Molecular and Biochemical Evidence of the Toxic Effects of Terbuthylazine and Malathion in Zebrafish
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Exposures
2.2. Antioxidants
2.3. Oxidative and Nitrosative Stress Markers
2.4. Nonspecific Markers of Lysosomal and Hepatocellular Injury
2.5. Hormonal and Immune Markers
2.6. Quantitative mRNA Expression Analysis
2.7. Statistical Data Processing
3. Results
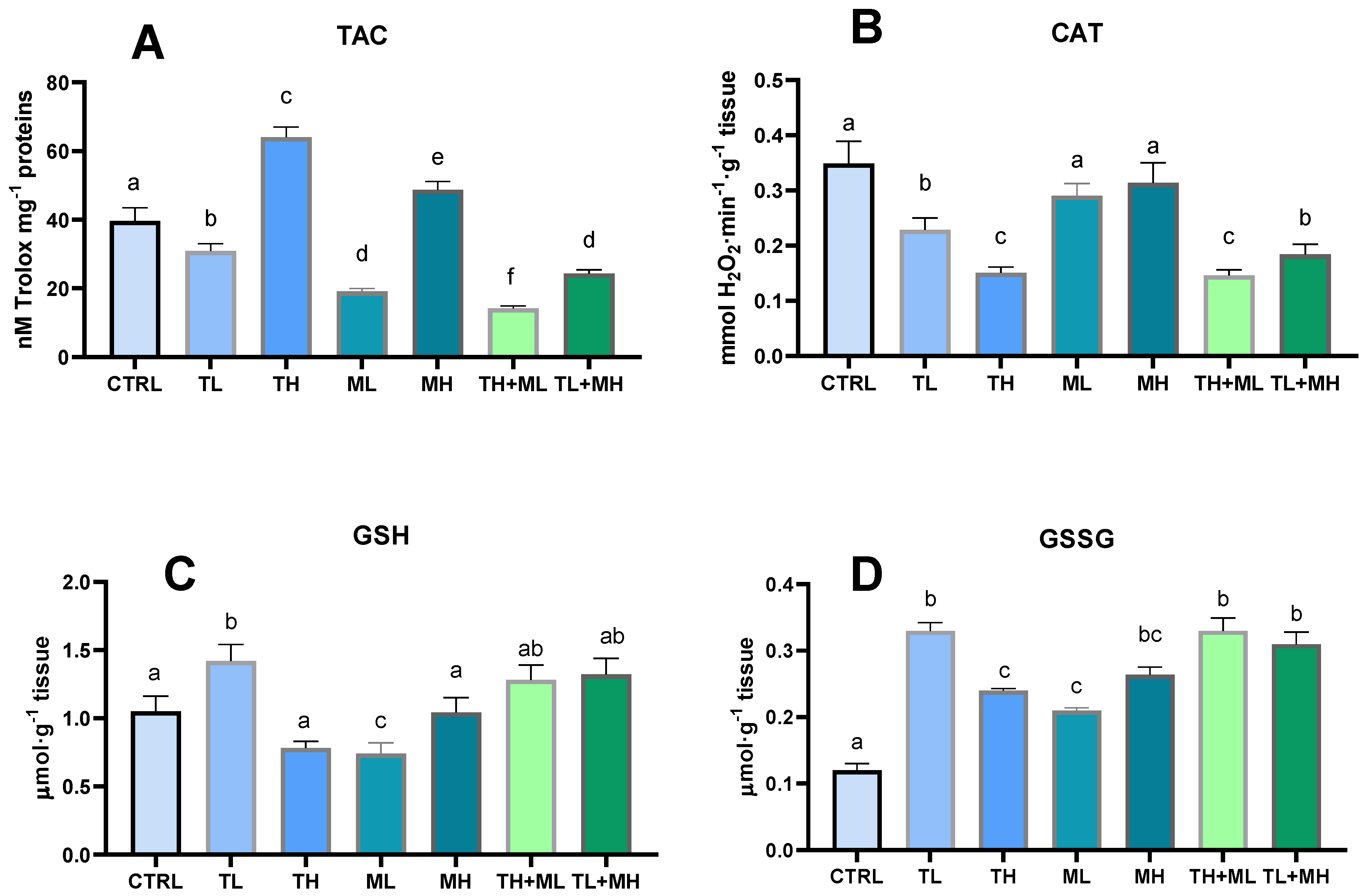
3.1. Antioxidants
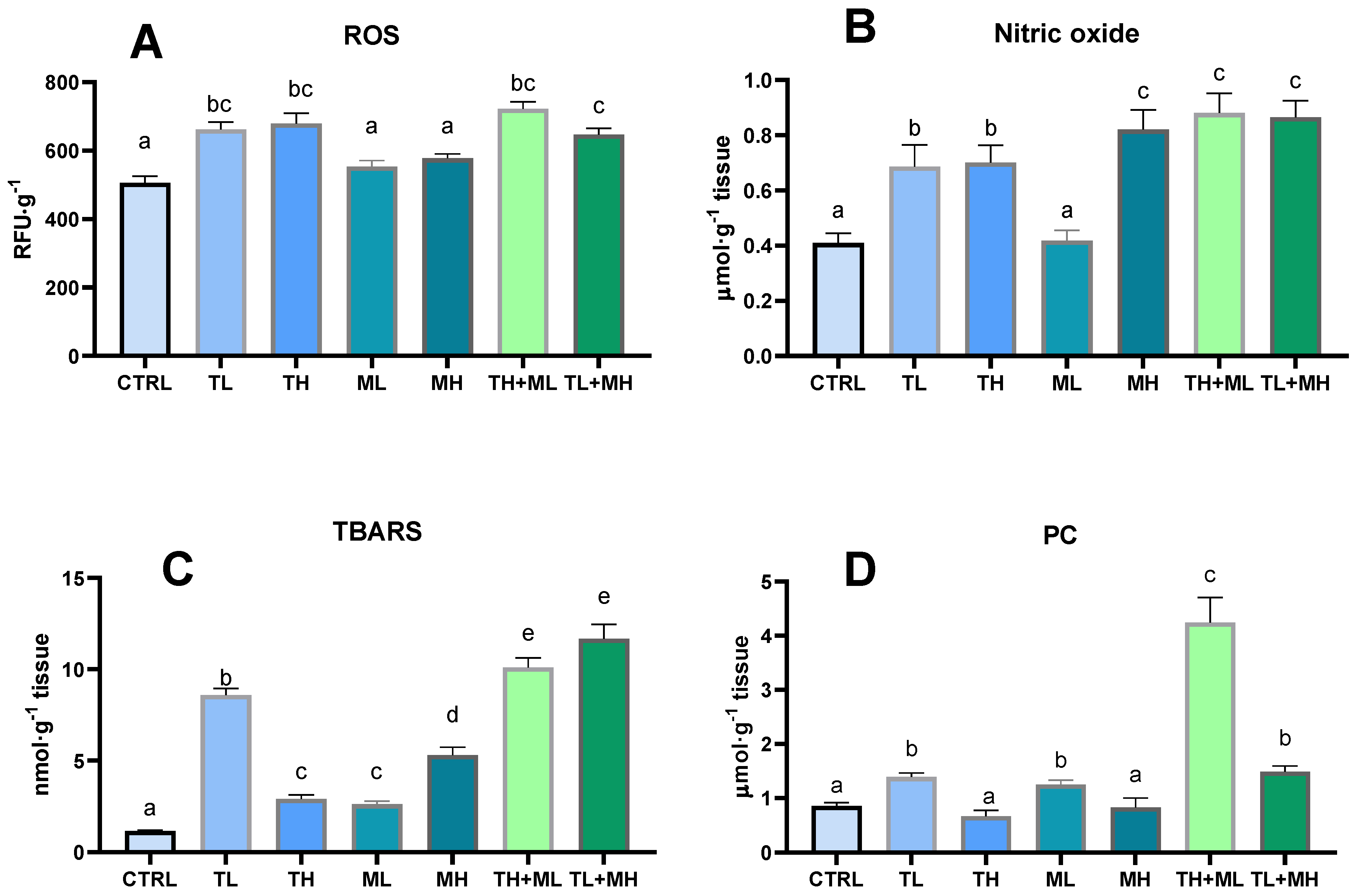
3.2. Prooxidant Events
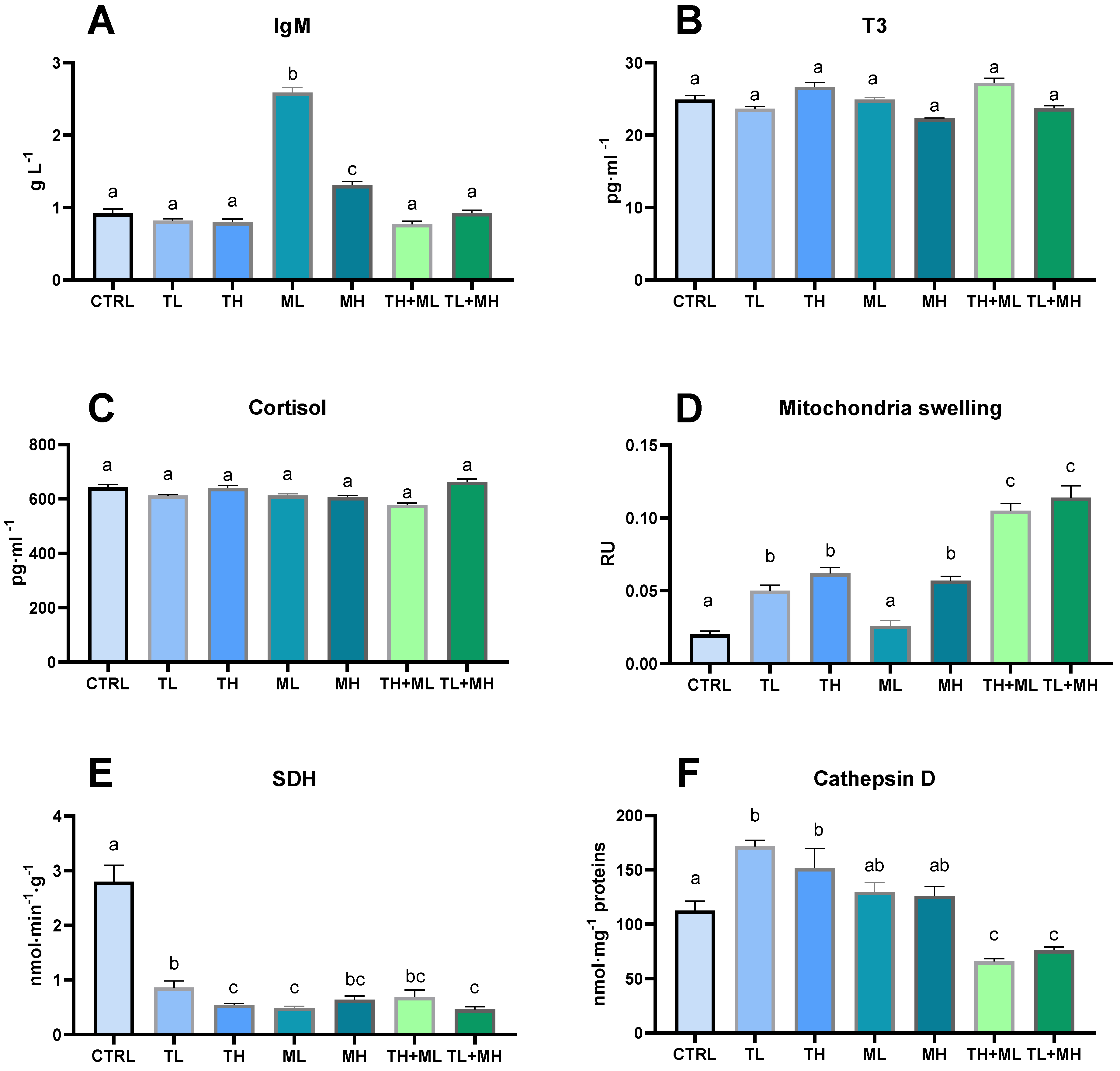
3.3. Immune Response
3.4. Mitochondrial and Lysosomal Response
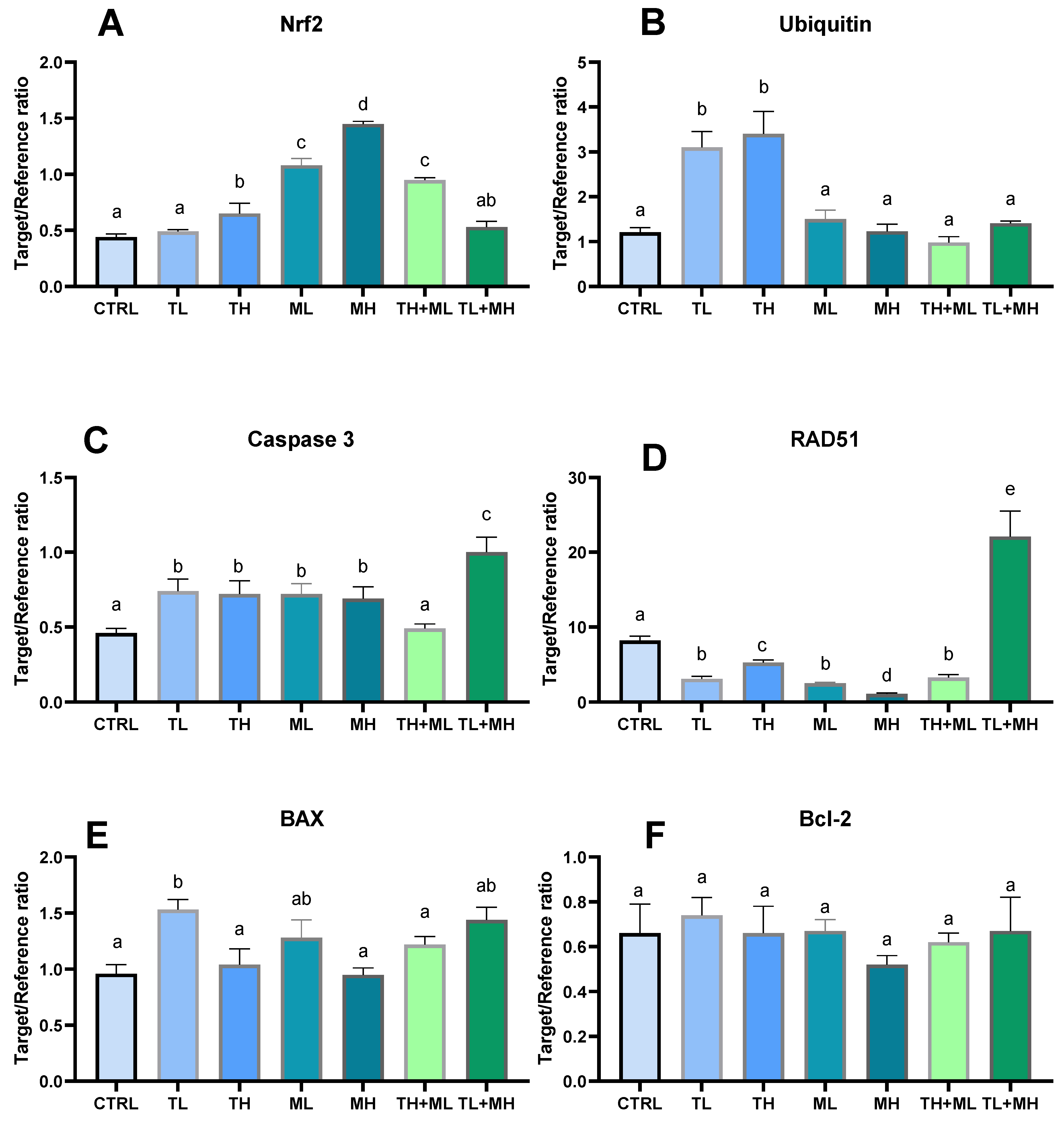
3.5. Signs of Geno- and Cytotoxicity
3.6. Expression of the Target Genes
