3. Results
Twenty-seven dogs with nonmineralized (NM) supraspinatus tendinopathy met the inclusion criteria. The breeds included were Boxer (five), American Staffordshire Bull Terrier (four), Beagle (two), Labrador Retriever (two), Pitbull (two), Border Collies (two), Argentine Dogo (one), Rhodesian Ridgeback (one), Black Russian Terrier (one), Central Asian Shepherd Dog (one), Pyrenean Mastiff (one), German Shepherd (one), Samoyed (one), Tibetan Mastiff (one), Great Swiss Cowherd (one) and mixed breed (one).
The patients’ ages ranged from 12 to 90 months (mean: 39.7 months). The patients included 18 males (1 neutered and 17 intact) and 9 females (7 spayed and 2 intact). The patients’ bodyweight ranged from 16 to 75 kg (mean: 33.5 kg).
3.1. Findings of the Orthopedic Evaluation
All dogs had unilateral thoracic limb lameness. The distribution of the affected limbs distribution was 16 on the right and 11 on the left. Intermittent or waxing–waning lameness was the admitting complaint for all dogs. The median lameness grade evaluated in the entire population was 3 (3.19 ± 0.62). The median duration of clinical signs was 4.7 months (range: 1.5–18 months). All dogs were previously treated with rest and administration of NSAIDs or corticosteroids for 7 to 14 days, without clinical improvement. Pain on flexion of the shoulder and on palpation of the insertion of the supraspinatus tendon was the most consistent finding during the orthopedic examination, occurring in every dog. Median pain grade was two (2.07 ± 0.55). The biceps tendon test in the German-speaking literature was subjectively graded as positive in 3 out of 27 and negative in 24 out of 27.
3.2. Imaging
The radiographs showed that 24 out of 27 pathologic shoulders had no radiographic abnormalities, 2 out of 27 had signs of slight shoulder osteoarthritis characterized by the presence of osteophytes in the caudal portion of the humeral head, 1 out of 27 had a deficit of the bicipital groove filling according to a positive-contrast radiographic study. Synovial fluid examination was performed in 16% of the cases and the results of the cytologic examination were compatible with degenerative joint disease.
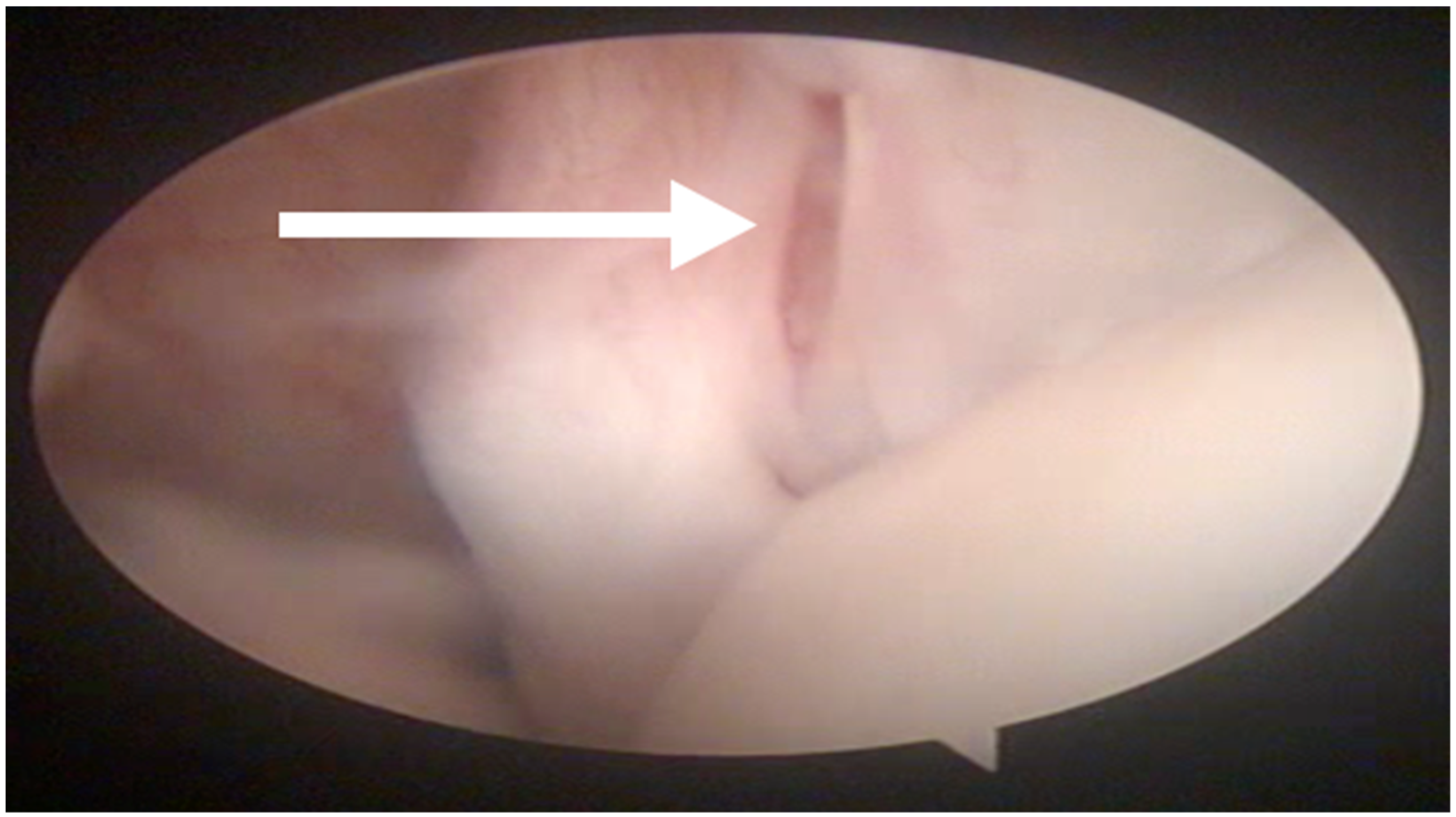
During the MRI evaluation, a marked hyperintense signal localized to the insertion of the supraspinatus muscle was found in all of the examined shoulders in the STIR images (
Figure 1).
