, J.O.; Bennett, E.; Maranger, R. Low buffering capacity and slow recovery of anthropogenic phosphorus pollution in watersheds. Nat. Geosci. 2018, 11, 921–925. [Google Scholar] [CrossRef]
- Fernández, S.R.; Chárraga, S.; Ávila-Gonzalez, E. Evaluation of a new generation phytase on phytate phosphorus release for egg production and tibia strength in hens fed a corn-soybean meal diet. Poult. Sci. 2019, 98, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Adeola, O.; Cowieson, A.J. Board-invited review: Opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 2011, 89, 3189–3218. [Google Scholar] [CrossRef]
- Taheri, H.R.; Jabbari, Z.; Adibnia, S.; Shahir, M.H.; Hosseini, S.A. Effect of high-dose phytase and citric acid, alone or in combination, on growth performance of broilers given diets severely limited in available phosphorus. Br. Poult. Sci. 2015, 56, 708–715. [Google Scholar] [CrossRef]
- Gautier, A.E.; Walk, C.L.; Dilger, R.N. Effects of a high level of phytase on broiler performance, bone ash, phosphorus utilization, and phytate dephosphorylation to inositol. Poult. Sci. 2018, 97, 211–218. [Google Scholar] [CrossRef]
- Ren, Z.; Sun, W.; Cheng, X.; Liu, Y.; Han, D.; Yan, J.; Pan, C.; Duan, Y.; Yang, X. The adaptability of Hy-Line Brown laying hens to low-phosphorus diets supplemented with phytase. Poult. Sci. 2020, 99, 3525–3531. [Google Scholar] [CrossRef]
- Wang, S.; Tang, C.H.; Zhang, J.M.; Wang, X.Q. The effect of dietary supplementation with phytase transgenic maize and different concentrations of non-phytate phosphorus on the performance of laying hens. Br. Poult. Sci. 2013, 54, 466–470. [Google Scholar] [CrossRef]
- Hughes, A.L.; Dahiya, J.P.; Wyatt, C.L.; Classen, H.L. Effect of quantum phytase on nutrient digestibility and bone ash in White Leghorn laying hens fed corn-soybean meal-based diets. Poult. Sci. 2009, 88, 1191–1198. [Google Scholar] [CrossRef]
- Punna, S.; Rol, D.A.S. Influence of supplemental microbial phytase on first cycle laying hens fed phosphorus-deficient diets from day one of age. Poult. Sci. 1999, 78, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Reich, T.; Gefen, A. Effect of trabecular bone loss on cortical strain rate during impact in an in vitro model of avian femur. Biomed. Eng. Online 2006, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, C.C.; Fleming, R.H. Osteoporosis in cage layers. Poult. Sci. 2000, 79, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, M.; Sun, H. Effects of dietary calcium to available phosphorus ratios on bone metabolism and osteoclast activity of the OPG /RANK/RANKL signalling pathway in piglets. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.; Dersjant-Li, Y.; Korver, D.R. Effects of dietary calcium and available phosphorus levels and phytase supplementation on performance, bone mineral density, and serum biochemical bone markers in aged white egg-laying hens. Poult. Sci. 2020, 99, 5792–5801. [Google Scholar] [CrossRef]