3.7. Data Integration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boedeker, W.; Watts, M.; Clausing, P.; Marquez, E. The global distribution of acute unintentional pesticide poisoning: Estimations based on a systematic review. BMC Public. Health 2020, 20, 1875. [Google Scholar] [CrossRef] [PubMed]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Worldometer. Pesticide Use by Country. 2022. Available online: https://www.worldometers.info/food-agriculture/pesticides-by-country/ (accessed on 7 July 2022).
- FAO. Pesticides Use. Global, Regional and Country Trends, 1990–2018. FAOSTAT Analytical Brief Series. No. 16. Rome. 2021. Available online: https://www.fao.org/3/cb3411en/cb3411en.pdf (accessed on 7 July 2022).
- Khatib, I.; Rychter, P.; Falfushynska, H. Pesticide Pollution: Detrimental outcomes and possible mechanisms of fish exposure to common organophosphates and triazines. J. Xenobiot. 2022, 12, 236–265. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.D.; Klaine, S.J.; Carvalho, F.P.; Barcelo, D. Pesticide Residues in Coastal Tropical Ecosystems: Distribution, Fate and Effects, 1st ed.; Everaarts, J., Ed.; CRC Press Boca Raton: London, UK, 2002; p. 576. [Google Scholar] [CrossRef]
- Tang, F.H.M.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of pesticide pollution at the global scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- Kattwinkel, M.; Kühne, J.V.; Foit, K.; Liess, M. Climate change, agricultural insecticide exposure, and risk for freshwater communities. Ecol. Appl. 2011, 21, 2068–2081. [Google Scholar] [CrossRef]
- Stipičević, S.; Galzina, N.; Udiković-Kolić, N.; Jurina, T.; Mendaš, G.; Dvoršćak, M.; Petrić, I.; Barić, K.; Drevenkar, V. Distribution of terbuthylazine and atrazine residues in crop-cultivated soil: The effect of herbicide application rate on herbicide persistence. Geoderma 2015, 259–260, 300–309. [Google Scholar] [CrossRef]
- Erickson, B.E. US EPA to Impose Atrazine Restrictions. 2022. Available online: https://cen.acs.org/environment/pesticides/US-EPA-impose-atrazine-restrictions/100/web/2022/07 (accessed on 4 February 2023).
- Terbuthylazine. R.E.D. Facts. 1995. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/fs_PC-080814_1-Jun-95.pdf (accessed on 4 February 2023).
- CLH Report for Terbuthylazine. 2008. Available online: https://echa.europa.eu/documents/10162/a765f0dd-a71c-f0df-decf-d7e4e1fb47ea (accessed on 4 February 2023).
