Variations of Supercooling Capacity in Intertidal Gastropods
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
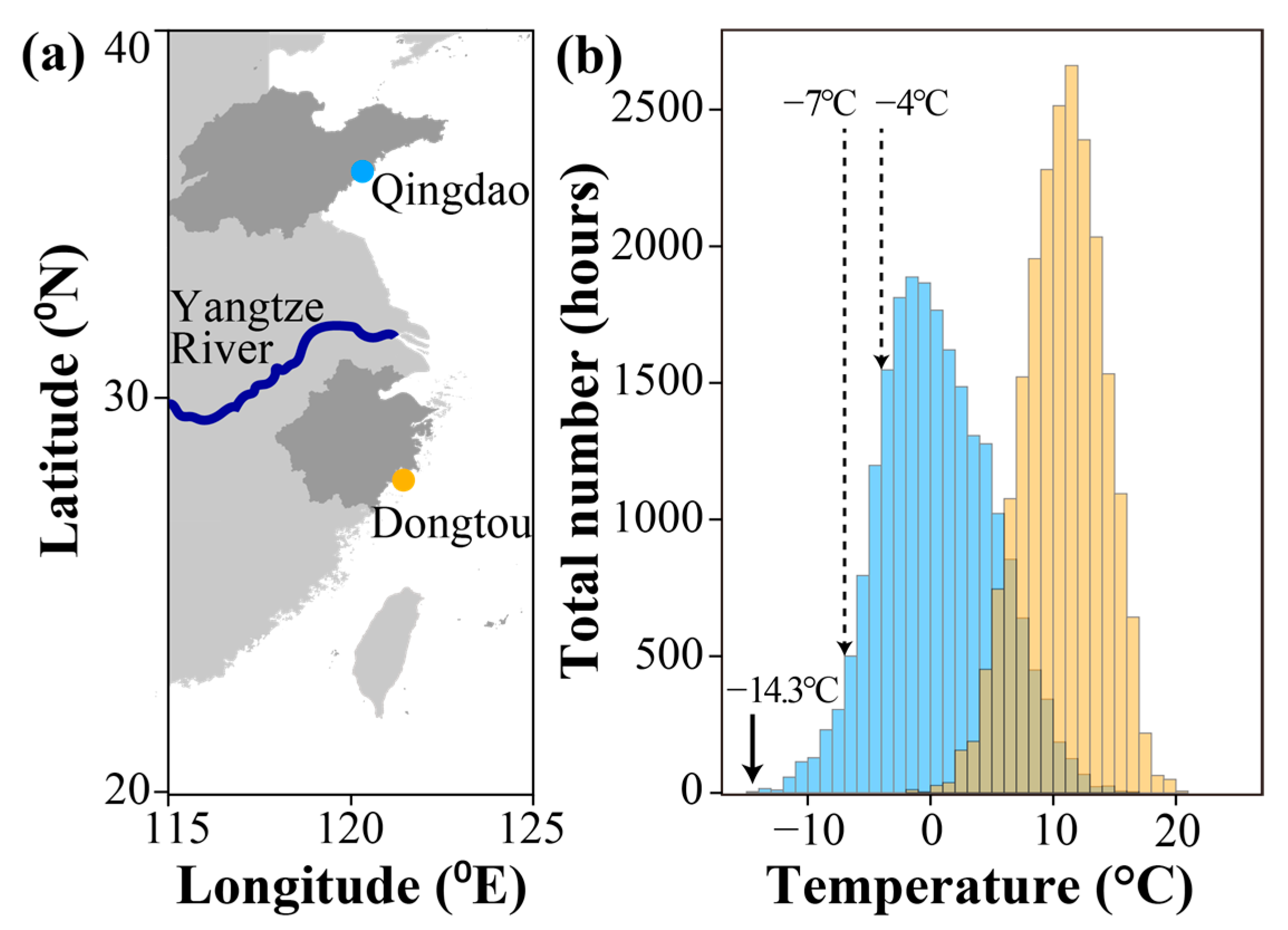
2.2. Environmental Temperature Variations
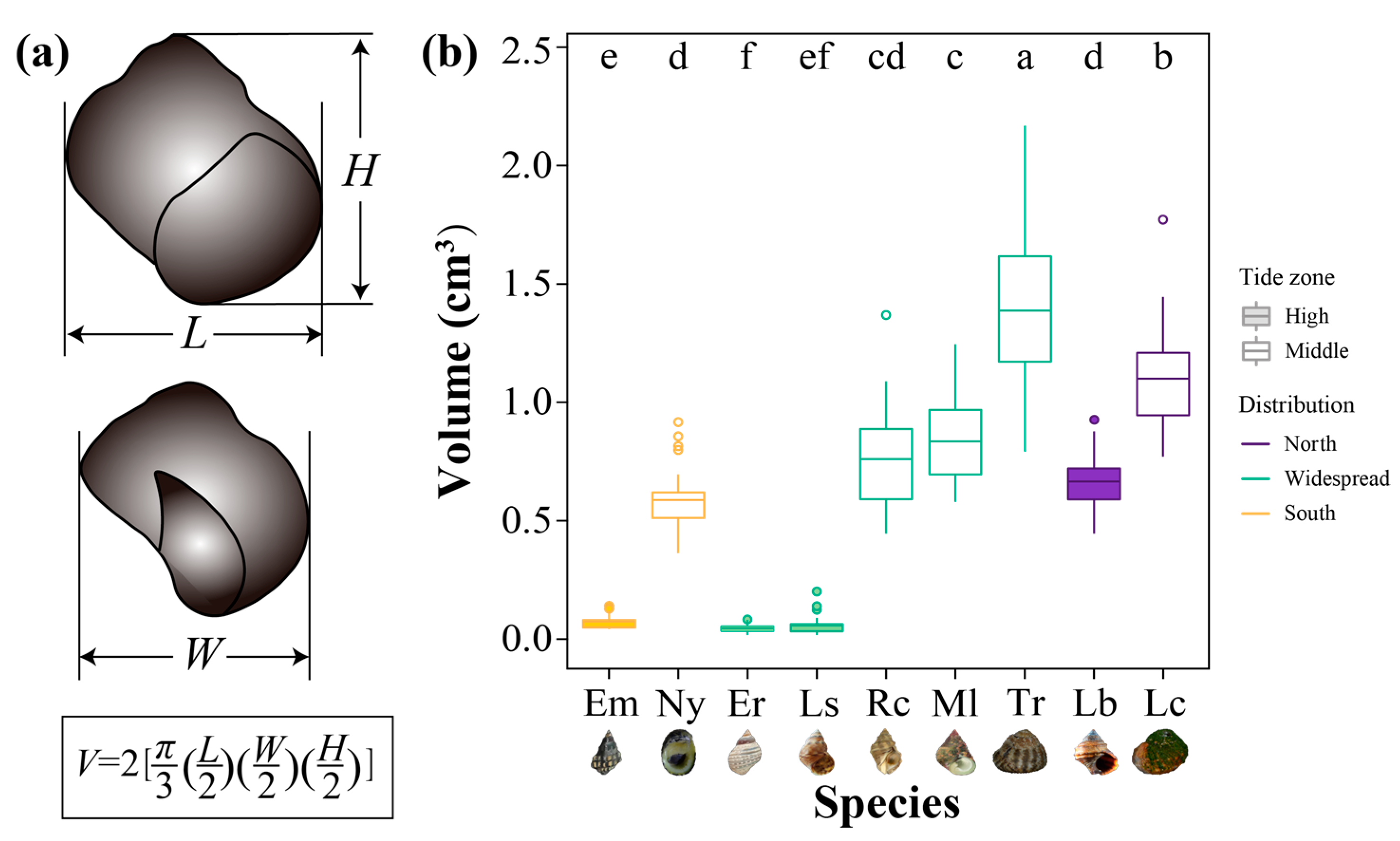
2.3. Measurement of Shell Size
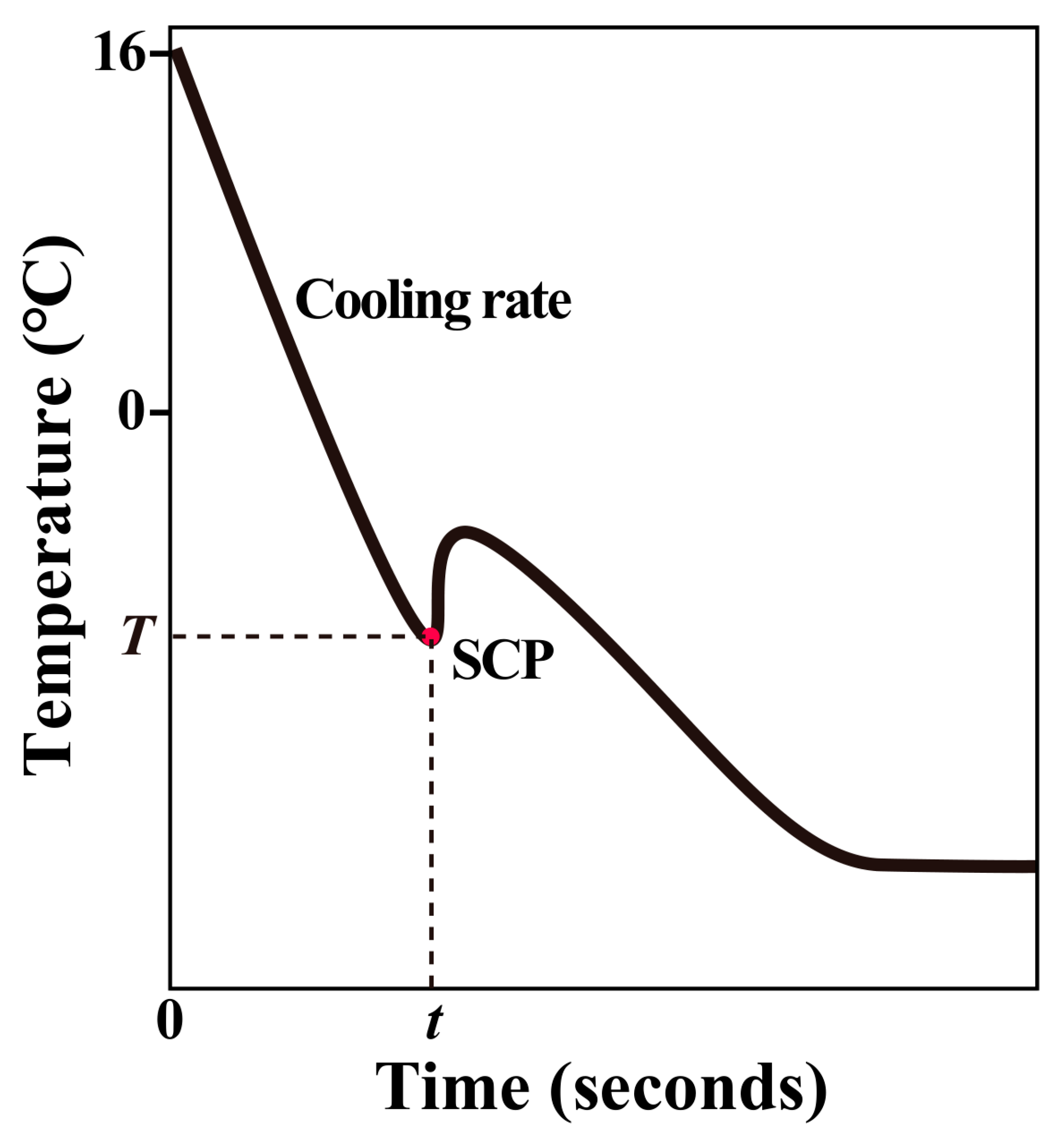
2.4. Freezing Curve Measurement
2.5. Statistical Analysis
3. Results
3.1. Air Temperatures in Winter
3.2. Body Size of Intertidal Snails
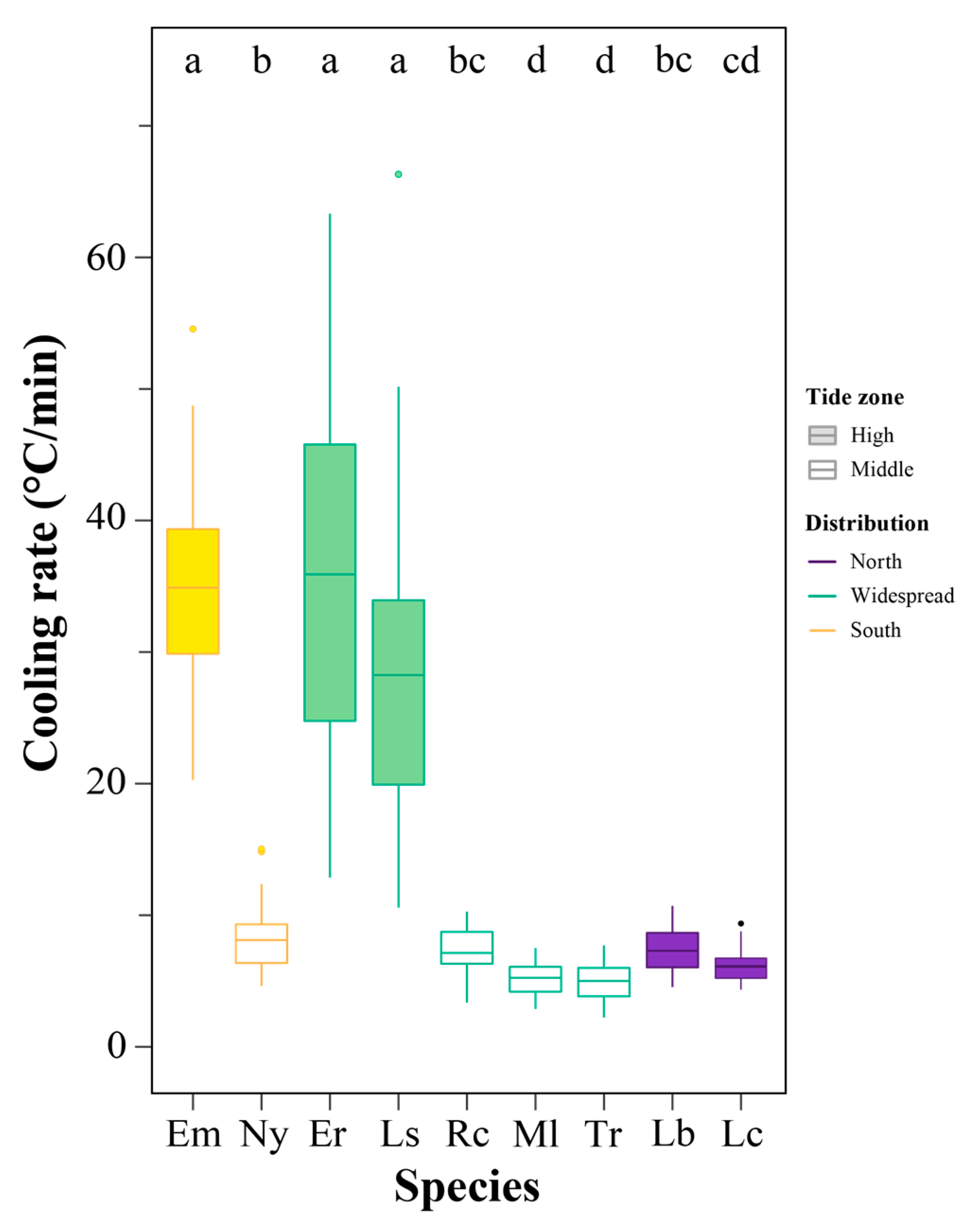
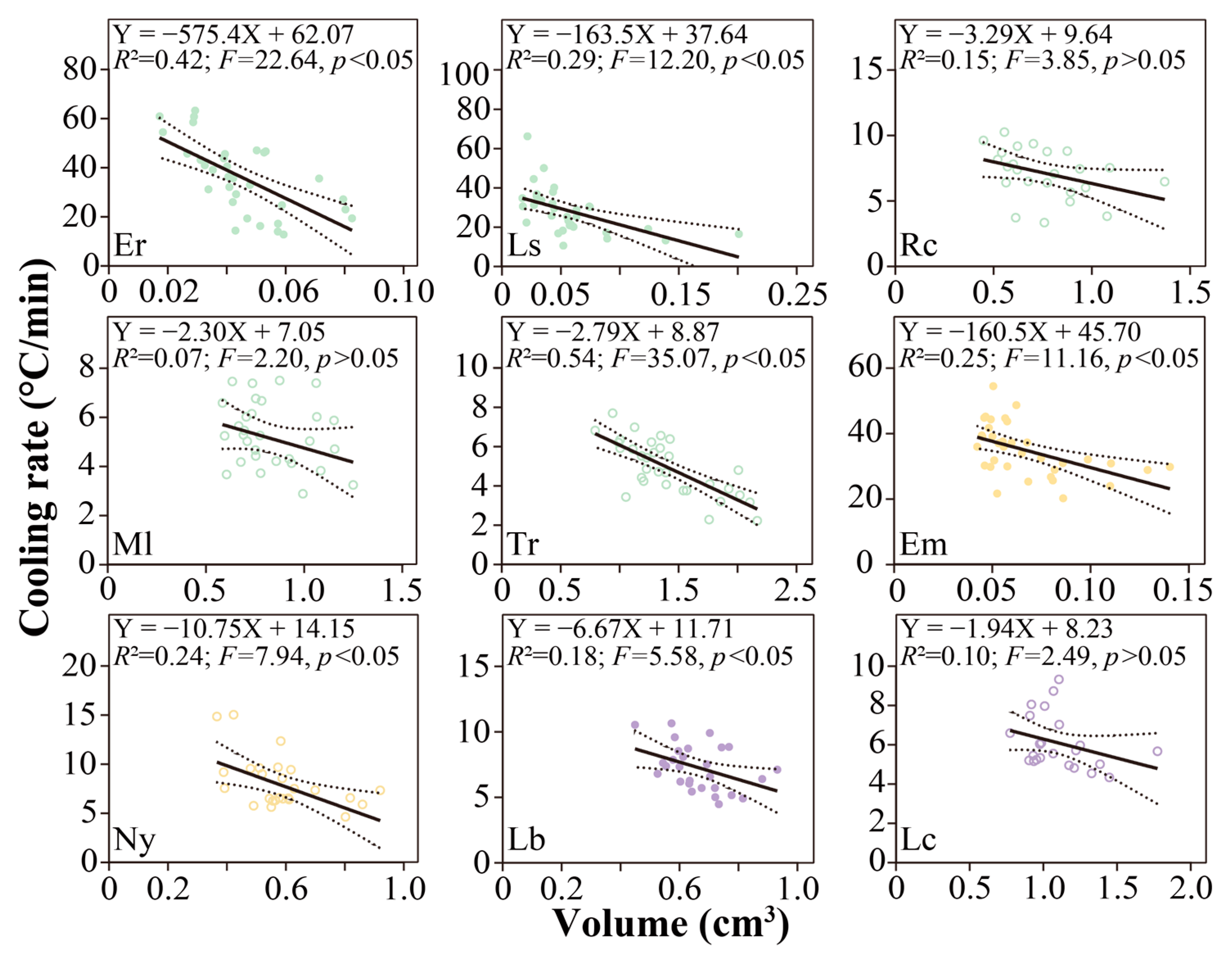
3.3. Cooling Rate Variations
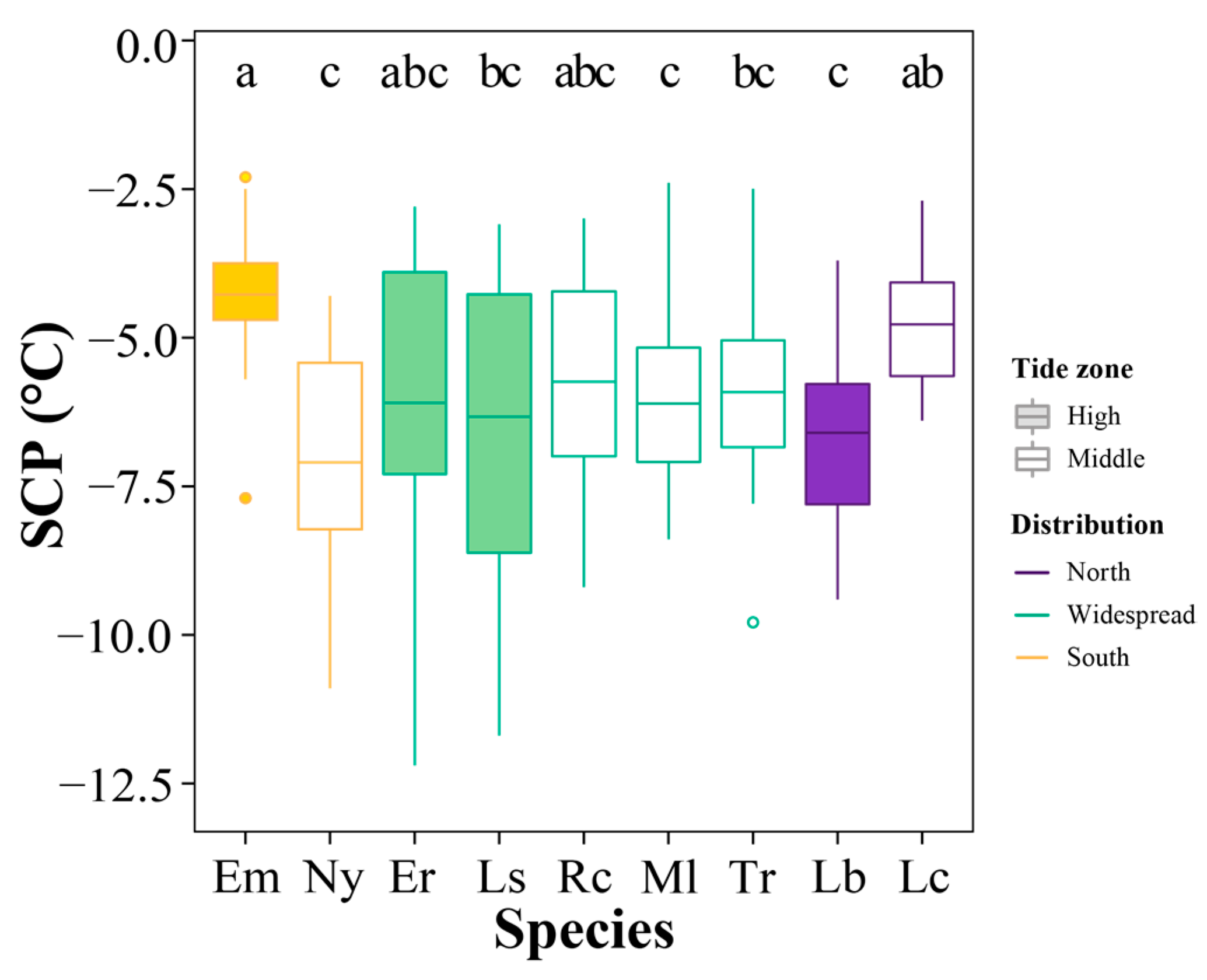
3.4. Supercooling Point Variations
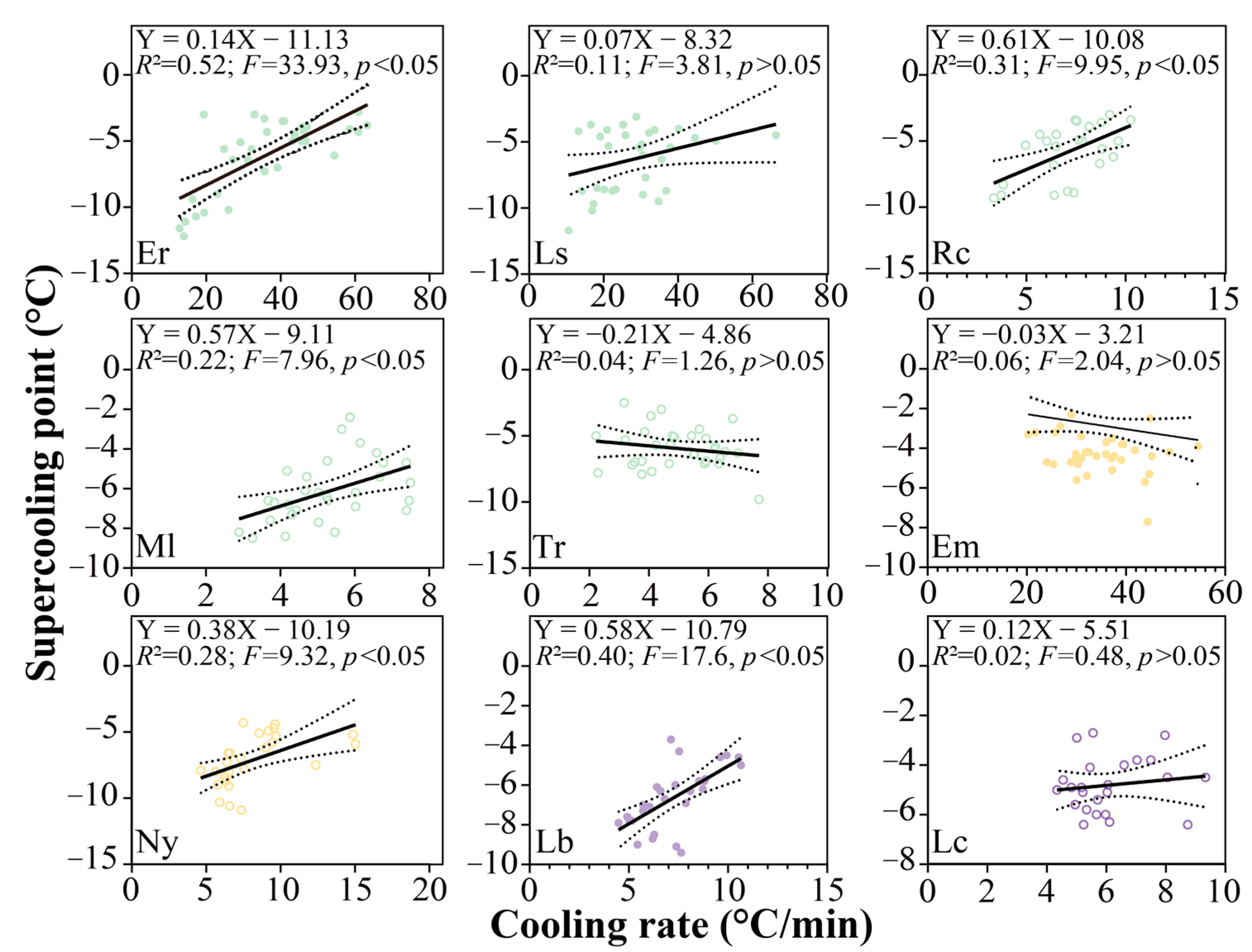
3.5. Factors Contributing to the Supercooling Points
4. Discussion
4.1. Limited Capacity to Supercool of Intertidal Gastropods
4.2. Variation in SCP at the Geographic and Local Scales
4.3. Cooling Rate Leads to Variation in SCPs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tomanek, L.; Helmuth, B. Physiological ecology of rocky intertidal organisms: A synergy of concepts. Integr. Comp. Biol. 2002, 42, 771–775. [Google Scholar] [CrossRef]
- Dong, Y.W.; Liao, M.L.; Han, G.D.; Somero, G.N. An integrated, multi-level analysis of thermal effects on intertidal molluscs for understanding species distribution patterns. Biol. Rev. 2022, 97, 554–581. [Google Scholar] [CrossRef]
- Helmuth, B.; Mieszkowska, N.; Moore, P.; Hawkins, S.J. Living on the edge of two changing worlds: Forecasting the responses of rocky intertidal ecosystems to climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 373–404. [Google Scholar] [CrossRef]
