Intestinal Microbiota of Anser fabalis Wintering in Two Lakes in the Middle and Lower Yangtze River Floodplain
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
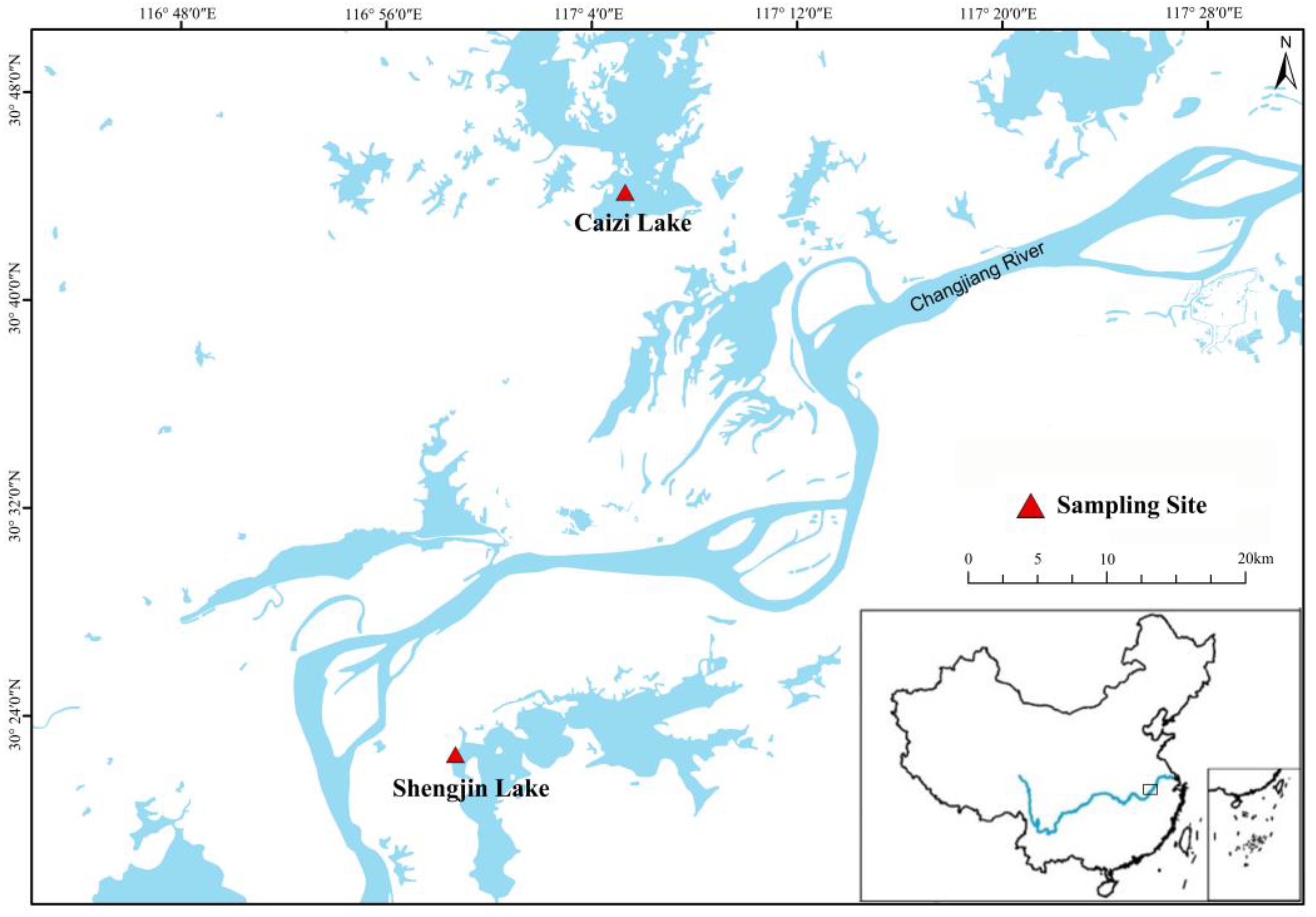
2.1. Study Areas
2.2. Sample Collection
2.3. DNA Extraction and Sequencing
2.4. Sequencing Data Analysis
2.5. Data Deposition
3. Results
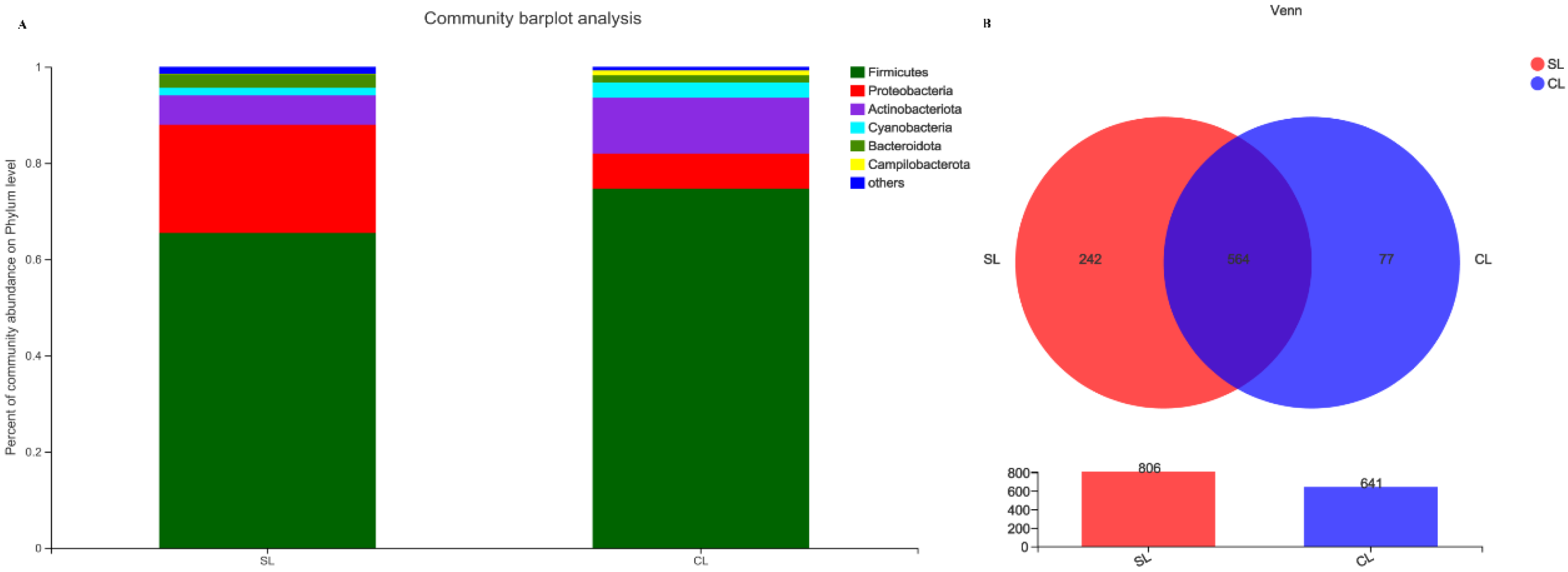
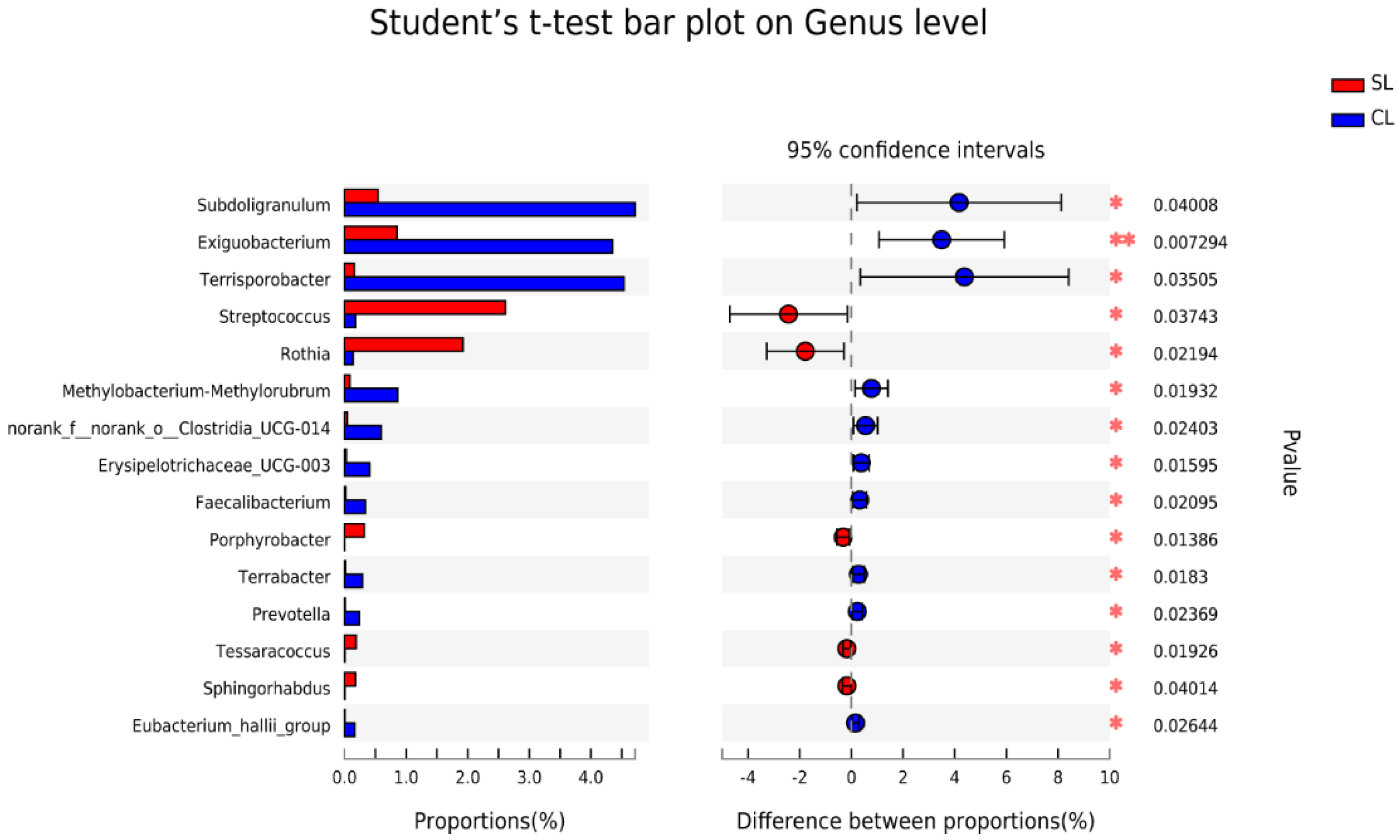
3.1. Comparative Analysis of Intestinal Microbiota Composition of SL and CL A. fabalis
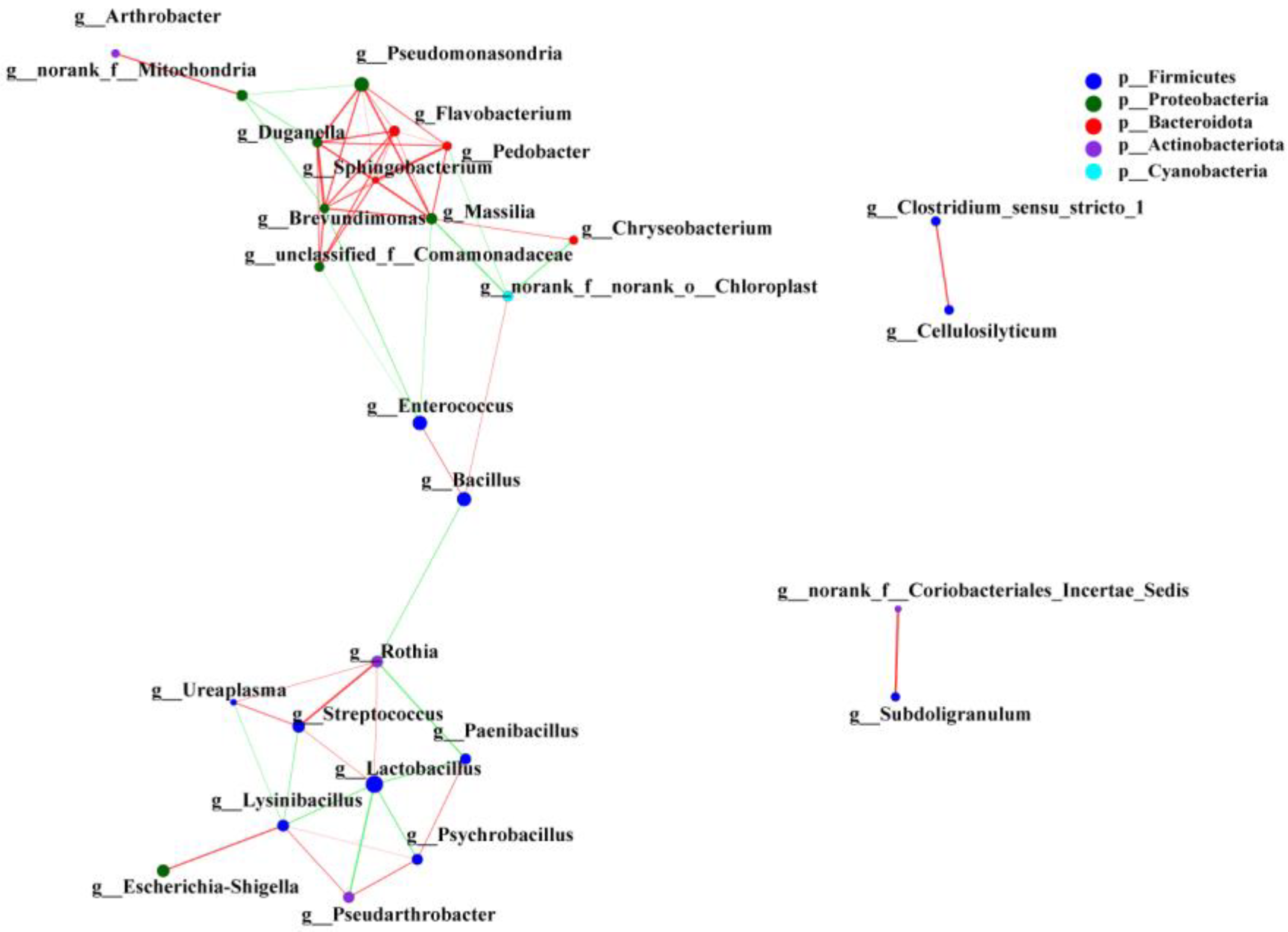
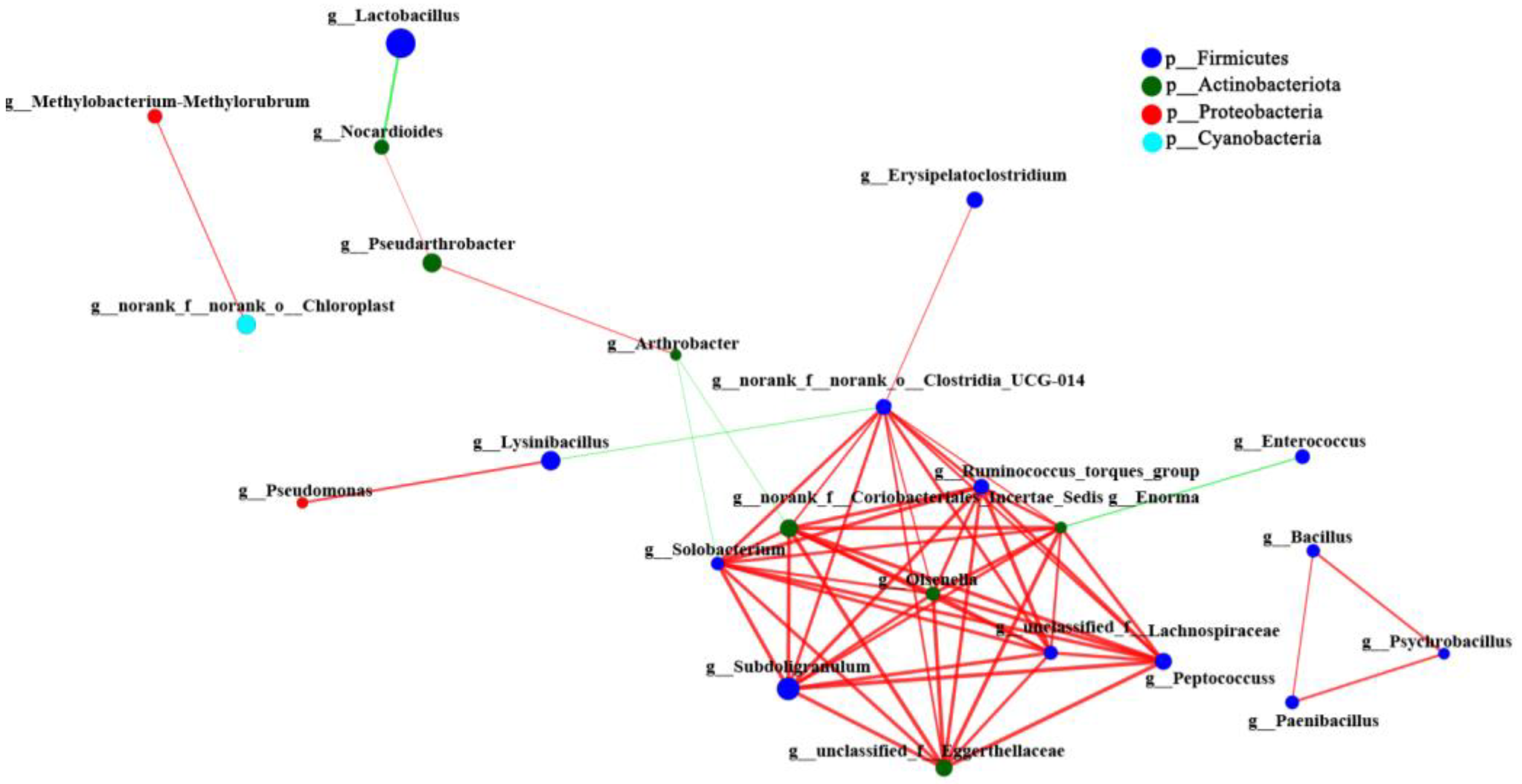
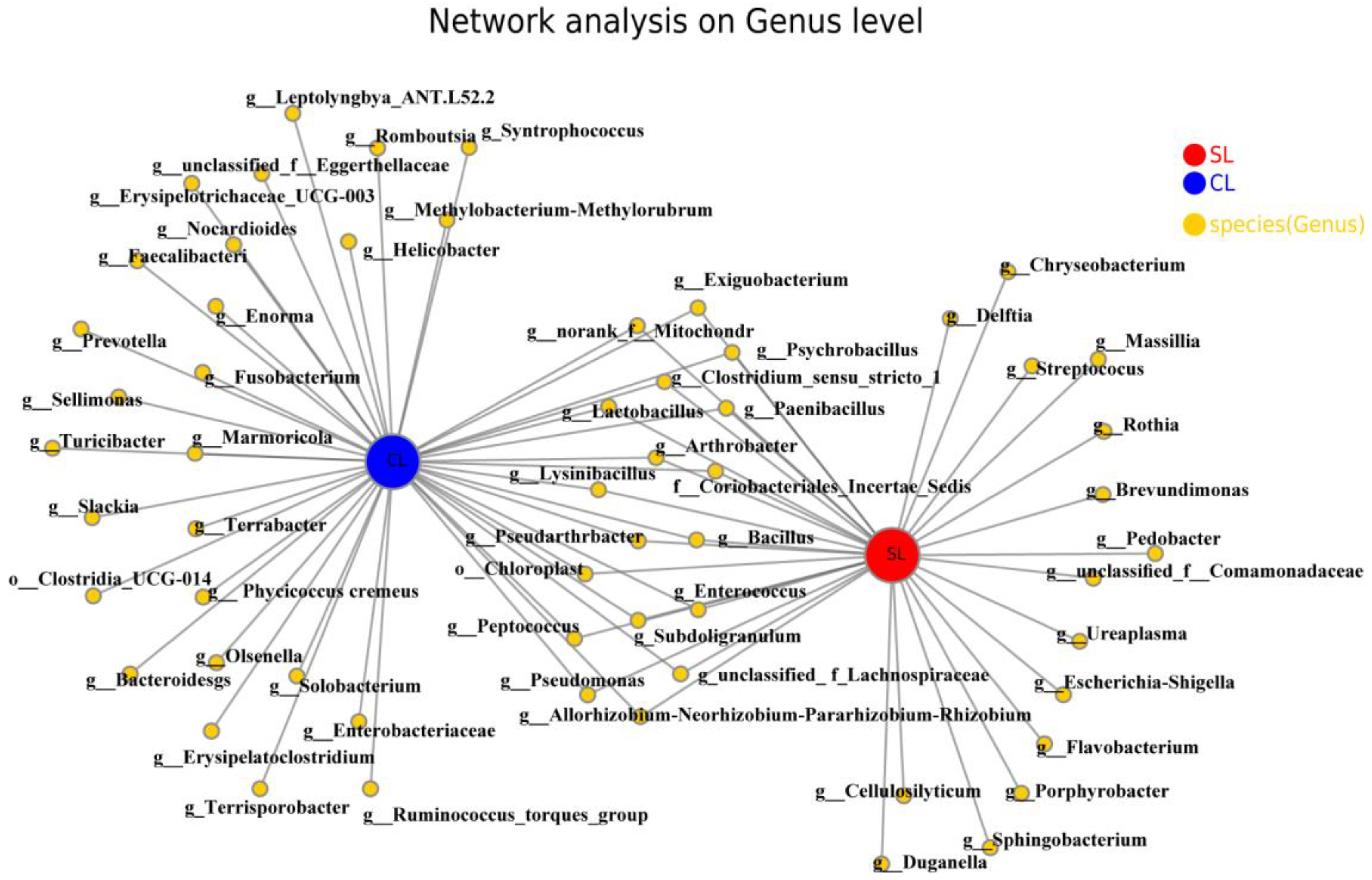
3.2. Interaction Analysis of Intestinal Microbiota Network of SL and CL A. fabalis
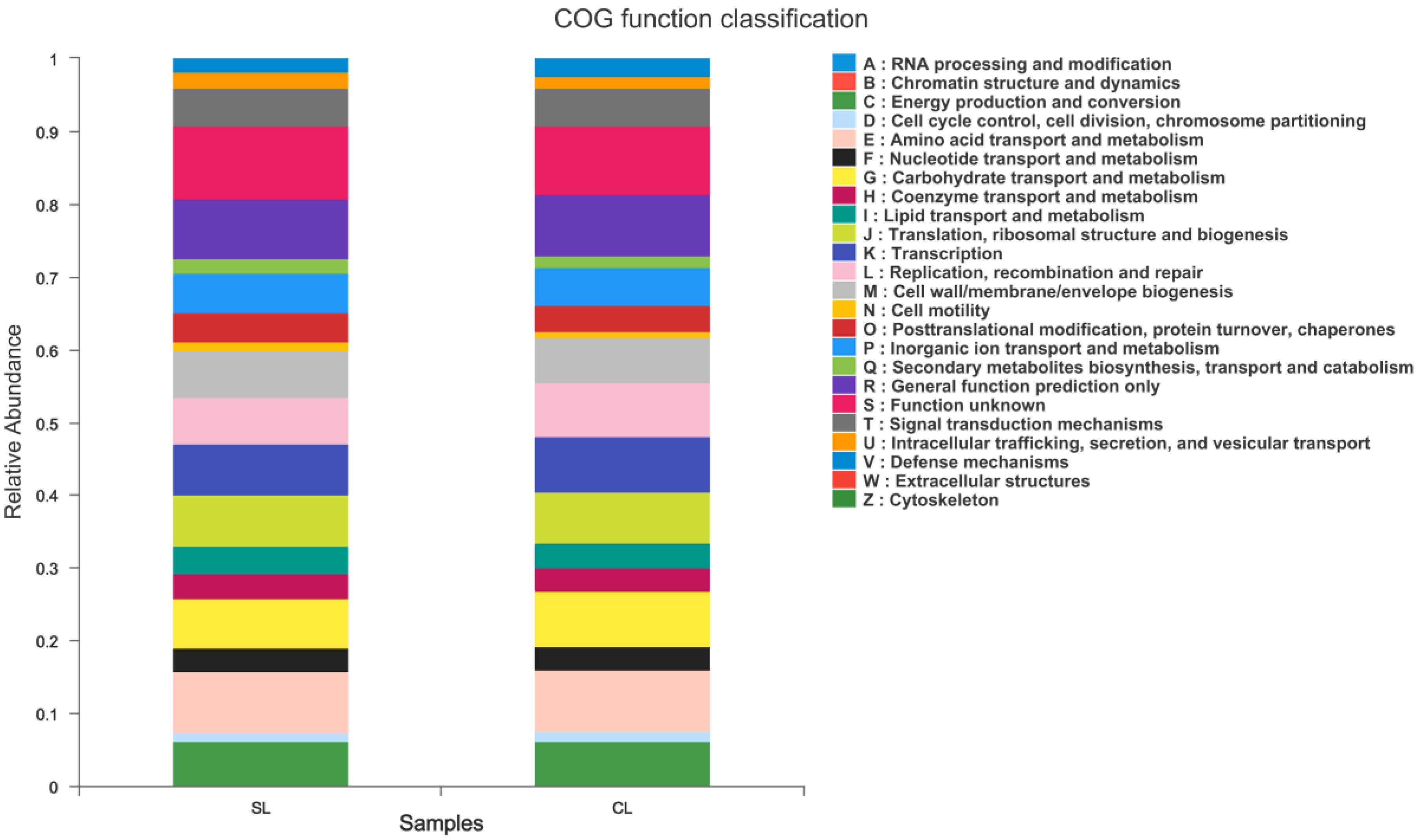
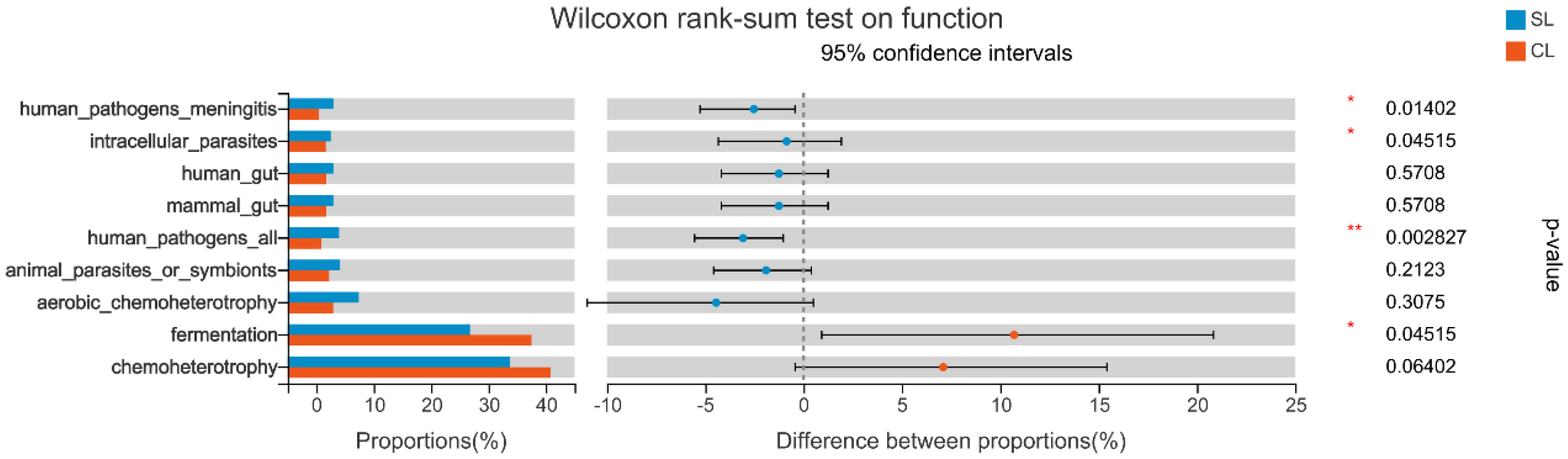
3.3. Functional Comparative Analysis of Intestinal Microbiota Genomes of SL and CL A. fabalis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, H.; Lee, W.Y. Interspecific comparison of the fecal microbiota structure in three Arctic migratory bird species. Ecol. Evol. 2020, 10, 5582–5594. [Google Scholar] [CrossRef] [PubMed]
