The Evolution of Coral Reef under Changing Climate: A Scientometric Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Scientometric Analysis
3. Objective of the Review
4. Systematic Data Collection
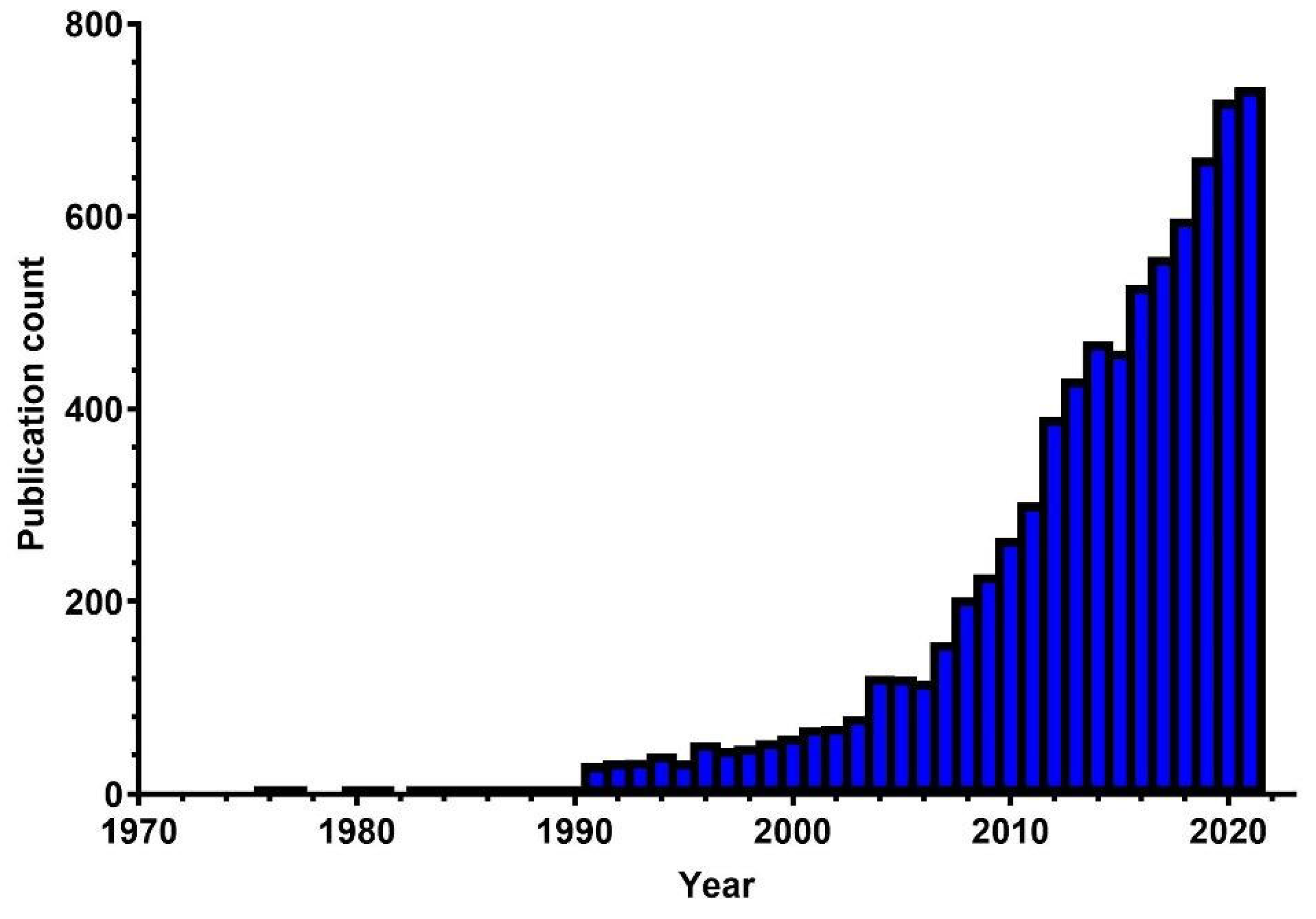
5. Evolution of the Literature
5.1. Global Publication
5.2. Leading Institutions, Funding, and Authorship Distribution
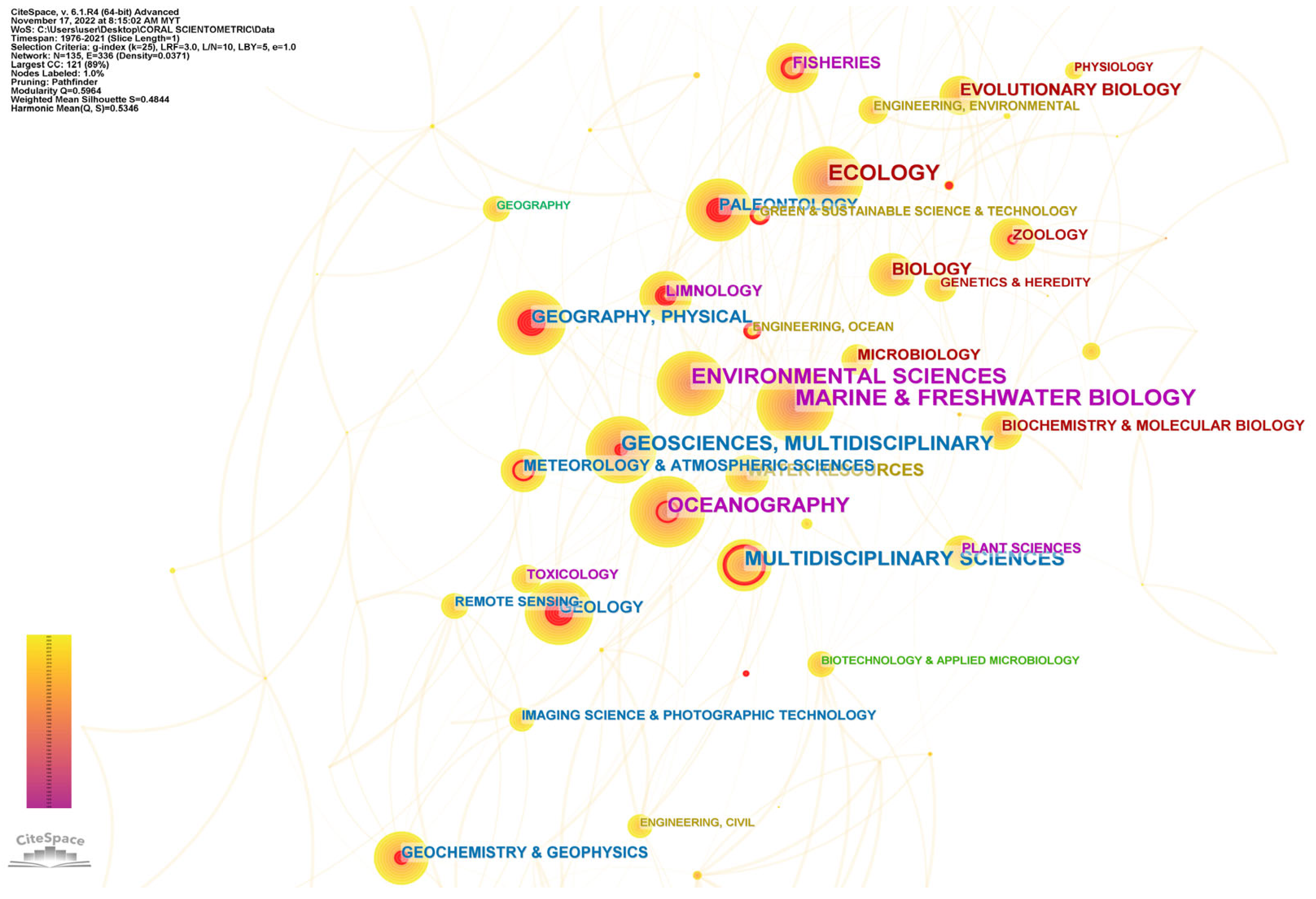
5.3. Emerging Research Disciplines
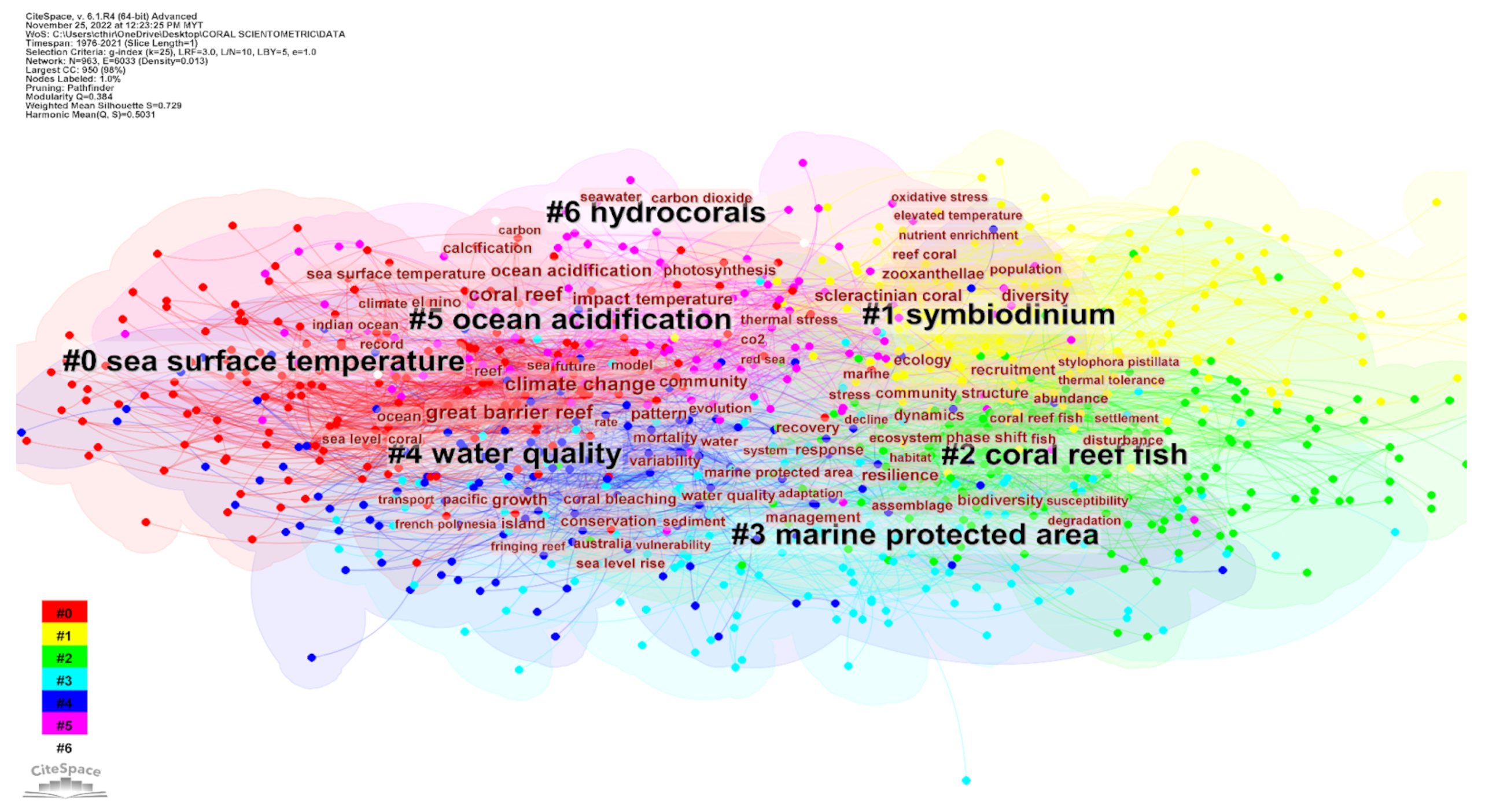
5.4. Research Cluster Analysis
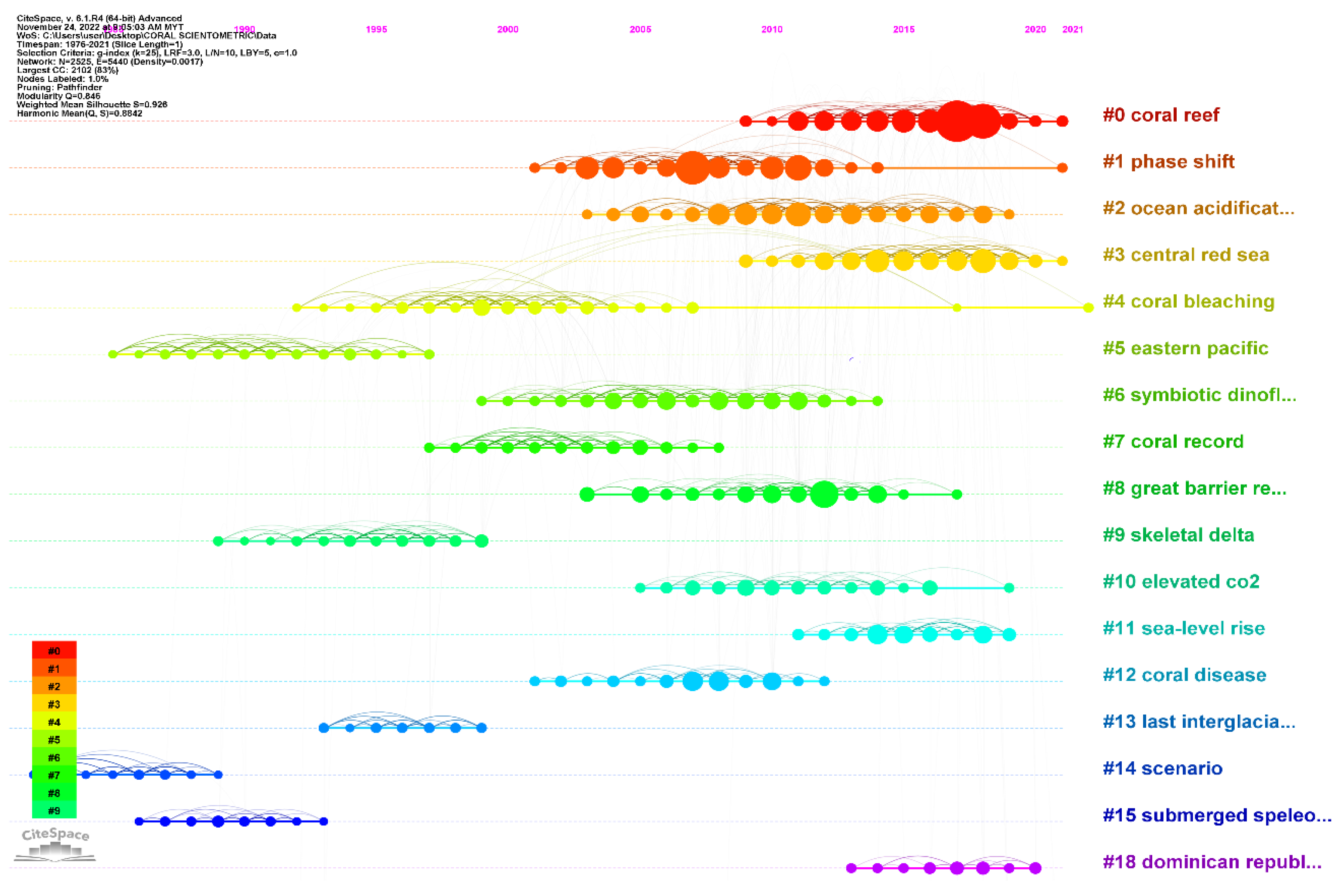
5.5. Timeline Co-citation Analysis
5.6. Highly Cited Articles in the Field
5.7. Distribution of Keywords
5.8. Dual Map Overlay
6. General Discussions
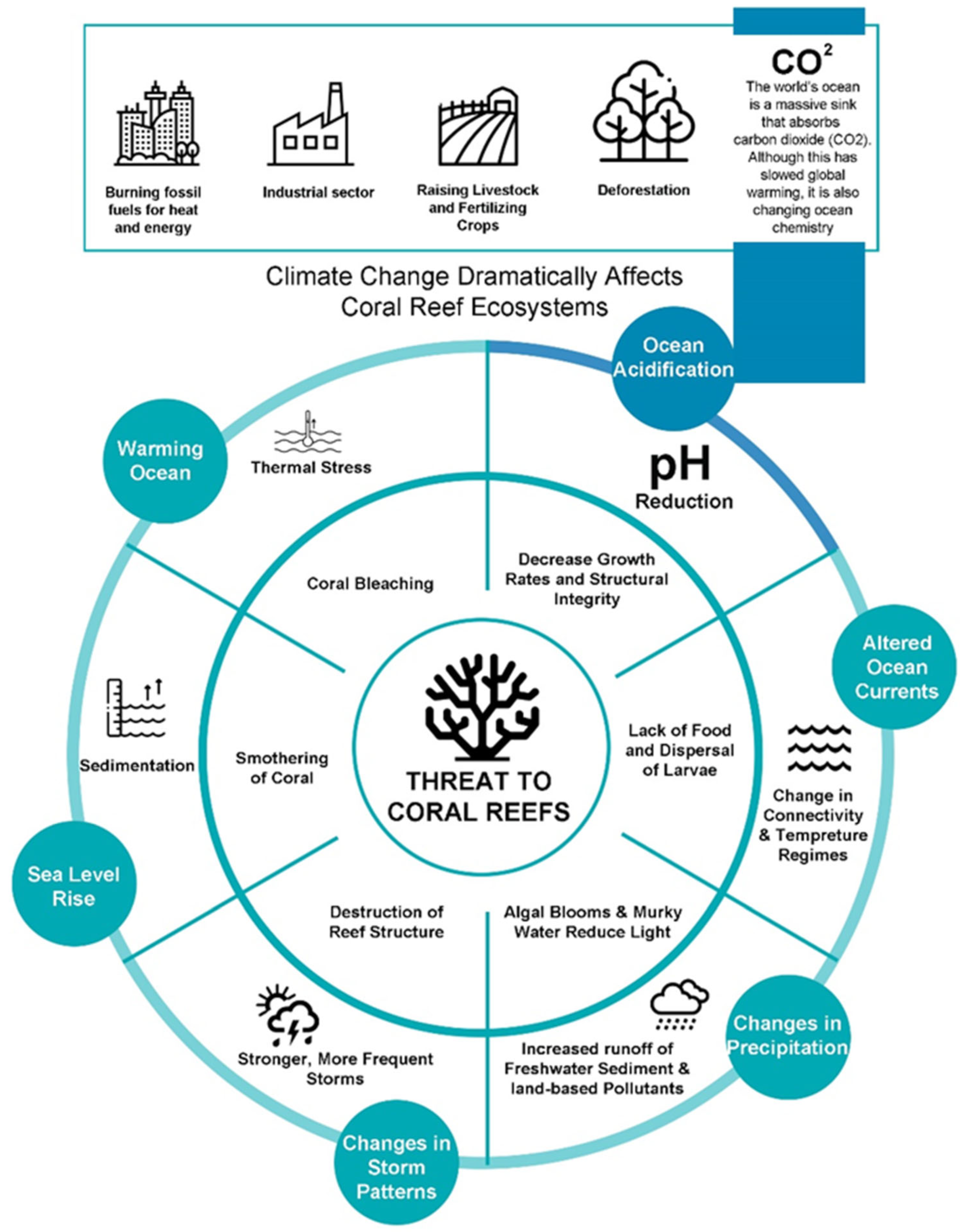
6.1. The Threat of Climate Change to Coral Reefs: Investigating the Impacts of Temperature and Ocean Acidification
6.2. Adaptive Strategies for Enhancing Coral Resistance and Resilience in the Face of Climate Change
7. Conclusions
- Monitoring: Regular coral reef monitoring can reveal vital details about the well-being and state of the reefs as well as the effects of climate change. These data can be used to pinpoint especially vulnerable regions and monitor long-term changes. Scientists and environmentalists can detect early warning signs of coral bleaching and other detrimental effects by monitoring coral reefs, which enables them to take action before it is too late;
- Research: Collaborative research efforts can contribute to a better understanding of the impacts of climate change on coral reefs and the mechanisms underlying these impacts and can also aid in developing and rigorously testing intervention and restoration techniques for coral reefs. Given that the preservation of coral reef ecosystems requires a range of interventions, including biological, ecological, and social strategies for mitigation and adaptation [19], such research can help create more efficient restoration, conservation and management strategies;
- Conservation and management: Collaborative conservation and management efforts can assist in mitigating the effects of climate change on coral reefs. Protected areas and marine reserves, for instance, can aid in mitigating the effects of overfishing and pollution, thereby making coral reefs more resilient to the effects of climate change;
- Mitigation: joint efforts can also aid in lowering atmospheric greenhouse gas concentrations, which are primarily responsible for climate change, for instance, by collaborating with regional organizations and authorities to advance sustainable development and lower carbon emissions;