- Mosbahi, N.; Pezy, J.-P.; Dauvin, J.-C.; Neifar, L. Spatial and Temporal Structures of the Macrozoobenthos from the Intertidal Zone of the Kneiss Islands (Central Mediterranean Sea). Open J. Mar. Sci. 2016, 06, 223–237. [Google Scholar] [CrossRef]
- Paine, R.T. A Note on Trophic Complexity and Community Stability. Am. Nat. 1969, 103, 91–93. [Google Scholar] [CrossRef]
- Savoca, S.; Grifó, G.; Panarello, G.; Albano, M.; Giacobbe, S.; Capillo, G.; Spanó, N.; Consolo, G. Modelling prey-predator interactions in Messina beachrock pools. Ecol. Model. 2020, 434, 109206. [Google Scholar] [CrossRef]
- Osland, M.J.; Stevens, P.W.; Lamont, M.M.; Brusca, R.C.; Hart, K.M.; Waddle, J.H.; Langtimm, C.A.; Williams, C.M.; Keim, B.D.; Terando, A.J.; et al. Tropicalization of temperate ecosystems in North America: The northward range expansion of tropical organisms in response to warming winter temperatures. Glob. Chang. Biol. 2021, 27, 3009–3034. [Google Scholar] [CrossRef]
- Smith, K.E.; Burrows, M.T.; Hobday, A.J.; King, N.G.; Moore, P.J.; Gupta, A.S.; Thomsen, M.S.; Wernberg, T.; Smale, D.A. Biological Impacts of Marine Heatwaves. Annu. Rev. Mar. Sci. 2023, 15, 119–145. [Google Scholar] [CrossRef]
- Boucek, R.E.; Gaiser, E.E.; Liu, H.; Rehage, J.S. A review of subtropical community resistance and resilience to extreme cold spells. Ecosphere 2016, 7, e01455:1–e01455:10. [Google Scholar] [CrossRef]
- Marchand, P.J. Life in the Cold: An Introduction to Winter Ecology, 4th ed.; University Press of New England: Lebanon, NH, USA, 2014. [Google Scholar]
- Studd, E.K.; Bates, A.E.; Bramburger, A.J.; Fernandes, T.; Hayden, B.; Henry, H.A.L.; Humphries, M.M.; Martin, R.; McMeans, B.C.; Moise, E.R.D.; et al. Nine Maxims for the Ecology of Cold-Climate Winters. BioScience 2021, 71, 820–830. [Google Scholar] [CrossRef]