- Brunthaler, R.; Teufelbauer, N.; Seaman, B.; Nedorost, N.; Bittermann, K.; Matt, J.; Weissenbacher-Lang, C.; Weissenböck, H. Trichomonosis in Austrian Songbirds-Geographic Distribution, Pathological Lesions and Genetic Characterization over Nine Years. Animals 2022, 12, 1306. [Google Scholar] [CrossRef] [PubMed]
- Basova, M.; Krasheninnikova, S.; Parrino, V. Intra-Decadal (2012–2021) Dynamics of Spatial Ichthyoplankton Distribution in Sevastopol Bay (Black Sea) Affected by Hydrometeorological Factors. Animals 2022, 12, 3317. [Google Scholar] [CrossRef]
- Wang, W.; Wang, A.; Yang, Y.; Wang, F.; Liu, Y.; Zhang, Y.; Sharshov, K.; Gui, L. Composition, diversity and function of gastrointestinal microbiota in wild red-billed choughs (Pyrrhocorax pyrrhocorax). Inte. Micr. 2019, 22, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Chen, J.; Liu, K.; Tang, M.; Yang, Y. The avian gut microbiota: Diversity, influencing factors, and future directions. Front. Microbiol. 2022, 13, 934272. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, C.K.; Chen, Y.Y.; Fang, A.; Shaw, G.T.; Hung, C.M.; Wang, D. Maternal gut microbes shape the early-life assembly of gut microbiota in passerine chicks via nests. Microbiome 2020, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Kirsten, G.; Sandercock, B.K.; Ari, J.; Zeglin, L.H. The avian gut microbiota: Community, physiology and function in wild birds. J. Avian Biol. 2018, 49, e01788. [Google Scholar]
- Dong, Y.; Xiang, X.; Zhao, G.; Song, Y.; Zhou, L. Variations in gut bacterial communities of hooded crane (Grus monacha) over spatial-temporal scales. PeerJ 2019, 7, e7045. [Google Scholar] [CrossRef]
- Góngora, E.; Elliott, K.H.; Whyte, L. Gut microbiome is affected by inter-sexual and inter-seasonal variation in diet for thick-billed murres (Uria lomvia). Sci. Rep. 2021, 11, 1200. [Google Scholar] [CrossRef]
- Liu, G.; Gong, Z.; Li, Q. Variations in gut bacterial communities between lesser white-fronted geese wintering at Caizi and Shengjin lakes in China. Microbiologyopen 2020, 9, e1037. [Google Scholar] [CrossRef]
- Dehgany-Asl, S.; Allymehr, M.; Talebi, A.; Yosefi, O.; Allahyari, E. Monitoring of aquatic birds and surveillance of avian influenza and Newcastle disease of waterfowls at the National Park of