Laterality in the Damaraland Mole-Rat: Insights from a Eusocial Mammal
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Data Acquisition
2.2. Animal Capture and Husbandry
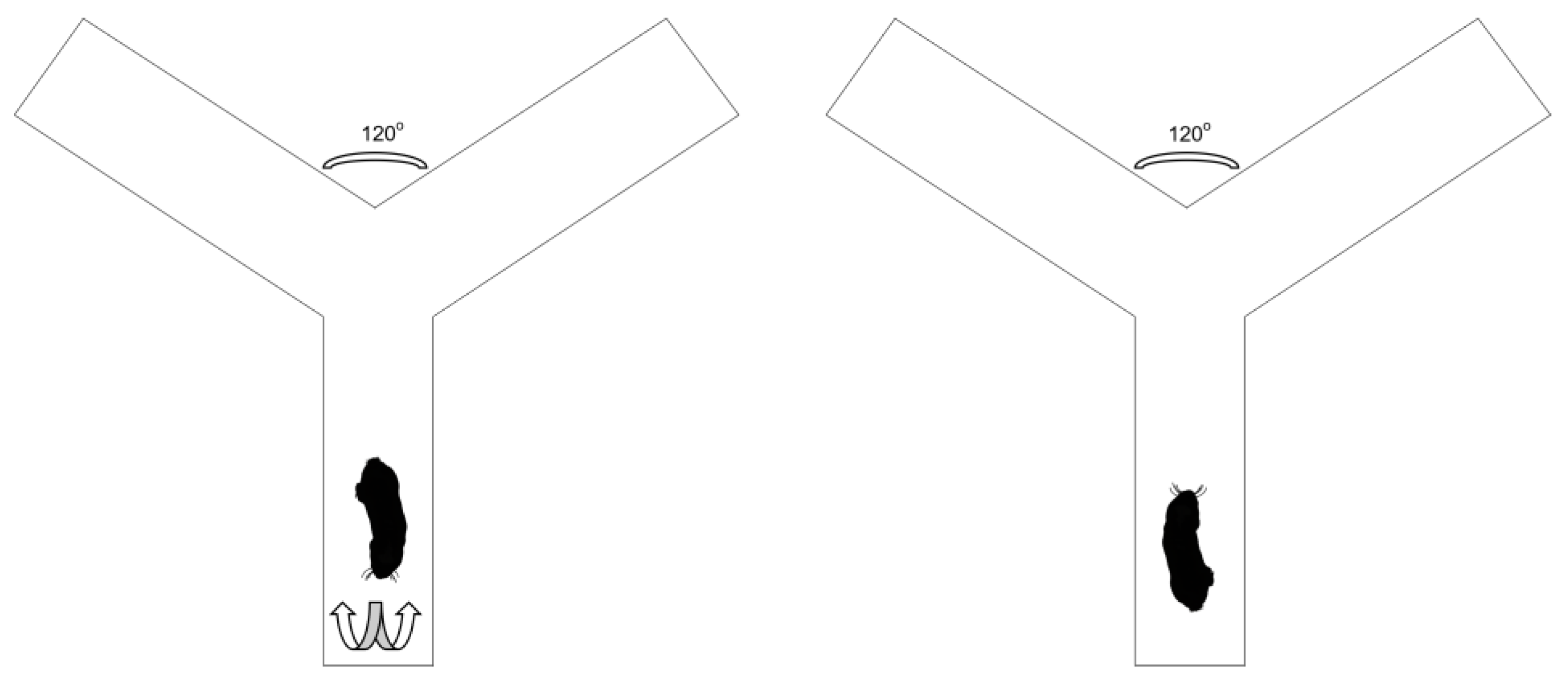
2.3. Experimental Design
2.4. Determination of Turning Biases, Laterality Index and Absolute Laterality
2.5. Statistical Analysis
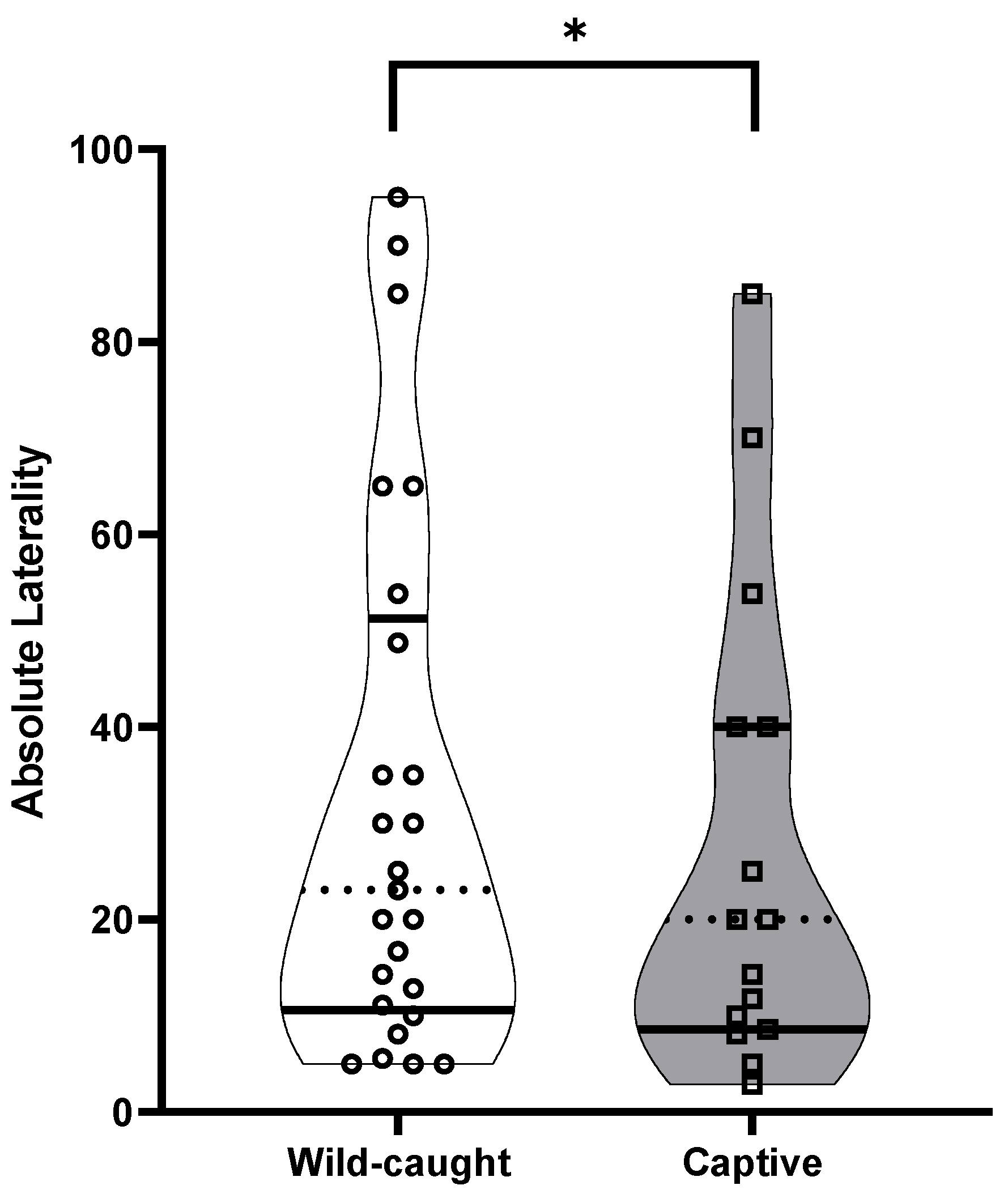
3. Results
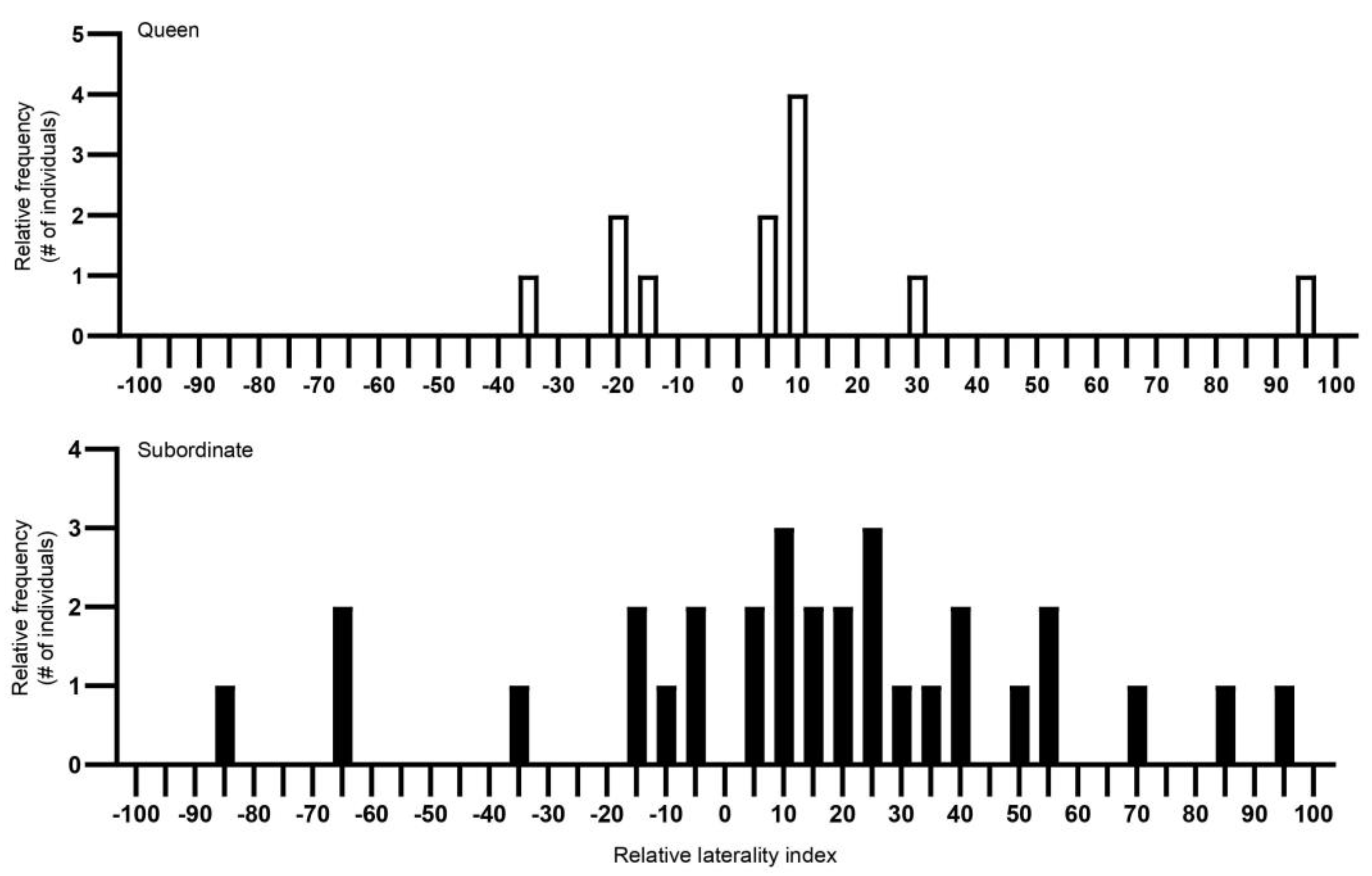
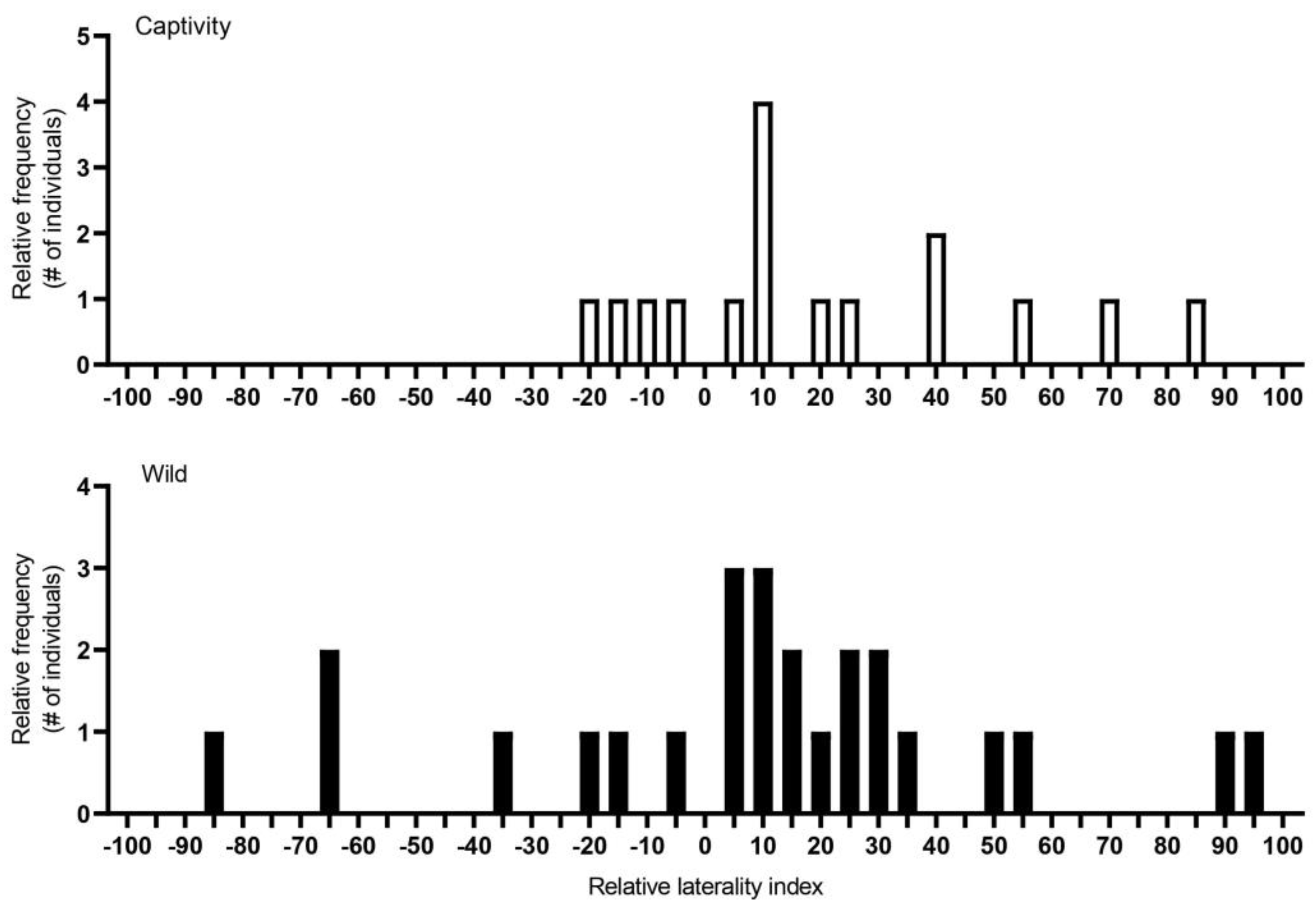
3.1. Individual Turning Biases
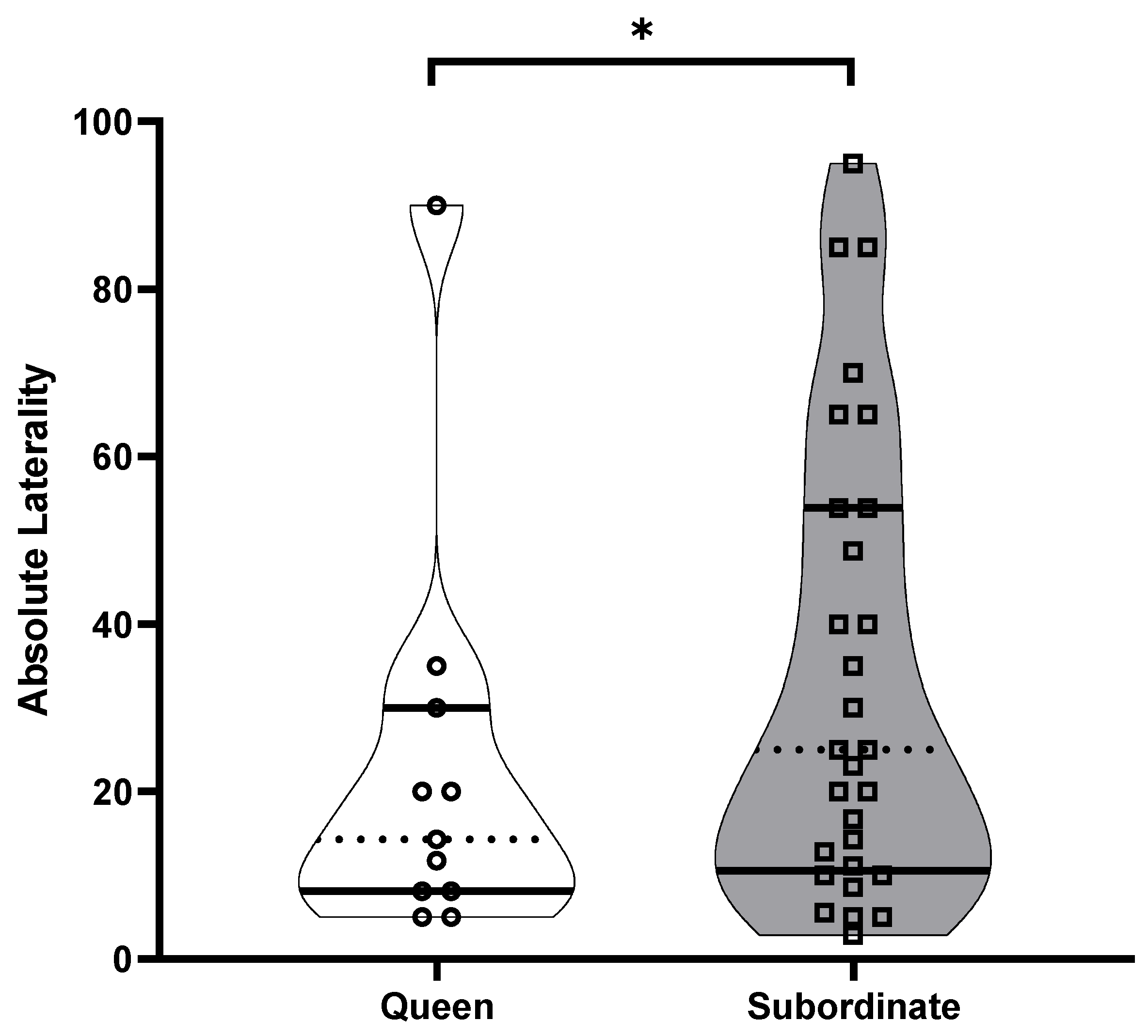
3.2. Individual Laterality, Population-Level Laterality and Strength of Direction of Laterality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonati, B.; Csermely, D.; Sovrano, V.A. Looking at a predator with the left or right eye: Asymmetry of response in lizards. Later. Asymmetries Body Brain Cogn. 2013, 18, 329–339. [Google Scholar] [CrossRef]
- George, I. Hemispheric asymmetry of songbirds. In The Two Halves of the Brain: Information Processing in the Cerebral Hemispheres; Boston Review: Boston, MA, USA, 2010; pp. 91–120. [Google Scholar]
- Bisazza, A.; Rogers, L.J.; Vallortigara, G. The origins of cerebral asymmetry: A review of evidence of behavioural and brain lateralization in fishes, reptiles and amphibians. Neurosci. Biobehav. Rev. 1998, 22, 411–426. [Google Scholar] [CrossRef]
- Petrazzini, M.E.M.; Sovrano, V.A.; Vallortigara, G.; Messina, A. Brain and behavioral asymmetry: A lesson from fish. Front. Neuroanat. 2020, 14, 11. [Google Scholar] [CrossRef]
- Frasnelli, E. Brain and behavioral lateralization in invertebrates. Front. Psychol. 2013, 4, 939. [Google Scholar] [CrossRef]
- Dadda, M.; Agrillo, C.; Bisazza, A.; Brown, C. Laterality enhances numerical skills in the guppy, Poecilia reticulata. Front. Behav. Neurosci. 2015, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Frasnelli, E.; Vallortigara, G.; Rogers, L.J. Left–right asymmetries of behaviour and nervous system in invertebrates. Neurosci. Biobehav. Rev. 2012, 36, 1273–1291. [Google Scholar] [CrossRef]
- Berlinghieri, F.; Panizzon, P.; Penry-Williams, I.L.; Brown, C. Laterality and fish welfare—A review. Appl. Anim. Behav. Sci. 2021, 236, 105239. [Google Scholar] [CrossRef]
- Versace, E.; Caffini, M.; Werkhoven, Z.; de Bivort, B.L. Individual, but not population asymmetries, are modulated by social environment and genotype in Drosophila melanogaster. Sci. Rep. 2020, 10, 4480. [Google Scholar] [CrossRef]
- Rogers, L.J. Relevance of brain and behavioural lateralization to animal welfare. Appl. Anim. Behav. Sci. 2010, 127, 1–11. [Google Scholar] [CrossRef]
- Chivers, D.P.; McCormick, M.I.; Allan, B.J.; Mitchell, M.D.; Gonçalves, E.J.; Bryshun, R.; Ferrari, M.C. At odds with the group: Changes in lateralization and escape performance reveal conformity and conflict in fish schools. Proc. R. Soc. B Biol. Sci. 2016, 283, 20161127. [Google Scholar] [CrossRef]
- McGreevy, P.; Rogers, L. Motor and sensory laterality in thoroughbred horses. Appl. Anim. Behav. Sci. 2005, 92, 337–352. [Google Scholar] [CrossRef]