- Sutton, A.O.; Studd, E.K.; Fernandes, T.; Bates, A.E.; Bramburger, A.J.; Cooke, S.J.; Hayden, B.; Henry, H.A.; Humphries, M.M.; Martin, R.; et al. Frozen out: Unanswered questions about winter biology. Environ. Rev. 2021, 29, 431–442. [Google Scholar] [CrossRef]
- Helmuth, B.; Hofmann, G.E. Microhabitats, thermal heterogeneity, and patterns of physiological stress in the rocky intertidal zone. Biol. Bull. 2001, 201, 374–384. [Google Scholar] [CrossRef]
- Serreze, M. Arctic Climate. In Encyclopedia of Atmospheric Sciences, 2nd ed.; North, G.R., Pyle, J., Zhang, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 107–115. [Google Scholar]
- Storey, K.; Storey, J.M. Freeze tolerance in animals. Physiol. Rev. 1988, 68, 27–84. [Google Scholar] [CrossRef]
- Aarset, A.V. Freezing tolerance in intertidal invertebrates (a review). Comp. Biochem. Physiol. Part A Physiol. 1982, 73, 571–580. [Google Scholar] [CrossRef]
- Ansart, A.; Vernon, P. Cold hardiness in molluscs. Acta Oecologica 2003, 24, 95–102. [Google Scholar] [CrossRef]
- Waller, C.L.; Worland, M.R.; Convey, P.; Barnes, D.K.A. Ecophysiological strategies of Antarctic intertidal invertebrates faced with freezing stress. Polar Biol. 2006, 29, 1077–1083. [Google Scholar] [CrossRef]
- Loomis, S.H. Freezing tolerance of marine invertebrates. Oceanogr. Mar. Biol. 1995, 33, 337–350. [Google Scholar]
- Li, X.X.; Tan, Y.; Sun, Y.X.; Wang, J.; Dong, Y.W. Microhabitat temperature variation combines with physiological variation to enhance thermal resilience of the intertidal mussel Mytilisepta virgata. Funct. Ecol. 2021, 35, 2497–2507. [Google Scholar] [CrossRef]