Urmia Lake. Vet. Med. Sci. 2022, 8, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- Parrino, V.; Costa, G.; Cannavà, C.; Fazio, E.; Bonsignore, M.; Concetta, S.; Piccione, G.; Fazio, F. Flow cytometry and micro-Raman spectroscopy: Identification of hemocyte populations in the mussel Mytilus galloprovincialis (Bivalvia: Mytilidae) from Faro Lake and Tyrrhenian Sea (Sicily, Italy). Fish Shellfish Immun. 2019, 87, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Capunitan, D.C.; Johnson, O.; Terrill, R.S.; Hird, S.M. Evolutionary signal in the gut microbiomes of 74 bird species from Equatorial Guinea. Mol. Ecol. 2020, 29, 829–847. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, Y.; Gong, M.; Zheng, C.; Zhang, C.; Li, H.; Wen, W.; Wang, Y.; Liu, G. Diet-induced microbiome shifts of sympatric overwintering birds. Appl. Microbiol. Biotechnol. 2021, 105, 5993–6005. [Google Scholar] [CrossRef]
- Stanley, C.Q.; Hallager, S.H.; Dudash, M.R.; Marra, P.P. Food limitation modulates the endogenous control of spring migratory behavior in a captive long-distance migratory bird population. Behavi. Ecol. Sociobiol. 2022, 76, 136. [Google Scholar] [CrossRef]
