What Influences the Prevalence and Intensity of Haemoparasites and Ectoparasites in an Insular Lizard?
Abstract
Simple Summary
Abstract
1. Introduction
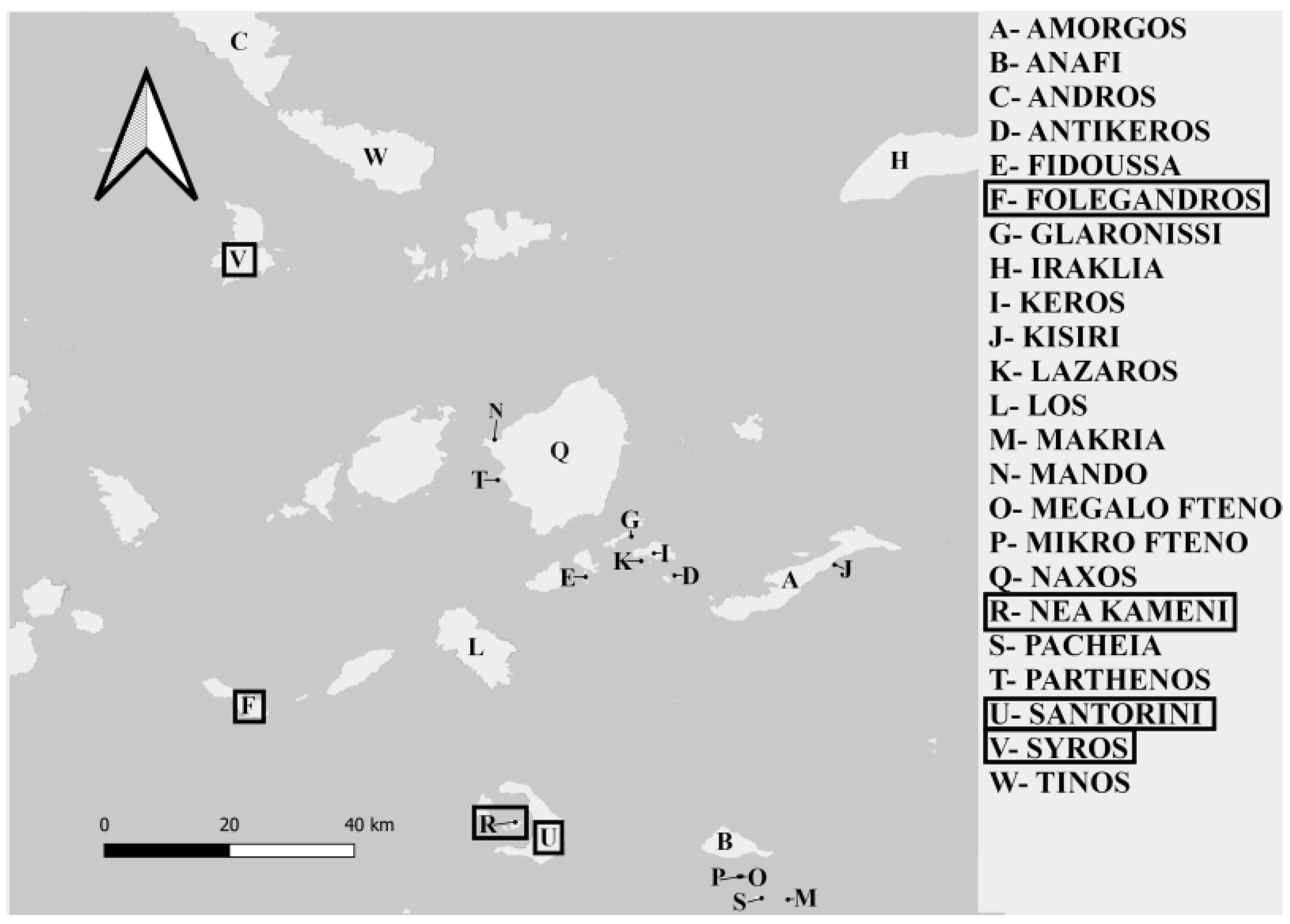
2. Materials and Methods
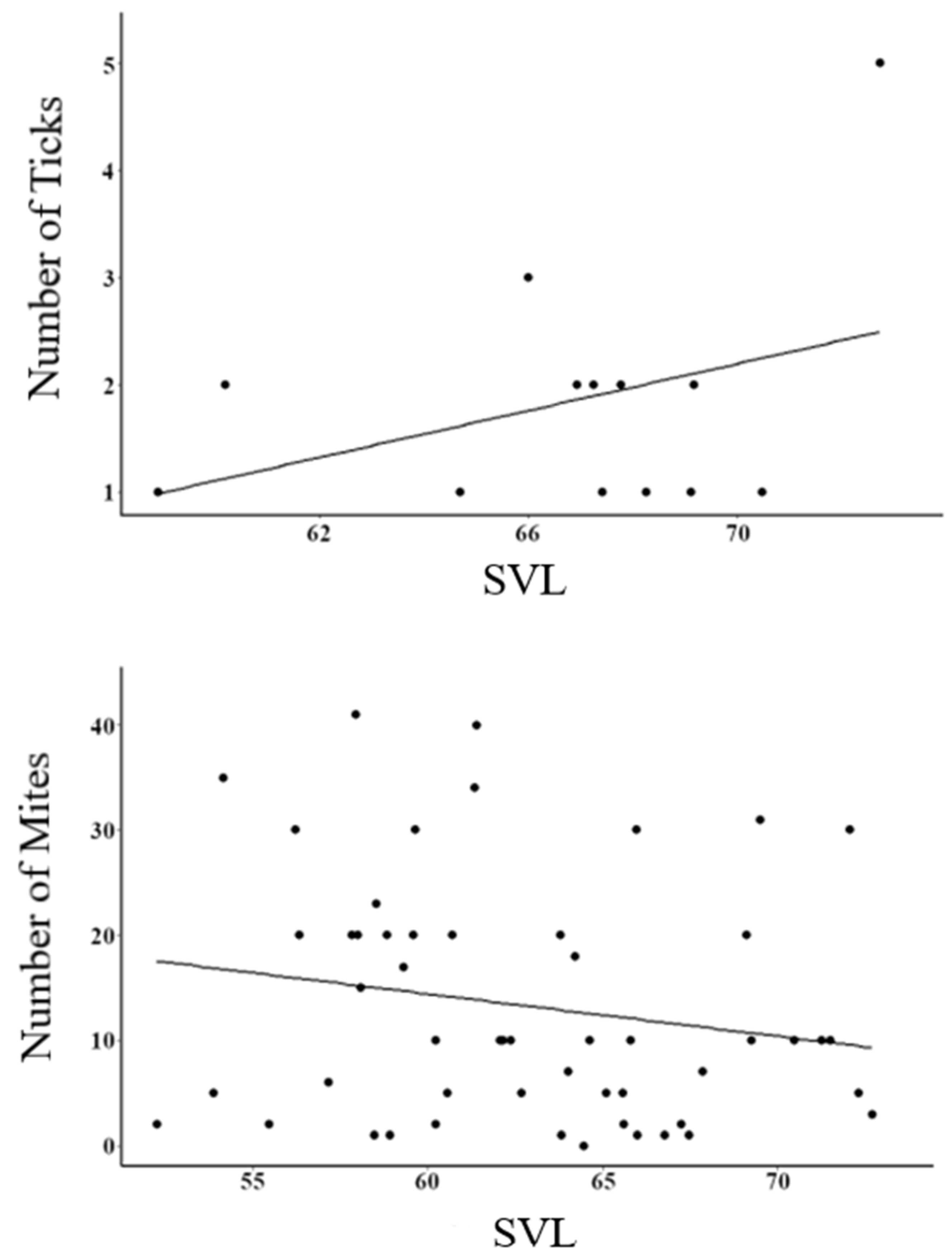
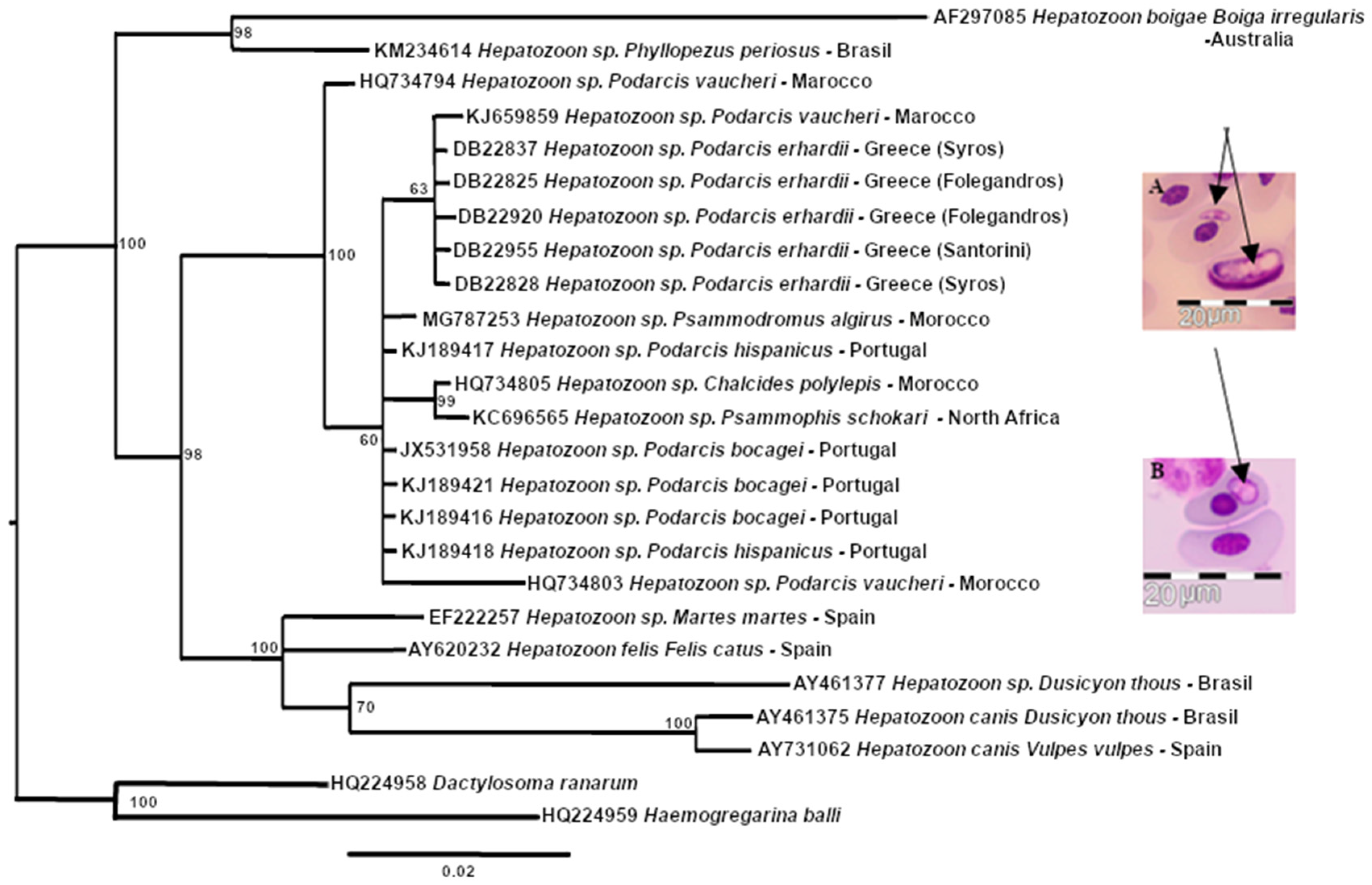
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Combes, C. Parasitism: The Ecology and Evolution of Intimate Interactions; University of Chicago Press: Chicago, IL, USA, 1996. [Google Scholar]
- Hudson, P.J.; Dobson, A.P.; Lafferty, K.D. Is a healthy ecosystem one that is rich in parasites? Trends Ecol. Evol. 2006, 21, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Poulin, R.; Morand, S. The diversity of parasites. Q. Rev. Biol. 2000, 75, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Fornberg, J.L.; Semegen, S.L. Biogeographic patterns of blood parasitism in the Aegean Wall Lizard across the cycladic islands. Front. Biogeogr. 2021, 13, 1–12. [Google Scholar] [CrossRef]
- Foufopoulos, J.; Roca, V.; White, K.A.; Pafilis, P.; Valakos, E.D. Effects of island characteristics on parasitism in a Mediterranean lizard (Podarcis erhardii): A role of population size and island history? North-West. J. Zool. 2017, 13, 70–76. [Google Scholar]
- Patiño, J.; Whittaker, R.J.; Borges, P.A.V.; Fernández-Palacios, J.M.; Ah-Peng, C.; Araújo, M.B.; Ávila, S.P.; Cardoso, P.; Cornuault, J.; Boer, E.J.; et al. A roadmap for island biology: 50 fundamental questions after 50 years of The Theory of Island Biogeography. J. Biogeogr. 2017, 44, 963–983. [Google Scholar] [CrossRef]
