Double-Fermented Soybean Meal Totally Replaces Soybean Meal in Broiler Rations with Favorable Impact on Performance, Digestibility, Amino Acids Transporters and Meat Nutritional Value
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fermentation and Characterization of Soybean Meal
2.1.1. Fermentation Process of Soybean Meal Was Carried out in Two Stages
2.1.2. Evaluation of DFSBM
Chemical Analysis
Amino Acids Quantification
Reduced Sugar Analysis
Measurement of pH
Phosphorus Analysis
Assessment of Phytase Enzyme Activity
Determination of Trypsin Inhibitor
2.2. Birds, Diets and Experimental Design
2.3. Growth Performnce Attributes and Digestibility Trial
2.4. Sample Collection and Analytical Procedures
2.5. Nutrient Digestibility and Pancreatic Digestive Enzymes
2.6. Serum Biochemical Analysis
2.7. Assesment of Liver Antioxidant
2.8. Chemical and Amino Acids Copmostion of Breast Meat
2.9. Cecal Bacterial Count and Duodenal pH
2.10. Nutrient Transporter-Encoding Genes Real-Time PCR
2.11. Statistical Analysis
3. Results
3.1. Growth Performance, Nutrient Digestibility and Digestive Enzyme Acivity
3.2. Serum Biochemical Parameters
3.3. Hepatic Antioxidant Potential
3.4. Chemical and Amino Acids Copmostion of Breast Meat
3.5. Cecal Bacterial Count and Duodenal pH
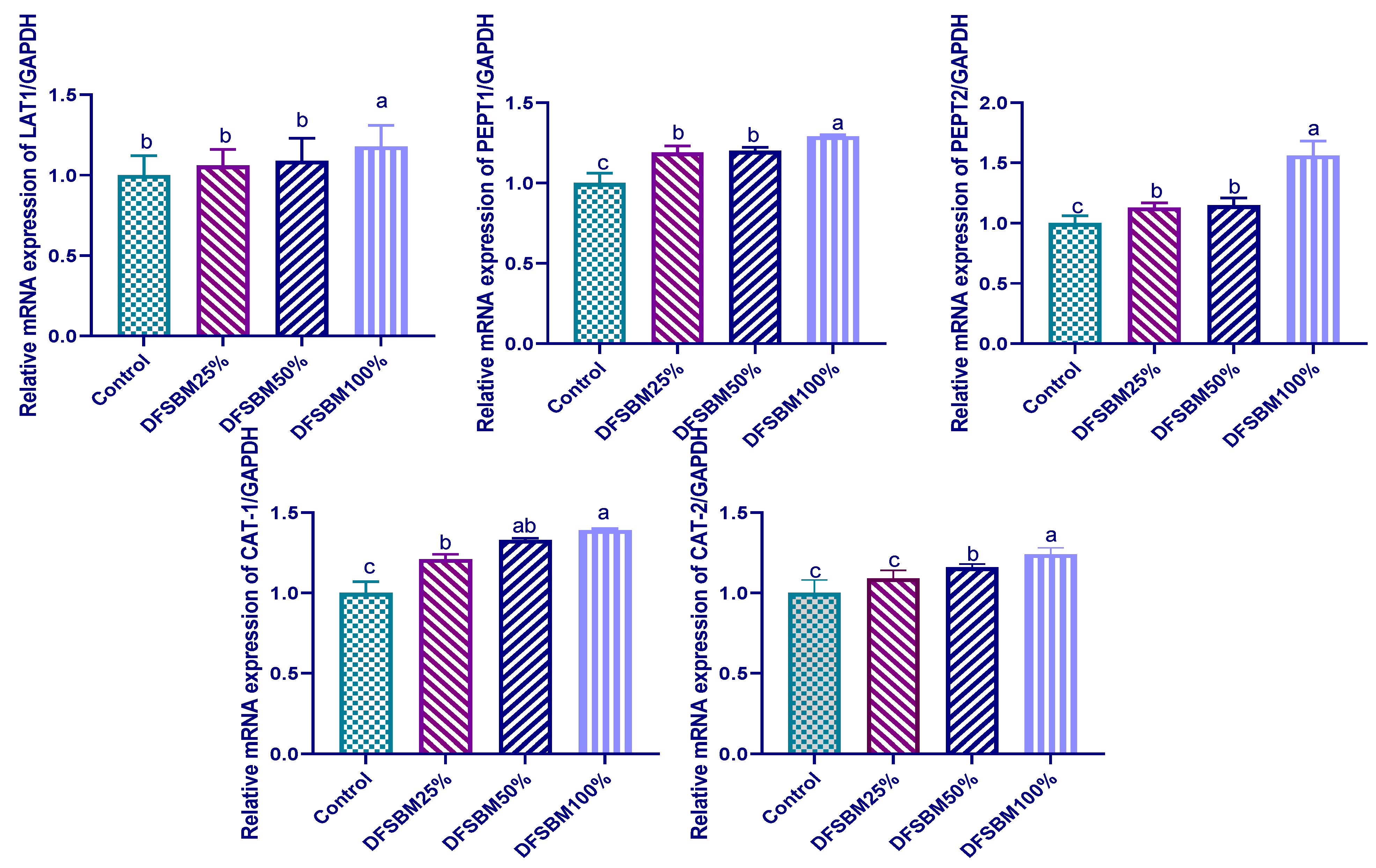
3.6. Gene Expression of Protein-Related Nutrient Transporters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Guo, B.; Wu, Z.; Wang, W.; Li, C.; Liu, G.; Cai, H. Effects of fermented soybean meal supplementation on the growth performance and cecal microbiota community of broiler chickens. Animals 2020, 10, 1098. [Google Scholar] [CrossRef]
- Jazi, V.; Boldaji, F.; Dastar, B.; Hashemi, S.; Ashayerizadeh, A. Effects of fermented cottonseed meal on the growth performance, gastrointestinal microflora population and small intestinal morphology in broiler chickens. Br. Poult. Sci. 2017, 58, 402–408. [Google Scholar] [CrossRef]
- Yeh, R.H.; Hsieh, C.W.; Chen, K.L. Screening lactic acid bacteria to manufacture two-stage fermented feed and pelleting to investigate the feeding effect on broilers. Poult. Sci. 2018, 97, 236–246. [Google Scholar] [CrossRef]
- Pinto, G.A.; Leite, S.G.; Terzi, S.C.; Couri, S. Selection of tannase-producing Aspergillus niger strains. Braz. J. Microbiol. 2001, 32, 24–26. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, Y.; Lohakare, J.; Yun, J.; Lee, J.; Kwon, M.; Park, J.; Choi, J.; Chae, B. Comparative efficacy of different soy protein sources on growth performance, nutrient digestibility and intestinal morphology in weaned pigs. Asian-Australas. J. Anim. Sci 2007, 20, 775–783. [Google Scholar] [CrossRef]
- Soomro, R.N.; Yao, J.; Gola, M.A.; Ali, A.; Htet, M.; Soomro, S. Beneficial aspects of feeding soybean meal (SBM) fermented with fungal and bacterial actions in broiler chickens. Am. J. Biol. Life Sci. 2017, 5, 21–29. [Google Scholar]