- Rogers, L.J.; Andrew, R. Comparative Vertebrate Lateralization; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Rogers, L.J. Brain lateralization and cognitive capacity. Animals 2021, 11, 1996. [Google Scholar] [CrossRef]
- Concha, M.L.; Bianco, I.H.; Wilson, S.W. Encoding asymmetry within neural circuits. Nat. Rev. Neurosci. 2012, 13, 832–843. [Google Scholar] [CrossRef]
- Rogers, L.J.; Vallortigara, G.; Andrew, R.J. Divided Brains: The Biology and Behaviour of Brain Asymmetries; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Vallortigara, G.; Rogers, L. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 2005, 28, 595–596. [Google Scholar] [CrossRef]
- Cameron, R.; Rogers, L. Hand preference of the common marmoset (Callithrix jacchus): Problem solving and responses in a novel setting. J. Comp. Psychol. 1999, 113, 149. [Google Scholar] [CrossRef]
- Vallortigara, G. The evolutionary psychology of left and right: Costs and benefits of lateralization. Dev. Psychobiol. J. Int. Soc. Dev. Psychobiol. 2006, 48, 418–427. [Google Scholar] [CrossRef]
- Ghirlanda, S.; Vallortigara, G. The evolution of brain lateralization: A game-theoretical analysis of population structure. Proc. R. Soc. London. Ser. B Biol. Sci. 2004, 271, 853–857. [Google Scholar] [CrossRef]
- Wiper, M.L. Evolutionary and mechanistic drivers of laterality: A review and new synthesis. Lateral. Asymmetries Body Brain Cogn. 2017, 22, 740–770. [Google Scholar] [CrossRef] [PubMed]
- Cowell, P.E.; Denenberg, V.H. Development of laterality and the role of the corpus callosum in rodents and humans. In Comparative Vertebrate Lateralization; Cambridge University Press: Cambridge, UK, 2002; pp. 274–305. [Google Scholar]
- Byrne, R.A.; Kuba, M.J.; Meisel, D.V. Lateralized eye use in Octopus vulgaris shows antisymmetrical distribution. Anim. Behav. 2004, 68, 1107–1114. [Google Scholar] [CrossRef]
- Frasnelli, E.; Vallortigara, G. Individual-level and population-level lateralization: Two sides of the same coin. Symmetry 2018, 10, 739. [Google Scholar] [CrossRef]
- Gatto, E.; Agrillo, C.; Brown, C.; Dadda, M. Individual differences in numerical skills are influenced by brain lateralization in guppies (Poecilia reticulata). Intelligence 2019, 74, 12–17. [Google Scholar] [CrossRef]
- D’antonio-Bertagnolli, A.J.; Anderson, M.J. Lateral asymmetry in the freely occurring behaviour of budgerigars (Melopsittacus undulatus) and its relation to cognitive performance. Lateral. Asymmetries Body Brain Cogn. 2018, 23, 344–363. [Google Scholar] [CrossRef]
- Piddington, T.; Rogers, L. Strength of hand preference and dual task performance by common marmosets. Anim. Cogn. 2013, 16, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, E.E.; Pouca, C.V.; Brown, C. Laterality strength is linked to stress reactivity in Port Jackson sharks (Heterodontus portusjacksoni). Behav. Brain Res. 2016, 305, 239–246. [Google Scholar] [CrossRef]
- Barnard, S.; Matthews, L.; Messori, S.; Podaliri-Vulpiani, M.; Ferri, N. Laterality as an indicator of emotional stress in ewes and lambs during a separation test. Anim Cogn 2016, 19, 207–214. [Google Scholar] [CrossRef]