- Hurston, H.; Voith, L.; Bonanno, J.; Foufopoulos, J.; Pafilis, P.; Valakos, E.; Anthony, N. Effects of fragmentation on genetic diversity in island populations of the Aegean wall lizard Podarcis erhardii (Lacertidae, Reptilia). Mol. Phylogenet. Evol. 2009, 52, 395–405. [Google Scholar] [CrossRef]
- Tomé, B.; Pereira, A.; Jorge, F.; Carretero, M.A.; Harris, D.J.; Perera, A. Along for the ride or missing it altogether: Exploring the host specificity and diversity of haemogregarines in the Canary Islands. Parasit. Vectors 2018, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Rodda, G.H.; Dean-Bradley, K. Excess density compensation of island herpetofaunal assemblages. J. Biogeogr. 2002, 29, 623–632. [Google Scholar] [CrossRef]
- Papkou, A.; Gokhale, C.S.; Traulsen, A.; Schulenburg, H. Host–parasite coevolution: Why changing population size matters. Zoology 2016, 119, 330–338. [Google Scholar] [CrossRef]
- Lymberakis, P.; Isailovic, J.C.; Ajtic, R.; Vogrin, M.; Böhme, W. Podarcis erhardii. IUCN Red List Assess. 2009, 1–7. [Google Scholar] [CrossRef]
- Brock, K.M.; Baeckens, S.; Donihue, C.M.; Martín, J.; Pafilis, P.; Edwards, D.L. Trait differences among discrete morphs of a color polymorphic lizard, Podarcis erhardii. PeerJ 2020, 8, e10284. [Google Scholar] [CrossRef] [PubMed]