- Public education and awareness: Raising public understanding of the effects of climate change on coral reefs can encourage support for management and conservation initiatives.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oliver, W.A. Origins and relationships of Paleozoic coral groups and the origin of the Scleractinia. Paleontol. Soc. Pap. 1996, 1, 107–134. [Google Scholar] [CrossRef]
- Spalding, M.D.; Ravilious, C.; Green, E.P. World Atlas of Coral Reefs. Mar. Pollut. Bull. 2002, 44, 350. [Google Scholar] [CrossRef]
- Dishon, G.; Grossowicz, M.; Krom, M.; Guy, G.; Gruber, D.F.; Tchernov, D. Evolutionary Traits that Enable Scleractinian Corals to Survive Mass Extinction Events. Sci. Rep. 2020, 10, 3903. [Google Scholar] [CrossRef]
- Rampino, M.R.; Shen, S.-Z. The end-Guadalupian (259.8 Ma) biodiversity crisis: The sixth major mass extinction? Hist. Biol. 2019, 33, 716–722. [Google Scholar] [CrossRef]
- Antonius, A. Coral reef pathology: A review. In Proceedings of the 4th International Coral Reef Symposium (ICRS), Manila, Phillipines, 27 May—1 June 1985; Volume 1981, pp. 155–160. [Google Scholar]
- Wilkinson, C.R.; Souter, D. Status of Caribbean Coral Reefs after Bleaching and Hurricanes in 2005; National Oceanic and Atmospheric Administration: Washington, DC, USA, 2008. [Google Scholar]
- Chung, F.C.; Komilus, C.F.; Mustafa, S. Effect of the creation of a marine protected area on populations of Coral Trout in the coral triangle region. Reg. Stud. Mar. Sci. 2017, 10, 1–9. [Google Scholar] [CrossRef]
- Kamil, T.T.M.; Jamal, A.A.; Fadzli, S.A.; Lananan, F.; Abd Ghani, A.F. Coral Reefs 3D Mapping using Low Cost Autonomous Water Surface Vehicle. Int. J. Appl. Eng. Res. 2017, 12, 14466–14470. [Google Scholar]
- Burt, J.A.; Camp, E.F.; Enochs, I.C.; Johansen, J.L.; Morgan, K.M.; Riegl, B.; Hoey, A.S. Insights from extreme coral reefs in a changing world. Coral Reefs 2020, 39, 495–507. [Google Scholar] [CrossRef]
- Mohammed, J.S. Applications of 3D printing technologies in oceanography. Methods Oceanogr. 2016, 17, 97–117. [Google Scholar] [CrossRef]
- Wake, D.B.; Vredenburg, V.T. Colloquium paper: Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl. Acad. Sci. USA 2008, 105, 11466–11473. [Google Scholar] [CrossRef]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef]
- McCallum, M.L. Vertebrate biodiversity losses point to a sixth mass extinction. Biodivers. Conserv. 2015, 24, 2497–2519. [Google Scholar] [CrossRef]
- RCT. The Evolutionary Survival of Coral Reefs. Available online: https://conservation.reefcause.com/the-evolutionary-survival-of-coral-reefs/ (accessed on 21 November 2022).