- Wei, H.; Chen, Y.; Nian, H.; Wang, J.; Liu, Y.; Wang, J.; Yang, K.; Zhao, Q.; Zhang, R.; Bao, J. Abnormal Bone Metabolism May Be a Primary Causative Factor of Keel Bone Fractures in Laying Hens. Animals 2021, 11, 3133. [Google Scholar] [CrossRef]
- Jiang, S.; Cheng, H.W.; Hester, P.Y.; Hou, J.F. Development of an enzyme-linked immunosorbent assay for detection of chicken osteocalcin and its use in evaluation of perch effects on bone remodeling in caged White Leghorns. Poult. Sci. 2013, 92, 1951–1961. [Google Scholar] [CrossRef]
- Li, T.T.; Xing, G.Z.; Shao, Y.X.; Zhang, L.Y.; Li, S.F.; Lu, L.; Liu, Z.P.; Liao, X.D.; Luo, X.G. Dietary calcium or phosphorus deficiency impairs the bone development by regulating related calcium or phosphorus metabolic utilization parameters of broilers. Poult. Sci. 2020, 99, 3207–3214. [Google Scholar] [CrossRef]
- Andreani, G.; Dalmonte, T.; Guerrini, A.; Lupini, C.; Fabbri, M.; Ferlizza, E.; Isani, G. Supplementation of Boswellia serrata and Salix alba extracts during the early laying phase: Effects on serum and albumen proteins, trace elements, and yolk cholesterol. Animals 2022, 12, 2014. [Google Scholar] [CrossRef]
- Carlos, A.B.; Edwards, H.M., Jr. The effects of 1,25-dihydroxycholecalciferol and phytase on the natural phytate phosphorus utilization by laying hens. Poult. Sci. 1998, 77, 850–858. [Google Scholar] [CrossRef]
- Kim, J.H.; Pitargue, F.M.; Jung, H.; Han, G.P.; Choi, H.S.; Kil, D.Y. Effect of superdosing phytase on productive performance and egg quality in laying hens. Asian-Australas. J. Anim. Sci. 2017, 30, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, K. Nonphytate phosphorus requirement of laying hens with and without phytase on a phase feeding program. Poult. Sci. 2000, 79, 748–763. [Google Scholar] [CrossRef]
- Ahmadi, H.; Rodehutscord, M. A meta-analysis of responses to dietary nonphytate phosphorus and phytase in laying hens. Poult. Sci. 2012, 91, 2072–2078. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Yang, Y.; Yuan, J.; Wang, Z.; Guo, Y. Effect of dietary nonphytate phosphorus on laying performance and small intestinal epithelial phosphate transporter expression in Dwarf pink-shell laying hens. J. Anim. Sci. Biotechnol. 2013, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.X.; Wang, J.P.; Zhang, Q.; Aureli, R.; Tschambser, A.; Faruk, M.U. Evaluation of the efficacy of a novel phytase in short-term digestibility and long-term egg production studies with laying hens. Poult. Sci. 2022, 101, 101894. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, M.M.; Pietilä, P.E.; Viljakainen, H.T.; Lamberg-Allardt, C.J. Prolonged increase in dietary phosphate intake alters bone mineralization in adult male rats. J. Nutr. Biochem. 2006, 17, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Miao, D.; Li, J.; Goltzman, D.; Karaplis, A.C. Transgenic mice overexpressing human fibroblast growth factor 23 (R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology 2004, 145, 5269–5279. [Google Scholar] [CrossRef]
- Jing, M.; Zhao, S.; Rogiewicz, A.; Slominski, B.A.; House, J.D. Effects of phytase supplementation on production performance, egg and bone quality, plasma biochemistry and mineral excretion of layers fed varying levels of phosphorus. Animal 2021, 15, 100010. [Google Scholar] [CrossRef]