- Somero, G.N. Thermal Physiology and Vertical Zonation of Intertidal Animals: Optima, Limits, and Costs of Living. Integr. Comp. Biol. 2002, 42, 780–789. [Google Scholar] [CrossRef]
- Finke, G.; Navarrete, S.; Bozinovic, F. Tidal regimes of temperate coasts and their influences on aerial exposure for intertidal organisms. Mar. Ecol. Prog. Ser. 2007, 343, 57–62. [Google Scholar] [CrossRef]
- Kennedy, J.R.; Harley, C.D.G.; Marshall, K.E. Drivers of plasticity in freeze tolerance in the intertidal mussel, Mytilus trossulus. J. Exp. Biol. 2020, 223, jeb.233478:1–jeb.233478:11. [Google Scholar] [CrossRef] [PubMed]
- Reid, H.; Harley, C. Low temperature exposure determines performance and thermal microhabitat use in an intertidal gastropod (Littorina Scutulata) during the winter. Mar. Ecol. Prog. Ser. 2021, 660, 105–118. [Google Scholar] [CrossRef]
- Stickle, W.B.; Carrington, E.; Hayford, H. Seasonal changes in the thermal regime and gastropod tolerance to temperature and desiccation stress in the rocky intertidal zone. J. Exp. Mar. Biol. Ecol. 2017, 488, 83–91. [Google Scholar] [CrossRef]
- Chiba, S.; Iida, T.; Tomioka, A.; Azuma, N.; Kurihara, T.; Tanaka, K. Population divergence in cold tolerance of the intertidal gastropod Littorina brevicula explained by habitat-specific lowest air temperature. J. Exp. Mar. Biol. Ecol. 2016, 481, 49–56. [Google Scholar] [CrossRef]
- Dennis, A.B.; Loomis, S.H.; Hellberg, M.E. Latitudinal Variation of Freeze Tolerance in Intertidal Marine Snails of the Genus Melampus (Gastropoda: Ellobiidae). Physiol. Biochem. Zoöl. 2014, 87, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, B.J.; Marshall, D.J.; Singh, S.; Chown, S.L. Cold tolerance of Littorinidae from southern Africa: Intertidal snails are not constrained to freeze tolerance. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2004, 174, 617–624. [Google Scholar] [CrossRef]