- Cahill, A.E.; Aiello-Lammens, M.E.; Fisher-Reid, M.C.; Hua, X.; Karanewsky, C.J.; Ryu, H.Y.; Sbeglia, G.C.; Spagnolo, F.; Waldron, J.B.; Warsi, O.; et al. How does climate change cause extinction? Proc. Biol. Sci. 2013, 280, 20121890. [Google Scholar] [CrossRef]
- Munstermann, M.J.; Heim, N.A.; McCauley, D.J.; Payne, J.L.; Upham, N.S.; Wang, S.C.; Knope, M.L. A global ecological signal of extinction risk in terrestrial vertebrates. Conserv. Biol. 2022, 36, e13852. [Google Scholar] [CrossRef]
- Spalding, C.; Hull, P.M. Towards quantifying the mass extinction debt of the Anthropocene. Proc. R. Soc. B Biol. Sci. 2021, 288, 20202332. [Google Scholar] [CrossRef]
- Da Silva, L.B.; Oliveira, G.L.; Frederico, R.G.; Loyola, R.; Zacarias, D.; Ribeiro, B.R.; Mendes-Oliveira, A.C. How future climate change and deforestation can drastically affect the species of monkeys endemic to the eastern Amazon, and priorities for conservation. Biodivers. Conserv. 2022, 31, 971–988. [Google Scholar] [CrossRef]
- Kleypas, J.; Allemand, D.; Anthony, K.; Baker, A.C.; Beck, M.W.; Hale, L.Z.; Hilmi, N.; Hoegh-Guldberg, O.; Hughes, T.; Kaufman, L.; et al. Designing a blueprint for coral reef survival. Biol. Conserv. 2021, 257, 109107. [Google Scholar] [CrossRef]
- Pandolfi, J.M.; Connolly, S.R.; Marshall, D.J.; Cohen, A.L. Projecting Coral Reef Futures Under Global Warming and Ocean Acidification. Science 2011, 333, 418–422. [Google Scholar] [CrossRef]
- Keegan, L.; White, R.; Macinnis-Ng, C. Current knowledge and potential impacts of climate change on New Zealand’s biological heritage. N. Z. J. Ecol. 2022, 46, 1–24. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral Reefs Under Rapid Climate Change and Ocean Acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- Rull, V. Biodiversity crisis or sixth mass extinction? Does the current anthropogenic biodiversity crisis really qualify as a mass extinction? Does the current anthropogenic biodiversity crisis really qualify as a mass extinction? EMBO Rep. 2022, 23, e54193. [Google Scholar] [CrossRef]
- Cowie, R.H.; Bouchet, P.; Fontaine, B. The Sixth Mass Extinction: Fact, fiction or speculation? Biol. Rev. Camb. Philos. Soc. 2022, 97, 640–663. [Google Scholar] [CrossRef]
- Rice, M.M.; Ezzat, L.; Burkepile, D.E. Corallivory in the Anthropocene: Interactive Effects of Anthropogenic Stressors and Corallivory on Coral Reefs. Front. Mar. Sci. 2019, 5, 525. [Google Scholar] [CrossRef]
- Clementi, G.M.; Babcock, E.A.; Valentin-Albanese, J.; Bond, M.E.; Flowers, K.I.; Heithaus, M.R.; Whitman, E.R.; Van Zinnicq Bergmann, M.P.M.; Guttridge, T.L.; O’Shea, O.R.; et al. Anthropogenic pressures on reef-associated sharks in jurisdictions with and without directed shark fishing. Mar. Ecol. Prog. Ser. 2021, 661, 175–186. [Google Scholar] [CrossRef]
- Hughes, T.P.; Anderson, K.D.; Connolly, S.R.; Heron, S.F.; Kerry, J.T.; Lough, J.M.; Baird, A.H.; Baum, J.K.; Berumen, M.L.; Bridge, T.C.; et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 2018, 359, 80–83. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Riahi, K.; Calvin, K.; Dellink, R.; Emmerling, J.; Fujimori, S.; Kc, S.; Kriegler, E.; O’Neill, B. The Shared Socio-economic Pathways: Trajectories for human development and global environmental change. Glob. Environ. Chang. 2017, 42, 148–152. [Google Scholar] [CrossRef]
- Cheng, L.; Abraham, J.; Hausfather, Z.; Trenberth, K.E. How fast are the oceans warming? Science 2019, 363, 128–129. [Google Scholar] [CrossRef]
- Doney, S.C.; Fabry, V.J.; Feely, R.A.; Kleypas, J.A. Ocean Acidification: The Other CO2 Problem. Annu. Rev. Mar. Sci. 2009, 1, 169–192. [Google Scholar] [CrossRef]
- Cornwall, C.E.; Comeau, S.; Kornder, N.A.; Perry, C.T.; van Hooidonk, R.; DeCarlo, T.M.; Pratchett, M.S.; Anderson, K.D.; Browne, N.; Carpenter, R.; et al. Global declines in coral reef calcium carbonate production under ocean acidification and warming. Biol. Sci. 2021, 118, e2015265118. [Google Scholar] [CrossRef]
- Riebesell, U.; Gattuso, J.-P. Lessons learned from ocean acidification research. Nat. Clim. Chang. 2014, 5, 12–14. [Google Scholar] [CrossRef]
- Goreau, T.J.; Hayes, R.L. Global change in ocean circulation from satellite sea surface temperature records: Implications for the future of coral-reefs, fisheries, and climate change. In Proceedings of the Oceans 2003. Celebrating the Past, Teaming Toward the Future (IEEE Cat. No.03CH37492), San Diego, CA, USA, 22–26 September 2003; p. 754. [Google Scholar]
- Dietzel, A.; Connolly, S.R.; Hughes, T.P.; Bode, M. The spatial footprint and patchiness of large-scale disturbances on coral reefs. Glob. Chang. Biol. 2021, 27, 4825–4838. [Google Scholar] [CrossRef]
- Baird, M.E.; Mongin, M.; Skerratt, J.; Margvelashvili, N.; Tickell, S.; Steven, A.D.; Robillot, C.; Ellis, R.; Waters, D.; Kaniewska, P.; et al. Impact of catchment-derived nutrients and sediments on marine water quality on the Great Barrier Reef: An application of the eReefs marine modelling system. Mar. Pollut. Bull. 2021, 167, 112297. [Google Scholar] [CrossRef]
- Kjerfve, B.; McField, M.; Thattai, D.; Giró, A. Coral reef health in the Gulf of Honduras in relation to fluvial runoff, hurricanes, and fishing pressure. Mar. Pollut. Bull. 2021, 172, 112865. [Google Scholar] [CrossRef]
- Tay, C.; Lindsey, E.O.; Chin, S.T.; McCaughey, J.W.; Bekaert, D.; Nguyen, M.; Hua, H.; Manipon, G.; Karim, M.; Horton, B.P.; et al. Sea-level rise from land subsidence in major coastal cities. Nat. Sustain. 2022, 5, 1049–1057. [Google Scholar] [CrossRef]
- Field, M.E.; Ogston, A.S.; Storlazzi, C.D. Rising sea level may cause decline of fringing coral reefs. Eos Trans. Am. Geophys. Union 2011, 92, 273–274. [Google Scholar] [CrossRef]
- Woodroffe, C.D.; Webster, J.M. Coral reefs and sea-level change. Mar. Geol. 2014, 352, 248–267. [Google Scholar] [CrossRef]
- Godoy, M.D.P.; Lacerda, L.D.d. Mangroves Response to Climate Change: A Review of Recent Findings on Mangrove Extension and Distribution. An. Da Acad. Bras. De Ciências 2015, 87, 651–667. [Google Scholar] [CrossRef]
- Rodrigues, E.; Cohen, M.C.L.; Liu, K.-b.; Pessenda, L.C.R.; Yao, Q.; Ryu, J.; Rossetti, D.; de Souza, A.; Dietz, M. The effect of global warming on the establishment of mangroves in coastal Louisiana during the Holocene. Geomorphology 2021, 381, 107648. [Google Scholar] [CrossRef]
- Hassan, I.; Musa, R.M.; Latiff Azmi, M.N.; Razali Abdullah, M.; Yusoff, S.Z. Analysis of climate change disinformation across types, agents and media platforms. Inf. Dev. 2023, 29, 5406–5414. [Google Scholar] [CrossRef]
- Zhou, Z.; Goh, Y.M.; Li, Q. Overview and analysis of safety management studies in the construction industry. Saf. Sci. 2015, 72, 337–350. [Google Scholar] [CrossRef]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to conduct a bibliometric analysis: An overview and guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Ellegaard, O.; Wallin, J.A. The bibliometric analysis of scholarly production: How great is the impact? Scientometrics 2015, 105, 1809–1831. [Google Scholar] [CrossRef]
- Li, W.; Zhao, Y. Bibliometric analysis of global environmental assessment research in a 20-year period. Environ. Impact Assess. Rev. 2015, 50, 158–166. [Google Scholar] [CrossRef]
- Van Nunen, K.; Li, J.; Reniers, G.; Ponnet, K. Bibliometric analysis of safety culture research. Saf. Sci. 2018, 108, 248–258. [Google Scholar] [CrossRef]
- Merigó, J.M.; Yang, J.-B. A bibliometric analysis of operations research and management science. Omega 2017, 73, 37–48. [Google Scholar] [CrossRef]
- Synnestvedt, M.B.; Chen, C.; Holmes, J.H. CiteSpace II: Visualization and knowledge discovery in bibliographic databases. In Proceedings of the AMIA Annual Symposium Proceedings, Washington, DC, USA, 22–26 October 2005; pp. 724–728. [Google Scholar]
- Ping, Q.; He, J.; Chen, C. How many ways to use CiteSpace? A study of user interactive events over 14 months. J. Assoc. Inf. Sci. Technol. 2017, 68, 1234–1256. [Google Scholar] [CrossRef]
- Chen, C. How to Use CiteSpace (6.1.R2); Lean Publishing: Victoria, BC, Canada, 2022; p. 137. [Google Scholar]
- Azra, M.N.; Aaqillah-Amr, M.A.; Ikhwanuddin, M.; Ma, H.; Waiho, K.; Ostrensky, A.; Tavares, C.P.d.S.; Abol-Munafi, A.B. Effects of climate-induced water temperature changes on the life history of brachyuran crabs. Rev. Aquac. 2020, 12, 1211–1216. [Google Scholar] [CrossRef]
- Mohan, V. Mapping of Coral Reef Research Literature: A Global Perspective. Ph.D. Thesis, Annamalai University, Annamalainagar, India, 2007. [Google Scholar]
- Clarivate. Web of Science: Emerging Sources Citation Index. Available online: https://clarivate.com/webofsciencegroup/solutions/webofscience-esci/ (accessed on 10 January 2023).
- Walpole, L.C.; Hadwen, W.L. Extreme events, loss, and grief—An evaluation of the evolving management of climate change threats on the Great Barrier Reef. Ecol. Soc. 2022, 27, 37. [Google Scholar] [CrossRef]
- CoralCOE. Terry Hughes, Emeritus Professor. Available online: https://www.coralcoe.org.au/legacy/person/terry-hughes (accessed on 24 November 2022).
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.C.; Kleypas, J.; et al. Climate Change, Human Impacts, and the Resilience of Coral Reefs. Science 2003, 301, 929–933. [Google Scholar] [CrossRef]
- CoralCOE. Ove Hoegh-Guldberg, Professor. Available online: https://www.coralcoe.org.au/legacy/person/ove-hoegh-guldberg (accessed on 24 November 2022).
- AIMS. Dr. Katharina Fabricius, Senior Principle Scientist. Available online: https://www.aims.gov.au/about/our-people/dr-katharina-fabricius (accessed on 24 November 2022).
- Sunday, J.M.; Fabricius, K.E.; Kroeker, K.J.; Anderson, K.M.; Brown, N.E.; Barry, J.P.; Connell, S.D.; Dupont, S.; Gaylord, B.; Hall-Spencer, J.M.; et al. Ocean acidification can mediate biodiversity shifts by changing biogenic habitat. Nat. Clim. Chang. 2017, 7, 81–85. [Google Scholar] [CrossRef]
- Gaylord, B.; Kroeker, K.J.; Sunday, J.M.; Anderson, K.M.; Barry, J.P.; Brown, N.E.; Connell, S.D.; Dupont, S.; Fabricius, K.E.; Hall-Spencer, J.M. Ocean acidification through the lens of ecological theory. Ecology 2015, 96, 3–15. [Google Scholar] [CrossRef]
- Connell, S.D.; Kroeker, K.J.; Fabricius, K.E.; Kline, D.I.; Russell, B.D. The other ocean acidification problem: CO2 as a resource among competitors for ecosystem dominance. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120442. [Google Scholar] [CrossRef] [PubMed]
- Fabricius, K.; De’ath, G.; Noonan, S.; Uthicke, S. Ecological effects of ocean acidification and habitat complexity on reef-associated macroinvertebrate communities. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132479. [Google Scholar] [CrossRef]
- Fabricius, K.E.; De’ath, G. Identifying Ecological Change and its Causes: A Case Study on Coral Reefs. Ecol. Appl. 2004, 14, 1448–1465. [Google Scholar] [CrossRef]
- Fabricius, K.E.; Cooper, T.F.; Humphrey, C.; Uthicke, S.; De’ath, G.; Davidson, J.; LeGrand, H.; Thompson, A.; Schaffelke, B. A bioindicator system for water quality on inshore coral reefs of the Great Barrier Reef. Mar. Pollut. Bull. 2012, 65, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Fabricius, K.E. Effects of terrestrial runoff on the ecology of corals and coral reefs: Review and synthesis. Mar. Pollut. Bull. 2005, 50, 125–146. [Google Scholar] [CrossRef]
- Fabricius, K.E.; Langdon, C.; Uthicke, S.; Humphrey, C.; Noonan, S.; De’ath, G.; Okazaki, R.; Muehllehner, N.; Glas, M.S.; Lough, J.M. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Chang. 2011, 1, 165–169. [Google Scholar] [CrossRef]
- Glynn, P.W. EL NIÑO—SOUTHERN OSCILLATION 1982–1983: NEARSHORE POPULATION, COMMUNITY, AND ECOSYSTEM RESPONSES. Annu. Rev. Ecol. Syst. 1988, 19, 309–346. [Google Scholar] [CrossRef]
- CoralCOE. Tim McClanahan, Senior Conservation Zoologist. Available online: https://www.coralcoe.org.au/legacy/person/tim-mcclanahan (accessed on 24 November 2022).