- Foufopoulos, J.; Ives, A.R. Reptile Extinctions on Land-Bridge Islands: Life-History Attributes and Vulnerability to Extinction. Am. Nat. 1999, 153, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. The Effects of Predation Risk, Shelter, and Food Availability on the Reproduction of Aegean Wall Lizards (Podarcis erhardii) in the Greek Islands. Master’s Thesis, University of Michigan, Ann Arbor, MI, USA, 2018. [Google Scholar]
- Telford, S.R. Hemoparasites of the Reptilia: Color Atlas and Textitle, 1; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Maia, J.P.; Harris, D.J.; Carranza, S.; Goméz-Díaz, E. Assessing the diversity, host-specificity and infection patterns of apicomplexan parasites in reptiles from Oman, Arabia. Parasitology 2016, 143, 1730–1747. [Google Scholar] [CrossRef] [PubMed]
- Barta, J.R.; Ogedengbe, J.D.; Martin, D.S.; Smith, T.G. Phylogenetic position of the Adeleorinid Coccidia (Myzozoa, Apicomplexa, Coccidia, Eucoccidiorida, Adeleorina) inferred using 18S rDNA sequences. J. Eukaryotic Microbiol. 2012, 59, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.J.; Borges-Nojosa, D.M.; Maia, J.P. Prevalence and diversity of Hepatozoon in native and exotic geckos from Brazil. J. Parasitol. 2015, 101, 80–85. [Google Scholar] [CrossRef]
- Smith, T.G.; Desser, S.S. Phylogenetic analysis of the genus Hepatozoon Miller, 1908 (Apicomplexa: Adeleorina). Syst. Parasitol. 1997, 36, 213–221. [Google Scholar] [CrossRef]