- Thyrring, J.; Tremblay, R.; Sejr, M.K. Local cold adaption increases the thermal window of temperate mussels in the Arctic. Conserv. Physiol. 2019, 7, coz098:1–coz098:10. [Google Scholar] [CrossRef]
- Sanford, E.; Kelly, M.W. Local Adaptation in Marine Invertebrates. Annu. Rev. Mar. Sci. 2011, 3, 509–535. [Google Scholar] [CrossRef]
- Sunday, J.; Bennett, J.M.; Calosi, P.; Clusella-Trullas, S.; Gravel, S.; Hargreaves, A.L.; Leiva, F.P.; Verberk, W.C.; Olalla-Tárraga, M.Á.; Morales-Castilla, I. Thermal tolerance patterns across latitude and elevation. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20190036:1–20190036:10. [Google Scholar] [CrossRef]
- Boroda, A.V.; Kipryushina, Y.O.; Odintsova, N.A. The effects of cold stress on Mytilus species in the natural environment. Cell Stress Chaperon. 2020, 25, 821–832. [Google Scholar] [CrossRef]
- Wallace, G.T.; Kim, T.L.; Neufeld, C.J. Interpopulational variation in the cold tolerance of a broadly distributed marine copepod. Conserv. Physiol. 2014, 2, cou04:1–cou04:12. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, J.; Choi, F.M.P.; Ding, P.; Li, X.X.; Han, G.D.; Ding, M.W.; Guo, M.; Huang, X.W.; Duan, W.X.; et al. Global warming and artificial shorelines reshape seashore biogeography. Glob. Ecol. Biogeogr. 2020, 29, 220–231. [Google Scholar] [CrossRef]
