Simple Summary
Recent findings of the nematode Eucoleus garfiai in wild boars across different countries, and more lately in southern Italy, have brought up the need for collecting epidemiological data on this parasite. In the present study, the prevalence of E. garfiai was analyzed in relation to altitude in different provinces of the Campania and Latium regions located, respectively, in southern and central Italy. Results showed that the parasite is more often found at altitudes higher than 900 m above sea level. Some species of earthworms are intermediate hosts of E. garfiai and it is well known that earthworms are more present in high quality soils, which are more likely found at high altitudes where anthropogenic interventions are less frequent. Therefore, we can suggest that the higher prevalence of E. garfiai above 900 m above sea level is probably linked to a higher presence of earthworms in the soil, due to its higher quality in these areas.
Abstract
Recent reports of Eucoleus garfiai in wild boars in southern Italy have highlighted the need for collecting epidemiological data on the presence of this parasite and understanding the role of possible interactions between wild boars, E. garfiai, and the environment. This study analyses, using histopathological and biomolecular techniques, the presence of E. garfiai in tongue samples of wild boars hunted in four provinces of the Campania and Latium regions (Italy), in areas located above and below 900 m above sea level (asl). Histopathological examinations revealed the presence of adults and eggs of nematodes, which were subsequently identified as E. garfiai by biomolecular analysis, in the tongue epithelium. The detection of the parasite was more frequent in samples collected from hunting areas located above 900 m asl than in those collected from areas located below 900 m asl (66.67% vs. 38.09%; p < 0.01). Some species of earthworms are intermediate hosts of E. garfiai and it is well known that earthworms are more present in high quality soils. Therefore, we can suggest that the higher prevalence of E. garfiai at higher altitudes is probably linked to a greater presence of earthworms in the soil, due to its higher quality in these areas.
1. Introduction
The lives of wild animals are strictly connected to the environment in which they live and to the other living beings with whom they share it [1,2,3]. The strong relationship between animals and the environment makes the contact of wild species with numerous saprophytic and/or pathogenic organisms particularly easy, especially with those living in waters and soil [4,5]. The modern approach to One Health obliges researchers to pay attention to the different environments that host biodiversity. Living beings are, in fact, conditioned by the size of living spaces, the availability of trophic resources, pathogens and anthropic pressures. In the case of large wild mammals, some studies have demonstrated relationships between oxidative stress markers and living environments both for individual populations and for the interspecific territory sharing [6,7]. Great interest nowadays is given to soil born-diseases [8,9], and, in wildlife, studies are mainly focused on soil pathogens which are of public health interest such as Francisella tularensis, Yersinia pestis, Bacillus anthracis, Coxiella burnetii, Avian influenza virus H5N1 [10], Swine flu H1N1 [11], Coronavirus [12], and different helminths [13] as they can negatively affect humans, livestock and domestic animals [14,15,16,17,18,19]. However, more and more studies have underlined the importance of pathogenic and saprophytic microorganisms and macrorganisms, such as earthworms, as a valuable tool for a variety of ecology-based applications, such as environmental bioindicators [20,21,22].
Wild boars (Sus scrofa) are extensively distributed worldwide and, starting from the mid-twentieth century, a significant increase in their population in Europe and Italy has been described with a consequent enlargement of their habitat [23,24,25] even though a reliable estimate of the number of individuals present is not known (unpublished data). As omnivores, wild boars can feed on a wide range of foods of plant origin (roots, rhizomes, crops) and of animal origin (insects, birds, snails), according to the availability of food sources [26,27]. Among the animal sources found in the stomachs of hunted wild boars, earthworms appear to be a significant component probably due to their availability in the first layers of soil, to their great energy and nutrient content, and to the rooting feeding habits of wild boars [28,29,30]. Nevertheless, ingestion of earthworms can also represent an important source of parasitic infections as these invertebrates are often intermediate hosts of nematodes which can parasitize the lungs of wild boars [31,32,33,34].
Recently, the nematode Eucoleus garfiai, synonym Capillaria garfiai, was detected in adult wild boars hunted in southern Italy [35]. Previous studies had already described the presence of E. garfiai in domestic and wild swine in Spain [36], Austria [37], Japan [38], central Italy [39] and Iran [40]. Adults and eggs can be observed in the tongue of wild boars after ingestion of infected earthworms, such as Lumbricus terrestris, Allolobophora caliginosa and Allolobophora rosea, which act as intermediate hosts [41]. Histopathological examination of infected tongues reveals moderate inflammation of the tissue characterized by infiltration of lymphocytes, plasma cells and eosinophils, suggesting reduced pathogenicity of the parasite [38]. Although E. garfiai can be considered a non-zoonotic and slightly pathogenetic parasite, data on the presence of this nematode in wild boars should be increased. Moreover, intraspecific relationships between wild boars–earthworms–environment should be explored further to better understand their epidemiological and ecological relevance. Therefore, considering the novelty of the presence in southern Italy and the lack of information about E. garfiai, the aim of this study was to assess by histopathological and biomolecular techniques the presence of E. garfiai in wild boars in different areas of the Campania and Latium regions (Italy) located at different altitudes.