- Khatlab, A.d.S.; Del Vesco, A.P.; de Oliveira Neto, A.R.; Fernandes, R.P.M.; Gasparino, E. Dietary supplementation with free methionine or methionine dipeptide mitigates intestinal oxidative stress induced by Eimeria spp. challenge in broiler chickens. J. Anim. Sci. Biotechnol. 2019, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Poncet, N.; Taylor, P.M. The role of amino acid transporters in nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Kheravii, S.; Morgan, N.; Swick, R.A.; Choct, M.; Wu, S.-B. Roles of dietary fibre and ingredient particle size in broiler nutrition. Worlds Poult. Sci. J. 2018, 74, 301–316. [Google Scholar] [CrossRef]
- Ruhnke, I.; Röhe, I.; Goodarzi Boroojeni, F.; Knorr, F.; Mader, A.; Hafeez, A.; Zentek, J. Feed supplemented with organic acids does not affect starch digestibility, nor intestinal absorptive or secretory function in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2015, 99, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Premathilaka, K.T.; Nawarathne, S.R.; Nambapana, M.N.; Macelline, S.P.; Wickramasuriya, S.S.; Ang, L.; Jayasena, D.D.; Heo, J.M. Partial or complete replacement of fishmeal with fermented soybean meal on growth performance, fecal composition, and meat quality in broilers. J. Anim. Sci. Technol. 2020, 62, 824. [Google Scholar] [CrossRef] [PubMed]
- Fotiadis, D.; Kanai, Y.; Palacín, M. The SLC3 and SLC7 families of amino acid transporters. Mol. Asp. Med. 2013, 34, 139–158. [Google Scholar] [CrossRef]
- Chen, H.; Mao, X.; Yin, J.; Yu, B.; He, J.; Che, L.; Yu, J.; Huang, Z.; Zheng, P.; Michiels, J. Comparison of jejunal digestive enzyme activities, expression of nutrient transporter genes, and apparent fecal digestibility in weaned piglets fed diets with varied sources of fiber. J. Anim. Feed Sci. 2015, 24, 41–47. [Google Scholar] [CrossRef]
- Röder, P.V.; Geillinger, K.E.; Zietek, T.S.; Thorens, B.; Koepsell, H.; Daniel, H. The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS ONE 2014, 9, e89977. [Google Scholar] [CrossRef] [PubMed]
- Teng, D.; Gao, M.; Yang, Y.; Liu, B.; Tian, Z.; Wang, J. Bio-modification of soybean meal with Bacillus subtilis or Aspergillus oryzae. Biocatal. Agric. Biotechnol. 2012, 1, 32–38. [Google Scholar] [CrossRef]
- Zhang, Y.; Ishikawa, M.; Koshio, S.; Yokoyama, S.; Dossou, S.; Wang, W.; Zhang, X.; Shadrack, R.S.; Mzengereza, K.; Zhu, K. Optimization of soybean meal fermentation for aqua-feed with Bacillus subtilis natto using the response surface methodology. Fermentation 2021, 7, 306. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Washington, DC, USA, 2012. [Google Scholar]
- Mabbott, G.A. Qualitative amino acid analysis of small peptides by GC/MS. J. Chem. Educ. 1990, 67, 441. [Google Scholar] [CrossRef]
- Tounsi, H.; Hadj Sassi, A.; Ben Romdhane, Z.; Lajnef, M.; Dupuy, J.-W.; Lapaillerie, D.; Lomenech, A.-M.; Bonneu, M.; Gargouri, A.; Hadj-Taieb, N. Catalytic properties of a highly thermoactive polygalacturonase from the mesophilic fungus Penicillium occitanis and use in juice clarification. J. Mol. Catal. B Enzym. 2016, 127, 56–66. [Google Scholar] [CrossRef]
- Pearson, D. Chemical Analysis of Foods; Churchill Livingstone: Edinburgh, UK, 1984. [Google Scholar]
- Jastrzȩbska, A.; Brudka, B.; Szymański, T.; Szłyk, E. Determination of phosphorus in food samples by X-ray fluorescence spectrometry and standard spectrophotometric method. Food Chem. 2003, 83, 463–467. [Google Scholar] [CrossRef]
- ISO 30024:2009; Animal Feeding Stuffs—Determination of Phytase Activity. ISO: Geneva, Switzerland, 2009.