- Ghirlanda, S.; Frasnelli, E.; Vallortigara, G. Intraspecific competition and coordination in the evolution of lateralization. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 861–866. [Google Scholar] [CrossRef]
- Denenberg, V.H. Hemispheric laterality in animals and the effects of early experience. Behav. Brain Sci. 1981, 4, 1–21. [Google Scholar] [CrossRef]
- Lehman, R.A. Lateralized asymmetry of behavior in animals at the population and individual level. Behav. Brain Sci. 1981, 4, 28. [Google Scholar] [CrossRef]
- Rogers, L.; Frasnelli, E.; Versace, E. Lateralized antennal control of aggression and sex differences in red mason bees, Osmia bicornis. Sci. Rep. 2016, 6, 29411. [Google Scholar] [CrossRef]
- Anfora, G.; Frasnelli, E.; Maccagnani, B.; Rogers, L.J.; Vallortigara, G. Behavioural and electrophysiological lateralization in a social (Apis mellifera) but not in a non-social (Osmia cornuta) species of bee. Behav. Brain Res. 2010, 206, 236–239. [Google Scholar] [CrossRef]
- Jacobs, P.J.; Oosthuizen, M.K. Laterality in the Cape mole-rat, Georychus capensis. Behav. Process. 2021, 185, 104346. [Google Scholar] [CrossRef] [PubMed]
- Faulkes, C.G.; Bennett, N.C. Social evolution in African mole-rats–a comparative overview. In Extraordinary Biology of the Naked Mole-Rat; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–33. [Google Scholar] [CrossRef]
- Bennett, N.C.; Faulkes, C.G. African Mole-Rats: Ecology and Eusociality; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Nowak, M.A.; Tarnita, C.E.; Wilson, E.O. The evolution of eusociality. Nature 2010, 466, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Crespi, B.J.; Yanega, D. The definition of eusociality. Behav. Ecol. 1995, 6, 109–115. [Google Scholar] [CrossRef]
- Wilson, E.O.; Hölldobler, B. Eusociality: Origin and consequences. Proc. Natl. Acad. Sci. USA 2005, 102, 13367–13371. [Google Scholar] [CrossRef]
- Hamilton, W.D. The evolution of altruistic behavior. Am. Nat. 1963, 97, 354–356. [Google Scholar] [CrossRef]
- Gardner, A.; Grafen, A. Capturing the superorganism: A formal theory of group adaptation. J. Evol. Biol. 2009, 22, 659–671. [Google Scholar] [CrossRef]
- Lovegrove, B. The evolution of eusociality in molerats (Bathyergidae): A question of risks, numbers, and costs. Behav. Ecol. Sociobiol. 1991, 28, 37–45. [Google Scholar] [CrossRef]
- Ratnieks, F.L.; Foster, K.R.; Wenseleers, T. Conflict resolution in insect societies. Annu. Rev. Entomol. 2006, 51, 581–608. [Google Scholar] [CrossRef]
- Burland, T.M.; Bennett, N.C.; Jarvis, J.U.; Faulkes, C.G. Eusociality in African mole-rats: New insights from patterns of genetic relatedness in the Damaraland mole-rat (Cryptomys damarensis). Proc. R. Soc. London Ser. B Biol. Sci. 2002, 269, 1025–1030. [Google Scholar] [CrossRef]
- Jarvis, J.U.; O’Riain, M.J.; Bennett, N.C.; Sherman, P.W. Mammalian eusociality: A family affair. Trends Ecol. Evol. 1994, 9, 47–51. [Google Scholar] [CrossRef]