- Allain, S.J.R.; Bateman, T.C.B. Ixodid ticks on Oertzen’s Rock Lizard (Anatololacerta oertzeni) on Ikaria, Greece, with notes on the island’s reptiles. Reptiles Amp. 2018, 25, 176–179. [Google Scholar] [CrossRef]
- Garrido, M.; Pérez-Mellado, V. Human pressure, parasitism and body condition in an insular population of a Mediterranean lizard. Eur. J. Wildl. Res. 2015, 61, 617–621. [Google Scholar] [CrossRef]
- Hurston, H. Historical Land Fragmentation and Its Effects on Genetic Diversity and Parasitism of Island Populations of Podarcis erhardii (Lacertidae, Reptilia). Master’s Thesis, Thesis and Dissertations University of New Orleans, New Orleans, LA, USA, 2007. [Google Scholar]
- Cheke, A.S.; Tsiakiris, R.; Christopoulos, A.; Ashcroft, R.E. The Breeding Birds of a Small Aegean Island (Amorgos, Cyclades, Greece) with Details of Changes over 25 Years. Wild Greece Ed. 2020. [Google Scholar]
- Handrinos, G.; Akriotis, T. The Birds of Greece; Helm Field Guides: London, UK, 1997. [Google Scholar]
- Langkilde, T.; Shine, R. How much stress do researchers inflict on their study animals? A case study using a scincid lizard, Eulamprus heatwolei. J. Exp. Biol. 2006, 209, 1035–1043. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ Part II. Biophotonics Int. 2005, 11, 36–43. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 6 March 2021).