The tendon of the supraspinatus muscle was severely enlarged in 3 out of 27 of the cases. Five MRI images revealed compression of the biceps brachii tendon sheaths, while 10 out of 27 of the biceps brachii tendon sheaths were distended. Moreover, an effusion was present in the sheath of the tendon of the infraspinatus muscle in 1 one out of 27 of the cases and in the sheath of the biceps brachii muscle in 1 out of 27 of the cases.
MRI abnormalities of the tendon of the subscapularis muscle were detected in one dog. No MRI abnormalities of the medial and lateral glenohumeral ligament were detected.
Signs of nonmineralized supraspinatus tendinopathy were present on the contralateral forelimb in 10 out of 27 dogs.
3.3. Surgical Findings
In all the dogs, surgery was performed by the same surgeon only in the shoulder affected by lameness. Intra-articular abnormalities were arthroscopically observed in 13 out of 27 shoulders and consisted of fibrillation of the medial glenohumeral ligament in 3 out of 27 cases, partial rupture of the tendon of the subscapularis muscle in 7 out of 27 cases and laxity of the tendon of the subscapularis muscle in 3 out of 27 cases (
Figure 2).
The arthroscopic and MRI findings were in agreement about the absence of intra-articular shoulders abnormalities in 10 out of 27 shoulders. In 13 out of 27 shoulders, the ectasia of biceps brachii tendon was detected with MRI but was not recognized with arthroscopy. In 9 out of 27 shoulders, lesions of the subscapularis tendon, not previously established with MRI, were diagnosed by arthroscopy. In 3 out of 27 shoulders, laxity of the medial glenohumeral ligament, not previously diagnosed with MRI, was observed by arthroscopy. In 27 out of 27 shoulders, both MRI and arthroscopy were in agreement about the lateral glenohumeral ligament integrity. No intra-operative complications were reported. No dogs required physical rehabilitation in the post-operative period.
3.4. Histopathological Findings
A biopsy sample of the supraspinatus tendon from all 27 dogs was submitted for histological evaluation. The sample was collected from the area of maximum thickness of the tendon, at the musculotendinous junction. The histological examination of the tissue specimens revealed discontinuous and disorganized collagen fibers that lacked reflectivity under polarized light in all specimens. The most prominent finding was severe myxomatous degeneration, with the remaining collagen separated by a palely basophilic myxoid matrix and edema.