- Kakade, M.; Rackis, J.; McGhee, J.; Puski, G. Determination of Trypsin Inhibitor Activity of Soy Products: A Collaborative Analysis of an Improved Procedure; USDA: Washington, DC, USA, 1974. [Google Scholar]
- Hamerstrand, G.; Black, L.; Glover, J. Trypsin Inhibitors in Soy Products: Modification of the standard Analytical Procedure; USDA: Washington, DC, USA, 1981. [Google Scholar]
- Aviagen, W. Ross 308: Broiler Nutrition Specification; Aviagen Inc.: Huntsville, AL, USA, 2022. [Google Scholar]
- Short, F.; Gorton, P.; Wiseman, J.; Boorman, K. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 1996, 59, 215–221. [Google Scholar] [CrossRef]
- Pollman, R.M. Atomic Absorption Spectrophotometric Determination of Calcium and Magnesium and Colorimetric Determination of Phosphorus in Cheese: Collaborative Study. J. Assoc. Off. Anal. Chem. 2020, 74, 27–31. [Google Scholar] [CrossRef]
- McDonald, P. Animal Nutrition; Pearson Education: London, UK, 2002. [Google Scholar]
- Somogyi, M. Modifications of two methods for the assay of amylase. Clin. Chem. 1960, 6, 23–35. [Google Scholar] [CrossRef]
- Tietz, N.; Fiereck, E.A. A specific method for serum lipase determination. Clin. Chim. Acta 1966, 13, 352–358. [Google Scholar] [CrossRef]
- Alandiyjany, M.N.; Kishawy, A.T.Y.; Abdelfattah-Hassan, A.; Eldoumani, H.; Elazab, S.T.; El-Mandrawy, S.A.M.; Saleh, A.A.; ElSawy, N.A.; Attia, Y.A.; Arisha, A.H.; et al. Nano-silica and magnetized-silica mitigated lead toxicity: Their efficacy on bioaccumulation risk, performance, and apoptotic targeted genes in Nile tilapia (Oreochromis niloticus). Aquat. Toxicol. 2022, 242, 106054. [Google Scholar] [CrossRef]
- Placer, Z.A.; Cushman, L.L.; Johnson, B.C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem. 1966, 16, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef]
- Cowell, D.; Dowman, A.; Lewis, R.; Pirzad, R.; Watkins, S. The rapid potentiometric detection of catalase positive microorganisms. Biosens. Bioelectron. 1994, 9, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Gindler, E.M.; King, J.D. Rapid colorimetric determination of calcium in biologic fluids with methylthymol blue. Am. J. Clin. Pathol. 1972, 58, 376–382. [Google Scholar] [CrossRef] [PubMed]
- EI-Merzabani, M.M.; Anwer-El-Aaser, A.; hkandar Zakhary, N. A New Method for Determination of Inorganic Phosphorus in Serum without Deproteinization. J. Clin. Chem. Clin. Biochem 1977, 15, 715–718. [Google Scholar]
- Langhout, D.; Schutte, J.; Van Leeuwen, P.; Wiebenga, J.; Tamminga, S. Effect of dietary high-and low-methylated citrus pectin on the activity of the ileal microflora and morphology of the small intestinal wall of broiler chicks. Br. Poult. Sci. 1999, 40, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Nkukwana, T.; Muchenje, V.; Masika, P.; Mushonga, B. Intestinal morphology, digestive organ size and digesta pH of broiler chickens fed diets supplemented with or without Moringa oleifera leaf meal. S. Afr. J. Anim. Sci. 2015, 45, 362–370. [Google Scholar] [CrossRef]
- Ibrahim, D.; Abdelfattah-Hassan, A.; Arisha, A.H.; Abd El-Aziz, R.M.; Sherief, W.R.; Adli, S.H.; El Sayed, R.; Metwally, A.E. Impact of feeding anaerobically fermented feed supplemented with acidifiers on its quality and growth performance, intestinal villi and enteric pathogens of mulard ducks. Livest. Sci. 2020, 242, 104299. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, Y.; Lu, Z.; Wang, Y. Solid-state fermentation of corn-soybean meal mixed feed with Bacillus subtilis and Enterococcus faecium for degrading antinutritional factors and enhancing nutritional value. J. Anim. Sci. Biotechnol. 2017, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R.; Chakraborty, R.; Dutta, A. Role of fermentation in improving nutritional quality of soybean meal—A review. Asian-Australas. J. Anim. Sci. 2016, 29, 1523. [Google Scholar] [CrossRef]
- Hirabayashi, M.; Matsui, T.; Yano, H.; Nakajima, T. Fermentation of soybean meal with Aspergillus usamii reduces phosphorus excretion in chicks. Poult. Sci. 1998, 77, 552–556. [Google Scholar] [CrossRef]
- Ilyas, A.; Hirabayasi, M.; Matsui, T.; Yano, H.; Yano, F.; Kikishima, T.; Takebe, M.; Hayakawa, K. A note on the removal of phytate in soybean meal using Aspergillus usami. Asian-Australas J. Anim. Sci. 1995, 8, 135–138. [Google Scholar] [CrossRef]