- Tian, H.; Solovyeva, D.; Danilov, G.; Vartanyan, S.; Wen, L.; Lei, J.; Lu, C.; Bridgewater, P.; Lei, G.; Zeng, Q. Combining modern tracking data and historical records improves understanding of the summer habitats of the Eastern Lesser White-fronted Goose Anser erythropus. Ecol. Evol. 2021, 11, 4126–4139. [Google Scholar] [CrossRef]
- Cao, L.; Fox, A.D. Birds and people both depend on China’s wetlands. Nature 2009, 4, 173. [Google Scholar] [CrossRef]
- Wang, W.; Cao, J.; Yang, F.; Wang, X.; Zheng, S.; Sharshov, K.; Li, L. High-throughput sequencing reveals the core gut microbiome of Bar-headed goose (Anser indicus) in different wintering areas in Tibet. Microbiologyopen 2016, 5, 287–295. [Google Scholar] [CrossRef]
- Eren, A.M.; Vineis, J.H.; Morrison, H.G.; Sogin, M.L. A Filtering Method to Generate High Quality Short Reads Using Illumina Paired-End Technology. PLoS ONE 2013, 8, e66643. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. Isme J. 2012, 6, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Faith, D.P.; Baker, A.M. Phylogenetic diversity (PD) and biodiversity conservation: Some bioinformatics challenges. Evol. Bioinform. 2006, 2, 121–128. [Google Scholar] [CrossRef]