- McAlpine-Bellis, E.; Stillman, J.H.; Tanner, R.L. Acclimation to future climate exposes vulnerability to cold extremes in intertidal sea hares. Integr. Comp. Biol. 2021, 61, 1741–1752. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.J. Freezing Resistance in Intertidal Invertebrates. Annu. Rev. Physiol. 1983, 45, 289–299. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Molecular Biology of Freezing Tolerance. Compr. Physiol. 2013, 3, 1283–1308. [Google Scholar] [CrossRef]
- Murphy, D.J. A Comparative Study of the Freezing Tolerances of the Marine Snails Littorina littorea (L.) and Nassarius obsoletus (Say). Physiol. Biochem. Zool. 1979, 52, 219–230. [Google Scholar] [CrossRef]
- Miller, L. Freezing tolerance in relation to cooling rate in an adult insect. Cryobiology 1978, 15, 345–349. [Google Scholar] [CrossRef]
- Murphy, D.J.; Johnson, L.C. Physical and temporal factors influencing the freezing tolerance of the marine snail Littorina littorea (L.). Biol. Bull. 1980, 158, 220–232. [Google Scholar] [CrossRef]
- Wang, J.; Russell, B.D.; Ding, M.W.; Dong, Y.W. Ocean acidification increases the sensitivity of and variability in physiological responses of an intertidal limpet to thermal stress. Biogeosciences 2018, 15, 2803–2817. [Google Scholar] [CrossRef]
- Li, H.T.K. Thermal Tolerance of Echinolittorina Species in Hong Kong: Implications for Their Vertical Distributions. Master’s Thesis, The University of Hong Kong, Pokfulam, Hong Kong, 2012. [Google Scholar]
- Takada, Y. Dimorphic migration, growth, and fecundity in a seasonally split population of Littorina brevicula (Mollusca: Gastropoda) on a boulder shore. Popul. Ecol. 2003, 45, 141–148. [Google Scholar] [CrossRef]
- Yeung, C.-Y. The Ecology of Nerita yoldii and N. albicilla on Hong Kong Rocky Shores. Master’s Thesis, The University of Hong Kong, Pokfulam, Hong Kong, 2006. [Google Scholar] [CrossRef]