2. Materials and Methods
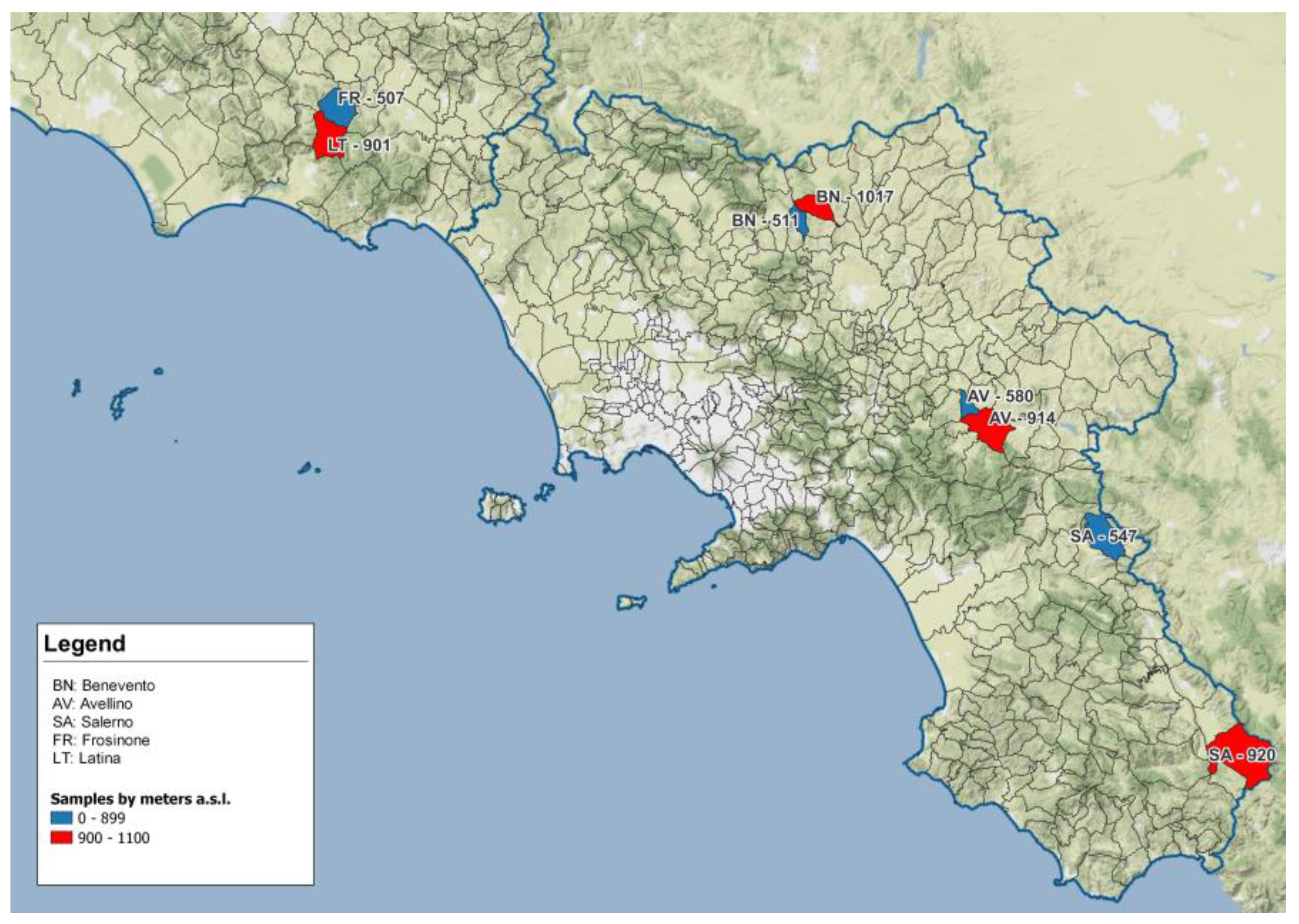
A total of 69 wild boars were collected in 8 different areas pertaining to 4 different provinces of 2 regions of southern and central Italy (3 in Campania and 1 in Latium) during the months of October, November and December of the 2021–2022 hunting season. Sampling locations are shown in Table 1 and Table 2 and identified on the map in Figure 1. Areas were selected according to altitude and to the presence of wild boar hunting teams which could ensure sample availability.

Table 1.
Sampling sites located below 900 m asl: sampling municipality/province, altitude, geographical coordinates, and acronyms used in the study are reported.

Table 2.
Sampling sites located above 900 m asl: sampling municipality/province, altitude, geographical coordinates and acronyms used in the study are reported.
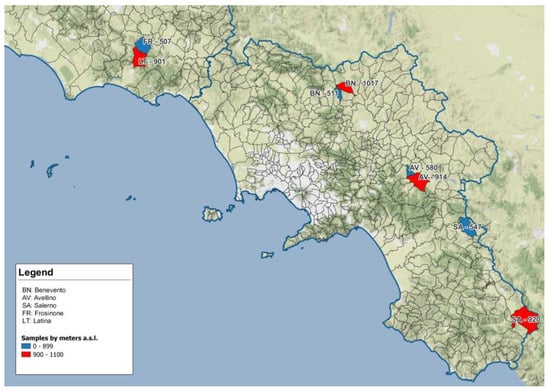
Figure 1.