- Cinner, J.E.; Huchery, C.; MacNeil, M.A.; Graham, N.A.J.; McClanahan, T.R.; Maina, J.; Maire, E.; Kittinger, J.N.; Hicks, C.C.; Mora, C.; et al. Bright spots among the world’s coral reefs. Nature 2016, 535, 416–419. [Google Scholar] [CrossRef]
- CoralCOE. Peter Mumby, Professor. Available online: https://www.coralcoe.org.au/legacy/person/peter-mumby (accessed on 24 November 2022).
- Mumby, P.J.; Hastings, A.; Edwards, H.J. Thresholds and the resilience of Caribbean coral reefs. Nature 2007, 450, 98–101. [Google Scholar] [CrossRef]
- JCU. Prof. David Bellwood, Marine & Aquaculture Sciences. Available online: https://research.jcu.edu.au/portfolio/david.bellwood/ (accessed on 10 January 2023).
- Bellwood, D.R.; Hoey, A.S.; Choat, J.H. Limited functional redundancy in high diversity systems: Resilience and ecosystem function on coral reefs. Ecol. Lett. 2003, 6, 281–285. [Google Scholar] [CrossRef]
- Hughes, T.P.; Rodrigues, M.J.; Bellwood, D.R.; Ceccarelli, D.; Hoegh-Guldberg, O.; McCook, L.; Moltschaniwskyj, N.; Pratchett, M.S.; Steneck, R.S.; Willis, B. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr. Biol. 2007, 17, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Anthony, K.R.; Kline, D.I.; Diaz-Pulido, G.; Dove, S.; Hoegh-Guldberg, O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. USA 2008, 105, 17442–17446. [Google Scholar] [CrossRef] [PubMed]
- Crawley, A.; Kline, D.I.; Dunn, S.; Anthony, K.E.N.; Dove, S. The effect of ocean acidification on symbiont photorespiration and productivity in Acropora formosa. Glob. Chang. Biol. 2010, 16, 851–863. [Google Scholar] [CrossRef]
- Anthony, K.R.; Maynard, J.A.; DIAZ-PULIDO, G.; Mumby, P.J.; Marshall, P.A.; Cao, L.; Hoegh-Guldberg, O. Ocean acidification and warming will lower coral reef resilience. Glob. Chang. Biol. 2011, 17, 1798–1808. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Bruno, J.F. The Impact of Climate Change on the World’s Marine Ecosystems. Science 2010, 328, 1523–1528. [Google Scholar] [CrossRef]
- Brown, B.E. Coral bleaching: Causes and consequences. Coral Reefs 1997, 16, S129–S138. [Google Scholar] [CrossRef]
- U-Miami. Andrew Baker, Professor. Available online: https://people.miami.edu/profile/93713336b906f39cffdc3f5699a72e54 (accessed on 10 January 2023).
- De’ath, G.; Fabricius, K.E.; Sweatman, H.; Puotinen, M. The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl. Acad. Sci. USA 2012, 109, 17995–17999. [Google Scholar] [CrossRef]
- Nerptropical. Dr. Ray Berkelmans. Available online: http://www.nerptropical.edu.au/people/ray-berkelmans (accessed on 10 January 2023).
- Kleypas, J.A.; Buddemeier, R.W.; Archer, D.; Gattuso, J.-P.; Langdon, C.; Opdyke, B.N. Geochemical Consequences of Increased Atmospheric Carbon Dioxide on Coral Reefs. Science 1999, 284, 118–120. [Google Scholar] [CrossRef]
- Kleypas, J.; Yates, K. Coral Reefs and Ocean Acidification. Oceanography 2009, 22, 108–117. [Google Scholar] [CrossRef]
- LU. Nick Graham. Available online: https://www.lancaster.ac.uk/lec/about-us/people/nick-graham (accessed on 10 January 2023).
- Lesser, M.P. Oxidative Stress in Marine Environments: Biochemistry and Physiological Ecology. Annu. Rev. Physiol. 2006, 68, 253–278. [Google Scholar] [CrossRef]
- Loya, Y. The Coral Reefs of Eilat—Past, Present and Future: Three Decades of Coral Community Structure Studies. In Coral Health and Disease; Rosenberg, E., Loya, Y., Eds.; Springer: Berlin, Germany, 2004; pp. 1–34. [Google Scholar]
- Wild, C.; Hoegh-Guldberg, O.; Naumann, M.S.; Colombo-Pallotta, M.F.; Ateweberhan, M.; Fitt, W.K.; Iglesias-Prieto, R.; Palmer, C.; Bythell, J.C.; Ortiz, J.-C.; et al. Climate change impedes scleractinian corals as primary reef ecosystem engineers. Mar. Freshw. Res. 2011, 62, 205–215. [Google Scholar] [CrossRef]
- Gates, R.D.; Edmunds, P.J. The Physiological Mechanisms of Acclimatization in Tropical Reef Corals. Am. Zool. 1999, 39, 30–43. [Google Scholar] [CrossRef]
- Gardner, T.A.; Côté, I.M.; Gill, J.A.; Grant, A.; Watkinson, A.R. Long-Term Region-Wide Declines in Caribbean Corals. Science 2003, 301, 958–960. [Google Scholar] [CrossRef]
- Chen, C.; Morris, S. Visualizing evolving networks: Minimum spanning trees versus pathfinder networks. In Proceedings of the IEEE Symposium on Information Visualization 2003 (IEEE Cat. No. 03TH8714), Seattle, Washington, DC, USA, 19–21 October 2003; pp. 67–74. [Google Scholar]
- Zhong, S.; Chen, R.; Song, F.; Xu, Y. Knowledge Mapping of Carbon Footprint Research in a LCA Perspective: A Visual Analysis Using CiteSpace. Processes 2019, 7, 818. [Google Scholar] [CrossRef]
- Olawumi, T.O.; Chan, D.W.M. A scientometric review of global research on sustainability and sustainable development. J. Clean. Prod. 2018, 183, 231–250. [Google Scholar] [CrossRef]
- Yang, H.; Shao, X.; Wu, M. A Review on Ecosystem Health Research: A Visualization Based on CiteSpace. Sustainability 2019, 11, 4908. [Google Scholar] [CrossRef]
- Gilmour, J.P.; Cook, K.L.; Ryan, N.M.; Puotinen, M.L.; Green, R.H.; Shedrawi, G.; Hobbs, J.-P.A.; Thomson, D.P.; Babcock, R.C.; Buckee, J.; et al. The state of Western Australia’s coral reefs. Coral Reefs 2019, 38, 651–667. [Google Scholar] [CrossRef]
- Done, T.J. Phase shifts in coral reef communities and their ecological significance. Hydrobiologia 1992, 247, 121–132. [Google Scholar] [CrossRef]
- Cruz, I.C.S.; Loiola, M.; Albuquerque, T.; Reis, R.; Nunes, J.d.A.C.C.; Reimer, J.D.; Mizuyama, M.; Kikuchi, R.K.P.; Creed, J.C. Effect of phase shift from corals to Zoantharia on reef fish assemblages. PLoS ONE 2015, 10, e0116944. [Google Scholar] [CrossRef]
- Brodie, J.E.; Kroon, F.J.; Schaffelke, B.; Wolanski, E.C.; Lewis, S.E.; Devlin, M.J.; Bohnet, I.C.; Bainbridge, Z.T.; Waterhouse, J.; Davis, A.M. Terrestrial pollutant runoff to the Great Barrier Reef: An update of issues, priorities and management responses. Mar. Pollut. Bull. 2012, 65, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P. Catastrophes, Phase Shifts, and Large-Scale Degradation of a Caribbean Coral Reef. Science 1994, 265, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- McManus, J.W.; Polsenberg, J.F. Coral–algal phase shifts on coral reefs: Ecological and environmental aspects. Prog. Oceanogr. 2004, 60, 263–279. [Google Scholar] [CrossRef]
- Idjadi, J.A.; Lee, S.C.; Bruno, J.F.; Precht, W.F.; Allen-Requa, L.; Edmunds, P.J. Rapid phase-shift reversal on a Jamaican coral reef. Coral Reefs 2006, 25, 209–211. [Google Scholar] [CrossRef]
- Norström, A.V.; Nyström, M.; Lokrantz, J.; Folke, C. Alternative states on coral reefs: Beyond coral–macroalgal phase shifts. Mar. Ecol. Prog. Ser. 2009, 376, 295–306. [Google Scholar] [CrossRef]
- Graham, N.A.J.; Bellwood, D.R.; Cinner, J.E.; Hughes, T.P.; Norström, A.V.; Nyström, M. Managing resilience to reverse phase shifts in coral reefs. Front. Ecol. Environ. 2013, 11, 541–548. [Google Scholar] [CrossRef]
- Crisp, S.K.; Tebbett, S.B.; Bellwood, D.R. A critical evaluation of benthic phase shift studies on coral reefs. Mar. Environ. Res. 2022, 178, 105667. [Google Scholar] [CrossRef]
- Beisner, B.E.; Haydon, D.T.; Cuddington, K. Alternative stable states in ecology. Front. Ecol. Environ. 2003, 1, 376–382. [Google Scholar] [CrossRef]
- Fong, C.R.; Gaynus, C.J.; Carpenter, R.C. Complex interactions among stressors evolve over time to drive shifts from short turfs to macroalgae on tropical reefs. Ecosphere 2020, 11, e3130. [Google Scholar] [CrossRef]
- Szmant, A.M. Nutrient enrichment on coral reefs: Is it a major cause of coral reef decline? Estuaries 2002, 25, 743–766. [Google Scholar] [CrossRef]
- Wolanski, E.; Richmond, R.; McCook, L.; Sweatman, H. Mud, Marine Snow and Coral Reefs. Am. Sci. 2003, 91, 44. [Google Scholar] [CrossRef]
- Bates, N.R. Twenty Years of Marine Carbon Cycle Observations at Devils Hole Bermuda Provide Insights into Seasonal Hypoxia, Coral Reef Calcification, and Ocean Acidification. Front. Mar. Sci. 2017, 4, 36. [Google Scholar] [CrossRef]
- Gattuso, J.P.; Magnan, A.; Billé, R.; Cheung, W.W.L.; Howes, E.L.; Joos, F.; Allemand, D.; Bopp, L.; Cooley, S.R.; Eakin, C.M.; et al. Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science 2015, 349, 4722. [Google Scholar] [CrossRef]
- Hill, T.S.; Hoogenboom, M.O. The indirect effects of ocean acidification on corals and coral communities. Coral Reefs 2022, 41, 1557–1583. [Google Scholar] [CrossRef]
- Zeebe, R.E. History of Seawater Carbonate Chemistry, Atmospheric CO2, and Ocean Acidification. Annu. Rev. Earth Planet. Sci. 2012, 40, 141–165. [Google Scholar] [CrossRef]
- IPCC. Climate Chang. 2014: Synthesis Report; Pachauri, R.K., Meyer, L., Eds.; Intergovermental Panel on Climate Change: Geneva, Switzerland, 2015; p. 151. [Google Scholar]
- Orr, J.C.; Fabry, V.J.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F.; et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef]
- Riebesell, U.; Zondervan, I.; Rost, B.; Tortell, P.D.; Zeebe, R.E.; Morel, F.M.M. Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature 2000, 407, 364–367. [Google Scholar] [CrossRef]
- Kleypas, J.A.; Buddemeier, R.W.; Gattuso, J.-P. The future of coral reefs in an age of global change. Int. J. Earth Sci. 2000, 90, 426–437. [Google Scholar] [CrossRef]
- Leclercq, N.I.c.; Gattuso, J.E.A.N.P.; Jaubert, J.E.A.N. CO2 partial pressure controls the calcification rate of a coral community. Glob. Chang. Biol. 2000, 6, 329–334. [Google Scholar] [CrossRef]