- Suprayogi, W.P.S.; Ratriyanto, A.; Akhirini, N.; Hadi, R.F.; Setyono, W.; Irawan, A. Changes in nutritional and antinutritional aspects of soybean meals by mechanical and solid-state fermentation treatments with Bacillus subtilis and Aspergillus oryzae. Bioresour. Technol. Rep. 2022, 17, 100925. [Google Scholar] [CrossRef]
- Ashayerizadeh, A.; Dastar, B.; Shargh, M.S.; Mahoonak, A.S.; Zerehdaran, S. Effects of feeding fermented rapeseed meal on growth performance, gastrointestinal microflora population, blood metabolites, meat quality, and lipid metabolism in broiler chickens. Livest. Sci. 2018, 216, 183–190. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef]
- Sarkar, P.; Jones, L.; Craven, G.; Somerset, S.; Palmer, C. Amino acid profiles of kinema, a soybean-fermented food. Food Chem. 1997, 59, 69–75. [Google Scholar] [CrossRef]
- Graham, A.; DeRouchey, J.M.; Goodband, R.D.; Tokach, M.D.; Dritz, S.S.; Thaler, R. Amino Acid Digestibility and Energy Concentration of Fermented Soybean Meal and Camelina Meal for Swine. In Swine Day 2013; Kansas State University: Manhattan, KS, USA, 2013. [Google Scholar]
- Lenis, N.P.; Jongbloed, A.W. New technologies in low pollution swine diets: Diet manipulation and use of synthetic amino acids, phytase and phase feeding for reduction of nitrogen and phosphorus excretion and ammonia emission-Review. Asian-Australas. J. Anim. Sci. 1999, 12, 305–327. [Google Scholar] [CrossRef]
- Bougouin, A.; Appuhamy, J.A.D.R.N.; Kebreab, E.; Dijkstra, J.; Kwakkel, R.P.; France, J. Effects of phytase supplementation on phosphorus retention in broilers and layers: A meta-analysis. Poult. Sci. 2014, 93, 1981–1992. [Google Scholar] [CrossRef]
- Chen, L.; Vadlani, P.V.; Madl, R.L. High-efficiency removal of phytic acid in soy meal using two-stage temperature-induced Aspergillus oryzae solid-state fermentation. J. Sci. Food Agric. 2014, 94, 113–118. [Google Scholar] [CrossRef]
- Sun, X.; Devi, N.D.; Urriola, P.E.; Tiffany, D.G.; Jang, J.-C.; Shurson, G.G.; Hu, B. Feeding value improvement of corn-ethanol co-product and soybean hull by fungal fermentation: Fiber degradation and digestibility improvement. Food Bioprod. Process. 2021, 130, 143–153. [Google Scholar] [CrossRef]
- Santos, F.R.; Hruby, M.; Pierson, E.E.M.; Remus, J.C.; Sakomura, N.K. Effect of Phytase Supplementation in Diets on Nutrient Digestibility and Performance in Broiler Chicks. J. Appl. Poult. Res. 2008, 17, 191–201. [Google Scholar] [CrossRef]
- Canibe, N.; Jensen, B.B. Fermented liquid feed—Microbial and nutritional aspects and impact on enteric diseases in pigs. Anim. Feed Sci. Technol. 2012, 173, 17–40. [Google Scholar] [CrossRef]
- Chiang, G.; Lu, W.; Piao, X.; Hu, J.; Gong, L.; Thacker, P. Effects of feeding solid-state fermented rapeseed meal on performance, nutrient digestibility, intestinal ecology and intestinal morphology of broiler chickens. Asian-Australas J. Anim. Sci. 2009, 23, 263–271. [Google Scholar] [CrossRef]
- Feng, J.; Liu, X.; Xu, Z.; Wang, Y.; Liu, J. Effects of fermented soybean meal on digestive enzyme activities and intestinal morphology in broilers. Poult. Sci. 2007, 86, 1149–1154. [Google Scholar] [CrossRef]
- Gilbert, E.; Wong, E.; Webb Jr, K. Board-invited review: Peptide absorption and utilization: Implications for animal nutrition and health. J. Anim. Sci. 2008, 86, 2135–2155. [Google Scholar] [CrossRef]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R. Real-time quantification of microRNAs by stem–loop RT–PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef]
- Ding, X.; Liu, P.; Zhang, K.; Wang, J.; Bai, S.; Zeng, Q.; Xuan, Y.; Su, Z.; Peng, H.; Li, D. Effects of enzyme-treated soy protein on performance, digestive enzyme activity and mRNA expression of nutrient transporters of laying hens fed different nutrient density diets. Animal 2021, 15, 100373. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Wang, D.; Wu, M.; Zheng, C.; Wu, Z.; Wang, C.; Liu, Q.; Zhang, J.; Guo, G. Effects of guanidinoacetic acid and coated folic acid supplementation on growth performance, nutrient digestion and hepatic gene expression in Angus bulls. Br. J. Nutr. 2021, 126, 510–517. [Google Scholar] [CrossRef]