3.5. Short Term Follow-Up
On Day 10, 21 out of 27 cases showed a lower lameness score compared with the pre-operative values. Lameness was classified subjectively as Grade II in 21 out of 27 cases, Grade III in 5 out of 27 cases and Grade IV in 1 out of 27 cases. There was a lack of improvement in lameness in 6 out of 27 cases: in 4 out of 27 cases, the lameness remained the same, while worsening lameness from Grade III to Grade IV and from Grade II to Grade III was observed in 2 out of 27 cases. A fluctuant mass the size of a golf ball was detected under the healed incision in 5 out of 27 cases (
Figure 3). It was presumed to be a seroma and was resolved through the administration of NSAIDS and rest for 10 days. In one dog, a recurrence of the seroma was reported on Day 20 and on Day 90, postoperatively.
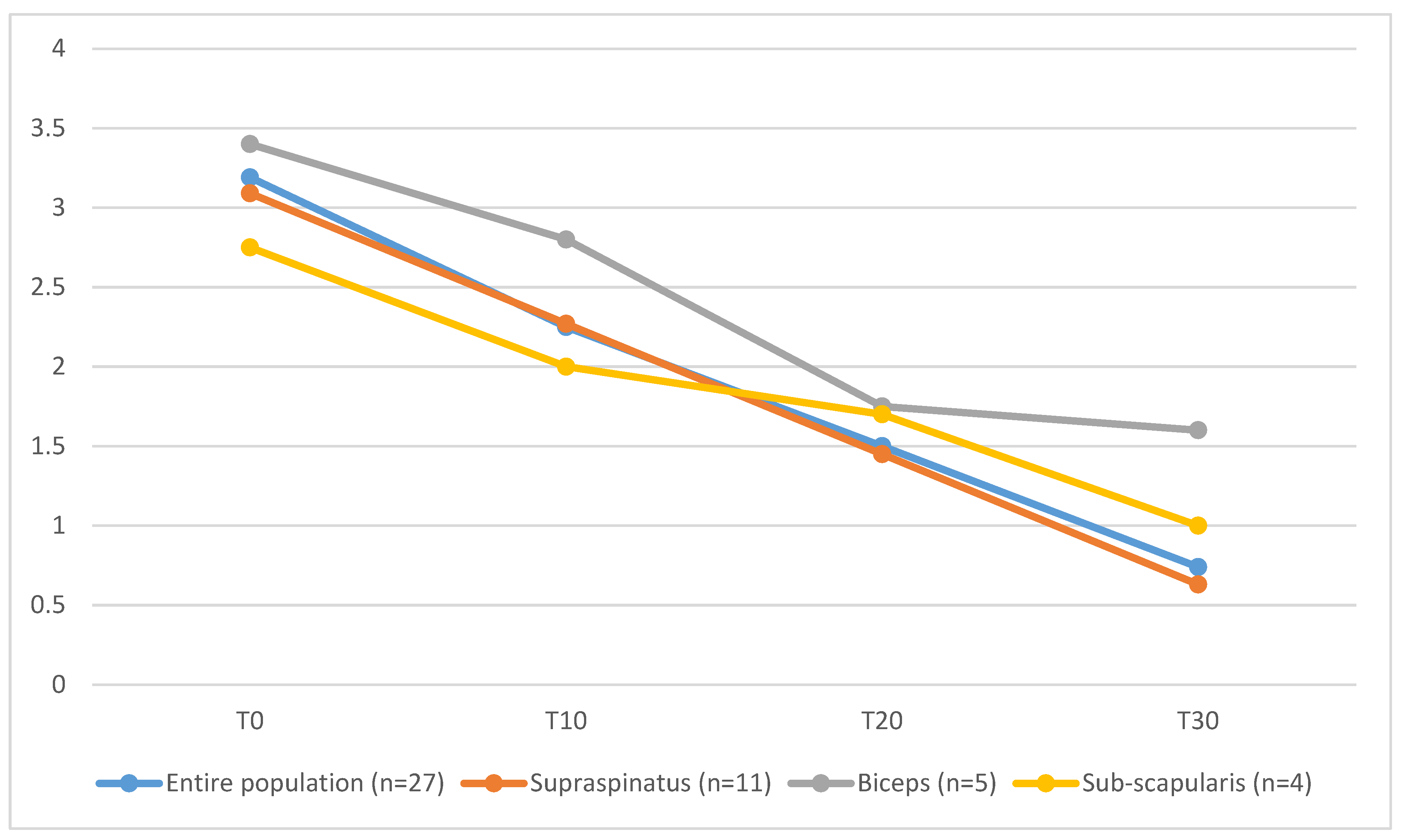
On Day 30, lameness was graded as absent in 7 out of 27 cases, as Grade I in 5 out of 27, as Grade II in 11 out of 27 cases, as Grade III in 3 out of 27 and Grade IV in one dog. Dogs affected by co-existing supraspinatus tendinopathy and biceps brachii tendinopathy (T30 = 1.6 ± 0.9) showed longer functional recovery, characterized by higher mean values of lameness score, compared to the entire population (T30 = 0.74 ± 1.3), to dogs affected only by ST tendinopathy (T30 = 0.63 ± 1.4) or dogs affected by ST tendinopathy and concomitant subscapular tendon lesion (T30 = 1 ± 0.8) (
Figure 4).