- Hoeksema, B.W. Delineation of the Indo-Malayan Centre of Maximum Marine Biodiversity: The Coral Triangle. In Biogeography, Time, and Place: Distributions, Barriers, and Islands; Renema, W., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 117–178. [Google Scholar]
- Veron, J.E.N.; Devantier, L.M.; Turak, E.; Green, A.L.; Kininmonth, S.; Stafford-Smith, M.; Peterson, N. Delineating the Coral Triangle. Galaxea J. Coral Reef Stud. 2009, 11, 91–100. [Google Scholar] [CrossRef]
- Lam, V.W.Y.; Chavanich, S.; Djoundourian, S.; Dupont, S.; Gaill, F.; Holzer, G.; Isensee, K.; Katua, S.; Mars, F.; Metian, M.; et al. Dealing with the effects of ocean acidification on coral reefs in the Indian Ocean and Asia. Reg. Stud. Mar. Sci. 2019, 28, 100560. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Hoegh-Guldberg, H.; Veron, J.E.; Green, A.; Gomez, E.D.; Lough, J.; King, M.; Ambariyanto, D.; Hansen, L.; Cinner, J. The Coral Triangle and Climate Change: Ecosystems, People and Societies at Risk; Hoegh-Guldberg, O., Hoegh-Guldberg, H., Veron, J.E., Eds.; WWF Australia: Sydney, Australia, 2009. [Google Scholar]
- Agostini, S.; Harvey, B.P.; Wada, S.; Kon, K.; Milazzo, M.; Inaba, K.; Hall-Spencer, J.M. Ocean acidification drives community shifts towards simplified non-calcified habitats in a subtropical− temperate transition zone. Sci. Rep. 2018, 8, 11354. [Google Scholar] [CrossRef] [PubMed]
- Speers, A.E.; Besedin, E.Y.; Palardy, J.E.; Moore, C. Impacts of climate change and ocean acidification on coral reef fisheries: An integrated ecological–economic model. Ecol. Econ. 2016, 128, 33–43. [Google Scholar] [CrossRef]
- Narita, D.; Rehdanz, K.; Tol, R.S.J. Economic costs of ocean acidification: A look into the impacts on global shellfish production. Clim. Chang. 2012, 113, 1049–1063. [Google Scholar] [CrossRef]
- Jones, G.P.; McCormick, M.I.; Srinivasan, M.; Eagle, J.V. Coral decline threatens fish biodiversity in marine reserves. Proc. Natl. Acad. Sci. USA 2004, 101, 8251–8253. [Google Scholar] [CrossRef]
- Simpson, S.D.; Munday, P.L.; Wittenrich, M.L.; Manassa, R.; Dixson, D.L.; Gagliano, M.; Yan, H.Y. Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol. Lett. 2011, 7, 917–920. [Google Scholar] [CrossRef]
- Osman, E.O.; Suggett, D.J.; Voolstra, C.R.; Pettay, D.T.; Clark, D.R.; Pogoreutz, C.; Sampayo, E.M.; Warner, M.E.; Smith, D.J. Coral microbiome composition along the northern Red Sea suggests high plasticity of bacterial and specificity of endosymbiotic dinoflagellate communities. Microbiome 2020, 8, 8. [Google Scholar] [CrossRef]
- Berman, T.; Paldor, N.; Brenner, S. Annual SST cycle in the Eastern Mediterranean, Red Sea and Gulf of Elat. Geophys. Res. Lett. 2003, 30, 65.1–65.4. [Google Scholar] [CrossRef]
- Furby, K.A.; Bouwmeester, J.; Berumen, M.L. Susceptibility of central Red Sea corals during a major bleaching event. Coral Reefs 2013, 32, 505–513. [Google Scholar] [CrossRef]
- Fine, M.; Cinar, M.; Voolstra, C.R.; Safa, A.; Rinkevich, B.; Laffoley, D.; Hilmi, N.; Allemand, D. Coral reefs of the Red Sea—Challenges and potential solutions. Reg. Stud. Mar. Sci. 2019, 25, 100498. [Google Scholar] [CrossRef]
- Cantin, N.E.; Cohen, A.L.; Karnauskas, K.B.; Tarrant, A.M.; McCorkle, D.C. Ocean Warming Slows Coral Growth in the Central Red Sea. Science 2010, 329, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Fine, M.; Gildor, H.; Genin, A. A coral reef refuge in the Red Sea. Glob. Chang. Biol. 2013, 19, 3640–3647. [Google Scholar] [CrossRef] [PubMed]
- Barshis, D.J.; Ladner, J.T.; Oliver, T.A.; Seneca, F.O.; Traylor-Knowles, N.; Palumbi, S.R. Genomic basis for coral resilience to climate change. Proc. Natl. Acad. Sci. USA 2013, 110, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Grimsditch, G.D.; Salm, R.V. Coral Reef Resilience and Resistance to Bleaching; IUCN: Gland, Switzerland, 2005; p. 50. [Google Scholar]
- Kleinhaus, K.; Al-Sawalmih, A.; Barshis, D.J.; Genin, A.; Grace, L.N.; Hoegh-Guldberg, O.; Loya, Y.; Meibom, A.; Osman, E.O.; Ruch, J.-D. Science, diplomacy, and the Red Sea’s unique coral reef: It’s time for action. Front. Mar. Sci. 2020, 7, 90. [Google Scholar] [CrossRef]
- Rich, W.A.; Carvalho, S.; Berumen, M.L. Coral bleaching due to cold stress on a central Red Sea reef flat. Ecol. Evol. 2022, 12, e9450. [Google Scholar] [CrossRef]
- Monroe, A.A.; Ziegler, M.; Roik, A.; Röthig, T.; Hardenstine, R.S.; Emms, M.A.; Jensen, T.; Voolstra, C.R.; Berumen, M.L. In situ observations of coral bleaching in the central Saudi Arabian Red Sea during the 2015/2016 global coral bleaching event. PLoS ONE 2018, 13, e0195814. [Google Scholar] [CrossRef]
- Spencer, T. Potentialities, uncertainties and complexities in the response of coral reefs to future sea-level rise. Earth Surf. Process. Landf. 1995, 20, 49–64. [Google Scholar] [CrossRef]
- Gischler, E.; Hudson, J.H. Holocene development of the Belize Barrier Reef. Sediment. Geol. 2004, 164, 223–236. [Google Scholar] [CrossRef]
- Lawman, A.E.; Dee, S.G.; DeLong, K.L.; Correa, A.M.S. Rates of Future Climate Change in the Gulf of Mexico and the Caribbean Sea: Implications for Coral Reef Ecosystems. J. Geophys. Res. Biogeosci. 2022, 127, e2022jg006999. [Google Scholar] [CrossRef]
- Donner, S.D.; Knutson, T.R.; Oppenheimer, M. Model-based assessment of the role of human-induced climate change in the 2005 Caribbean coral bleaching event. Proc. Natl. Acad. Sci. USA 2007, 104, 5483–5488. [Google Scholar] [CrossRef]
- Miller, J.; Muller, E.; Rogers, C.; Waara, R.; Atkinson, A.; Whelan, K.R.T.; Patterson, M.; Witcher, B. Coral disease following massive bleaching in 2005 causes 60% decline in coral cover on reefs in the US Virgin Islands. Coral Reefs 2009, 28, 925–937. [Google Scholar] [CrossRef]
- Hopley, D.; Smithers, S.G.; Parnell, K. The Geomorphology of the Great Barrier Reef; Cambridge University Press: New York, NY, USA, 2007; p. 548. [Google Scholar]
- Smith, G.; Claire, S. Ocean Temperature Outlooks—Coral Bleaching Risk: Great Barrier Reef and Australian waters; Bureau of Meteorology, Australia: Victoria, Australia, 2020. [Google Scholar]
- Reaser, J.K.; Pomerance, R.; Thomas, P.O. Coral Bleaching and Global Climate Change: Scientific Findings and Policy Recommendations. Conserv. Biol. 2000, 14, 1500–1511. [Google Scholar] [CrossRef]
- Douglas, A.E. Coral bleaching––how and why? Mar. Pollut. Bull. 2003, 46, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Stat, M.; Carter, D.; Hoeghguldberg, O. The evolutionary history of Symbiodinium and scleractinian hosts—Symbiosis, diversity, and the effect of climate change. Perspect. Plant Ecol. Evol. Syst. 2006, 8, 23–43. [Google Scholar] [CrossRef]
- Gordon, B.R.; Leggat, W. Symbiodinium-invertebrate symbioses and the role of metabolomics. Mar. Drugs 2010, 8, 2546–2568. [Google Scholar] [CrossRef]
- Fujise, L.; Yamashita, H.; Suzuki, G.; Sasaki, K.; Liao, L.M.; Koike, K. Moderate Thermal Stress Causes Active and Immediate Expulsion of Photosynthetically Damaged Zooxanthellae (Symbiodinium) from Corals. PLoS ONE 2014, 9, e114321. [Google Scholar] [CrossRef]
- Maire, J.; van Oppen, M.J.H. A role for bacterial experimental evolution in coral bleaching mitigation? Trends Microbiol. 2022, 30, 217–228. [Google Scholar] [CrossRef]
- Kushmaro, A.; Loya, Y.; Fine, M.; Rosenberg, E. Bacterial infection and coral bleaching. Nature 1996, 380, 396. [Google Scholar] [CrossRef]
- Lesser, M.P. Coral Bleaching: Causes and Mechanisms. In Coral Reefs: An Ecosystem in Transition; Dubinsky, Z., Stambler, N., Eds.; Springer: New York, NY, USA, 2010; pp. 405–419. [Google Scholar]
- Coles, S.L.; Jokiel, P.L. Effects of Salinity on Coral Reefs. In Pollution in Tropical Aquatic Systems; Connell, D.W., Hawker, D.W., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 147–166. [Google Scholar]
- Bessell-Browne, P.; Negri, A.P.; Fisher, R.; Clode, P.L.; Jones, R. Cumulative impacts: Thermally bleached corals have reduced capacity to clear deposited sediment. Sci. Rep. 2017, 7, 2716. [Google Scholar] [CrossRef]
- Donovan, M.K.; Adam, T.C.; Shantz, A.A.; Speare, K.E.; Munsterman, K.S.; Rice, M.M.; Schmitt, R.J.; Holbrook, S.J.; Burkepile, D.E. Nitrogen pollution interacts with heat stress to increase coral bleaching across the seascape. Proc. Natl. Acad. Sci. USA 2020, 117, 5351–5357. [Google Scholar] [CrossRef]
- Glynn, P.W. Coral reef bleaching: Facts, hypotheses and implications. Glob. Chang. Biol. 1996, 2, 495–509. [Google Scholar] [CrossRef]
- Baird, A.; Marshall, P. Mass bleaching of corals on the Great Barrier Reef. Coral Reefs 1998, 17, 376. [Google Scholar] [CrossRef]
- Rosenberg, E.; Ben-Haim, Y. Microbial diseases of corals and global warming. Environ. Microbiol. 2002, 4, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, T.; Fine, M.; Roff, G.; Hoegh-Guldberg, O. Bacteria are not the primary cause of bleaching in the Mediterranean coral Oculina patagonica. ISME J. 2008, 2, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Eakin, C.M.; Sweatman, H.P.A.; Brainard, R.E. The 2014–2017 global-scale coral bleaching event: Insights and impacts. Coral Reefs 2019, 38, 539–545. [Google Scholar] [CrossRef]
- Aronson, R.; Precht, W.; Toscano, M.; Koltes, K. The 1998 bleaching event and its aftermath on a coral reef in Belize. Mar. Biol. 2002, 141, 435–447. [Google Scholar] [CrossRef]
- Gleeson, M.W.; Strong, A.E. Applying MCSST to coral reef bleaching. Adv. Space Res. 1995, 16, 151–154. [Google Scholar] [CrossRef]