Geographical map presenting localization of sampling sites in Campania and Latium region: sites located >900 m asl are in red, sites located <900 m asl are in blue. Blue line indicates the geographical border between regions.
Three samples (1 from CF AV < 900 m and 2 from PL BN > 900 m) were excluded as they were in a bad state of conservation; therefore, 66 wild boar tongue samples were isolated and transported by members of the laboratory of Animal Husbandry of the Department of Veterinary Medicine and Animal Productions, University of Naples “Federico II” in containers filled with 10% buffered formalin to the laboratory of Veterinary General Pathology and Anatomical Pathology of the Department of Veterinary Medicine and Animal Productions for further histopathological processing. Samples of 2 cm width were cut from each tongue and routinely processed for histopathological examination as previously described [42]. Briefly, they were fixed in 10% buffered formalin, paraffin-embedded, stained with haematoxylin and eosin (HE). After, tissue preparations were observed by light microscopy (Microscope Nikon Eclipse E-600, Tokyo, Japan) to identify the possible presence of parasites and morphological alterations. Parasite egg size was measured using a free image analysis software (Image J). Two samples were also stored at −20 °C and two adult parasites (one for each sample) were isolated under a stereomicroscope and stored at −20 °C for biomolecular analysis. DNA extraction and Polymerase chain reaction (PCR were performed as previously described [43,44]. The following primer pair: E. garfiai 18s_FW: 5′-GTCGTCGTCGAGATGAGTCG-3′, E. garfiai 18s_REV: 5′-TCTCTCCGGAATCGAACCCT-3′ (annealing T 60 °C), designed on the sequence available on GenBank (MW947272.1), was employed for amplification of a specific fragment (180 bp) of 18s ribosomal RNA gene of E. garfiai. A positive control mimicking E. garfiai 18s intended amplicon was synthesized according to gBlocks Gene Fragments technology (Integrated DNA Technologies Coralville, IA, USA) and run along with PCR reactions. Moreover, one no template control (NTC) was included as negative control. Amplification products were then migrated by electrophoresis on 2.5% agarose gel in TAE buffer (Tris-Acetate-EDTA) along with a 100 bp molecular marker (Bioline), stained with ethidium bromide and observed under UV with the ChemiDoc gel scanner (Bio-Rad). One representative PCR product was purified using the QIAquick PCR Purification Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol, and submitted for sequencing at BMR Genomics (Padova, Italy). The obtained sequence was aligned with available sequences of E. garfiai from GenBank.
In order to evaluate the possible relationship between the presence/absence of the parasite in wild boars and altitude (below and above 900 m asl), comparison of the percentages was carried out with the Chi-squared test of independence using the JMP® PRO 14 software.
3. Results
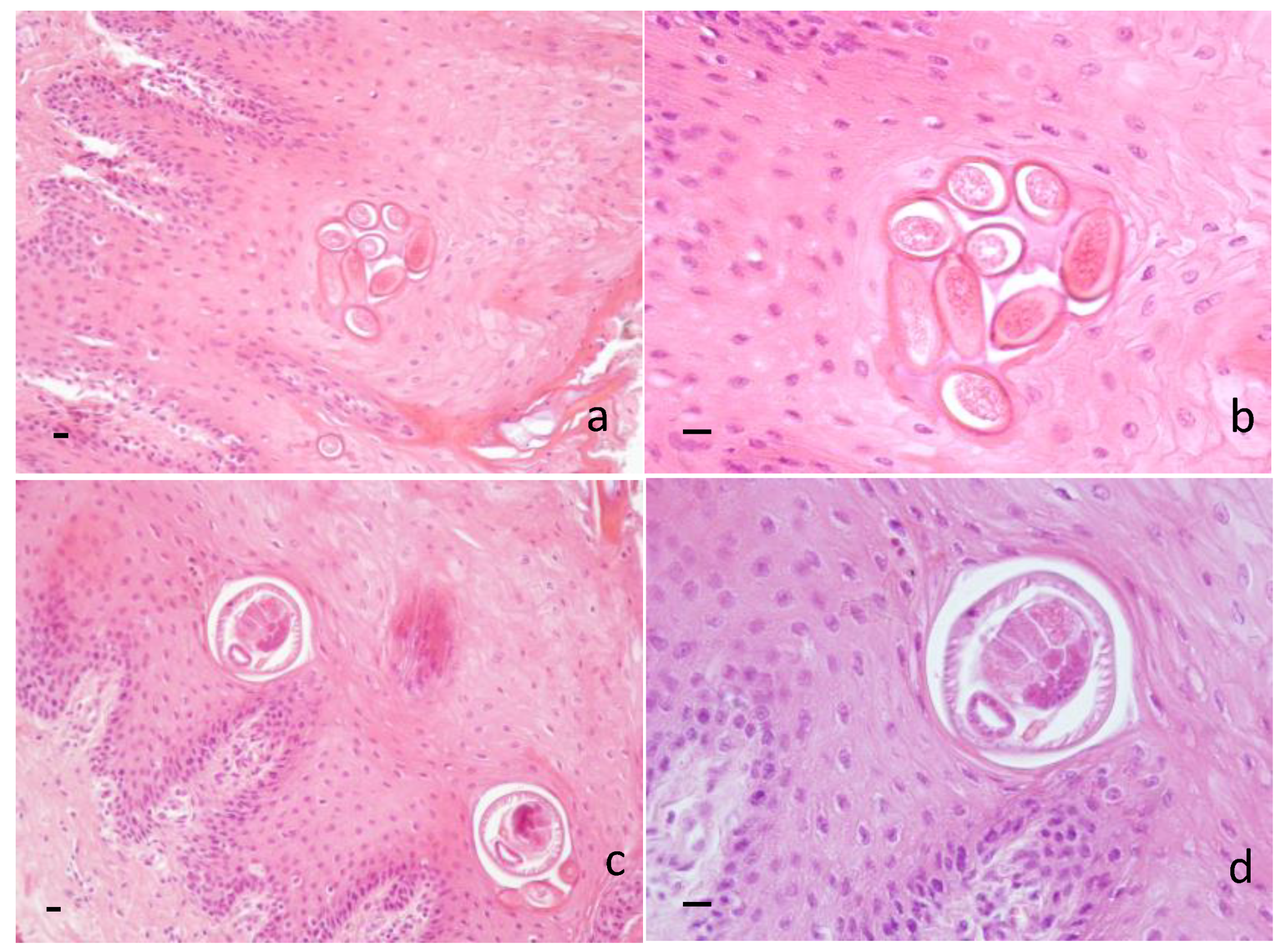
Histopathological examination revealed the presence of several sections of adults and/or eggs of helminths in the dorsal lingual epithelium of 32/66 samples (48.48%) (for a comprehensive view of results, see Table S1). Barrel-shaped eggs, showing two protruding polar plugs and measuring approximately 50 µm in the longitudinal direction, were observed throughout the corneal layer and the prickle layer (Figure 2a,b), while fragments of E. garfiai adults and/or whole adults, measuring approximately 70 µm in the transversal direction, were identified in the prickle layer, often in proximity to the basal layer (Figure 2c,d). Histopathological examination revealed hyperkeratosis, parakeratosis and strong exfoliation of the corneal layer of the lingual epithelium; few lymphocytes, plasma cells and eosinophils were noticed below the basal cell layer. Histological localization of adults and eggs and microscopic morphological appearance suggested identification of the parasite.