- Use of Malathion for Vector Control: Report of a WHO Meeting, Geneva. 2016. Available online: http://apps.who.int/iris/bitstream/handle/10665/207475/9789241510578_eng.pdf?sequence=1 (accessed on 4 February 2023).
- Malathion: Human Health Draft Risk Assessment for Registration Review. 2016. Available online: https://www3.epa.gov/pesticides/Malathion-Human-Health-Draft-Risk-Assessment-for-Registration-Review.pdf (accessed on 4 February 2023).
- Redondo-López, S.; León, A.C.; Jiménez, K.; Solano, K.; Blanco-Peña, K.; Mena, F. Transient exposure to sublethal concentrations of a pesticide mixture (chlorpyrifos-difenoconazole) caused different responses in fish species from different trophic levels of the same community. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 251, 109208. [Google Scholar] [CrossRef] [PubMed]
- Trimble, A.J.; Lydy, M.J. Effects of triazine herbicides on organophosphate insecticide toxicity in Hyalella azteca. Arch. Environ. Contam. Toxicol. 2006, 51, 29–34. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Z.; Yao, H.; Cao, Y.; Xing, H.; Xu, S. Pro- and anti-inflammatory cytokine expression in immune organs of the common carp exposed to atrazine and chlorpyrifos. Pestic. Biochem. Physiol. 2014, 114, 8–15. [Google Scholar] [CrossRef]
- Cowie, A.M.; Sarty, K.I.; Mercer, A.; Koh, J.; Kidd, K.A.; Martyniuk, C.J. Molecular networks related to the immune system and mitochondria are targets for the pesticide dieldrin in the zebrafish (Danio rerio) central nervous system. J. Proteomics. 2017, 157, 71–82. [Google Scholar] [CrossRef]
- Bodnar, O.; Horyn, O.; Khatib, I.; Falfushynska, H. Multibiomarker assessment in zebrafish Danio rerio after the effects of malathion and chlorpyrifos. Toxicol. Environ. Health. Sci. 2021, 13, 165–174. [Google Scholar] [CrossRef]
- Falfushynska, H.; Khatib, I.; Kasianchuk, N.; Lushchak, O.; Horyn, O.; Sokolova, I.M. Toxic effects and mechanisms of common pesticides (Roundup and chlorpyrifos) and their mixtures in a zebrafish model (Danio rerio). Sci. Total. Environ. 2022, 833, 155236. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Wang, H.; Wan, Y.; Zhou, L.; Wang, Q.; Wang, M. Combined toxicities of cadmium and five agrochemicals to the larval zebrafish (Danio rerio). Sci. Rep. 2022, 12, 16045. [Google Scholar] [CrossRef]
- Sule, R.O.; Condon, L.; Gomes, A.V. A common feature of pesticides: Oxidative stress-the role of oxidative stress in pesticide-induced toxicity. Oxid. Med. Cell. Longev. 2022, 2022, 5563759. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.W.; Beach, S.R.; Brody, G.H. Microtrial methods for translating gene-environment dynamics into preventive interventions. Prev. Sci. 2010, 11, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Karyab, H.; Mahvi, A.H.; Nazmara, S.; Bahojb, A. Determination of water sources contamination to diazinon and malathion and spatial pollution patterns in Qazvin, Iran. Bull. Environ. Contam. Toxicol. 2013, 90, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Baillie, B.R. Herbicide concentrations in waterways following aerial application in a steepland planted forest in New Zealand. N. Z. J. For. Sci. 2016, 46, 16. [Google Scholar] [CrossRef]
- Rodrigo-Ilarri, J.; Rodrigo-Clavero, M.E.; Cassiraga, E.; Ballesteros-Almonacid, L. Assessment of groundwater contamination by terbuthylazine using vadose zone numerical models. Case study of Valencia Province (Spain). Int. J. Environ. Res. Public. Health. 2020, 17, 3280. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Dragoi, E.N.; Almomani, F.; Golzadeh, N.; Vo, D.N. A global systematic review of the concentrations of Malathion in water matrices: Meta-analysis, and probabilistic risk assessment. Chemosphere. 2022, 291 Pt 2, 132789. [Google Scholar] [CrossRef]
- Katalinic, V.; Modun, D.; Music, I.; Boban, M. Gender differences in antioxidant capacity of rat tissues determined by 2,2’-azinobis (3-ethylbenzothiazoline 6-sulfonate; ABTS) and ferric reducing antioxidant power (FRAP) assays. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 140, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H.; Wyss, S.R.; Scherz, B.; Skvaril, F. Heterogeneity of erythrocyte catalase II. Isolation and characterization of normal and variant erythrocyte catalase and their subunits. Eur. J. Biochem. 1974, 48, 137–145. [Google Scholar] [CrossRef]