- Donner, S.D.; Skirving, W.J.; Little, C.M.; Oppenheimer, M.; Hoegh-Guldberg, O.V.E. Global assessment of coral bleaching and required rates of adaptation under climate change. Glob. Chang. Biol. 2005, 11, 2251–2265. [Google Scholar] [CrossRef] [PubMed]
- Sully, S.; Burkepile, D.E.; Donovan, M.K.; Hodgson, G.; van Woesik, R. A global analysis of coral bleaching over the past two decades. Nat. Commun. 2019, 10, 1264. [Google Scholar] [CrossRef]
- Coles, S.L.; Riegl, B.M. Thermal tolerances of reef corals in the Gulf: A review of the potential for increasing coral survival and adaptation to climate change through assisted translocation. Mar. Pollut. Bull. 2013, 72, 323–332. [Google Scholar] [CrossRef]
- Bowden-Kerby, A.; Carne, L. Thermal tolerance as a factor in Caribbean Acropora restoration. In Proceedings of the 12th International Coral Reef Symposium (ICRS), Cairns, Australia, 9–13 July 2012; pp. 1–5. [Google Scholar]
- Berkelmans, R.; van Oppen, M.J.H. The role of zooxanthellae in the thermal tolerance of corals: A ‘nugget of hope’ for coral reefs in an era of climate change. Proc. Biol. Sci. 2006, 273, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.; Loya, Y. Coral Health and Disease; Springer: Berlin, Germany, 2004. [Google Scholar]
- Squires, D.F. Neoplasia in a Coral? Science 1965, 148, 503–505. [Google Scholar] [CrossRef] [PubMed]
- Harvell, D.; Aronson, R.; Baron, N.; Connell, J.; Dobson, A.; Ellner, S.; Gerber, L.; Kim, K.; Kuris, A.; McCallum, H.; et al. The rising tide of ocean diseases: Unsolved problems and research priorities. Front. Ecol. Environ. 2004, 2, 375–382. [Google Scholar] [CrossRef]
- Lafferty, K.D.; Porter, J.W.; Ford, S.E. Are Diseases Increasing in the Ocean? Annu. Rev. Ecol. Evol. Syst. 2004, 35, 31–54. [Google Scholar] [CrossRef]
- Weil, E. Coral Reef Diseases in the Wider Caribbean. In Coral Health and Disease; Rosenberg, E., Loya, Y., Eds.; Springer: Cham, Switzerland, 2004; pp. 35–68. [Google Scholar]
- Aronson, R.B.; Precht, W.F.; Macintyre, I.G. Extrinsic control of species replacement on a Holocene reef in Belize: The role of coral disease. Coral Reefs 1998, 17, 223–230. [Google Scholar] [CrossRef]
- Aronson, R.B.; Precht, W.F. White-band disease and the changing face of Caribbean coral reefs. In The Ecology and Etiology of Newly Emerging Marine Diseases; Porter, J.W., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 25–38. [Google Scholar]
- Moriarty, T.; Leggat, W.; Huggett, M.J.; Ainsworth, T.D. Coral Disease Causes, Consequences, and Risk within Coral Restoration. Trends Microbiol. 2020, 28, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, X. Research on the Literature of Green Building Based on the Web of Science: A Scientometric Analysis in CiteSpace (2002–2018). Sustainability 2019, 11, 3716. [Google Scholar] [CrossRef]
- Brandes, U. A faster algorithm for betweenness centrality. J. Math. Sociol. 2001, 25, 163–177. [Google Scholar] [CrossRef]
- LaJeunesse, T.C.; Parkinson, J.E.; Gabrielson, P.W.; Jeong, H.J.; Reimer, J.D.; Voolstra, C.R.; Santos, S.R. Systematic Revision of Symbiodiniaceae Highlights the Antiquity and Diversity of Coral Endosymbionts. Curr. Biol. 2018, 28, 2570–2580. [Google Scholar] [CrossRef]
- Zeebe, R.E.; Wolf-Gladrow, D. CO2 in Seawater: Equilibrium, Kinetics, Isotopes; Elsevier: Amsterdam, The Netherlands, 2001; p. 149. [Google Scholar]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.; Hendriks, I.E.; Ramajo, L.; Singh, G.S.; Duarte, C.M.; Gattuso, J.-P. Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Glob. Chang. Biol. 2013, 19, 1884–1896. [Google Scholar] [CrossRef]
- Baker, A.C.; Correa, A.M.; Cunning, R. Diversity, distribution and stability of Symbiodinium in reef corals of the eastern tropical pacific. In Coral Reefs of the Eastern Tropical Pacific: Persistence and Loss in a Dynamic Environment; Glynn, P., Manzello, D., Enochs, I.C., Eds.; Springer: Miami, FL, USA, 2017; Volume 8, pp. 405–420. [Google Scholar]
- Bruno, J.F.; Selig, E.R. Regional decline of coral cover in the Indo-Pacific: Timing, extent, and subregional comparisons. PLoS ONE 2007, 2, e711. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Barnes, M.L.; Bellwood, D.R.; Cinner, J.E.; Cumming, G.S.; Jackson, J.B.C.; Kleypas, J.; van de Leemput, I.A.; Lough, J.M.; Morrison, T.H.; et al. Coral reefs in the Anthropocene. Nature 2017, 546, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Graham, N.A.J.; Jackson, J.B.C.; Mumby, P.J.; Steneck, R.S. Rising to the challenge of sustaining coral reef resilience. Trends Ecol. Amp. Evol. 2010, 25, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Heron, S.F.; Maynard, J.A.; van Hooidonk, R.; Eakin, C.M. Warming Trends and Bleaching Stress of the World’s Coral Reefs 1985–2012. Sci. Rep. 2016, 6, 38402. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, T.D.; Heron, S.F.; Ortiz, J.C.; Mumby, P.J.; Grech, A.; Ogawa, D.; Eakin, C.M.; Leggat, W. Climate change disables coral bleaching protection on the Great Barrier Reef. Science 2016, 352, 338–342. [Google Scholar] [CrossRef]
- Palumbi, S.R.; Barshis, D.J.; Traylor-Knowles, N.; Bay, R.A. Mechanisms of reef coral resistance to future climate change. Science 2014, 344, 895–898. [Google Scholar] [CrossRef]
- Jackson, J.B.C.; Donovan, M.K.; Cramer, K.L.; Lam, W. Status and Trends of Caribbean Coral Reefs: 1970–2012; Global Coral Reef Monitoring Network: Gland, Switzerland, 2014. [Google Scholar]
- Andréfouët, S.; Adjeroud, M. Chapter 38: French Polynesia. In World Seas: An Environmental Evaluation; Sheppard, C., Ed.; Elsevier: Cambridge, MA, USA, 2019; Volume 2: The Indian Ocean to the Pacific, pp. 827–854. [Google Scholar]
- Pérez-Rosales, G.; Pichon, M.; Rouzé, H.; Villéger, S.; Torda, G.; Bongaerts, P.; Carlot, J.; Parravicini, V.; Hédouin, L.; Bardout, G.; et al. Mesophotic coral ecosystems of French Polynesia are hotspots of alpha and beta generic diversity for scleractinian assemblages. Divers. Distrib. 2022, 28, 1391–1403. [Google Scholar] [CrossRef]
- Sully, S.; Hodgson, G.; van Woesik, R. Present and future bright and dark spots for coral reefs through climate change. Glob. Chang. Biol. 2022, 28, 4509–4522. [Google Scholar] [CrossRef]
- IPCC. The Ocean and Cryosphere in a Changing Climate; Cambridge University Press: New York, NY, USA, 2022; p. 716. [Google Scholar]
- Graham, T.; Idechong, N. Reconciling customary and constitutional law. Ocean Coast. Manag. 1998, 40, 143–164. [Google Scholar] [CrossRef]
- Wabnitz, C.C.C.; Cisneros-Montemayor, A.M.; Hanich, Q.; Ota, Y. Ecotourism, climate change and reef fish consumption in Palau: Benefits, trade-offs and adaptation strategies. Mar. Policy 2018, 88, 323–332. [Google Scholar] [CrossRef]
- Barnett, J. Climate Change and Food Security in the Pacific Islands. In Food Security in Small Island States; Connell, J., Lowitt, K., Eds.; Springer: Gateway East, Singapore, 2019; pp. 25–38. [Google Scholar]
- Greenstein, B.J.; Pandolfi, J.M. Escaping the heat: Range shifts of reef coral taxa in coastal Western Australia. Glob. Chang. Biol. 2007, 14, 513–528. [Google Scholar] [CrossRef]
- Precht, W.F.; Aronson, R.B. Climate flickers and range shifts of reef corals. Front. Ecol. Environ. 2004, 2, 307–314. [Google Scholar] [CrossRef]
- Yamano, H.; Sugihara, K.; Nomura, K. Rapid poleward range expansion of tropical reef corals in response to rising sea surface temperatures. Geophys. Res. Lett. 2011, 38, L04601. [Google Scholar] [CrossRef]
- Lauria, V.; Massi, D.; Fiorentino, F.; Milisenda, G.; Cillari, T. Habitat suitability mapping of the black coral Leiopathes glaberrima to support conservation of vulnerable marine ecosystems. Sci. Rep. 2021, 11, 15661. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.; Paris, C.B.; Ridgwell, A.; Hendy, E.J. Modelling dispersal and connectivity of broadcast spawning corals at the global scale. Glob. Ecol. Biogeogr. 2013, 23, 1–11. [Google Scholar] [CrossRef]
- Van Hooidonk, R.; Maynard, J.A.; Manzello, D.; Planes, S. Opposite latitudinal gradients in projected ocean acidification and bleaching impacts on coral reefs. Glob. Chang. Biol. 2013, 20, 103–112. [Google Scholar] [CrossRef]
- Muir, P.R.; Wallace, C.C.; Done, T.; Aguirre, J.D. Limited scope for latitudinal extension of reef corals. Science 2015, 348, 1135–1138. [Google Scholar] [CrossRef]
- Azra, M.N.; Noor, M.I.M.; Eales, J.; Sung, Y.Y.; Ghaffar, M.A. What evidence exists for the impact of climate change on the physiology and behaviour of important aquaculture marine crustacean species in Asia? A systematic map protocol. Environ. Evid. 2022, 11, 1–8. [Google Scholar] [CrossRef]
- Eakin, C.M.; Liu, G.; Gomez, A.M.; De la Couri, J.L.; Heron, S.F.; Skirving, W.J.; Strong, A.E. Unprecedented three years of global coral bleaching 2014–2017. Sidebar 3.1.(in state of the climate in 2017). Bull. Am. Meteorol. Soc. 2018, 99, S74–S75. [Google Scholar]
- Authority, G.B.R.M.P. 2016 Coral Bleaching Event on the Great Barrier Reef; Great Barrier Reef Marine Park Authority: Townsville, QLD, Australia, 2017; ISBN 9780995373167. [Google Scholar]
- Kim, C.J.S.; Roelfsema, C.; Dove, S.; Hoegh-Guldberg, O. The Condition of Four Coral Reefs in Timor-Leste before and after the 2016–2017 Marine Heatwave. Oceans 2022, 3, 147–171. [Google Scholar] [CrossRef]