The clinical outcome was judged to be unsatisfactory in the dog affected by Grade IV lameness, revision surgery was discussed with the owner, and supraspinatus tenectomy was performed.
On Day 60, 17 out of 27 patients did not show any sign of lameness, 3 out of 27 still had Grade I lameness, 5 out of 27 had Grade II lameness, 1 out of 27 had Grade III lameness and I out of 27 had Grade IV lameness.
3.6. Long-Term Follow-Up
All owners agreed to a telephone interview but only 13 out of 27 agreed to a clinical consultation, radiographic study of the shoulder joint and force plate analysis.
Of the 13 dogs that had a long-term clinical evaluation, all had a complete range of motion of the joint and did not show any sign of pain during flexion or extension of the joint or under palpation of the supraspinatus tendon. The symmetry index was between 0.01 and 14.34, with a mean value of 3.19.
A radiographic recheck of the surgically treated shoulder showed mild mineralization in 4 out of 13 dogs and mild degenerative joint disease in 2 out of 13 cases.
Twenty-three owners declared that they were satisfied with the recovery of their dog after surgery, 2 out of 27 were mostly satisfied and 2 out of 27 remained unsatisfied.
Twenty-two of the dogs did not show any sign of lameness on the treated limb, but 9 out of 27 showed some episodes of lameness, which were classified as mild.
When the owners were asked if their pets presented any episodes of lameness on the other forelimb, 25 out of 27 owners answered that no alteration in the gait was noted in that limb.
Gait was judged by the owners as normal in 19 out of 27 dogs, 3 out of 27 had a nearly normal gait, 3 out of 27 had slight but frequent difficulties, and in 2 out of 27 dogs, the difficulty was evident.
The ability to stand up from a sitting position was normal in 12 out of 27 dogs, 8 out of 27 had slight and infrequent difficulties, 5 out of 27 had slight but frequent difficulties, and discomfort was evident in only one dog.
The ability to run was normal in 19 out of 27 dogs, 3 out of 27 had slight and infrequent difficulties, 2 out of 27 had slight but frequent difficulties and 3 out of 27 owners were unable to answer this question.
Nineteen of the dogs did not have difficulty during intense activity and play, 5 out of 27 had slight and infrequent difficulties, and only 3 out of 27 dogs had slight but frequent difficulties.
Twenty-one dogs did not have any difficulty going downstairs and 5 out of 27 had slight and infrequent difficulties; one owner was not able to answer.
After we added the scores of the different activities evaluated by the owners, the functional recovery was judged to be very good in 15 out of 27 dogs, good in 8 out of 27 and sufficient in 4 out of 27 dogs.
4. Discussion
In the literature, a breed-related predisposition to supraspinatus tendinopathy has been reported in Rottweilers and Labrador Retrievers [
1,
3,
7,
9]. In the present retrospective study, only 7% of the affected dogs were represented by Labrador Retrievers, and no Rottweilers presented at our institution for nonmineralized supraspinatus tendinopathy. The breeds most represented in our population were Boxers and American Staffordshire Bull Terriers. We can argue that a breed-oriented approach (BOA) is not helpful for a diagnosis of the nonmineralized form of the supraspinatus tendinopathy because a clear breed predisposition has still not been proved.