- Anderson, M.E. Determination of glutathione and glutathione disulfide in biological samples. Methods. Enzymol. 1985, 113, 548–555. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, e351–e358. [Google Scholar] [CrossRef]
- Reznick, A.Z.; Packer, L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods. Enzymol. 1994, 233, e357–e363. [Google Scholar] [CrossRef]
- Viarengo, A.; Burlando, B.; Cavaletto, M.; Marchi, B.; Ponzano, E.; Blasco, J. Role of metallothionein against oxidative stress in the mussel Mytilus galloprovincialis. Am. J. Physiol. 1999, 277, e1612–e1619. [Google Scholar] [CrossRef]
- Olive, P.L. DNA precipitation assay: A rapid and simple method for detecting DNA damage in mammalian cells. Environ. Molec. Mutagen. 1988, 11, 487–495. [Google Scholar] [CrossRef]
- Bester, M.J.; Potgieter, H.C.; Vermaak, W.J.H. Cholate and pH reduce interference by sodium dodecyl sulfate in the determination of DNA with hoechst. Anal. Biochem. 1994, 223, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Sessa, W.C.; Pritchard, K.; Seyedi, N.; Wang, J.; Hintze, T.H. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ. Res. 1994, 74, 349–353. [Google Scholar] [CrossRef]
- Bergmeyer, H.U.; Bernt, E. Lactate dehydrogenase U.V. assay with pyruvate and NADH. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Gawehn, K., Eds.; Academic Press: New York, NY, USA, 1974; Volume II, pp. 574–579. [Google Scholar] [CrossRef]
- Barrett, A.J. Lysosomal acid proteinase of rabbit liver. Biochem. J. 1967, 104, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Falfushynska, H.; Poznanskyi, D.; Kasianchuk, N.; Horyn, O.; Bodnar, O. Multimarker Responses of Zebrafish to the Effect of Ibuprofen and Gemfibrozil in Environmentally Relevant Concentrations. Bull. Environ. Contam. Toxicol. 2022, 109, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, B.D.; Seth, V.; Ahmed, R.S. Pesticide-induced oxidative stress: Perspectives and trends. Rev. Environ. Health 2001, 16, 1–40. [Google Scholar] [CrossRef]
- Abass, K.; Pelkonen, O.; Rautio, A. Chloro-s-triazines-toxicokinetic, Toxicodynamic, Human Exposure, and Regulatory Considerations. Curr. Drug. Metab. 2021, 22, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Gervais, J.A.; Luukinen, B.; Buhl, K.; Stone, D. Malathion Technical Fact Sheet; National Pesticide Information Center, Oregon State University Extension Services. 2009. Available online: http://npic.orst.edu/factsheets/archive/malatech.html (accessed on 4 February 2023).
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World. Allergy. Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Velisek, J.; Stara, A.; Koutnik, D.; Machova, J. Effect of terbuthylazine-2-hydroxy at environmental concentrations on early life stages of common carp (Cyprinus carpio L.). Biomed. Res. Int. 2014, 2014, 621304. [Google Scholar] [CrossRef] [PubMed]
- Stara, A.; Zuskova, E.; Kouba, A.; Velisek, J. Effects of terbuthylazine-desethyl, a terbuthylazine degradation product, on red swamp crayfish (Procambarus clarkii). Sci. Total. Environ. 2016, 566–567, 733–740. [Google Scholar] [CrossRef]
- Marsillach, J.; Costa, L.G.; Furlong, C.E. Protein adducts as biomarkers of exposure to organophosphorus compounds. Toxicology 2013, 307, 46–54. [Google Scholar] [CrossRef]
- Blahová, J.; Plhalová, L.; Hostovský, M.; Divišová, L.; Dobšíková, R.; Mikulíková, I.; Stěpánová, S.; Svobodová, Z. Oxidative stress responses in zebrafish Danio rerio after subchronic exposure to atrazine. Food. Chem. Toxicol. 2013, 61, 82–85. [Google Scholar] [CrossRef]
- Abhijith, B.D.; Ramesh, M.; Poopal, R.K. Responses of metabolic and antioxidant enzymatic activities in gill, liver and plasma of Catla catla during methyl parathion exposure. J. Basic. Appl. Zool. 2016, 77, 31–40. [Google Scholar] [CrossRef]
- Kaur, M.; Jindal, R. Oxidative stress response in liver, kidney and gills of ctenopharyngodon idellus (cuvier & valenciennes) exposed to chlorpyrifos. MOJ Biol. Med. 2017, 1, 103–112. [Google Scholar] [CrossRef]