- Michener, C.D. Comparative social behavior of bees. Annu. Rev. Entomol. 1969, 14, 299–342. [Google Scholar] [CrossRef]
- Sherman, P.W.; Lacey, E.A.; Reeve, H.K.; Keller, L. The eusociality continuum. Behav. Ecol. 1995, 6, 102–108. [Google Scholar] [CrossRef]
- Oosthuizen, M.K. Exploratory behaviour, memory and neurogenesis in the social Damaraland mole-rat (Fukomys damarensis). J. Exp. Biol. 2020, 223, jeb221093. [Google Scholar] [CrossRef] [PubMed]
- Francioli, Y.; Thorley, J.; Finn, K.; Clutton-Brock, T.; Zöttl, M. Breeders are less active foragers than non-breeders in wild Damaraland mole-rats. Biol. Lett. 2020, 16, 20200475. [Google Scholar] [CrossRef] [PubMed]
- Hickman, G. A live-trap and trapping technique for fossorial mammals. S. Afr. J. Zool. 1979, 14, 9–12. [Google Scholar] [CrossRef]
- Bisazza, A.; Cantalupo, C.; Capocchiano, M.; Vallortigara, G. Population lateralisation and social behaviour: A study with 16 species of fish. Lateral. Asymmetries Body Brain Cogn. 2000, 5, 269–284. [Google Scholar] [CrossRef] [PubMed]
- McLean, S.; Morrell, L.J. Consistency in the strength of laterality in male, but not female, guppies across different behavioural contexts. Biol. Lett. 2020, 16, 20190870. [Google Scholar] [CrossRef] [PubMed]
- Team, R. R Programming; R Development Core Team: Vienna, Austria, 2021. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. Package ‘lmertest’. R Package Version 2015, 2, 734. [Google Scholar]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated marginal means, aka least-squares means. R Package Version 2018, 1, 3. [Google Scholar]
- O’Shea-Wheller, T.A. Honeybees show a context-dependent rightward bias. Biol. Lett. 2019, 15, 20180877. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J.; Rigosi, E.; Frasnelli, E.; Vallortigara, G. A right antenna for social behaviour in honeybees. Sci. Rep. 2013, 3, 2045. [Google Scholar] [CrossRef] [PubMed]
- Leedale, A.E.; Thorley, J.; Clutton-Brock, T. Odour-based social recognition in Damaraland mole-rats, Fukomys damarensis. Anim. Behav. 2021, 179, 83–96. [Google Scholar] [CrossRef]
- Hetling, J.R.; Baig-Silva, M.S.; Comer, C.M.; Pardue, M.T.; Samaan, D.Y.; Qtaishat, N.M.; Pepperberg, D.R.; Park, T.J. Features of visual function in the naked mole-rat Heterocephalus glaber. J. Comp. Physiol. A 2005, 191, 317–330. [Google Scholar] [CrossRef]
- Costanzo, M.S. Aspects of Memory in the Damaraland Mole-Rat, Cryptomys damarensis: Spatial Learning and Kin Recognition. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2007. [Google Scholar]
- Pyott, S.J.; van Tuinen, M.; Screven, L.A.; Schrode, K.M.; Bai, J.-P.; Barone, C.M.; Price, S.D.; Lysakowski, A.; Sanderford, M.; Kumar, S. Functional, morphological, and evolutionary characterization of hearing in subterranean, eusocial African mole-rats. Curr. Biol. 2020, 30, 4329–4341.e4. [Google Scholar] [CrossRef] [PubMed]
- Rickard, C.; Bennett, N. Recrudescence of sexual activity in a reproductively quiescent colony of the Damaraland mole-rat (Cryptomys damarensis), by the introduction of an unfamiliar and genetically unrelated male—A case of incest avoidance in ‘queenless’ colonies. J. Zool. 1997, 241, 185–202. [Google Scholar] [CrossRef]