- Osmanyan, A.K.; Ghazi Harsini, S.; Mahdavi, R.; Fisinin, V.I.; Arkhipova, A.L.; Glazko, T.T.; Kovalchuk, S.N.; Kosovsky, G.Y. Intestinal amino acid and peptide transporters in broiler are modulated by dietary amino acids and protein. Amino Acids 2018, 50, 353–357. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, Y.; Cheng, Q.; Xv, J.; Hou, Y.; Wu, X.; Du, E.; Ding, B. Partial substitution of fermented soybean meal for soybean meal influences the carcass traits and meat quality of broiler chickens. Animals 2020, 10, 225. [Google Scholar] [CrossRef]
- Mehri, M.; Bagherzadeh-Kasmani, F.; Rokouei, M. Growth responses of breast and leg muscles to essential amino acids in broiler chicks. Animal 2016, 10, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Sembratowicz, I.; Chachaj, R.; Krauze, M.; Ognik, K. The effect of diet with fermented soybean meal on blood metabolites and redox status of chickens. Ann. Anim. Sci. 2020, 20, 599–611. [Google Scholar] [CrossRef]
- Liong, M.; Shah, N. Bile salt deconjugation and BSH activity of five bifidobacterial strains and their cholesterol co-precipitating properties. Food Res. Int. 2005, 38, 135–142. [Google Scholar] [CrossRef]
- Seifi, K.; Torshizi, M.A.K.; Rahimi, S.; Kazemifard, M. Efficiency of early, single-dose probiotic administration methods on performance, small intestinal morphology, blood biochemistry, and immune response of Japanese quail. Poult. Sci. 2017, 96, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Yin, X.; Yao, J.; Cheng, J.; Zhang, J.; Xu, W.; Mu, Y.; Xu, J. Fermented Soybean Meal Affects the Reproductive Performance and Oxidative Status of Sows, and the Growth of Piglets. Animals 2021, 11, 597. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.; Lin, L.; Wang, C.; Tsai, C.; Chang, S.; Lee, T. Effects of fermented soybean meal with Bacillus velezensis, Lactobacillus spp. or their combination on broiler performance, gut antioxidant activity and microflora. Anim. Biosci. 2022, 35, 1892–1903. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, A.; Neu, J.; Riezzo, G.; Raimondi, F.; Martinelli, D.; Francavilla, R.; Indrio, F. Gastrointestinal function development and microbiota. Ital. J. Pediatr. 2013, 39, 1–7. [Google Scholar] [CrossRef]
- Emami, N.K.; Calik, A.; White, M.B.; Kimminau, E.A.; Dalloul, R.A. Effect of probiotics and multi-component feed additives on microbiota, gut barrier and immune responses in broiler chickens during subclinical necrotic enteritis. Front. Vet. Sci. 2020, 7, 572142. [Google Scholar] [CrossRef]
- Rehman, A.; Arif, M.; Sajjad, N.; Al-Ghadi, M.Q.; Alagawany, M.; Abd El-Hack, M.E.; Alhimaidi, A.R.; Elnesr, S.S.; Almutairi, B.O.; Amran, R.A.; et al. Dietary effect of probiotics and prebiotics on broiler performance, carcass, and immunity. Poult. Sci. 2020, 99, 6946–6953. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Toghyani, M.; Kheravii, S.K.; Pineda, L.; Han, Y.; Swick, R.A.; Wu, S.-B. Organic acid blends improve intestinal integrity, modulate short-chain fatty acids profiles and alter microbiota of broilers under necrotic enteritis challenge. Anim. Nutr. 2022, 8, 82–90. [Google Scholar] [CrossRef]
- Jazi, V.; Ashayerizadeh, A.; Toghyani, M.; Shabani, A.; Tellez, G.; Toghyani, M. Fermented soybean meal exhibits probiotic properties when included in Japanese quail diet in replacement of soybean meal. Poult. Sci. 2018, 97, 2113–2122. [Google Scholar] [CrossRef]
- Xie, Z.; Hu, L.; Li, Y.; Geng, S.; Cheng, S.; Fu, X.; Zhao, S.; Han, X. Changes of gut microbiota structure and morphology in weaned piglets treated with fresh fermented soybean meal. World J. Microbiol. Biotechnol. 2017, 33, 213. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Normal SBM | DFSBM | SEM | p-Value |
| DM% | 87.50 b | 89.11 a | 0.36 | <0.001 |
| CP% | 47.5 b | 49.23 a | 0.39 | <0.001 |
| EE% | 1.48 a | 0.913 b | 0.13 | <0.001 |
| Ash% | 6.07 b | 7.12 a | 0.24 | <0.001 |
| CF% | 3.69 a | 3.18 b | 0.16 | <0.001 |
| Reduced suger (mg/g) | 165 a | 30 b | 0.58 | <0.001 |
| Phosphorus% | 0.67 b | 0.93 a | 0.06 | <0.001 |
| pH | 7.37 a | 5.25 b | 0.48 | <0.001 |
| Phytase activity (FTU/kg) | 26 b | 883.33 a | 0.88 | <0.001 |