Lafuente and colleagues [
7] found a difference in the body weight of dogs affected by a nonmineralized supraspinatus tendinopathy (mean weight: 35 kg) and mineralized supraspinatus tendinopathy (mean weight: 26.2 kg). Our results did not confirm these findings because, in our population, the mean weight of the dogs affected by the nonmineralized form of supraspinatus tendinopathy was 33.5 kg. We can suppose that body weight is not a predisposing factor for this pathology, but it is typical of a medium and large dog, because it has never been described in a small or toy breed of dog [
1].
The dogs included in this study were affected by chronic lameness that was intermittent and worsened after physical activity. NSAIDs had been administered previously to all dogs, in association with steroids in some cases, for 7 to 14 days without clinical improvement. The cause of this nonresponse was associated with the degeneration and metaplasia of the tendon, without inflammation [
7,
31]. In our population, histopathological examination of a portion of the tendon was performed in each case. In all of the samples, the histopathologic changes observed were myxomatous degeneration and chondroid metaplasia, which are characteristics of tendon degeneration, without tissue inflammation. During the physical examination, most dogs included were affected by a severe chronic lameness secondary to shoulder pain. Dogs affected by the nonmineralized supraspinatus tendinopathy included in our study presented lameness characterized by a marked abduction of the limb during the swing phase. We suppose that this lameness can be explained by an attempt by the dog to reduce the pain evoked by compression of the biceps tendon from the impingement of the pathologic supraspinatus tendon [
32] during extension of the shoulder. Affected dogs aim to advance the limb, limiting the extension and abducting the shoulder in order to decrease the pain. In our experience, this type of lameness is characteristic of supraspinatus tendinopathy and can add the suspect of the pathology.
The biceps test (in the German-speaking literature) was performed, and it was judged to be positive in 3 out of 27 cases but partial or complete rupture of the biceps brachii tendon was not subsequently confirmed neither by MRI nor arthroscopy. This can be explained by the fact that the biceps test is an extremely subjective evaluation, and the response can be operator-dependent. Moreover, many of the dogs included in our study had a straight poise of the forelimbs, so the flexion of the shoulder determined the extension of the elbow, even if the biceps tendon was intact. This can lead one to erroneously suspect its breakage.
In human medicine, the Hawkins test is described as a specific test for evaluating the supraspinatus tendon. The patient first flexes her/his arm by 90 degrees, then flexes her/his elbow by 90 degrees and intrarotates his/her shoulder. Pain indicates that the test is positive, and the pain is due to the compression of the biceps tendon by the tendon of the supraspinatus [
15]. In our experience, we observed a progressive increase in the operator’s capacity to identify the supraspinatus tendon through palpation during the clinical examination and to evoke pain by exerting digital pressure in its insertion on the major tubercle. However, it is difficult to differentiate the pain evoked by the compression of the supraspinatus tendon from pain evoked during the execution of the biceps test in the English-speaking literature. We can argue that many lesions in the past were considered biceps tendinopathy because of a positive response to biceps tendon test in the English-speaking literature. A great number of patients included in the study presented with muscle atrophy, and this can help to identify the insertion of the tendon. We must emphasize that the significance of tendon palpation for correctly formulating [
33] the diagnosis has never been evaluated in the literature and we do not know its sensitivity and specificity.
In adult patients referred for shoulder lameness, it is always necessary to carry out a orthogonal comparative radiographic study even if the majority of shoulder pathologies involve the tenoligamentous structures [
7,
34]. A radiographic study can exclude some pathologies such as tendon avulsion of the biceps brachii muscle or the mineralized form of supraspinatus tendinopathy. In dogs affected by the nonmineralized form of supraspinatus tendinopathy, the only visible alteration is the presence of osteophytes in the bicipital groove visible in the skyline projection [
7]. Some authors attribute the presence of osteophytes to arthritis determined by irritation of the biceps tendon and its sheath, and by the compression applied by a thickened supraspinatus tendon [
7].
In our study, we examined the synovial fluid in only 16% of the cases, but we considered this to be a mistake during the diagnostic process. It is advisable to include a cytologic examination of the synovial fluid in the diagnostic work out in order to rule out pathologies such as neutrophilic arthrosynovitis before considering MRI of the shoulder.