- Lovegrove, B.G.; Körtner, G.; Körtner, G. The magnetic compass orientation of the burrows of the Damara mole-rat Cryptomys damarensis (Bathyergidae). J. Zool. 1992, 226, 631–633. [Google Scholar] [CrossRef]
- Glick, S.D.; Ross, D.A. Right-sided population bias and lateralization of activity in normal rats. Brain Res. 1981, 205, 222–225. [Google Scholar] [CrossRef]
- Creed, R.; Miller, J. Interpreting animal wall-following behavior. Experientia 1990, 46, 758–761. [Google Scholar] [CrossRef]
- Lucon-Xiccato, T.; Nati, J.J.; Blasco, F.R.; Johansen, J.L.; Steffensen, J.F.; Domenici, P. Severe hypoxia impairs lateralization in a marine teleost fish. J. Exp. Biol. 2014, 217, 4115–4118. [Google Scholar]
- Arteni, N.; Pereira, L.; Rodrigues, A.; Lavinsky, D.; Achaval, M.; Netto, C. Lateralized and sex-dependent behavioral and morphological effects of unilateral neonatal cerebral hypoxia-ischemia in the rat. Behav. Brain Res. 2010, 210, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Lateralised brain function in anurans: Comparison to lateralisation in other vertebrates. Lateral. Asymmetries Body Brain Cogn. 2002, 7, 219–239. [Google Scholar] [CrossRef] [PubMed]
- Tyler-Julian, K.; Chapman, K.M.; Frances, C.; Bauer, G.B. Behavioral lateralization in the Florida manatee (Trichechus manatus latirostris). Int. J. Comp. Psychol. 2016, 29. [Google Scholar] [CrossRef]
- Brown, C.; Magat, M. The evolution of lateralized foot use in parrots: A phylogenetic approach. Behav. Ecol. 2011, 22, 1201–1208. [Google Scholar] [CrossRef]
- Hopkins, W.; Russell, J.; Schaeffer, J.; Gardner, M.; Schapiro, S. Handedness for tool use in captive chimpanzees (Pan troglodytes): Sex differences, performance, heritability and comparison to the wild. Behaviour 2009, 146, 1463–1483. [Google Scholar]
- Lhota, S.; Jůnek, T.; Bartoš, L. Patterns and laterality of hand use in free-ranging aye-ayes (Daubentonia madagascariensis) and a comparison with captive studies. J. Ethol. 2009, 27, 419–428. [Google Scholar] [CrossRef]
- Bibost, A.-L.; Brown, C. Laterality influences schooling position in rainbowfish, Melanotaenia spp. PLoS ONE 2013, 8, e80907. [Google Scholar] [CrossRef]
- Ariyomo, T.O.; Watt, P.J. Aggression and sex differences in lateralization in the zebrafish. Anim. Behav. 2013, 86, 617–622. [Google Scholar] [CrossRef]
- Austin, N.; Rogers, L. Limb preferences and lateralization of aggression, reactivity and vigilance in feral horses, Equus caballus. Anim. Behav. 2012, 83, 239–247. [Google Scholar] [CrossRef]
- Rohlfs, P.; Ramírez, J.M. Aggression and brain asymmetries: A theoretical review. Aggress. Violent Behav. 2006, 11, 283–297. [Google Scholar] [CrossRef]
- Hews, D.K.; Castellano, M.; Hara, E. Aggression in females is also lateralized: Left-eye bias during aggressive courtship rejection in lizards. Anim. Behav. 2004, 68, 1201–1207. [Google Scholar] [CrossRef]