| Trypsin inhibitor level mg/g (dry weight basis) | 19.96 a | 5.29 b | 3.28 | <0.001 |
| Essential amino acids (% of DM) | ||||
| Argenine | 3.45 b | 3.66 a | 0.05 | <0.001 |
| Histidine | 1.24 a | 1.15 b | 0.02 | <0.001 |
| Isoleucine | 2.25 b | 2.44 a | 0.04 | <0.001 |
| Leucine | 3.78 b | 4.01 a | 0.05 | <0.001 |
| Lysine | 3.10 b | 4.34 a | 0.28 | <0.001 |
| Methionine | 0.67 b | 0.81 a | 0.03 | <0.001 |
| Phenylalanine | 2.47 b | 2.80 a | 0.07 | <0.001 |
| Threonine | 1.82 b | 1.88 a | 0.01 | <0.001 |
| Tryptophan | 0.71 b | 0.89 a | 0.04 | <0.001 |
| Valine | 2.35 b | 2.68 a | 0.07 | <0.001 |
| Total essential amino acids | 21.86 b | 24.66 a | 0.63 | <0.001 |
| Non-essential amino acids (% of DM) | ||||
| Alanine | 2.17 b | 2.32 a | 0.03 | <0.001 |
| Aspartic acid | 5.40 b | 6.32 a | 0.20 | <0.001 |
| Cystine | 0.68 b | 0.77 a | 0.02 | <0.001 |
| Glutamic acid | 8.55 b | 9.33 a | 0.17 | <0.001 |
| Glycine | 2.03 b | 2.12 a | 0.02 | <0.001 |
| Proline | 2.37 b | 2.51 a | 0.03 | <0.001 |
| Serine | 2.11 b | 2.23 a | 0.03 | <0.001 |
| Tyrosine | 1.72 b | 1.98 a | 0.06 | <0.001 |
| Total nonessential amino acids | 25.03 b | 27.57 a | 0.56 | <0.001 |
| Total free amino acids | 0.73 b | 4.76 a | 0.91 | <0.001 |
| Probiotic bacteria counts | ||||
| Lactic acid bacteria log cfu/g feed | 4.25 b | 5.70 a | 0.32 | <0.001 |
| Bacillus spp log cfu/g feed | 3.21 b | 6.26 a | 0.68 | <0.001 |
| Ingredients | Starter Rations | Grower Rations | Finisher Rations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | DFSBM Replacement % | Control | DFSBM Replacement % | Control | DFSBM Replacement % | |||||||
| 25% | 50% | 100% | 25% | 50% | 100% | 25% | 50% | 100% | ||||
| Yellow corn | 55.4 | 55.4 | 55.4 | 55.4 | 58 | 58 | 58 | 58 | 62.9 | 62.9 | 62.9 | 62.9 |
| SBM, 47.5% | 39.14 | 29.35 | 19.57 | 0 | 35.6 | 26.7 | 17.8 | 0 | 30.5 | 22.88 | 15.25 | 0 |
| DFSBM | 0 | 9.79 | 19.57 | 39.14 | 0 | 8.9 | 17.8 | 35.6 | 0 | 7.63 | 15.25 | 30.5 |
| Soybean oil | 1.66 | 1.66 | 1.66 | 1.66 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Calcium carbonate | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 |
| Calcium dibasic phosphate | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Common salt | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Premix * | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| DL-Methionine, 98% | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| L-Lysine HCL, 78% | 0 | 0 | 0 | 0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 | 0.3 | 0.3 | 0.3 |
| Anti-mycotoxin | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Calculated Composition | ||||||||||||
| ME, Kcal/Kg | 2979.07 | 2979.46 | 2979.27 | 2979.85 | 3056.97 | 3057.15 | 3057.32 | 3057.68 | 3103.94 | 3104.34 | 3104.25 | 3104.55 |
| CP, % | 23.29 | 23.17 | 23.10 | 23.32 | 21.67 | 21.73 | 21.80 | 21.93 | 19.86 | 19.92 | 19.98 | 20.09 |
| CF% | 2.72 | 2.62 | 2.67 | 2.52 | 2.65 | 2.60 | 2.56 | 2.47 | 2.58 | 2.54 | 2.50 | 2.42 |
| EE% | 4.53 | 4.42 | 4.48 | 4.31 | 5.41 | 5.36 | 5.31 | 5.21 | 5.54 | 5.50 | 5.46 | 5.37 |
| Ca, % | 0.97 | 1.02 | 0.99 | 1.07 | 0.96 | 0.98 | 1.01 | 1.05 | 0.95 | 0.97 | 0.99 | 1.03 |
| Available phosphorus, % | 0.67 | 0.72 | 0.69 | 0.77 | 0.64 | 0.67 | 0.69 | 0.74 | 0.61 | 0.63 | 0.65 | 0.69 |
| Phytase activity (FTU/kg) | 59 | 368 | 575 | 960 | 54 | 351 | 534 | 917 | 48 | 337 | 502 | 884 |
| Ingredients | Starter Rations | Grower Rations | Finisher Rations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | DFSBM Replacement % | Control | DFSBM Replacement % | Control | DFSBM Replacement % | |||||||
| 25% | 50% | 100% | 25% | 50% | 100% | 25% | 50% | 100% | ||||
| Essential amino acids (% of DM) | ||||||||||||
| Argenine | 1.60 | 1.64 | 1.62 | 1.68 | 1.49 | 1.50 | 1.52 | 1.56 | 1.33 | 1.35 | 1.36 | 1.39 |
| Histidine | 0.62 | 0.60 | 0.61 | 0.58 | 0.58 | 0.57 | 0.56 | 0.55 | 0.53 | 0.52 | 0.52 | 0.50 |
| Isoleucine | 1.02 | 1.06 | 1.04 | 1.09 | 0.95 | 0.97 | 0.98 | 1.01 | 0.85 | 0.86 | 0.87 | 0.90 |
| Leucine | 2.00 | 2.05 | 2.02 | 2.09 | 1.89 | 1.91 | 1.93 | 1.97 | 1.74 | 1.76 | 1.78 | 1.81 |
| Lysine | 1.36 | 1.60 | 1.48 | 1.84 | 1.33 | 1.44 | 1.55 | 1.77 | 1.34 | 1.44 | 1.53 | 1.72 |
| Methionine | 0.54 | 0.56 | 0.56 | 0.60 | 0.52 | 0.53 | 0.55 | 0.57 | 0.49 | 0.51 | 0.52 | 0.54 |