- Yu, X.; Yu, K.; Huang, W.; Liang, J.; Qin, Z.; Chen, B.; Yao, Q.; Liao, Z. Thermal acclimation increases heat tolerance of the scleractinian coral Acropora pruinosa. Sci. Total Environ. 2020, 733, 139319. [Google Scholar] [CrossRef] [PubMed]
- Jokiel, P.L.; Coles, S.L. Response of Hawaiian and other Indo-Pacific reef corals to elevated temperature. Coral Reefs 1990, 8, 155–162. [Google Scholar] [CrossRef]
- De’ath, G.; Lough, J.M.; Fabricius, K.E. Declining coral calcification on the Great Barrier Reef. Science 2009, 323, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Anthony, K.R. Coral reefs under climate change and ocean acidification: Challenges and opportunities for management and policy. Annu. Rev. Environ. Resour. 2016, 41, 59–81. [Google Scholar] [CrossRef]
- Lough, J.M.; Anderson, K.D.; Hughes, T.P. Increasing thermal stress for tropical coral reefs: 1871–2017. Sci. Rep. 2018, 8, 6079. [Google Scholar] [CrossRef] [PubMed]
- Rodolfo-Metalpa, R.; Hoogenboom, M.O.; Rottier, C.; Ramos-Esplá, A.; Baker, A.C.; Fine, M.; Ferrier-Pagès, C. Thermally tolerant corals have limited capacity to acclimatize to future warming. Glob. Chang. Biol. 2014, 20, 3036–3049. [Google Scholar] [CrossRef]
- Kavousi, J.; Tavakoli-Kolour, P.; Mohammadizadeh, M.; Bahrami, A.; Barkhordari, A. Mass coral bleaching in the Northern Persian Gulf, 2012. Sci. Mar. 2014, 78, 397–404. [Google Scholar] [CrossRef]
- Camp, E.F.; Schoepf, V.; Mumby, P.J.; Hardtke, L.A.; Rodolfo-Metalpa, R.; Smith, D.J.; Suggett, D.J. The future of coral reefs subject to rapid climate change: Lessons from natural extreme environments. Front. Mar. Sci. 2018, 5, 4. [Google Scholar] [CrossRef]
- Weis, V.M. Cellular mechanisms of Cnidarian bleaching: Stress causes the collapse of symbiosis. J. Exp. Biol. 2008, 211, 3059–3066. [Google Scholar] [CrossRef]
- Krueger, T.; Horwitz, N.; Bodin, J.; Giovani, M.E.; Escrig, S.; Meibom, A.; Fine, M. Common reef-building coral in the Northern Red Sea resistant to elevated temperature and acidification. R. Soc. Open Sci. 2017, 4, 170038. [Google Scholar] [CrossRef]
- Evensen, N.R.; Fine, M.; Perna, G.; Voolstra, C.R.; Barshis, D.J. Remarkably high and consistent tolerance of a Red Sea coral to acute and chronic thermal stress exposures. Limnol. Oceanogr. 2021, 66, 1718–1729. [Google Scholar] [CrossRef]
- Littman, R.; Willis, B.L.; Bourne, D.G. Metagenomic analysis of the coral holobiont during a natural bleaching event on the Great Barrier Reef. Environ. Microbiol. Rep. 2011, 3, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Doni, L.; Oliveri, C.; Lasa, A.; Di Cesare, A.; Petrin, S.; Martinez-Urtaza, J.; Coman, F.; Richardson, A.; Vezzulli, L. Large-scale impact of the 2016 Marine Heatwave on the plankton-associated microbial communities of the Great Barrier Reef (Australia). Mar. Pollut. Bull. 2023, 188, 114685. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.F.; Selig, E.R.; Casey, K.S.; Page, C.A.; Willis, B.L.; Harvell, C.D.; Sweatman, H.; Melendy, A.M. Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biol. 2007, 5, e124. [Google Scholar] [CrossRef]
- Tignat-Perrier, R.; van de Water, J.A.J.M.; Guillemain, D.; Aurelle, D.; Allemand, D.; Ferrier-Pagès, C. The Effect of Thermal Stress on the Physiology and Bacterial Communities of Two Key Mediterranean Gorgonians. Appl. Environ. Microbiol. 2022, 88, e02340-21. [Google Scholar] [CrossRef]
- Hoey, A.S.; Howells, E.; Johansen, J.L.; Hobbs, J.P.A.; Messmer, V.; McCowan, D.M.; Wilson, S.K.; Pratchett, M.S. Recent advances in understanding the effects of climate change on coral reefs. Diversity 2016, 8, 12. [Google Scholar] [CrossRef]
- Feely, R.A.; Doney, S.C.; Cooley, S.R. Ocean acidification: Present conditions and future changes in a high-CO₂ world. Oceanography 2009, 22, 36–47. [Google Scholar] [CrossRef]
- Albright, R.; Langdon, C. Ocean acidification impacts multiple early life history processes of the Caribbean coral Porites astreoides. Glob. Chang. Biol. 2011, 17, 2478–2487. [Google Scholar] [CrossRef]
- Mera, H.; Bourne, D.G. Disentangling causation: Complex roles of coral-associated microorganisms in disease. Environ. Microbiol. 2018, 20, 431–449. [Google Scholar] [CrossRef]
- Ferrari, M.C.; Munday, P.L.; Rummer, J.L.; McCormick, M.I.; Corkill, K.; Watson, S.A.; Allan, B.J.M.; Meekan, M.G.; Chivers, D.P. Interactive effects of ocean acidification and rising sea temperatures alter predation rate and predator selectivity in reef fish communities. Glob. Chang. Biol. 2015, 21, 1848–1855. [Google Scholar] [CrossRef]
- Mora, C.; Wei, C.-L.; Rollo, A.; Amaro, T.; Baco, A.R.; Billett, D.; Bopp, L.; Chen, Q.; Collier, M.; Danovaro, R. Biotic and human vulnerability to projected changes in ocean biogeochemistry over the 21st century. PLoS Biol. 2013, 11, e1001682. [Google Scholar] [CrossRef] [PubMed]
- Folke, C.; Carpenter, S.; Walker, B.; Scheffer, M.; Elmqvist, T.; Gunderson, L.; Holling, C.S. Regime Shifts, Resilience, and Biodiversity in Ecosystem Management. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 557–581. [Google Scholar] [CrossRef]
- Crook, E.D.; Cohen, A.L.; Rebolledo-Vieyra, M.; Hernandez, L.; Paytan, A. Reduced calcification and lack of acclimatization by coral colonies growing in areas of persistent natural acidification. Proc. Natl. Acad. Sci. USA 2013, 110, 11044–11049. [Google Scholar] [CrossRef] [PubMed]
- Shamberger, K.E.; Cohen, A.L.; Golbuu, Y.; McCorkle, D.C.; Lentz, S.J.; Barkley, H.C. Diverse coral communities in naturally acidified waters of a Western Pacific reef. Geophys. Res. Lett. 2014, 41, 499–504. [Google Scholar] [CrossRef]
- Manzello, D.P. Ocean acidification hot spots: Spatiotemporal dynamics of the seawater CO2 system of eastern Pacific coral reefs. Limnol. Oceanogr. 2010, 55, 239–248. [Google Scholar] [CrossRef]
- McCulloch, M.; Trotter, J.; Montagna, P.; Falter, J.; Dunbar, R.; Freiwald, A.; Försterra, G.; Correa, M.L.; Maier, C.; Rüggeberg, A.; et al. Resilience of cold-water scleractinian corals to ocean acidification: Boron isotopic systematics of pH and saturation state up-regulation. Geochim. Et Cosmochim. Acta 2012, 87, 21–34. [Google Scholar] [CrossRef]
- Howells, M.; Hermann, S.; Welsch, M.; Bazilian, M.; Segerström, R.; Alfstad, T.; Gielen, D.; Rogner, H.; Fischer, G.; van Velthuizen, H.; et al. Integrated analysis of climate change, land-use, energy and water strategies. Nat. Clim. Chang. 2013, 3, 621–626. [Google Scholar] [CrossRef]
- Drollet, J.H.; Glaziou, P.; Martin, P.M.V. A study of mucus from the solitary coral Fungia fungites (Scleractinia: Fungiidae) in relation to photobiological UV adaptation. Mar. Biol. 1993, 115, 263–266. [Google Scholar] [CrossRef]
- Weston, A.J.; Dunlap, W.C.; Shick, J.M.; Klueter, A.; Iglic, K.; Vukelic, A.; Starcevic, A.; Ward, M.; Wells, M.L.; Trick, C.G.; et al. A profile of an endosymbiont-enriched fraction of the coral Stylophora pistillata reveals proteins relevant to microbial-host interactions. Mol. Cell. Proteom. MCP 2012, 11, M111.015487. [Google Scholar] [CrossRef]
- Levin, R.A.; Beltran, V.H.; Hill, R.; Kjelleberg, S.; McDougald, D.; Steinberg, P.D.; van Oppen, M.J.H. Sex, Scavengers, and Chaperones: Transcriptome Secrets of Divergent Symbiodinium Thermal Tolerances. Mol. Biol. Evol. 2016, 33, 2201–2215. [Google Scholar] [CrossRef]
- D’Angelo, C.; Hume, B.C.; Burt, J.; Smith, E.G.; Achterberg, E.P.; Wiedenmann, J. Local adaptation constrains the distribution potential of heat-tolerant Symbiodinium from the Persian/Arabian Gulf. ISME J. 2015, 9, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Pochon, X.; Gates, R.D. A new Symbiodinium clade (Dinophyceae) from soritid foraminifera in Hawai’i. Mol. Phylogenet. Evol. 2010, 56, 492–497. [Google Scholar] [CrossRef] [PubMed]
- LaJeunesse, T.C.; Pettay, D.T.; Sampayo, E.M.; Phongsuwan, N.; Brown, B.; Obura, D.O.; Fitt, W.K. Long-standing environmental conditions, geographic isolation and host–symbiont specificity influence the relative ecological dominance and genetic diversification of coral endosymbionts in the genus Symbiodinium. J. Biogeogr. 2010, 37, 785–800. [Google Scholar] [CrossRef]
- Stat, M.; Gates, R.D. Clade D Symbiodinium in scleractinian corals: A “nugget” of hope, a selfish opportunist, an ominous sign, or all of the above? J. Mar. Biol. 2011, 2011, 730715. [Google Scholar] [CrossRef]
- Abrego, D.; Van Oppen, M.J.; Willis, B.L. Highly infectious symbiont dominates initial uptake in coral juveniles. Mol. Ecol. 2009, 18, 3518–3531. [Google Scholar] [CrossRef]
- LaJeunesse, T.C.; Loh, W.K.; Van Woesik, R.; Hoegh-Guldberg, O.; Schmidt, G.W.; Fitt, W.K. Low symbiont diversity in southern Great Barrier Reef corals, relative to those of the Caribbean. Limnol. Oceanogr. 2003, 48, 2046–2054. [Google Scholar] [CrossRef]
- McCulloch, M.; Falter, J.; Trotter, J.; Montagna, P. Coral resilience to ocean acidification and global warming through pH up-regulation. Nat. Clim. Chang. 2012, 2, 623–627. [Google Scholar] [CrossRef]
- Chollett, I.; Mumby, P.J.; Cortés, J. Upwelling areas do not guarantee refuge for coral reefs in a warming ocean. Mar. Ecol. Prog. Ser. 2010, 416, 47–56. [Google Scholar] [CrossRef]
- Porter, S.N.; Sink, K.J.; Schleyer, M.H. The Third Global Coral Bleaching Event on the Marginal Coral Reefs of the Southwestern Indian Ocean and Factors That Contribute to Their Resistance and Resilience. Diversity 2021, 13, 464. [Google Scholar] [CrossRef]