- Bisazza, A.; de Santi, A. Lateralization of aggression in fish. Behav. Brain Res. 2003, 141, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Ocklenburg, S.; Korte, S.M.; Peterburs, J.; Wolf, O.T.; Güntürkün, O. Stress and laterality–The comparative perspective. Physiol. Behav. 2016, 164, 321–329. [Google Scholar] [CrossRef]
- Santin, L.; Begega, A.; Rubio, S.; Arias, J. Behaviour laterality in male rats: Influence of practice and stress. Physiol. Behav. 1996, 60, 161–164. [Google Scholar] [CrossRef]
- Herbst, M.; Bennett, N. Burrow architecture and burrowing dynamics of the endangered Namaqua dune mole rat (Bathyergus janetta) (Rodentia: Bathyergidae). J. Zool. 2006, 270, 420–428. [Google Scholar] [CrossRef]
- Herbst, M.; Jarvis, J.; Bennett, N. A field assessment of reproductive seasonality in the threatened wild Namaqua dune mole-rat (Bathyergus janetta). J. Zool. 2004, 263, 259–268. [Google Scholar] [CrossRef]
- Cockrem, J.F. Individual variation in glucocorticoid stress responses in animals. Gen. Comp. Endocrinol. 2013, 181, 45–58. [Google Scholar] [CrossRef]
- Angelier, F.; Wingfield, J.C. Importance of the glucocorticoid stress response in a changing world: Theory, hypotheses and perspectives. Gen. Comp. Endocrinol. 2013, 190, 118–128. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 2016, 6, 603. [Google Scholar]
- Neveu, P.J.; Moya, S. In the mouse, the corticoid stress response depends on lateralization. Brain Res. 1997, 749, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, A.P.; Leigh, A.E.; Man, M.-S.; Kendrick, K.M. Face pictures reduce behavioural, autonomic, endocrine and neural indices of stress and fear in sheep. Proc. R. Soc. London. Ser. B Biol. Sci. 2004, 271, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cruz, C.; Simon, M.; Czéh, B.; Flügge, G.; Fuchs, E. Hemispheric differences in basilar dendrites and spines of pyramidal neurons in the rat prelimbic cortex: Activity-and stress-induced changes. Eur. J. Neurosci. 2009, 29, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R. Hemispheric asymmetry in stress processing in rat prefrontal cortex and the role of mesocortical dopamine. Stress 2004, 7, 131–143. [Google Scholar] [CrossRef]
- Medger, K.; Bennett, N.C.; Lutermann, H.; Ganswindt, A. Non-invasive assessment of glucocorticoid and androgen metabolite levels in cooperatively breeding Damaraland mole-rats (Fukomys damarensis). Gen. Comp. Endocrinol. 2018, 266, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.W.; Bennett, N.C.; Best, C.; van Jaarsveld, B.; Cheng, H.; Ivy, C.M.; Kirby, A.M.; Munro, D.; Sprenger, R.J.; Storey, K.B.; et al. Acute hypoxia induces plasma cortisol increases in social, but not solitary subterranean African mole-rat species. Gen. Comp. Endocrinol. 2023; under revision. [Google Scholar]
- Johnston, R.A.; Vullioud, P.; Thorley, J.; Kirveslahti, H.; Shen, L.; Mukherjee, S.; Karner, C.M.; Clutton-Brock, T.; Tung, J. Morphological and genomic shifts in mole-rat ‘queens’ increase fecundity but reduce skeletal integrity. Elife 2021, 10, e65760. [Google Scholar] [CrossRef]