- Nunes, M.E.M.; Müller, T.E.; Murussi, C.; do Amaral, A.M.B.; Gomes, J.L.C.; Marins, A.T.; Leitemperger, J.; Rodrigues, C.C.R.; Fiuza, T.L.; Costa, M.D.; et al. Oxidative effects of the acute exposure to a pesticide mixture of cypermethrin and chlorpyrifos on carp and zebrafish—A comparative study. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2018, 206–207, 48–53. [Google Scholar] [CrossRef]
- Destro, A.L.F.; Silva, S.B.; Gregório, K.P.; de Oliveira, J.M.; Lozi, A.A.; Zuanon, J.A.S.; Salaro, A.L.; da Matta, S.L.P.; Gonçalves, R.V.; Freitas, M.B. Effects of subchronic exposure to environmentally relevant concentrations of the herbicide atrazine in the Neotropical fish Astyanax altiparanae. Ecotoxicol. Environ. Saf. 2021, 208, 111601. [Google Scholar] [CrossRef] [PubMed]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Lim, S.R.; Choi, H.K.; Bae, J. Triazine herbicides inhibit relaxin signaling and disrupt nitric oxide homeostasis. Toxicol. Appl. Pharmacol. 2016, 307, 10–18. [Google Scholar] [CrossRef]
- Sokolova, I. Mitochondrial adaptations to variable environments and their role in animals’ stress tolerance. Integr. Comp. Biol. 2018, 58, 519–531. [Google Scholar] [CrossRef]
- Reddam, A.; McLarnan, S.; Kupsco, A. Environmental chemical exposures and mitochondrial dysfunction: A review of recent literature. Curr. Environ. Health Rpt. 2022, 9, 631–649. [Google Scholar] [CrossRef] [PubMed]
- Phillips, N.R.; Sprouse, M.L.; Roby, R.K. Simultaneous quantification of mitochondrial DNA copy number and deletion ratio: A multiplex real-time PCR assay. Sci. Rep. 2014, 4, 3887. [Google Scholar] [CrossRef]
- Moosavi, B.; Zhu, X.L.; Yang, W.C.; Yang, G.F. Genetic, epigenetic and biochemical regulation of succinate dehydrogenase function. Biol. Chem. 2020, 401, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.; Santos, R.M.; Burns, R.; Zhang, W.C. Succinate dehydrogenase and ribonucleic acid networks in cancer and other diseases. Cancers 2020, 12, 3237. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life. Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Chenxu, G.; Minxuan, X.; Yuting, Q.; Tingting, G.; Jing, F.; Jinxiao, L.; Sujun, W.; Yongjie, M.; Deshuai, L.; Qiang, L.; et al. Loss of RIP3 initiates annihilation of high-fat diet initialized nonalcoholic hepatosteatosis: A mechanism involving Toll-like receptor 4 and oxidative stress. Free. Radic. Biol. Med. 2019, 134, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Alu, A.; Han, X.; Ma, X.; Wu, M.; Wei, Y.; Wei, X. The role of lysosome in regulated necrosis. Acta Pharm. Sin. B 2020, 10, 1880–1903. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.Z.; Crawford, N.; Longley, D.B. The role of ubiquitination in apoptosis and necroptosis. Cell Death. Differ. 2022, 29, 272–284. [Google Scholar] [CrossRef]
- Giron-Perez, M.I.; Santerre, A.; Gonzalez-Jaime, F.; Casas-Solis, J.; Hernandez-Coronado, M.; Peregrina-Sandoval, J.; Takemura, A.; Zaitseva, G. Immunotoxicity and hepatic function evaluation in Nile tilapia (Oreochromis niloticus) exposed to diazinon. Fish. Shellfish. Immunol. 2007, 23, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, L.; Zhang, Y.; Fang, Q.; Li, Y. Toxic effects of chlorpyrifos on lysozyme activities, the contents of complement C3 and IgM, and IgM and complement C3 expressions in common carp (Cyprinus carpio L.). Chemosphere 2013, 93, 428–433. [Google Scholar] [CrossRef]
- Díaz-Resendiz, K.J.G.; Girón-Pérez, M.I. Effect of chlorpyrifos on the immune response of Nile tilapia (Oreochromis niloticus). Revista. Bio. Ciencias. 2014, 3, 59–64. [Google Scholar] [CrossRef]
- Dezfuli, B.S.; Simoni, E.; Giari, L.; Manera, M. Effects of experimental terbuthylazine exposure on the cells of Dicentrarchus labrax (L.). Chemosphere 2006, 64, 1684–1694. [Google Scholar] [CrossRef] [PubMed]
- Plumb, J.A.; Areechon, N. Effect of malathion on humoral immune response of channel catfish. Dev. Comp. Immunol. 1990, 14, 355–358. [Google Scholar] [CrossRef]
- Güven, M.; Bayram, F.; Unlühizarci, K.; Keleştimur, F. Endocrine changes in patients with acute organophosphate poisoning. Hum. Exp. Toxicol. 1999, 18, 598–601. [Google Scholar] [CrossRef]
- Trang, A.; Khandhar, P.B. Physiology, Acetylcholinesterase. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539735/ (accessed on 4 February 2023).