- Apolinário, M. Temporal variations in community structure in and around intertidal barnacle (Chthamalus challengeri Hoek) patches on a plebby shore in Japan. Braz. J. Biol. 1999, 59, 43–53. [Google Scholar] [CrossRef]
- Linse, K.; Roterman, C.N.; Chen, C. A New Vent Limpet in the Genus Lepetodrilus (Gastropoda: Lepetodrilidae) from Southern Ocean Hydrothermal Vent Fields Showing High Phenotypic Plasticity. Front. Mar. Sci. 2019, 6, 381. [Google Scholar] [CrossRef]
- McClain, C.R.; Nekola, J.C. The role of local-scale processes on terrestrial and deep-sea gastropod body size distributions across multiple scales. Evol. Ecol. Res. 2008, 10, 129–146. [Google Scholar]
- Matsukura, K.; Tsumuki, H.; Izumi, Y.; Wada, T. Physiological response to low temperature in the freshwater apple snail, Pomacea canaliculata (Gastropoda: Ampullariidae). J. Exp. Biol. 2009, 212, 2558–2563. [Google Scholar] [CrossRef] [PubMed]
- Toxopeus, J.; Sinclair, B.J. Mechanisms underlying insect freeze tolerance: Mechanisms of insect freeze tolerance. Biol. Rev. 2018, 93, 1891–1914. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/index.html (accessed on 24 December 2022).
- Wharton, D.A. Supercooling and freezing tolerant animals. In Supercooling; Wilson, P., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 17–28. [Google Scholar]
- Currie-Olsen, D.; Hesketh, A.V.; Grimm, J.; Kennedy, J.; Marshall, K.E.; Harley, C.D.G. Lethal and sublethal implications of low temperature exposure for three intertidal predators. Available online: https://ssrn.com/abstract=4130896 (accessed on 20 December 2022).