- Voigt, C.; Bennett, N.C. Reproductive status-dependent kisspeptin and RF amide-related peptide (Rfrp) gene expression in female Damaraland mole-rats. J. Neuroendocrinol. 2018, 30, e12571. [Google Scholar] [CrossRef]
- Young, A.J.; Oosthuizen, M.K.; Lutermann, H.; Bennett, N.C. Physiological suppression eases in Damaraland mole-rat societies when ecological constraints on dispersal are relaxed. Horm. Behav. 2010, 57, 177–183. [Google Scholar] [CrossRef]
- Young, A.J.; Bennett, N.C. Morphological divergence of breeders and helpers in wild damaraland mole-rat societies. Evol. Int. J. Org. Evol. 2010, 64, 3190–3197. [Google Scholar] [CrossRef]
- Pfannkuche, K.A.; Bouma, A.; Groothuis, T.G. Does testosterone affect lateralization of brain and behaviour? A meta-analysis in humans and other animal species. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 929–942. [Google Scholar] [CrossRef]
- Beking, T.; Geuze, R.H.; Groothuis, T.G. Investigating effects of steroid hormones on lateralization of brain and behavior. In Lateralized Brain Functions; Springer: Berlin/Heidelberg, Germany, 2017; pp. 633–666. [Google Scholar]
- Hausmann, M.; GuÈntuÈrkuÈn, O.; Corballis, M. Age-related changes in hemispheric asymmetry depend on sex. Lateral. Asymmetries Body Brain Cogn. 2003, 8, 277–290. [Google Scholar] [CrossRef]
- Dolcos, F.; Rice, H.J.; Cabeza, R. Hemispheric asymmetry and aging: Right hemisphere decline or asymmetry reduction. Neurosci. Biobehav. Rev. 2002, 26, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol. Aging 2002, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Daselaar, S.; Cabeza, R. Age-Related Changes in Hemispheric Organization. In Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging; Oxford University Press: Oxford, UK, 2005; p. 325. [Google Scholar]
- Przybyla, A.; Haaland, K.Y.; Bagesteiro, L.B.; Sainburg, R.L. Motor asymmetry reduction in older adults. Neurosci. Lett. 2011, 489, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.M.; Jarvis, J.U.; Bennett, N.C. The long-lived queen: Reproduction and longevity in female eusocial Damaraland mole-rats (Fukomys damarensis). Afr. Zool. 2013, 48, 193–196. [Google Scholar] [CrossRef]
- Bennett, N.C.; Faulkes, C.G.; Molteno, A.J. Reproductive suppression in subordinate, non-breeding female Damaraland mole-rats: Two components to a lifetime of socially induced infertility. Proc. Biol. Sci. 1996, 263, 1599–1603. [Google Scholar] [CrossRef]
- Bennett, N.; Jarvis, J.; Faulkes, C.; Millar, R. LH responses to single doses of exogenous GnRH by freshly captured Damaraland mole-rats, Cryptomys damarensis. Reproduction 1993, 99, 81–86. [Google Scholar] [CrossRef]
- Molteno, A.; Bennett, N. Anovulation in non-reproductive female Damaraland mole-rats (Cryptomys damarensis). J Reprod Fertil 2000, 119, 35–42. [Google Scholar] [CrossRef]
- Voigt, C.; Gahr, M.; Leitner, S.; Lutermann, H.; Bennett, N. Breeding status and social environment differentially affect the expression of sex steroid receptor and aromatase mRNA in the brain of female Damaraland mole-rats. Front. Zool. 2014, 11, 38. [Google Scholar] [CrossRef]