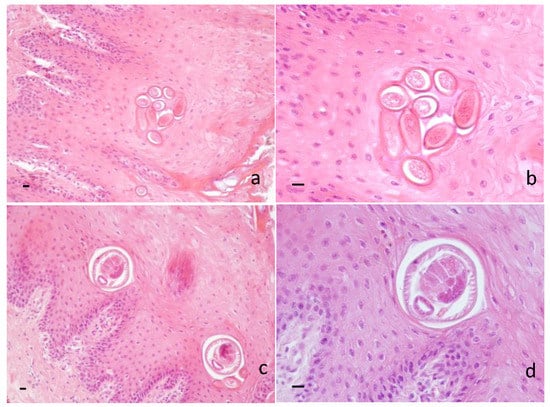
Figure 2.
Wild boar. Histological section of tongue. (a) Barrel-shaped eggs of E. garfiai showing protruding polar plugs in the prickle layer of the epithelium. 20 × HE. Scale bar 10 µm; (b) eggs of E. garfiai 40 × HE. Scale bar 10 µm; (c) transversal section of adults of E. garfiai in the prickle layer of the epithelium. 20 × HE. Scale bar 10 µm; (d) transversal section of adult of E. garfiai 40 × HE. Scale bar 10 µm.
A fragment of the expected size (180 bp) of E. garfiai was successfully amplified from both the analyzed samples and, as expected, from the positive control but not in NTC. Sequence analysis confirmed the identity of the amplicon, in agreement with the histopathological identification of the parasites. Statistical analysis highlighted that samples collected from hunting areas located above 900 m asl presented a higher prevalence of the parasite compared to those collected from areas located below 900 m asl (66.67% vs. 38.09%; p < 0.01) (Table 3). This result was consistent in all areas (Table 3).

Table 3.
Percentages of positive samples according to sampling area.
4. Discussion
The growth in population of wild ungulates and the expansion of their living habitat towards more anthropogenic areas pose numerous concerns under an ecological and sanitary point of view. Increased use of lands for agricultural purposes, deforestation and inhabitation of suburban areas have modified the extension of areas inhabited by humans and wild boars, causing overlapping between the two populations, and developing more chances of contact exposure among wild boars and humans and livestock [45]. Wild boars can harbor many infectious agents which are transmissible to livestock, domestic animals and humans, and parasites represent a noteworthy category [46,47]. However, not all parasites infecting wild boars are zoonotic and pathogenic. As previously reported, E. garfiai is a non-zoonotic non-pathogenic helminth which can be found in the tongue of wild boars without causing severe lesions. Histopathological results of our study confirmed the presence of eggs and adults in tongue samples associated with mild inflammatory alterations, as previously described [35,38]. Recent findings of E. garfiai in southern Italy have raised interest in collecting more data to better understand the ecology and epidemiology of this particular parasite. In this study, we investigated the prevalence of E. garfiai in different areas located below and above 900 m asl and the results showed a higher prevalence of E. garfiai in sampling areas located above 900 m asl. Altitude has frequently been used as a parameter for studying biodiversity richness and it was previously reported that earthworms’ communities adapt well to different gradients of altitude, increasing in number and variety of population, probably due to lower anthropogenic influence and higher soil quality [48,49,50,51]. The presence of higher densities of intermediate hosts has already been linked to abundance of parasite communities [52]; therefore, we suggest that the higher prevalence of this parasite at higher altitudes could probably be connected to a higher presence of earthworms (Lumbricus spp. and Allolobophora spp.), which act as intermediate hosts.