- Hargens, A.R.; Shabica, S.V. Protection against lethal freezing temperatures by mucus in an Antarctic limpet. Cryobiology 1973, 10, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Storey, K.B.; Storey, J.M. Natural freezing survival in animals. Annu. Rev. Ecol. Syst. 1996, 27, 365–386. [Google Scholar] [CrossRef]
- Johnston, I.A. Cold adaptation in marine organisms. Philos. T. R. Soc. B. 1990, 326, 655–667. [Google Scholar] [CrossRef]
- Marshall, K.E.; Dowle, E.J.; Petrunina, A.; Kolbasov, G.; Chan, B.K.K. Transcriptional dynamics following freezing stress reveal selection for mechanisms of freeze tolerance at the poleward range margin in the cold water intertidal barnacle Semibalanus Balanoides. BioRxiv 2018, 449330. [Google Scholar] [CrossRef]
- Wang, J.; Yan, H.-Y.; Cheng, Z.-Y.; Huang, X.-W.; Wang, W.; Ding, M.-W.; Dong, Y.-W. Recent northward range extension of Nerita yoldii (Gastropoda: Neritidae) on artificial rocky shores in China. J. Molluscan Stud. 2018, 84, 345–353. [Google Scholar] [CrossRef]
- Stickle, W.B.; Lindeberg, M.; Rice, S.D. Comparative freeze tolerance and physiological adaptations of three species of vertically distributed rocky intertidal gastropods from Southeast Alaska. J. Exp. Mar. Biol. Ecol. 2015, 463, 17–21. [Google Scholar] [CrossRef]
- Hellberg, M.E. Gene Flow and Isolation among Populations of Marine Animals. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 291–310. [Google Scholar] [CrossRef]
- Bar Dolev, M.; Braslavsky, I.; Davies, P.L. Ice-Binding Proteins and Their Function. Annu. Rev. Biochem. 2016, 85, 515–542. [Google Scholar] [CrossRef] [PubMed]
- Hengherr, S.; Worland, M.R.; Reuner, A.; Brümmer, F.; Schill, R.O. Freeze tolerance, supercooling points and ice formation: Comparative studies on the subzero temperature survival of limno-terrestrial tardigrades. J. Exp. Biol. 2009, 212, 802–807. [Google Scholar] [CrossRef]
- Fokina, N.N.; Ruokolainen, T.R.; Nemova, N.N. The effect of intertidal habitat on seasonal lipid composition changes in blue mussels, Mytilus edulis L., from the White Sea. Polar Rec. 2018, 54, 133–151. [Google Scholar] [CrossRef]
- Somero, G.N. Solutions: How adaptive changes in cellular fluids enable marine life to cope with abiotic stressors. Mar. Life Sci. Technol. 2022, 4, 389–413. [Google Scholar] [CrossRef]
- Box, I.C.H.; Matthews, B.J.; Marshall, K.E. Molecular evidence of intertidal habitats selecting for repeated ice-binding protein evolution in invertebrates. J. Exp. Biol. 2022, 225, jeb243409:1–jeb243409:13. [Google Scholar] [CrossRef]
| Species | Geographic Distribution | Vertical Distribution (Height above CD) ¶ | References | ||
|---|---|---|---|---|---|
| Echinolittorina malaccana | 20° S~30° N | Southern species | 3.00 m (1.75~3.25 m) | High-shore species | [42] |
| E. radiata | 12° N~43° N | Widespread species | 2.75 m (1.75~3.25 m) | High-shore species | [42] |
| Littoraria sinensis | 19° N~40° N | Widespread species | Between E. radiata and L. brevicula | High-shore species | Present study |
| Littorina brevicula | 22° N~45° N | Northern species | 2.30 m (1.00~2.60 m) | High-shore species | [43] |
| Nerita yoldii | 21° N~33° N | Southern species | 1.75 m (1.00~2.50 m) | Mid-shore species | [44] |
| Reishia clavigera | 6° S~40° N | Widespread species | 1.00 m (1.00~2.25 m) | Mid-shore species | [44] |
| Monodonta labio | 25° S~42° N | Widespread species | 1.50 m (1.00~2.00 m) | Mid-shore species | [44] |
| Tegula rustica | 17° S~44° N | Widespread species | Lower than M. labio | Mid-shore species | [45] |
| Lunella coreensis | 30° N~42° N | Northern species | 1.50 m (1.00~1.75 m) | Mid-shore species | [44] |
| Added Term | rdf | rd | df | edf | de | cde | AIC |
|---|---|---|---|---|---|---|---|
| Null | 283 | 1177.16 | / | / | / | / | / |
| +Geographic distribution | 281 | 1128.03 | 2 | / | 4.17% | 4.17% | 1205 |
| +Vertical distribution | 280 | 1102.44 | 1 | / | 2.18% | 6.35% | 1201 |
| +Species | 275 | 981.39 | 8 | / | 10.25% | 16.60% | 1178 |
| +Volume | 268 | 947.42 | / | 0.92 | −0.70% | 15.90% | 1164 |
| +Cooling rate | 247 | 644.49 | / | 3.99 | 23.10% | 39.00% | 1024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, S. Variations of Supercooling Capacity in Intertidal Gastropods. Animals 2023, 13, 724. https://doi.org/10.3390/ani13040724
Wang J, Wang S. Variations of Supercooling Capacity in Intertidal Gastropods. Animals. 2023; 13(4):724. https://doi.org/10.3390/ani13040724
Chicago/Turabian StyleWang, Jie, and Shuo Wang. 2023. "Variations of Supercooling Capacity in Intertidal Gastropods" Animals 13, no. 4: 724. https://doi.org/10.3390/ani13040724
APA StyleWang, J., & Wang, S. (2023). Variations of Supercooling Capacity in Intertidal Gastropods. Animals, 13(4), 724. https://doi.org/10.3390/ani13040724