- Cobilinschi, C.; Tincu, R.C.; Băetu, A.E.; Deaconu, C.O.; Totan, A.; Rusu, A.; Neagu, P.T.; Grințescu, I.M. Endocrine disturbances induced by low-dose organophosphate exposure in male wistar rats. Acta. Endocrinol. 2021, 17, 177–185. [Google Scholar] [CrossRef]
- Anderson, J.C.; Marteinson, S.C.; Prosser, R.S. Prioritization of pesticides for assessment of risk to aquatic ecosystems in Canada and identification of knowledge gaps. Rev. Environ. Contam. Toxicol. 2021, 259, 171–231. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Srinivasan, K.; Abdisalaam, S.; Su, F.; Raj, P.; Dozmorov, I.; Mishra, R.; Wakeland, E.K.; Ghose, S.; Mukherjee, S.; et al. RAD51 interconnects between DNA replication, DNA repair and immunity. Nucleic. Acids. Res. 2017, 45, 4590–4605. [Google Scholar] [CrossRef]
- Hernández, A.F.; Gil, F.; Lacasaña, M. Toxicological interactions of pesticide mixtures: An update. Arch. Toxicol. 2017, 91, 3211–3223. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, C.; Rossi, A.; Ale, A.; Campana, M.; Parma, M.J.; Cazenave, J. Combined toxicological effects of pesticides: A fish multi-biomarker approach. Ecol. Indic. 2014, 36, 532–538. [Google Scholar] [CrossRef]


| Gene | Primers | Primer (5′–3′) | Tm | Ta | NCBI Accession No. |
|---|---|---|---|---|---|
| Caspase 3a | Forward | GACGCAAAGCGTGTGGATAC | 58.4 | 58.4 | NM_131877.3 |
| Reverse | GCCGATGTTGGGGTAGTTCA | 59.6 | |||
| Bcl-2 | Forward | GCGGAGGGAACAACTCTGAA | 59.8 | 59.8 | AY695820.1 |
| Reverse | ATCCCGTAACACCCGGTAGA | 60.0 | |||
| BAX | Forward | GACTTGGGAGCTGCACTTCT | 59.8 | 59.8 | NM_131562.2 |
| Reverse | CTGACTCCGGGTCACTTCAG | 60.1 | |||
| Nrf2 | Forward | CGGGAGATTTCAGCTCAGGG | 60.4 | 59.8 | NM_182889.1 |
| Reverse | CGAAGGATCCGTCTTCGGTT | 59.8 | |||
| RAD51 | Forward | CCGCTATGATGACCGAGTCC | 60.0 | 59.9 | NM_213206.2 |
| Reverse | CAGCATACGCAGAAAGCGTC | 59.9 | |||
| Ubiquitin | Forward | GTCTCCGAGGAGGCTCAGAT | 60.4 | 59.8 | NM_001013272.2 |
| Reverse | GTGAGTGCATAAGCAGGGGA | 59.8 | |||
| Tubulin | Forward | GTTGGAGCTGAGAGTGTGGAA | 59.9 | 59.0 | NM_194388.2 |
| Reverse | CAATGGACAGGAAACACAGCA | 59.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatib, I.; Horyn, O.; Bodnar, O.; Lushchak, O.; Rychter, P.; Falfushynska, H. Molecular and Biochemical Evidence of the Toxic Effects of Terbuthylazine and Malathion in Zebrafish. Animals 2023, 13, 1029. https://doi.org/10.3390/ani13061029
Khatib I, Horyn O, Bodnar O, Lushchak O, Rychter P, Falfushynska H. Molecular and Biochemical Evidence of the Toxic Effects of Terbuthylazine and Malathion in Zebrafish. Animals. 2023; 13(6):1029. https://doi.org/10.3390/ani13061029
Chicago/Turabian StyleKhatib, Ihab, Oksana Horyn, Oksana Bodnar, Oleh Lushchak, Piotr Rychter, and Halina Falfushynska. 2023. "Molecular and Biochemical Evidence of the Toxic Effects of Terbuthylazine and Malathion in Zebrafish" Animals 13, no. 6: 1029. https://doi.org/10.3390/ani13061029
APA StyleKhatib, I., Horyn, O., Bodnar, O., Lushchak, O., Rychter, P., & Falfushynska, H. (2023). Molecular and Biochemical Evidence of the Toxic Effects of Terbuthylazine and Malathion in Zebrafish. Animals, 13(6), 1029. https://doi.org/10.3390/ani13061029