| Mole # | Colony | Wild/Captive | Breeding Status | Left Turns | Right Turns | Total Turns | Binomial Probability |
|---|---|---|---|---|---|---|---|
| WB1 | 1 | Wild | Queen | 38 | 2 | 40 | p < 0.05 |
| WB2 | 1 | Wild | Subordinate | 7 | 33 | 40 | p < 0.05 |
| WB3 | 2 | Wild | Queen | 13 | 27 | 40 | p = 0.04 |
| WB4 | 2 | Wild | Subordinate | 7 | 33 | 40 | p < 0.05 |
| WB5 | 2 | Wild | Subordinate | 19 | 21 | 40 | ns |
| WB6 | 3 | Wild | Queen | 16 | 24 | 40 | ns |
| WB7 | 3 | Wild | Subordinate | 21 | 19 | 40 | ns |
| WB8 | 3 | Wild | Subordinate | 39 | 1 | 40 | p < 0.05 |
| WB9 | 3 | Wild | Subordinate | 3 | 37 | 40 | p < 0.05 |
| WT1 | 8 | Wild | Subordinate | 24 | 15 | 39 | ns |
| WT2 | 8 | Wild | Subordinate | 27 | 13 | 40 | p = 0.04 |
| WT3 | 8 | Wild | Subordinate | 26 | 14 | 40 | ns |
| WT4 | 8 | Wild | Subordinate | 22 | 18 | 40 | ns |
| WT5 | 8 | Wild | Queen | 26 | 14 | 40 | ns |
| WT6 | 9 | Wild | Subordinate | 30 | 9 | 39 | p < 0.05 |
| WT7 | 9 | Wild | Queen | 21 | 19 | 40 | ns |
| WT8 | 9 | Wild | Subordinate | 25 | 15 | 40 | ns |
| WT9 | 9 | Wild | Subordinate | 22 | 17 | 39 | ns |
| WT10 | 9 | Wild | Subordinate | 24 | 16 | 40 | ns |
| WT11 | 9 | Wild | Subordinate | 29 | 10 | 39 | p < 0.05 |
| LE1 | 16 | Wild | Subordinate | 21 | 15 | 36 | ns |
| LE2 | 16 | Wild | Subordinate | 20 | 16 | 36 | ns |
| LE3 | 16 | Wild | Subordinate | 19 | 17 | 36 | ns |
| LE4 | 16 | Wild | Subordinate | 15 | 20 | 35 | ns |
| LE5 | 16 | Wild | Subordinate | 20 | 17 | 37 | ns |
| L9 | 11 | Captive | Queen | 21 | 19 | 40 | ns |
| L10 | 11 | Captive | Subordinate | 24 | 16 | 40 | ns |
| L11 | 11 | Captive | Subordinate | 30 | 9 | 39 | p < 0.05 |
| L12 | 11 | Captive | Subordinate | 37 | 3 | 40 | p < 0.05 |
| L13 | 12 | Captive | Queen | 16 | 24 | 40 | ns |
| L14 | 12 | Captive | Subordinate | 34 | 6 | 40 | p < 0.05 |
| L15 | 12 | Captive | Subordinate | 25 | 15 | 40 | ns |
| L16 | 12 | Captive | Subordinate | 18 | 22 | 40 | ns |
| L17 | 12 | Captive | Subordinate | 28 | 12 | 40 | p = 0.02 |
| L18 | 13 | Captive | Queen | 19 | 15 | 34 | ns |
| L19 | 13 | Captive | Subordinate | 28 | 12 | 40 | p = 0.02 |
| LE13 | 19 | Captive | Queen | 20 | 17 | 37 | ns |
| LE14 | 19 | Captive | Subordinate | 17 | 18 | 35 | ns |
| LE15 | 20 | Captive | Subordinate | 19 | 16 | 35 | ns |
| LE16 | 20 | Captive | Queen | 15 | 20 | 35 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobs, P.J.; Oosthuizen, M.K. Laterality in the Damaraland Mole-Rat: Insights from a Eusocial Mammal. Animals 2023, 13, 627. https://doi.org/10.3390/ani13040627
Jacobs PJ, Oosthuizen MK. Laterality in the Damaraland Mole-Rat: Insights from a Eusocial Mammal. Animals. 2023; 13(4):627. https://doi.org/10.3390/ani13040627
Chicago/Turabian StyleJacobs, Paul J., and Maria K. Oosthuizen. 2023. "Laterality in the Damaraland Mole-Rat: Insights from a Eusocial Mammal" Animals 13, no. 4: 627. https://doi.org/10.3390/ani13040627
APA StyleJacobs, P. J., & Oosthuizen, M. K. (2023). Laterality in the Damaraland Mole-Rat: Insights from a Eusocial Mammal. Animals, 13(4), 627. https://doi.org/10.3390/ani13040627