Moreover, infection of earthworms with E. garfiai could also be higher in areas located above 900 m asl due to the presence of a higher number of wild boars in these areas and the positive correlation of final host abundance to parasitic infection of intermediate hosts [53]. The association between Metastrongylus spp. larval infection of earthworms and wild boar density was previously demonstrated by Nagy et al. [54], and further studies could also demonstrate the validity of this theory for E. garfiai.
5. Conclusions
Soil quality and sustainability can be evaluated by using its micro and macrofauna. In particular, earthworms have been proven to be valuable bioindicators and biomonitors due to the abundance and variety of species composition of the earthworm fauna, the behavior of these invertebrates in contact with the soil substrate, and the accumulation of chemicals from the environment into their bodies [55,56,57,58,59]. Soil bacteria and parasites have also frequently been used to assess the quality of soil and of the wider ecosystems connected to it [60,61,62,63]. The present study shows a higher prevalence of E. garfiai in tongues of wild boars collected at higher altitudes where a higher presence of earthworms is found. Therefore, it could be useful for the evaluation of the investigated environments to add to routine tests for the identification of zoonotic parasites (Trichinella spp.) the assessment of E. garfiai occurrence in samples of tongues from wild boars hunted during the hunting season, as an indicator of earthworm presence and consequently of soil quality. However, significant research gaps still exist, and more studies should be carried out to understand the variations in parasite abundance and the possible use of E. garfiai as a bioindicator.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani13040706/s1, Table S1: Eucoleus garfiai in wild boars in Campania and Latium regions (Italy).
Author Contributions
Conceptualization, L.E. and N.P.; biomolecular analysis G.A.; histopathological analysis K.P. and M.M.; writing—original draft preparation, K.P.; writing—review and editing, V.V., P.V. and A.C.U.; project administration, L.E.; funding acquisition, L.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by PSR 14/20 Campania. Tipologia intervento 16.1.1 “sostegno per costituzione e funzionamento dei GO del PEI in materia di produttività e sostenibilità dell’agricoltura”. Azione 2 “Sostegno ai POI”. “Uso tecnologico e muove pratiche a carattere innovativo per la gestione, il controllo e la valorizzazione economica del cinghiale (Sus scrofa) in maniera sostenibile in Regione Campania”. S.U.S Campania (CUP B58H19004460009).
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the non-invasive sampling method. The meat was sampled by dead animals shot in compliance with the 157/92 national law. All the procedures were performed in compliance to European rules (Directive 2010/63/UE).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to thank Anna Matrone for participating to the study and Raffaele Delli Gatti of hunting house “Invidiabili”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dorazio, R.M.; Connor, E.F.; Askins, R.A. Estimating the Effects of Habitat and Biological Interactions in an Avian Community. PLoS ONE 2015, 10, e0135987. [Google Scholar] [CrossRef]
- Cabon, V.; Bùi, M.; Kühne, H. Endangered animals and plants are positively or neutrally related to wild boar (Sus scrofa) soil disturbance in urban grasslands. Sci. Rep. 2022, 12, 16649. [Google Scholar] [CrossRef] [PubMed]
- Barasona, J.A.; Carpio, A.; Boadella, M.; Gortazar, C.; Piñeiro, X.; Zumalacárregui, C.; Vicente, J.; Viñuela, J. Expansion of native wild boar populations is a new threat for semi-arid wetland areas. Ecol. Indic. 2021, 125, 107563. [Google Scholar] [CrossRef]
- Carpio, A.J.; Castro-Lopez, J.; Guerrero-Casado, J.M.; Ruiz-Aizpurua, L.; Vicente, J.; Tortosa, F.S. Effect of wild ungulate density on invertebrates in a Maditerranean ecosystem. Anim. Biodivers. Conserv. 2014, 37, 115–125. [Google Scholar] [CrossRef]
- Carpio, A.J.; García, M.; Hillström, L.; Lönn, M.; Carvalho, J.; Acevedo, P.; Bueno, C.G. Wild Boar Effects on Fungal Abundance and Guilds from Sporocarp Sampling in a Boreal Forest Ecosystem. Animals 2022, 12, 2521. [Google Scholar] [CrossRef] [PubMed]