In the dogs included in this study, given the negative results of the radiographic and synovial liquid exams, we advanced nonmineralized tendinopathy of the supraspinatus as a probable diagnosis, and we informed the owners that this diagnosis could be confirmed through ultrasound examination or magnetic resonance imaging [
7]. With the possibility of choosing between ultrasound and MRI, the authors always prefer MRI. The most common alterations highlighted by an ultrasound examination are the enlargement of the tendon with or without axial deviation of the biceps tendon and inhomogeneities in the tendon [
1,
33]. However, the alterations highlighted by the ultrasound exam are subjective and their interpretation is strongly influenced by the operator’s experience [
7]. MRI is a powerful diagnostic modality, commonly used for diagnoses of human musculoskeletal diseases and providing information on the intra- and extra-articular structures simultaneously with high soft tissue contrast, and high-resolution and multiplanar imaging capabilities [
35]. Compared with ultrasound, it allows the evaluation of teno-ligamentous structures such as the subscapular muscle and collateral ligaments that are not fully reached by ultrasound. This can provide the surgeons with more information on the status of the shoulder joint compared with ultrasound alone. For this reason, we prefer to advise for MRI, which represents the gold standard for diagnosing extra-articular pathologies of the shoulder [
1,
34]. Despite this, it is the opinion of the authors that, in further studies, a comparison between MRI and ultrasound findings could be very useful in order to better define diagnostic criteria of the nonmineralized supraspinatus tendinopathy.
The common alteration revealed by MRI in patients affected by nonmineralized tendinopathy is an enlargement and a marked increase in the signal in the insertion area of the supraspinatus tendon above the greater tubercle of the humerus in the STIR sequences. In a recent study, it was noticed that pathologic tendons, in addition to an alteration in the signal, also present an increase in volume [
22]. In our population, only 3 cases out of 27 had an increase in the volume in addition to an alteration in the signal.
The alterations indicated by MRI must always be associated with the clinical data.
In our case studies, it was interesting to note that there was some discrepancy in the evaluation of the shoulder joint when we compared the findings of MRI and arthroscopy in the biceps and subscapularis tendons and the medial collateral ligament. Regarding the biceps tendon, MRI highlighted an ectasia in 10 out of 27 cases that was not confirmed by arthroscopy. Arthroscopy indicated a partial rupture and laxity of the subscapularis tendon in 10 out of 27 cases that was not highlighted in 9 out of 27 cases through MRI. Finally, arthroscopy highlighted laxity of the medial collateral ligament in 3 out of 27 cases that was not displayed in MRI in 0 out of 3 cases. For this reason, as arthroscopy is a minimally invasive technique which requires a short prolongation of the patient’s anesthesia with a very low incidence of complications, it is considered useful to always carry it out before surgery, as it often provides useful information for understanding the pathology and the prognosis of the patient. As well as ultrasound, evaluation by MRI imaging presents minimal variability caused by the operator’s experience. Having a good evaluation of the means of intra-articular stability is also fundamental for evaluating the prognosis and for the functional recovery of the patients treated surgically. In our case studies, 37% of cases had a subscapularis tendon injury that can lead to the persistence of lameness in the post-operative period, which must be discussed with the owners.
Dogs at our institution affected by supraspinatus tendinopathy had a mechanical alteration of the gait that led them to abduction of the limb. We argue that this gait alteration may be the cause of the torn subscapularis tendon or the partial rupture of the medial collateral ligament, which were often observed in our population; otherwise, these lesions can be considered to be concomitant to the nonmineralized supraspinatus tendinopathy. It is reasonable to consider those lesions as of lesser regard because the lameness often resolves after the treatment of the supraspinatus tendinopathy alone. Dogs included in this study that presented a concomitant injury to the subscapularis tendon or medial collateral ligament were treated in the post-operative period [
36] using dedicated hobbles. The use of hobbles was recommended for at least 3 months.
In our cases, slower functional recovery with a protracted lameness was reported in patients affected by concomitant supraspinatus and biceps brachii tendinopathy. It is supposed that, if not treated, the biceps brachii tendinopathy might have a consistent influence on the post-operative lameness score compared with the other concomitant lesions observed by means of arthroscopy or by MRI.