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar]
- Oksanen, A.J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.R.; Hara, R.B.O.; Simpson, G.L.; Solymos, P.; et al. Package ‘Vegan’ 2.5-7. Community Ecology Package; CRAN: Vienna, Austria, 2020. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
- Ujvari, B.; Madsen, T.; Olsson, M. High Prevalence of Hepatozoon spp. (Apicomplexa, Hepatozoidae) Infection in Water Pythons (Liasis fuscus) From Tropical Australia. J. Parasitol. 2004, 90, 670–672. [Google Scholar] [CrossRef]
- Maia, J.P.M.C.; Harris, D.J.; Perera, A. Molecular survey of Hepatozoon Species in Lizards from North Africa. J. Parasitol. 2011, 97, 513–517. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Senfeld, T.; Calcott, B. PartitionFinder 2: New Methods for Selecting Partitioned Models of Evolution for Molecular and Morphological Phylogenetic Analyses. Mol. Biol. Evol. 2016, 34, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Megía-Palma, R.; Barja, I.; Barrientos, R. Fecal glucocorticoid metabolites and ectoparasites as biomarkers of heat stress close to roads in a Mediterranean lizard. Sci. Total Environ. 2021, 802, 149919. [Google Scholar] [CrossRef]
- Huyghe, K.; Oystaeyen, A.V.; Pasmans, F.; Tadić, Z.; Vanhooydonck, B.; Damme, R. Seasonal changes in parasite load and a cellular immune response in a colour polymorphic lizard. Oecologia 2010, 163, 867–874. [Google Scholar] [CrossRef]
- Amo, L.; López, P.; Martín, J. Prevalence and intensity of haemogregarine blood parasites and their mite vectors in the common wall lizard, Podarcis muralis. Parasitol. Res. 2005, 96, 378–381. [Google Scholar] [CrossRef]
- Drechsler, R.M.; Belliure, J.; Megía-Palma, R. Phenological and intrinsic predictors of mite and haemacoccidian infection dynamics in a Mediterranean community of lizards. Parasitology 2021, 148, 1328–1338. [Google Scholar] [CrossRef]
- Hamilton, K.; Goulet, C.T.; Drummond, E.M.; Senior, A.F.; Schroder, M.; Gardner, M.G.; While, G.M.; Chapple, D.G. Decline in lizard species diversity, abundance and ectoparasite load across an elevational gradient in the Australian alps. Austral. Ecol. 2021, 46, 8–19. [Google Scholar] [CrossRef]
- Poulakakis, N.; Lymberakis, P.; Antoniou, A.; Chalkia, D.; Zouros, E.; Mylonas, M.; Valakos, E. Molecular phylogeny and biogeography of the wall-lizard Podarcis erhardii (Squamata: Lacertidae). Mol. Phylogenet. Evol. 2003, 28, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.L.A.; Philpot, K.E.; Damas-Moreira, I.; Stevens, M. Intraspecific Colour Variation among Lizards in Distinct Island Environments Enhances Local Camouflage. PLoS ONE 2015, 10, e0135241. [Google Scholar] [CrossRef] [PubMed]
- Meiri, S. Evolution and ecology of lizard body sizes. Glob. Ecol. Biogeogr. 2008, 17, 724–734. [Google Scholar] [CrossRef]