| Phenylalanine | 1.17 | 1.24 | 1.20 | 1.30 | 1.09 | 1.12 | 1.15 | 1.21 | 0.99 | 1.01 | 1.04 | 1.09 |
| Threonine | 0.88 | 0.89 | 0.88 | 0.90 | 0.82 | 0.83 | 0.83 | 0.84 | 0.74 | 0.75 | 0.75 | 0.76 |
| Tryptophane | 0.31 | 0.35 | 0.33 | 0.38 | 0.29 | 0.30 | 0.32 | 0.35 | 0.25 | 0.27 | 0.28 | 0.31 |
| Valine | 1.19 | 1.25 | 1.22 | 1.31 | 1.12 | 1.14 | 1.17 | 1.23 | 1.02 | 1.04 | 1.07 | 1.12 |
| Non-Essential amino acids (% of DM) | ||||||||||||
| Alanine | 1.09 | 1.12 | 1.11 | 1.15 | 1.03 | 1.04 | 1.05 | 1.08 | 0.94 | 0.95 | 0.96 | 0.98 |
| Aspartic acid | 2.43 | 2.61 | 2.52 | 2.79 | 2.26 | 2.34 | 2.42 | 2.58 | 2.01 | 2.08 | 2.15 | 2.29 |
| Cystine | 0.38 | 0.39 | 0.39 | 0.41 | 0.36 | 0.37 | 0.37 | 0.39 | 0.33 | 0.34 | 0.35 | 0.36 |
| Glutamic acid | 4.09 | 4.24 | 4.17 | 4.39 | 3.82 | 3.89 | 3.96 | 4.10 | 3.45 | 3.51 | 3.57 | 3.69 |
| Glycine | 0.97 | 0.98 | 0.98 | 1.00 | 0.90 | 0.91 | 0.92 | 0.93 | 0.81 | 0.82 | 0.83 | 0.84 |
| Proline | 1.35 | 1.38 | 1.37 | 1.40 | 1.29 | 1.30 | 1.31 | 1.33 | 1.20 | 1.21 | 1.22 | 1.24 |
| Serine | 1.03 | 1.05 | 1.04 | 1.08 | 0.97 | 0.98 | 0.99 | 1.01 | 0.88 | 0.89 | 0.89 | 0.91 |
| Tyrosine | 0.76 | 0.81 | 0.79 | 0.86 | 0.71 | 0.73 | 0.75 | 0.80 | 0.63 | 0.65 | 0.66 | 0.70 |
| Gene | Gene Full Name | Primer Sequence (5′-3′) | Accession No |
|---|---|---|---|
| LAT1 | Na+-independent cationic and neutral amino acid | F: CTCTCTCTCATCATCTGGGC R: TCATTCCTGGGTCTGTTGCT | XM_415975 |
| CAT-1 | Na+-independent cationic amino acid transporter-1 | F: ATGTAGGTTGGGATGGAGCC R: AACGAGTAAGCCAGGAGGGT | XM_015277949.1 |
| CAT-2 | Na+-independent cationic amino acid transporter-2 | F: CAAGTCTTCTCGGCTCTAT R: GTGCCTGCCTCTTACTCA | XM_015285435.1 |
| PepT-1 | Oligopeptide transporter-1 | F:TTTCCTTTACATCCCTCTCC R:TCACTTCTACTCTCACTC | NM-204365 |
| PepT-2 | Oligopeptide transporter-2 | F:TGACTGGGCATCGGAACAA R:ACCCGTGTCACCATTTTAACCT | NM_001319028.1 |
| GAPDH | Glyceraldahyde -3-phosphate dehydrogenase | F-GGTGGTGCTAAGCGTGTTA R-CCCTCCACAATGCCAA | NM205518 |
| Parameter | Control | Replacement % with DFSBM | SEM | p-Value | ||
|---|---|---|---|---|---|---|
| 25 | 50 | 100 | ||||
| Overall performance | ||||||
| Initial weight (g/bird) | 44.67 | 44.33 | 44.00 | 43.83 | 0.23 | 0.635 |
| Final BWT (g/bird) | 2269 c | 2293 c | 2448 b | 2559 a | 36.10 | <0.001 |
| Total BWG (g/bird) | 2224 c | 2248 c | 2404 b | 2515 a | 36.20 | <0.001 |
| Total FI (g/bird) | 3838 a | 3679 b | 3815 a | 3904 a | 27.50 | 0.003 |
| Total FCR | 1.73 a | 1.64 b | 1.59 c | 1.55 d | 0.02 | <0.001 |
| Apparent Nutrient digestibility % | ||||||
| DM | 77.38 c | 80.39 b | 81.74 a | 81.71 a | 0.54 | <0.001 |
| CP | 72.31 c | 74.05 b | 75.13 a | 75.71 a | 0.41 | <0.001 |
| EE | 82.33 b | 82.39 b | 84.30 a | 84.61 a | 0.32 | <0.001 |
| Phosphorus | 50.68 d | 53.14 c | 59.01 b | 63.37 a | 1.50 | <0.001 |
| Calcium | 38.16 d | 40.56 c | 43.21 b | 46.36 a | 3.21 | <0.001 |
| Pancreatic digestive enzyme activity | ||||||
| Amylase (u/mg prot) | 161.97 c | 164.63 b | 166.49 a | 167.93 a | 0.70 | <0.001 |
| Lipase (u/mg prot) | 403.95 c | 405.36 bc | 406.80 ab | 409.08 a | 0.62 | 0.002 |
| Trypsin (u/mg prot) | 483.33 b | 483.95 ab | 484.86 a | 485.06 a | 0.24 | 0.005 |
| Parameter | Control | Replacement % with DFSBM | SEM | p-Value | ||
|---|---|---|---|---|---|---|
| 25 | 50 | 100 | ||||
| Liver function tests | ||||||
| ALT, U/L | 17.89 | 17.81 | 17.57 | 17.82 | 0.10 | 0.740 |
| AST, U/L | 17.98 | 17.70 | 17.53 | 17.60 | 0.16 | 0.811 |
| Kidney function tests | ||||||
| Creatinine, mg/dL | 0.94 b | 1.04 ab | 1.10 a | 1.08 a | 0.02 | 0.018 |
| Uric acid, mg/dL | 4.07 | 4.07 | 4.14 | 4.29 | 0.07 | 0.666 |
| Serum lipid profile | ||||||
| Total Cholesterol, mg/dL | 132.44 a | 129.54 b | 124.90 c | 119.89 d | 1.45 | <0.001 |
| TAGs, mg/dL | 62.52 a | 57.88 b | 55.73 c | 50.85 d | 1.28 | <0.001 |
| HDL-C, mg/dL | 89.85 c | 92.56 b | 94.03 b | 98.62 a | 0.99 | <0.001 |
| LDL-C, mg/dL | 30.09 a | 25.41 b | 19.72 c | 11.10 d | 2.17 | <0.001 |
| VLDL-C, mg/dL | 12.50 a | 11.58 b | 11.15 c | 10.17 d | 0.26 | <0.001 |
| Parameter | Control | Replacement % with DFSBM | SEM | p-Value | ||
|---|---|---|---|---|---|---|
| 25 | 50 | 100 | ||||
| MDA nmol/mg | 0.417 a | 0.409 b | 0.402 c | 0.397 d | 0.002 | <0.001 |