In the literature, the craniomedial surgical approach has been reported for executing splitting of the supraspinatus tendon [
7,
37]. In our case studies, we decided to adopt the dorsal recumbency position in the patients, which allowed the operators to perform a very small surgical approach and to limit the dissection of soft tissues. The most frequent complication found in our cases was seroma, which occurred in 18% of cases. To avoid this complication, we adopted the quilting suturing technique [
38].
The execution of tendon splitting is easy and involves making longitudinal release incisions at the level of the muscle–tendon junction [
7,
37]. This technique induces a reduction in the intra-tendinous pressure, an increase in the critical zone’s vascularization and a reduction in the healing time of the tendon [
7]. However, it is sometimes difficult to macroscopically identify the pathological area and the incisions may remain localized in the tendinous portion. If lameness persists in the post-operative period, it may be secondary to the execution of incisions in a nonpathological portion of the tendon or to the formation of excessive scar tissue, as we observed in one case [
19].
The histopathologic examination of collected samples highlighted severe myxomatous degeneration, with the remaining collagen separated by a palely basophilic myxoid matrix and edema. Pownder et al. [
23] suggested that histologic evidence of mucinous or chondroid degeneration in focal biopsy specimens should be interpreted cautiously because the identification of well-organized structures during examination of the entire tendon might refute such a diagnosis. In our study all samples were obtained from the area of maximum thickness at the musculotendinous junction where mineralization foci are commonly localized in the mineralized form of the pathology. This portion of the tendon was characterized by a thickened appearance and, during surgical splitting, was characterized by a “crunchy” sound. Control specimens of normal tendons were unavailable for comparison purposes; considering those findings it is possible that for some cases to have been incorrectly interpreted as evidence of mucinous or chondroid degeneration indicative of maladaptive or pathological lesions. More studies are still required in order to better define the histopathological features of the non-mineralized form of supraspinatus tendinopathy.
The visual and histological examination of some tendinous portions highlighted the presence of mineralized foci in the tendon samples of subjects affected by the nonmineralized form of the pathology. This may support the theory that the nonmineralized tendinopathy is an initial phase of the more common mineralized form, so we can suppose that in dogs with the nonmineralized form, the mineralized form could develop over time if surgery is not carried out. Some authors affirm that degeneration of the tendon can follow a cycle of degeneration and regeneration. Interruption of this cycle can cause the progressive formation of mineralized foci and the development of clinical signs [
18,
39,
40,
41].
In 21 out of 27 (77%) patients, there was a marked improvement in lameness after the removal of stitches 10 days after surgery. The 30-day follow-up was satisfactory in 81% of cases, but in only one case, we decided, given the persistence of marked lameness, in favor of surgical re-intervention. During the surgery, fibrosis was observed in the portion where the tendon splitting had been performed, so we executed a partial tenectomy of the tendon, which led to rapid remission of the symptoms.
To our knowledge, long-term follow-up of patients affected by the mineralized or nonmineralized forms of supraspinatus tendinopathy has never been reported in the literature. Anecdotally, according to some surgeons, it would be better to perform a complete tenectomy to avoid the risk of recurrence. In our study, we performed a long-term follow-up between 6 and 9 months after the surgery. The long-term follow-up included a telephone interview, and clinical and radiological examinations. In 85% of patients, the result was considered to be satisfactory by the owners and, in 81% of the cases, lameness was no longer reported. These data support the efficacy of the tendon splitting technique for the treatment of nonmineralized supraspinatus tendinopathy.
Furthermore, the long-term follow-up allowed us to highlight how although the MRI had shown an alteration in the STIR signal in the contralateral limb in 11% of cases, none of these patients ever became symptomatic. This shows how the alterations found by MRI must always be supported by clinical data to have value; therefore, it is not necessary to perform surgery for preventive purposes in the absence of symptoms.
For cases in which it was possible to execute the force plate examination in the long-term clinical examination, we observed a low symmetry index, with an average value of 1.58. As demonstrated by Grandjean e Fanchon, this value, being less than 3.2, can be considered physiological [
30,
42,
43].
This study has the implicit limitations of retrospective studies. One limit is related to the fact that the information was extracted retrospectively from the medical records. Another limitation is related to the impossibility of executing a long-term clinical follow-up in some patients; therefore, the data obtained have a limited value because they are based on the subjective judgement of the owners. However, it is believed that the owners are able to effectively evaluate their dog in the home environment, which is why the owner’s opinion should not be underestimated as part of the evaluation of the results [
44].