- Riegl, B.; Glynn, P.W.; Banks, S.; Keith, I.; Rivera, F.; Vera-Zambrano, M.; D’Angelo, C.; Wiedenmann, J. Heat attenuation and nutrient delivery by localized upwelling avoided coral bleaching mortality in northern Galapagos during 2015/2016 ENSO. Coral Reefs 2019, 38, 773–785. [Google Scholar] [CrossRef]
- Hsu, P.C.; Lee, H.J.; Zheng, Q.; Lai, J.W.; Su, F.C.; Ho, C.R. Tide-Induced Periodic Sea Surface Temperature Drops in the Coral Reef Area of Nanwan Bay, Southern Taiwan. J. Geophys. Res. Ocean. 2020, 125, e2019JC015226. [Google Scholar] [CrossRef]
- Finelli, C.M.; Helmuth, B.S.; Pentcheff, N.D.; Wethey, D.S. Water flow influences oxygen transport and photosynthetic efficiency in corals. Coral Reefs 2006, 25, 47–57. [Google Scholar] [CrossRef]
- Skirving, W.; Guinotte, J. The Sea Surface Temperature Story on the Great Barrier Reef during the Coral Bleaching Event of 1998. In Oceanographic Processes of Coral Reefs; CRC Press: Boca Raton, FL, USA, 2000; pp. 301–313. [Google Scholar]
- McLean, R.; Kench, P. Destruction or persistence of coral atoll islands in the face of 20th and 21st century sea-level rise? Wiley Interdiscip. Rev. Clim. Chang. 2015, 6, 445–463. [Google Scholar] [CrossRef]


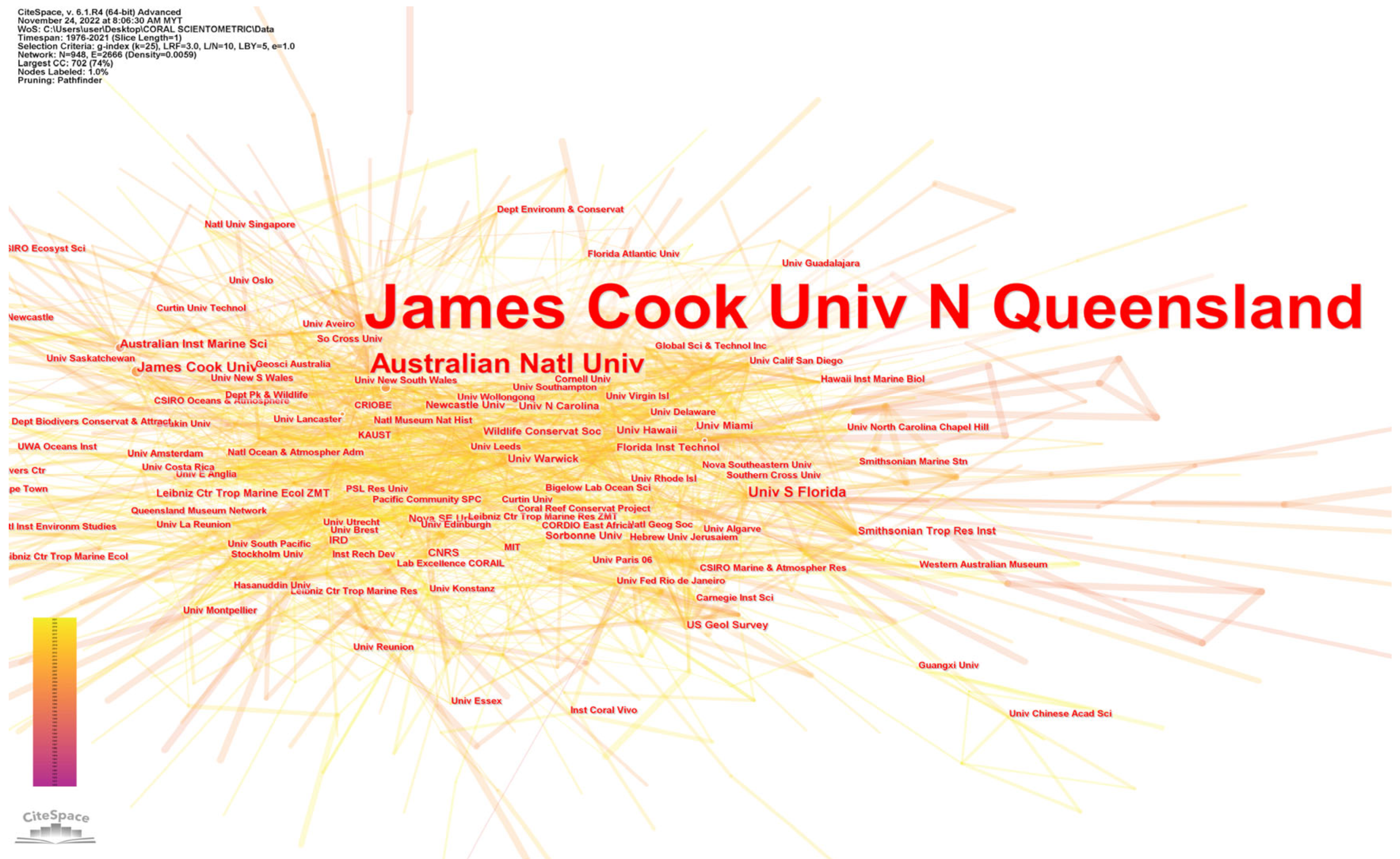


| Institution | Record Count |
|---|---|
| James Cook University (Australia) | 1119 |
| Australian Institute of Marine Science (Australia) | 727 |
| University of Queensland (Australia) | 643 |
| University of California System (USA) | 449 |
| Centre National de la Recherche Scientifique, CNRS (France) | 443 |
| UDICE French Research Universities (France) | 400 |
| National Oceanic Atmospheric Admin NOAA (USA) | 379 |
| University of Hawaii System (USA) | 345 |
| University of Western Australia (Australia) | 335 |
| Commonwealth Scientific Industrial Research Organization CSIRO (Australia) | 312 |
| Institut de Recherche pour le Développement (France) | 279 |
| State University System of Florida (USA) | 270 |
| University of Miami (USA) | 242 |
| United States Department of the Interior (USA) | 199 |
| University of Hawaii Manoa (USA) | 191 |
| King Abdullah University of Science Technology (Saudi Arabia) | 185 |
| Smithsonian Institution (USA) | 182 |
| United States Geological Survey (USA) | 178 |
| University of California San Diego (USA) | 177 |
| Australian National University (Australia) | 176 |
| Funding Agency | Record Count |
|---|---|
| Australian Research Council (Australia) | 1001 |
| National Science Foundation (USA) | 807 |
| Australian Government (Australia) | 307 |
| National Oceanic and Atmospheric Administration (USA) | 258 |
| UK Research Innovation (United Kingdom) | 248 |
| Natural Environment Research Council (United Kingdom) | 231 |
| National Natural Science Foundation (China) | 201 |
| European Commission of the European Union (EU) | 187 |
| National Science Foundation (NSF) Directorate for Geosciences (USA) | 177 |
| Ministry of Education Culture, Sports Science, and Technology (Japan) | 161 |
| Japan Society for The Promotion of Science (Japan) | 142 |
| Australian Institute of Marine Science (Australia) | 134 |
| CGIAR (United Nations) | 126 |
| German Research Foundation (Germany) | 124 |
| Consejo Nacional de Ciencia y Tecnología (Mexico) | 117 |
| National Council for Scientific and Technological Development (Brazil) | 114 |
| Natural Sciences and Engineering Research Council of Canada (Canada) | 102 |
| King Abdullah University of Science Technology (Saudi Arabia) | 92 |
| Agence Nationale de la Recherche (France) | 84 |
| Grants-in-Aid for Scientific Research (Kakenhi) (Japan) | 78 |
| Institution | Affiliation | Citation Count |
|---|---|---|
| Terry Hughes | James Cook University ARC Centre of Excellence for Coral Reef Studies | 2605 |
| Ove Hoegh-Guldberg | The University of Queensland ARC Centre of Excellence for Coral Reef Studies | 2441 |
| Katharina Fabricius | Australian Institute of Marine Science | 1108 |
| Peter W. Glynn | University of Miami | 979 |
| Tim McClanahan | The Wildlife Conservation Society ARC Centre of Excellence for Coral Reef Studies | 960 |
| Peter Mumby | The University of Queensland ARC Centre of Excellence for Coral Reef Studies | 907 |
| David R. Bellwood | James Cook University | 891 |
| John Pandolfi | The University of Queensland ARC Centre of Excellence for Coral Reef Studies | 866 |
| Kenneth R.N. Anthony | Australian Institute of Marine Science | 841 |
| John Bruno | The University of North Carolina | 781 |
| Barbara E. Brown | Newcastle University | 773 |
| Andrew C. Baker | University of Miami | 749 |
| Glenn De’ath | The Australian Institute of Marine Science | 746 |
| Joan Kleypas | National Center for Atmospheric Research | 729 |
| Ray Berkelmans | Australian Institute of Marine Science | 705 |
| Nick Graham | Lancaster University | 687 |
| Michael Lesser | The University of New Hampshire | 661 |
| Joseph Loya | Tel Aviv University | 636 |
| Peter J. Edmunds | California State University | 596 |
| Toby Gardner | Stockholm Environment Institute | 570 |
| Reference | Citations | Burst Index * | Burst Period | Journal | Centrality ** | Cluster |
|---|---|---|---|---|---|---|
| Hughes et al. [185] | 546 | 157.67 | 2018–2021 | Nature | 0.7 | #0, #4 |
| Hughes et al. [28] | 359 | 125.52 | 2018–2021 | Science | 0 | #4 |
| Hoegh-Guldberg et al. [22] | 351 | 166.14 | 2008–2012 | Science | 0.3 | #0, #2 |
| Hughes et al. [185] | 252 | 87.69 | 2018–2021 | Nature | 0.4 | #0, #7 |
| Hughes et al. [23,28] | 246 | 94.42 | 2019–2021 | Nature | 0.01 | #8 |
| De’Ath et al. [83] | 204 | 79.65 | 2013–2017 | PNAS | 0.6 | #7, #8 |
| Pandolfi et al. [20] | 175 | 68.19 | 2012–2016 | Science | 0.4 | #0, #2 |
| Fabricius et al. [68] | 162 | 63.09 | 2012–2016 | Nature Climate Change | 0.05 | #2 |
| LaJeunesse et al. [180] | 134 | 68.39 | 2013–2017 | Current Biology | 0 | #6 |
| Hughes et al. [186] | 122 | 48.71 | 2011–2015 | Trends in Ecology & Evolution | 0.01 | #0 |
| Heron et al. [187] | 118 | 33.85 | 2018–2021 | Scientific Reports | 0.6 | #4 |
| Ainsworth et al. [188] | 117 | 28.75 | 2017–2021 | Science | 0.4 | #0, #4 |
| Palumbi et al. [189] | 116 | 39.87 | 2015–2019 | Science | 0.05 | #0 |
| Baker et al. [183] | 108 | 50.7 | 2010–2013 | CETP | 0.04 | #5, #6 |
| Anthony et al. [79] | 105 | 45.6 | 2009–2013 | Global Change Biology | 0.4 | #10 |
| Kroeker et al. [182] | 104 | 39.22 | 2014–2018 | Global Change Biology | 0.4 | #2, #10 |
| Zeebe and Wolf-Gladrow [181] | 97 | 39.97 | 2010–2014 | Gulf Professional Publishing | 0.6 | #4, #10 |
| Jackson et al. [190] | 96 | 34.08 | 2016–2019 | Global Coral Reef Monitoring Network | 0.4 | #7, #18 |
| Bruno and Selig [184] | 95 | 44.45 | 2008–2012 | PLoS one | 0.05 | #0, #7 |
| Keyword | Year | Strength | Begin | End | 1976–2021 |
|---|---|---|---|---|---|
| French Polynesia | 1992 | 24.51 | 1992 | 2006 | ▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▃▃▃▃▃▃▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂ |
| Record | 1992 | 24.36 | 1992 | 2005 | ▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▃▃▃▃▃▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂ |
| El Niño | 1995 | 22.08 | 1995 | 2006 | ▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▃▃▃▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂ |
| Australia | 1992 | 21.33 | 1992 | 2007 | ▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▃▃▃▃▃▃▃▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂▂▂ |
| Indian Ocean | 1990 | 17.72 | 1998 | 2010 | ▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▃▃▃▃▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂ |
| Continental shelf | 1993 | 17.14 | 1993 | 2010 | ▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▃▃▃▃▃▃▃▃▃▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂ |
| Sea level | 1991 | 16.43 | 1991 | 2005 | ▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▃▃▃▃▃▃▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂ |
| Sea surface temperature | 1996 | 15.91 | 1996 | 2006 | ▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▃▃▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂ |
| Ocean warming | 2017 | 15.09 | 2018 | 2021 | ▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▃▃▃▃ |
| Island | 1991 | 14.65 | 1991 | 2005 | ▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▃▃▃▃▃▃▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thirukanthan, C.S.; Azra, M.N.; Lananan, F.; Sara’, G.; Grinfelde, I.; Rudovica, V.; Vincevica-Gaile, Z.; Burlakovs, J. The Evolution of Coral Reef under Changing Climate: A Scientometric Review. Animals 2023, 13, 949. https://doi.org/10.3390/ani13050949
Thirukanthan CS, Azra MN, Lananan F, Sara’ G, Grinfelde I, Rudovica V, Vincevica-Gaile Z, Burlakovs J. The Evolution of Coral Reef under Changing Climate: A Scientometric Review. Animals. 2023; 13(5):949. https://doi.org/10.3390/ani13050949
Chicago/Turabian StyleThirukanthan, Chandra Segaran, Mohamad Nor Azra, Fathurrahman Lananan, Gianluca Sara’, Inga Grinfelde, Vite Rudovica, Zane Vincevica-Gaile, and Juris Burlakovs. 2023. "The Evolution of Coral Reef under Changing Climate: A Scientometric Review" Animals 13, no. 5: 949. https://doi.org/10.3390/ani13050949
APA StyleThirukanthan, C. S., Azra, M. N., Lananan, F., Sara’, G., Grinfelde, I., Rudovica, V., Vincevica-Gaile, Z., & Burlakovs, J. (2023). The Evolution of Coral Reef under Changing Climate: A Scientometric Review. Animals, 13(5), 949. https://doi.org/10.3390/ani13050949