| SOD u/mg prot | 973.34 d | 976.74 c | 983.92 b | 994.33 a | 2.44 | <0.001 |
| CAT u/g prot | 34.37 d | 38.51 c | 41.70 b | 46.55 a | 1.35 | <0.001 |
| GSH-PX u/g prot | 37.07 d | 41.87 c | 46.19 b | 52.11 a | 1.68 | <0.001 |
| Parameter | Control | Replacement % with DFSBM | SEM | p-Value | ||
|---|---|---|---|---|---|---|
| 25 | 50 | 100 | ||||
| Chemical composition of breast muscle (% of DM) | ||||||
| DM | 25.66 | 25.66 | 25.86 | 25.66 | 0.14 | 0.959 |
| CP | 85.60 b | 86.07 ab | 86.62 a | 86.31 a | 0.13 | 0.011 |
| EE | 6.53 a | 6.26 b | 6.11 c | 6.00 c | 0.06 | <0.001 |
| Ash | 4.26 b | 4.33 ab | 4.39 a | 4.45 a | 0.02 | 0.008 |
| Calcium | 0.20 b | 0.21 b | 0.22 a | 0.23 a | 0.004 | 0.001 |
| Phosphorus | 0.89 c | 0.91 b | 0.92 a | 0.93 a | 0.004 | <0.001 |
| Amino acids composition of breast muscle (% of DM) | ||||||
| Essential amino acids (% of DM) | ||||||
| Argenine | 1.61 | 1.61 | 1.61 | 1.61 | 0.003 | 1.00 |
| Histidine | 0.77 | 0.77 | 0.77 | 0.76 | 0.003 | 0.482 |
| Isoleucin | 1.24 | 1.23 | 1.23 | 1.23 | 0.003 | 0.813 |
| Leucine | 2.25 | 2.25 | 2.25 | 2.25 | 0.002 | 0.752 |
| Lysine | 2.44 | 2.44 | 2.42 | 2.60 | 0.03 | 0.090 |
| Methionine | 0.47 | 0.46 | 0.47 | 0.47 | 0.002 | 0.532 |
| Phenylalanine | 1.21 a | 1.21 a | 1.19 b | 1.18 c | 0.005 | 0.001 |
| Therionine | 0.86 | 0.87 | 0.87 | 0.86 | 0.002 | 0.201 |
| Tryptophan | 2.52 | 2.54 | 2.54 | 2.54 | 0.005 | 0.162 |
| Valine | 1.27 | 1.27 | 1.27 | 1.27 | 0.002 | 0.951 |
| Non-essential amino acids (% of DM) | ||||||
| Alanine | 1.36 | 1.37 | 1.36 | 1.38 | 0.003 | 0.403 |
| Aspartic acid | 2.21 b | 2.23 a | 2.23 a | 2.23 a | 0.004 | 0.007 |
| Cysteine | 0.28 | 0.27 | 0.27 | 0.28 | 0.002 | 0.287 |
| Glutamic acid | 3.52 b | 3.54 a | 3.53 ab | 3.54 a | 0.003 | 0.021 |
| Glycine | 1.11 | 1.12 | 1.11 | 1.12 | 0.003 | 0.802 |
| Proline | 0.85 | 0.85 | 0.85 | 0.85 | 0.002 | 0.672 |
| Serine | 0.81 | 0.81 | 0.81 | 0.81 | 0.002 | 0.951 |
| Tyrosine | 0.95 d | 0.95 c | 0.95 b | 0.95 a | 0.002 | 0.752 |
| Parameter | Control | Replacement % with DFSBM | SEM | p-Value | ||
|---|---|---|---|---|---|---|
| 25 | 50 | 100 | ||||
| Cecal bacterial count (log10 CFU/g cecal content) | ||||||
| Total bacterial count | 4.54 b | 5.32 a | 5.47 a | 5.51 a | 0.12 | <0.001 |
| Coliform count | 2.59 a | 2.11 b | 1.93 b | 1.36 c | 0.13 | <0.001 |
| Lactobacillus count | 2.89 d | 3.19 c | 3.88 b | 4.46 a | 0.18 | <0.001 |
| Duodenal pH | ||||||
| pH | 5.67 a | 5.12 b | 4.85 c | 4.69 d | 0.11 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Raheem, S.M.; Mohammed, E.S.Y.; Mahmoud, R.E.; El Gamal, M.F.; Nada, H.S.; El-Ghareeb, W.R.; Marzok, M.; Meligy, A.M.A.; Abdulmohsen, M.; Ismail, H.; et al. Double-Fermented Soybean Meal Totally Replaces Soybean Meal in Broiler Rations with Favorable Impact on Performance, Digestibility, Amino Acids Transporters and Meat Nutritional Value. Animals 2023, 13, 1030. https://doi.org/10.3390/ani13061030
Abdel-Raheem SM, Mohammed ESY, Mahmoud RE, El Gamal MF, Nada HS, El-Ghareeb WR, Marzok M, Meligy AMA, Abdulmohsen M, Ismail H, et al. Double-Fermented Soybean Meal Totally Replaces Soybean Meal in Broiler Rations with Favorable Impact on Performance, Digestibility, Amino Acids Transporters and Meat Nutritional Value. Animals. 2023; 13(6):1030. https://doi.org/10.3390/ani13061030
Chicago/Turabian StyleAbdel-Raheem, Sherief M., El Said Yehia Mohammed, Rania Elsaid Mahmoud, Mahmoud Fathy El Gamal, Hend S. Nada, Waleed Rizk El-Ghareeb, Mohamed Marzok, Ahmed M. A. Meligy, Mohamad Abdulmohsen, Hesham Ismail, and et al. 2023. "Double-Fermented Soybean Meal Totally Replaces Soybean Meal in Broiler Rations with Favorable Impact on Performance, Digestibility, Amino Acids Transporters and Meat Nutritional Value" Animals 13, no. 6: 1030. https://doi.org/10.3390/ani13061030
APA StyleAbdel-Raheem, S. M., Mohammed, E. S. Y., Mahmoud, R. E., El Gamal, M. F., Nada, H. S., El-Ghareeb, W. R., Marzok, M., Meligy, A. M. A., Abdulmohsen, M., Ismail, H., Ibrahim, D., & Kishawy, A. T. Y. (2023). Double-Fermented Soybean Meal Totally Replaces Soybean Meal in Broiler Rations with Favorable Impact on Performance, Digestibility, Amino Acids Transporters and Meat Nutritional Value. Animals, 13(6), 1030. https://doi.org/10.3390/ani13061030