- Neufeld, J.D.; Mohn, W.W. Unexpectedly high bacterial diversity in arctic tundra relative to boreal forest soils, revealed by serial analysis of ribosomal sequence tags. Appl. Environ. Microbiol. 2005, 71, 5710–5718. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Geier, M.S.; Hughes, R.J.; Denman, S.E.; Moore, R.J. Highly Variable Microbiota Development in the Chicken Gastrointestinal Tract. PLoS ONE 2013, 8, e84290. [Google Scholar] [CrossRef]
- Schiff, S.L.; Tsuji, J.M.; Wu, L.; Venkiteswaran, J.J.; Molot, L.A.; Elgood, R.J.; Paterson, M.J.; Neufeld, J.D. Millions of Boreal Shield Lakes can be used to Probe Archaean Ocean Biogeochemistry. Sci. Rep. 2017, 7, 46708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, Z.; Zhu, L. Gut microbiome of migratory shorebirds: Current status and future perspectives. Ecol. Evol. 2021, 11, 3737–3745. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, S.; Sharshov, K.; Sun, H.; Yang, F.; Wang, X.; Li, L.; Xiao, Z. Metagenomic profiling of gut microbial communities in both wild and artificially reared Bar-headed goose (Anser indicus). Microbiologyopen 2017, 6, e00429. [Google Scholar] [CrossRef]