- Cheng, X.; Yan, J.K.; Sun, W.Q.; Chen, Z.Y.; Wu, S.R.; Ren, Z.Z.; Yang, X.J. Effect of inorganic phosphate supplementation on egg production in Hy-Line Brown layers fed 2000 FTU/kg phytase. Animal 2020, 14, 2246–2252. [Google Scholar] [CrossRef]
- Moshtaghian, J.; Parsons, C.M.; Leeper, R.W.; Harrison, P.C.; Koelkebeck, K.W. Effect of sodium aluminosilicate on phosphorus utilization by chicks and laying hens. Poult. Sci. 1991, 70, 955–962. [Google Scholar] [CrossRef]
- Viveros, A.; Brenes, A.; Arija, I.; Centeno, C. Effects of microbial phytase supplementation on mineral utilization and serum enzyme activities in broiler chicks fed different levels of phosphorus. Poult. Sci. 2002, 81, 1172–1183. [Google Scholar] [CrossRef]
- Boorman, K.N.; Gunaratne, S.P. Dietary phosphorus supply, egg-shell deposition and plasma inorganic phosphorus in laying hens. Br. Poult. Sci. 2001, 42, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.A. Novel insights into the regulation of systemic phosphate homeostasis and renal phosphate excretion. J. Nephrol. 2007, 20, 130–134. [Google Scholar] [PubMed]
- Fukumoto, S. Phosphate metabolism and vitamin D. BoneKEy Rep. 2014, 3, 497. [Google Scholar] [CrossRef] [PubMed]
- Crenshaw, T.D.; Rortvedt, L.A.; Hassen, Z. Triennial Growth Symposium: A novel pathway for vitamin D-mediated phosphate homeostasis: Implications for skeleton growth and mineralization. J. Anim. Sci. 2011, 89, 1957–1964. [Google Scholar] [CrossRef]
- Kearns, A.E.; Khosla, S.; Kostenuik, P.J. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr. Rev. 2008, 29, 155–192. [Google Scholar] [CrossRef]
- Kerschnitzki, M.; Zander, T.; Zaslansky, P.; Fratzl, P.; Shahar, R.; Wagermaier, W. Rapid alterations of avian medullary bone material during the daily egg-laying cycle. Bone 2014, 69, 109–117. [Google Scholar] [CrossRef]
- Boling, S.D.; Douglas, M.W.; Johnson, M.L.; Wang, X.; Parsons, C.M.; Koelkebeck, K.W.; Zimmerman, R.A. The effects of dietary available phosphorus levels and phytase on performance of young and older laying hens. Poult. Sci. 2000, 79, 224–230. [Google Scholar] [CrossRef]
- Teng, X.; Zhang, W.; Xu, D.; Liu, Z.; Yang, N.; Luo, D.; Wang, H.; Ge, M.; Zhang, R. Effects of low dietary phosphorus on tibia quality and metabolism in caged laying hens. Prev. Vet. Med. 2020, 181, 105049. [Google Scholar] [CrossRef]
- Khosla, S.; Arrighi, H.M.; Melton, L.J., 3rd; Atkinson, E.J.; O’Fallon, W.M.; Dunstan, C.; Riggs, B.L. Correlates of osteoprotegerin levels in women and men. Osteoporos. Int. 2002, 13, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Tsurukai, T.; Udagawa, N.; Matsuzaki, K.; Takahashi, N.; Suda, T. Roles of macrophage-colony stimulating factor and osteoclast differentiation factor in osteoclastogenesis. J. Bone Miner. Metab. 