- Megía-Palma, R.; Martínez, J.; Cuervo, J.J.; Belliure, J.; Jiménez-Robles, O.; Gomes, V.; Cabido, C.; Pausas, J.G.; Fitze, P.S.; Martín, J.; et al. Molecular evidence for host–parasite co-speciation between lizards and Schellackia parasites. Int. J. Parasitol 2018, 48, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Marzal, A.; Ibáñez, A.; González-Blázquez, M.; López, P.; Martín, J. Prevalence and genetic diversity of blood parasite mixed infections in Spanish terrapins, Mauremys leprosa. Parasitology 2017, 144, 1449–1457. [Google Scholar] [CrossRef]
- Damas-Moreira, I.; Maia, J.P.; Tomé, B.; Salvi, D.; Perera, A.; Harris, D.J. Blood parasites in sympatric lizards: What is their impact on hosts’ immune system? Amphibia-Reptilia 2022, 43, 37–49. [Google Scholar] [CrossRef]
| Island Characteristics | Lizard Characteristics | Parasite Prevalence | Parasite Intensity | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area (km2) | Number of Inhabitants | Type of Habitat | Avian Predators | Sex | SVL (mm) | Mass (g) | Haemogregarines | Schellackia | Ticks | Mites | Haemogregarines | Schellackia | Ticks | Mites | |
| Syros | 101.9 | 21,507 | Rocky shrubland | 5 | 35 M | 69.6 ± 4.7 | 8.8 ± 1.7 | 54.3 ± 11.2 | 14.3 ± 1.1 | - | - | 6.2 ± 11.2 | 0.4 ± 1.1 | - | - |
| 22 F | 67.0 ± 5.5 | 6.6 ± 1.6 | 31.8 ± 14.4 | 4.5 ± 3.2 | - | - | 6.2 ± 14.4 | 0.7 ± 3.2 | - | - | |||||
| Folegandros | 32.38 | 800 | Rocky shrubland | 2 | 37 M | 67.3 ± 4.3 | 7.8 ± 1.5 | 84.2 ± 24.8 | 2.6 ± 0.2 | 50 ± 1.4 | 23.7 ± 3.9 | 9.8 ± 24.8 | 0 ± 0.2 | 0.9 ± 1.4 | 1.3 ± 3.9 |
| 26 F | 64.2 ± 4.7 | 5.5 ± 0.9 | 57.7 ± 10.6 | 7.7 ± 0.4 | 11.5 ± 0.6 | 15.4 ± 1.9 | 5.9 ± 10.6 | 0.1 ± 0.4 | 0.2 ± 0.6 | 0.5 ± 1.9 | |||||
| Santorini | 76.19 | 13,500 | Rocky shrubland | 5 | 25 M | 64.4 ± 4.9 | 7.1 ± 1.8 | 69.2 ± 7.7 | 38.5 ± 0.9 | - | 92.3 ± 11.5 | 5.1 ± 7.7 | 0.6 ± 0.9 | - | 15.1 ± 11.5 |
| 25 F | 59.4 ± 4.6 | 4.6 ± 1.0 | 64 ± 4.8 | 36 ± 4.8 | - | 88 ± 1.8 | 7 ± 4.8 | 0.9 ± 4.8 | - | 13.8 ± 1.8 | |||||
| Nea Kameni | 3.4 | - | Lava dome | 5 | 11 M | 61.7 ± 4.2 | 5.7 ± 1.2 | 9 ± 0.6 | 27.3 ± 1.5 | - | - | 0.2 ± 0.6 | 0.6 ± 1.5 | - | - |
| 10 F | 61.3 ± 2.8 | 4.4 ± 0.8 | 10 ± 0.3 | 10 ± 0.6 | - | - | 0.1 ± 0.3 | 0.2 ± 0.6 | - | - | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, A.I.; Damas-Moreira, I.; Marshall, K.L.A.; Perera, A.; Harris, D.J. What Influences the Prevalence and Intensity of Haemoparasites and Ectoparasites in an Insular Lizard? Animals 2023, 13, 723. https://doi.org/10.3390/ani13040723
Ferreira AI, Damas-Moreira I, Marshall KLA, Perera A, Harris DJ. What Influences the Prevalence and Intensity of Haemoparasites and Ectoparasites in an Insular Lizard? Animals. 2023; 13(4):723. https://doi.org/10.3390/ani13040723
Chicago/Turabian StyleFerreira, A. Isabel, Isabel Damas-Moreira, Kate L. A. Marshall, Ana Perera, and D. James Harris. 2023. "What Influences the Prevalence and Intensity of Haemoparasites and Ectoparasites in an Insular Lizard?" Animals 13, no. 4: 723. https://doi.org/10.3390/ani13040723
APA StyleFerreira, A. I., Damas-Moreira, I., Marshall, K. L. A., Perera, A., & Harris, D. J. (2023). What Influences the Prevalence and Intensity of Haemoparasites and Ectoparasites in an Insular Lizard? Animals, 13(4), 723. https://doi.org/10.3390/ani13040723