- Esposito, L.; Tafuri, S.; Cocchia, N.; Iorio, E.; Ciani, F. Assessment of living conditions in wild boars by analysis of oxidative stress markers. J. Appl. Anim. Welf. Sci. 2020, 24, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, N.; Gentile, L.; Scioli, E.; Alberti, J.P.; Esposito, L. Management models applied to the human-wolf conflict in agro-forestry-pastoral territories of two Italian protected areas and one Spanish game area. Animals 2021, 11, 1141. [Google Scholar] [CrossRef]
- Steffan, J.J.; Derby, J.A.; Brevik, E.C. Soil pathogens that may potentially cause pandemics, including severe acute respiratory syndrome (SARS) coronaviruses. Curr. Opin. Environ. Sci. Health 2020, 17, 35–40. [Google Scholar] [CrossRef]
- Santarém, V.A.; Rubinsky-Elefant, G.; Ferreira, M.U. Soil-Transmitted Helminthic Zoonoses in Humans and Associated Risk Factors. In Soil Contamination; Pascucci, S., Ed.; Intech Open: London, UK, 2011; pp. 43–66. [Google Scholar] [CrossRef]
- Gutiérrez, R.A.; Buchy, P. Contaminated soil and transmission of influenza virus (H5N1). Emerg. Infect. Dis. 2012, 18, 1530–1532. [Google Scholar] [CrossRef]
- Carlson, J.; Fischer, M.; Zani, L.; Eschbaumer, M.; Fuchs, W.; Mettenleiter, T.; Beer, M.; Blome, S. Stability of African Swine Fever Virus in Soil and Options to Mitigate the Potential Transmission Risk. Pathogens 2020, 9, 977. [Google Scholar] [CrossRef]
- Anand, U.; Bianco, F.; Suresh, S.; Tripathi, V.; Núñez-Delgado, A.; Race, M. SARS-CoV-2 and other viruses in soil: An environmental outlook. Environ. Res. 2021, 198, 111297. [Google Scholar] [CrossRef] [PubMed]
- Majewska, A.A.; Huang, T.; Han, B.; Drake, J.M. Predictors of zoonotic potential in helminths. Filos. Trans. Soc. R. Lond. B Biol. Sci. 2021, 376, 20200356. [Google Scholar] [CrossRef] [PubMed]
- Bevins, S.N.; Chandler, J.C.; Barrett, N.; Schmit, B.S.; Wiscomb, G.W.; Shriner, S.A. Plague Exposure in Mammalian Wildlife Across the Western United States. Vector Borne Zoonotic Dis. 2021, 21, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Samia, N.I.; Kausrud, K.L.; Heesterbeek, H.; Ageyev, V.; Begon, M.; Chan, K.S.; Stenseth, N.C. Dynamics of the plague-wildlife-human system in Central Asia are controlled by two epidemiological thresholds. Proc. Natl. Acad. Sci. USA 2011, 108, 14527–14532. [Google Scholar] [CrossRef]
- Hestvik, G.; Uhlhorn, H.; Koene, M.; Åkerström, S.; Malmsten, A.; Dahl, F.; Åhlén, P.A.; Dalin, A.M.; Gavier-Widén, D. Francisella tularensis in Swedish predators and scavengers. Epidemiol. Infect. 2019, 147, e293. [Google Scholar] [CrossRef]
- Mukarati, N.L.; Matope, G.; de Garine-Wichatitsky, M.; Ndhlovu, D.N.; Caron, A.; Pfukenyi, D.M. The pattern of anthrax at the wildlife-livestock-human interface in Zimbabwe. PLoS Negl. Trop. Dis. 2020, 14, e0008800. [Google Scholar] [CrossRef]
- Hugh-Jones, M.E.; de Vos, V. Anthrax and wildlife. Rev.—Spento Int. Epizoot. 2002, 21, 359–383. [Google Scholar] [CrossRef]
- González-Barrio, D.; Ruiz-Fons, F. Coxiella burnetii in wild mammals: A systematic review. Transbound. Emerg. Dis. 2019, 66, 662–671. [Google Scholar] [CrossRef]
- Sumampouw, O.; Risjani, Y. Bacteria as Indicators of Environmental Pollution: Review. Int. J. Ecosyst. Ecol. Sci. 2014, 4, 251–258. [Google Scholar] [CrossRef]
- Vidal-Martínez, V.M.; Wunderlich, A.C. Parasites as bioindicators of environmental degradation in Latin America: A meta-analysis. J. Helminthol. 2017, 91, 165–173. [Google Scholar] [CrossRef]
- Zaghloul, A.; Saber, M.; Gadow, S.; Awad, F. Biological indicators for pollution detection in terrestrial and aquatic ecosystems. Bull. Natl. Res. Cent. 2020, 44, 127. [Google Scholar] [CrossRef]
- Massei, G.; Kindberg, J.; Licoppe, A.; Gačić, D.; Šprem, N.; Kamler, J.; Baubet, E.; Hohmann, U.; Monaco, A.; Ozoliņš, J.; et al. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest. Manag. Sci. 2015, 71, 492–500. [Google Scholar] [CrossRef]
- Kamieniarz, R.; Panek, M. Game Animals in Poland at the Turn of the 20th and 21st Century; Stacja Badawcza OHZ PZŁ: Czempiń, Poland, 2008; ISBN 978-83-904442-9-1. (In Polish) [Google Scholar]