- Ryu, H.; Grond, K.; Verheijen, B.; Elk, M.; Buehler, D.M.; Santo Domingo, J.W. Intestinal microbiota and species diversity of Campylobacter and Helicobacter spp. in migrating shorebirds in Delaware Bay. Appl. Environ. Microbiol. 2014, 80, 1838–1847. [Google Scholar] [CrossRef]
- Zhao, M.; Cong, P.; Barter, M.; Fox, A.D.; Cao, L. The changing abundance and distribution of Greater White-fronted Geese Anser albifrons in the Yangtze River floodplain: Impacts of recent hydrological changes. Bird Conserv. Int. 2012, 22, 135–143. [Google Scholar] [CrossRef]
- Wu, N.; Yang, X.; Zhang, R.; Li, J.; Xiao, X.; Ecology, Y.H.J.M. Dysbiosis Signature of Fecal Microbiota in Colorectal Cancer Patients. Microb. Ecol. 2013, 66, 462–470. [Google Scholar] [CrossRef]

| Sampling Period | Species | Sampling Place | Sampling Habitat | Number |
|---|---|---|---|---|
| 10 November 2021–4 March 2022 | Anser fabalis | Yang etou | vast expanse of grassland at waterside | 60 |
| 16 November 2021–12 March 2022 | Anser fabalis | Caizi Lake | vast expanse of grassland at waterside | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Zhou, D.; Ge, M.; Zhang, Y.; Li, W.; Han, Y.; He, G.; Shi, S. Intestinal Microbiota of Anser fabalis Wintering in Two Lakes in the Middle and Lower Yangtze River Floodplain. Animals 2023, 13, 707. https://doi.org/10.3390/ani13040707
Zhao K, Zhou D, Ge M, Zhang Y, Li W, Han Y, He G, Shi S. Intestinal Microbiota of Anser fabalis Wintering in Two Lakes in the Middle and Lower Yangtze River Floodplain. Animals. 2023; 13(4):707. https://doi.org/10.3390/ani13040707
Chicago/Turabian StyleZhao, Kai, Duoqi Zhou, Mengrui Ge, Yixun Zhang, Wenhui Li, Yu Han, Guangyu He, and Shuiqin Shi. 2023. "Intestinal Microbiota of Anser fabalis Wintering in Two Lakes in the Middle and Lower Yangtze River Floodplain" Animals 13, no. 4: 707. https://doi.org/10.3390/ani13040707
APA StyleZhao, K., Zhou, D., Ge, M., Zhang, Y., Li, W., Han, Y., He, G., & Shi, S. (2023). Intestinal Microbiota of Anser fabalis Wintering in Two Lakes in the Middle and Lower Yangtze River Floodplain. Animals, 13(4), 707. https://doi.org/10.3390/ani13040707