2000, 18, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.S.; Wu, Y.Y.; Janckila, A.J.; Ku, C.H.; Hsieh, A.T.; Ho, C.L.; Lee, S.H.; Chao, T.Y. Serum tartrate-resistant acid phosphatase 5b (TRACP5b) activity as a biomarker for bone metastasis in non-small cell lung cancer patients. Clin. Chim. Acta 2011, 412, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Reithmeier, A.; Panizza, E.; Krumpel, M.; Orre, L.M.; Branca, R.M.M.; Lehtiö, J.; Ek-Rylander, B.; Andersson, G. Tartrate-resistant acid phosphatase (TRAP/ACP5) promotes metastasis-related properties via TGFβ2/TβR and CD44 in MDA-MB-231 breast cancer cells. BMC Cancer 2017, 17, 650. [Google Scholar] [CrossRef]
- Kučukalić-Selimović, E.; Valjevac, A.; Hadžović-Džuvo, A.; Skopljak-Beganović, A.; Alimanovic-Alagić, R.; Brković, A. Evaluation of bone remodelling parameters after one year treatment with alendronate in postmenopausal women with osteoporosis. Bosn. J. Basic Med. Sci. 2011, 11, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Raymond, B.; Johansson, A.M.; McCormack, H.A.; Fleming, R.H.; Schmutz, M.; Dunn, I.C.; De Koning, D.J. Genome-wide association study for bone strength in laying hens. J. Anim. Sci. 2018, 96, 2525–2535. [Google Scholar] [CrossRef] [PubMed]
- Huber, K.; Zeller, E.; Rodehutscord, M. Modulation of small intestinal phosphate transporter by dietary supplements of mineral phosphorus and phytase in broilers. Poult. Sci. 2015, 94, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, R.; Jiao, H.; Wang, X.; Zhao, J.; Lin, H. Effects of dietary phosphorus level on the expression of calcium and phosphorus transporters in laying hens. Front. Physiol. 2018, 9, 627. [Google Scholar] [CrossRef] [PubMed]
- Lötscher, M.; Wilson, P.; Nguyen, S.; Kaissling, B.; Biber, J.; Murer, H.; Levi, M. New aspects of adaptation of rat renal Na-Pi cotransporter to alterations in dietary phosphate. Kidney Int. 1996, 49, 1012–1018. [Google Scholar] [CrossRef] [PubMed]

| Item | % (Unless Stated Otherwise) |
|---|---|
| Corn | 60.5 |
| Soybean meal, 46% | 22 |
| Soybean oil | 1 |
| Calcium carbonate | 8.5 |
| Palmmeal | 5 |
| Dicalcium phosphate | - |
| Premix 1 | 3 |
| Total | 100 |
| Nutrient contents | |
| Metabolic energy (Kcal/kg) | 2537 |
| Crude protein | 15.79 |
| Phytase (FTU/kg) | 1470 |
| Calcium | 3.81 |
| Total phosphorus (analyzed) | 0.34 |
| NPP | 0.12 |
| Lysine | 0.81 |
| Methionine | 0.42 |
| Treatment | Phytase (mg/kg) | Dietary Phytase Activity (FTU/kg) | Calcium % | NPP Supplementation Levels % | Dietary NPP Levels % |
|---|---|---|---|---|---|
| 1 | 29.1 | 1470 | 3.81 | 0 | 0.12 |
| 2 | 29.1 | 1470 | 3.81 | 0.10 | 0.22 |
| 3 | 29.1 | 1470 | 3.81 | 0.15 | 0.27 |
| 4 | 29.1 | 1470 | 3.81 | 0.20 | 0.32 |
| 5 | 29.1 | 1470 | 3.81 | 0.25 | 0.37 |
| 6 | 29.1 | 1470 | 3.81 | 0.30 | 0.42 |
| Target | Primer | Primer Sequence | Predicted Size (bp) | Annealing Temperature (°C) |
|---|---|---|---|---|
| Gene | ||||
| NaPi-Ⅱa | upstream | 5′-TTGGCCAGCACCAGACTCAG-3′ | 118 | 60 |
| downstream | 5′-GCAGTAGGCAAACGGGGTC-3′ | 60 | ||
| β-actin | upstream | 5′-CTGGGGCTCATCTGAAGGGT-3′ | 308 | 60 |
| downstream | 5′-GGACGCTGGGATGATGTTCT-3′ | 60 |
| NPP Supplementation Levels (%) | |||||||