- Johann, F.; Handschuh, M.; Linderoth, P.; Dormann, C.F.; Arnold, J. Adaptation of wild boar (Sus scrofa) activity in a human-dominated landscape. BMC Ecol. 2020, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Anaya, A.; Herrero, J.; Rosell, C.; Couto, S.; García-Serrano, A. Food habits of wild boars (Sus scrofa) in a mediterranean coastal wetland. Wetlands 2008, 28, 197–203. [Google Scholar] [CrossRef]
- Ballari, S.; Barrios-García, M.N. A review of wild boar Sus scrofa diet and factors affecting food selection in native and introduced ranges. Mamm. Rev. 2014, 44, 124–134. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, E.J. Diet of the wild boar (Sus scrofa): Implications for management in forest-agricultural and urban environments in South Korea. PeerJ 2019, 7, e7835. [Google Scholar] [CrossRef]
- Baubet, E.; Roper-Coudert, Y.; Brandt, S. Seasonal and annual variation in earthworm consumption by wild boar (Sus scrofa scrofa L.). Eur. J. Wildl. Res. 2003, 30, 179–186. [Google Scholar] [CrossRef]
- Baubet, E.; Bonenfant, C.; Brandt, S. Diet of the wild boar in the French Alps. Galemys 2004, 16, 101–113. [Google Scholar]
- Humbert, J.; Henry, C. Studies on the prevalence and the transmission of lung and stomach nematodes of the wild boar (Sus scrofa) in France. J. Wildl. Dis. 1989, 25, 335–341. [Google Scholar] [CrossRef]
- Popiołek, M.; Knecht, D.; Szczęsna-Staśkiewicz, J.; Czerwińska-Rożałow, A. Helminths of the wild boar (Sus scrofa L.) in natural and breeding conditions. Bull. Vet. Inst. Pulawy 2010, 54, 161–166. [Google Scholar]
- Fernandez-de-Mera, I.G.; Gortazar, C.; Vicente, J.; Höfle, U.; Fierro, Y. Wild boar helminths: Risks in animal translocations. Vet. Parasitol. 2003, 115, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Nosal, P.; Kowal, J.; Nowosad, B. Structure of Metastrongylidae in wild boars from southern Poland. Helminthologia 2010, 47, 212–218. [Google Scholar] [CrossRef]
- Pacifico, L.; Sgadari, M.F.; D’Alessio, N.; Buono, F.; Restucci, B.; Sgroi, G.; Ottaviano, M.; Antoniciello, M.; Fioretti, A.; Tamponi, C.; et al. First description of Eucoleus garfiai (Gallego and Mas-Coma, 1975) in wild boar (Sus scrofa) in Italy. Parasitol. Res. 2022, 121, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Gállego, J.; Mas-Coma, S. Capillaria garfiai n. sp. (Nematoda-Trichuridae), a Parásito de la Mucosa Lingual del Jabalí, Sus scrofa Linnaeus, 1758 (Mammalia-artiodactyla). Vie Milieu 1975, 25, 237–248. [Google Scholar]
- Lowenstein, M.; Kutzer, E. Die Capillarien (Nematoda, Trichuridae) des Wildschweines (Sus scrofa) in Osterreich [Capillaria (Nematoda, Trichuridae) of wild swine (Sus scrofa) in Austria]. Angew. Parasitol. 1989, 30, 221–237. [Google Scholar]
- Masuda, A.; Kameyama, K.; Goto, M.; Narasaki, K.; Kondo, H.; Shibuya, H.; Matsumoto, J. Eucoleus garfiai (Gallego et Mas-Coma, 1975) (Nematoda: Capillariidae) infection in wild boars (Sus scrofa leucomystax) from the Amakusa Islands. J. Parasitol. 2019, 73, 101972. [Google Scholar] [CrossRef] [PubMed]
- Moretta, I.; Veronesi, F.; Di Paola, R.; Battistacci, L.; Moretti, A. Parasitological survey on wild boar (Sus scrofa) shot in the hunting season 2009–2010 in Umbria (central Italy). Large Anim. Rev. 2011, 17, 187–192. [Google Scholar]
- Maleki, B.; Dalimi, A.; Majidiani, H.; Badri, M.; Gorgipur, M.; Khorshidi, A. Parasitic Infections of Wild Boars (Sus scrofa) in Iran: A Literature Review. Infect. Disord. Drug Targets 2020, 20, 585–597. [Google Scholar] [CrossRef]
- Lowenstein, M.; Kutzer, E. Zur Verbreitung und Biologie von Capillaria garfiai aus Sus scrofa [The distribution and biology of Capillaria garfiai from Sus scrofa]. Appl Parasitol. 1993, 34, 51–62. [Google Scholar]
- Martano, M.; Altamura, G.; Power, K.; Liguori, P.; Restucci, B.; Borzacchiello, G.; Maiolino, P. Beclin 1, LC3 and P62 Expression in Equine Sarcoids. Animals 2021, 12, 20. [Google Scholar] [CrossRef]
- Altamura, G.; Power, K.; Martano, M.; Degli Uberti, B.; Galiero, G.; De Luca, G.; Maiolino, P.; Borzacchiello, G. Felis catus papillomavirus type-2 E6 binds to E6AP, promotes E6AP/p53 binding and enhances p53 proteasomal degradation. Sci. Rep. 2018, 8, 17529. [Google Scholar] [CrossRef] [PubMed]
- Eleni, C.; Corteggio, A.; Altamura, G.; Meoli, R.; Cocumelli, C.; Rossi, G.; Friedrich, K.G.; Di Cerbo, P.; Borzacchiello, G. Detection of Papillomavirus DNA in Cutaneous Squamous Cell Carcinoma and Multiple Papillomas in Captive Reptiles. J. Comp. Pathol. 2017, 57, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.J.; Lindsay, D.S.; Sriranganathan, N. Wild boars as sources for infectious diseases in livestock and humans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2697–2707. [Google Scholar] [CrossRef] [PubMed]
- Gazzonis, A.L.; Villa, L.; Riehn, K.; Hamedy, A.; Minazzi, S.; Olivieri, E.; Zanzani, S.A.; Manfredi, M.T. Occurrence of selected zoonotic food-borne parasites and first molecular identification of Alaria alata in wild boars (Sus scrofa) in Italy. Parasitol. Res. 2018, 117, 2207–2215. [Google Scholar] [CrossRef]
- Gazzonis, A.L.; Gjerde, B.; Villa, L.; Minazzi, S.; Zanzani, S.A.; Riccaboni, P.; Sironi, G.; Manfredi, M.T. Prevalence and molecular characterisation of Sarcocystis miescheriana and Sarcocystis suihominis in wild boars (Sus scrofa) in Italy. Parasitol Res. 2019, 118, 1271–1287. [Google Scholar] [CrossRef]
- Perry, I. Altitudinal Adaptations of Earthworms. PhD Thesis, Cardiff University, Cardiff, UK, 2020. [Google Scholar]
- Reynolds, J.W. Contribution to North American Earthworms (Anellida), No. 2. The activity and distribution of earthworms in tulip poplar stands in the Great Smoky Mountains National Park, Sevier County, Tennessee (Acanthodrilidae, Lumbricidae and Megascolecidae). Bull. Tall Timbers Res. Stn. 1972, 11, 41–54. [Google Scholar]
- González, G.; García, E.; Cruz, V.; Borges, S.; Zalamea, M.; Rivera, M.M. Earthworm communities along an elevation gradient in Northeastern Puerto Rico. Eur. J. Soil Biol. 2007, 43, S24–S32. [Google Scholar] [CrossRef]
- Darmi, D.; Rizwar, R.; Helmiyetti, H. Abundance and Distribution Patterns of Megadrilli Earthworms at Different Altitude in Kabawetan Tea Plantation, Bengkulu. In Proceedings of the 3rd KOBI Congress, International and National Conferences (KOBICINC 2020), Bengkulu, Indonesia, 24–25 November 2020. [Google Scholar]
- Santiago Bass, C.; Weis, J.S. Increased abundance of snails and trematode parasites of Fundulus heteroclitus (L.) in restored New Jersey wetlands. Wetl. Ecol. Manag. 2008, 16, 173–182. [Google Scholar] [CrossRef]
- Arneberg, P.; Skorping, A.; Grenfell, B.; Read, A. Host densities as determinants of abundance in parasite communities. Proc. R. Soc. Lond. B Biol. Sci. 1998, 265, 1283–1289. [Google Scholar] [CrossRef]
- Nagy, G.; Csivincsik, A.; Sugár., L. Wild boar density drives Metastrongylus infection in earthworm. Acta Parasitol. 2015, 60, 35–39. [Google Scholar] [CrossRef]
- Fründ, H.C.; Osnabrück, H.; Graefe, U. Earthworms as Bioindicators of Soil Quality. In Biology of Earthworms. Soil Biology; Karaca, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 24. [Google Scholar] [CrossRef]
- Ahmed, N.; Al-Mutairi, K.A. Earthworms Effect on Microbial Population and Soil Fertility as Well as Their Interaction with Agriculture Practices. Sustainability 2022, 14, 7803. [Google Scholar] [CrossRef]
- Römbke, J.; Jänsch, S.; Didden, W. The use of earthworms in ecological soil classification and assessment concepts. Ecotoxicol. Environ. Saf. 2005, 62, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, R.J.; Paoletti, M.G. Australian earthworms as a natural agroecological resource. Ann. Arid Zone 2006, 45, 309–330. [Google Scholar]
- Falco, L.B.; Sandler, R.; Momo, F.; Di Ciocco, C.; Saravia, L.; Coviella, C. Earthworm assemblages in a different intensity of agricultural uses and their relation to edaphic variables. PeerJ 2015, 3, e979. [Google Scholar] [CrossRef]
- Stockdale, E.; Watson, C. Biological indicators of soil quality in organic farming systems. Agric. Food Sci. 2009, 24, 308–318. [Google Scholar] [CrossRef]
- Ezzat, S.M.; El Bouraie, M.M. Role of Bacteria as Bioindicators for Organochlorine Pesticides Residues in Groundwater. Life Sci. J. 2014, 11, 895–910. [Google Scholar]
- Vega-Ávila, A.; Medina, E.; Paroldi, H.; Toro, M.; Baigori, M.; Vázquez, F. Bioindicators of soil quality of open shrubland and vineyards. J. Soil Sci. Plant Nutr. 2018, 18, 1065–1079. [Google Scholar] [CrossRef]
- Jaafar, R.S. The Potential Role of Soil Bacteria as an Indicator of Heavy Metal Pollution in Southern, Iraq. Baghdad Sci. J. 2022, 19, 0753. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).