|---|---|---|---|---|---|---|---|
| Items | 0 | 0.1 | 0.15 | 0.20 | 0.25 | 0.30 | p-value |
| Serum parameters | |||||||
| Calcium (mmol/L) | 4.36 ± 0.40 | 4.96 ± 0.36 | 4.52 ± 0.38 | 4.64 ± 0.43 | 4.63 ± 0.33 | 4.77 ± 0.42 | 0.1880 |
| Phosphorus (mmol/L) | 1.46 ± 0.18 | 1.52 ± 0.10 | 1.64 ± 0.17 | 1.50 ± 0.16 | 1.68 ± 0.17 | 1.66 ± 0.11 | 0.1309 |
| ALP (U/L) | 381.00 ± 27.00 | 407.00 ± 46.89 | 394.67 ± 50.54 | 340.00 ± 35.17 | 377.00 ± 41.07 | 423.67 ± 42.62 | 0.2445 |
| Serum micronutrients | |||||||
| Copper (pmol/L) | 32.74 ± 2.83 | 34.30 ± 2.19 | 32.81 ± 2.81 | 33.55 ± 4.42 | 34.63 ± 2.34 | 32.72 ± 2.32 | 0.7700 |
| Iron (pmol/L) | 20.05 ± 1.05 | 20.25 ± 1.11 | 19.80 ± 2.27 | 19.55 ± 1.40 | 20.87 ± 2.67 | 20.86 ± 3.21 | 0.8436 |
| Zinc (pmol/L) | 60.38 ± 4.97 | 58.83 ± 5.34 | 60.24 ± 3.69 | 57.70 ± 4.68 | 63.58 ± 5.82 | 60.98 ± 6.78 | 0.5260 |
| Manganese (pmol/L) | 10.11 ± 1.08 | 9.71 ± 0.86 | 10.37 ± 0.69 | 9.82 ± 1.34 | 10.25 ± 0.55 | 10.58 ± 0.45 | 0.5383 |
| NPP Supplementation Levels (%) | |||||||
|---|---|---|---|---|---|---|---|
| Items | 0 | 0.1 | 0.15 | 0.20 | 0.25 | 0.30 | p-value |
| Breaking strength (N/mm²) | 13.21 c ± 2.68 | 15.49 b ± 1.48 | 15.33 b ± 1.09 | 15.41 b ± 1.89 | 17.46 a ± 1.01 | 17.98 a ± 2.82 | 0.0061 |
| Ash content (%) | 58.56 d ± 0.41 | 59.83 bc ± 0.67 | 59.75b c ± 0.51 | 60.40 ab ± 0.42 | 60.61 a ± 0.41 | 61.02 a ± 0.46 | <0.0001 |
| Calcium (%) | 19.62 d ± 0.32 | 20.16 cd ± 0.30 | 20.13 cd ± 0.39 | 20.50 bc ± 0.28 | 20.88 ab ± 0.39 | 21.15 a ± 0.29 | <0.0001 |
| Phosphorus (%) | 6.91 d ± 0.65 | 7.62 c ± 0.39 | 7.80 bc ± 0.50 | 8.47 ab ± 0.47 | 8.80 a ± 0.52 | 9.03 a ± 0.75 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Liu, Y.; Jiang, K.; Li, L.; Jiao, N.; Zhu, Z.; Zhang, K.; Jiang, S.; Yang, W.; Li, Y. Effects of Low-Phosphorus Diets Supplemented with Phytase on the Production Performance, Phosphorus-Calcium Metabolism, and Bone Metabolism of Aged Hy-Line Brown Laying Hens. Animals 2023, 13, 1042. https://doi.org/10.3390/ani13061042
Ren Y, Liu Y, Jiang K, Li L, Jiao N, Zhu Z, Zhang K, Jiang S, Yang W, Li Y. Effects of Low-Phosphorus Diets Supplemented with Phytase on the Production Performance, Phosphorus-Calcium Metabolism, and Bone Metabolism of Aged Hy-Line Brown Laying Hens. Animals. 2023; 13(6):1042. https://doi.org/10.3390/ani13061042
Chicago/Turabian StyleRen, Yuechang, Yaping Liu, Kexin Jiang, Linkui Li, Ning Jiao, Zhengqi Zhu, Kaiying Zhang, Shuzhen Jiang, Weiren Yang, and Yang Li. 2023. "Effects of Low-Phosphorus Diets Supplemented with Phytase on the Production Performance, Phosphorus-Calcium Metabolism, and Bone Metabolism of Aged Hy-Line Brown Laying Hens" Animals 13, no. 6: 1042. https://doi.org/10.3390/ani13061042
APA StyleRen, Y., Liu, Y., Jiang, K., Li, L., Jiao, N., Zhu, Z., Zhang, K., Jiang, S., Yang, W., & Li, Y. (2023). Effects of Low-Phosphorus Diets Supplemented with Phytase on the Production Performance, Phosphorus-Calcium Metabolism, and Bone Metabolism of Aged Hy-Line Brown Laying Hens. Animals, 13(6), 1042. https://doi.org/10.3390/ani13061042






