Effects of Partial Replacement of Soybean Meal with Defatted Hermetia illucens Meal in the Diet of Laying Hens on Performance, Dietary Egg Quality, and Serum Biochemical and Redox Indices
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Design, and Diets
2.2. Sample Collection
2.3. Analytical Procedure
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Secci, G.; Bovera, F.; Nizza, S.; Baronti, N.; Gasco, L.; Conte, G.; Serra, A.; Bonelli, A.; Parisi, G. Quality of Eggs from Lohmann Brown Classic Laying Hens Fed Black Soldier Fly Meal as Substitute for Soya Bean. Animal 2018, 12, 2191–2197. [Google Scholar] [CrossRef] [PubMed]
- Sogari, G.; Amato, M.; Biasato, I.; Chiesa, S.; Gasco, L. The Potential Role of Insects as Feed: A Multi-Perspective Review. Animals 2019, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- De Marco, M.; Martínez, S.; Hernandez, F.; Madrid, J.; Gai, F.; Rotolo, L.; Belforti, M.; Bergero, D.; Katz, H.; Dabbou, S.; et al. Nutritional Value of Two Insect Larval Meals (Tenebrio molitor and Hermetia illucens) for Broiler Chickens: Apparent Nutrient Digestibility, Apparent Ileal Amino Acid Digestibility and Apparent Metabolizable Energy. Anim. Feed Sci. Technol. 2015, 209, 211–218. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Wang, H.; Yang, Q.; ur Rehman, K.; Li, W.; Cai, M.; Li, Q.; Mazza, L.; Zhang, J.; et al. Dynamic Changes of Nutrient Composition throughout the Entire Life Cycle of Black Soldier Fly. PLoS ONE 2017, 12, e0182601. [Google Scholar] [CrossRef]
- Schiavone, A.; De Marco, M.; Martínez, S.; Dabbou, S.; Renna, M.; Madrid, J.; Hernandez, F.; Rotolo, L.; Costa, P.; Gai, F.; et al. Nutritional Value of a Partially Defatted and a Highly Defatted Black Soldier Fly Larvae (Hermetia illucens L.) Meal for Broiler Chickens: Apparent Nutrient Digestibility, Apparent Metabolizable Energy and Apparent Ileal Amino Acid Digestibility. J. Anim. Sci. Biotechnol. 2017, 8, 51. [Google Scholar] [CrossRef]
- Ibáñez, M.A.; de Blas, C.; Cámara, L.; Mateos, G.G. Chemical Composition, Protein Quality and Nutritive Value of Commercial Soybean Meals Produced from Beans from Different Countries: A Meta-Analytical Study. Anim. Feed Sci. Technol. 2020, 267, 114531. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional Composition of Black Soldier Fly (Hermetia illucens) Prepupae Reared on Different Organic Waste Substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Ge, C.; Yao, H. Antimicrobial Peptides from Black Soldier Fly (Hermetia illucens) as Potential Antimicrobial Factors Representing an Alternative to Antibiotics in Livestock Farming. Animals 2021, 11, 1937. [Google Scholar] [CrossRef]
- Park, S.-I.; Kim, J.-W.; Yoe, S.M. Purification and Characterization of a Novel Antibacterial Peptide from Black Soldier Fly (Hermetia illucens) Larvae. Dev. Comp. Immunol. 2015, 52, 98–106. [Google Scholar] [CrossRef]
- Józefiak, A.; Engberg, R. Insect Proteins as a Potential Source of Antimicrobial Peptides in Livestock Production. A Review. J. Anim. Feed Sci. 2017, 26, 87–99. [Google Scholar] [CrossRef]
- Chatzidimitriou, E.; Davis, H.; Maurer, V.; Leiber, F.; Leifert, C.; Stergiadis, S.; Butler, G. Egg Fatty Acid Profiles and Potential Health Risk from Defatted Insect Meal in Laying Hens’ Diets. J. Insects Food Feed 2022, 8, 1085–1095. [Google Scholar] [CrossRef]
- Heuel, M.; Kreuzer, M.; Sandrock, C.; Leiber, F.; Mathys, A.; Gold, M.; Zurbrügg, C.; Gangnat, I.D.M.; Terranova, M. Transfer of Lauric and Myristic Acid from Black Soldier Fly Larval Lipids to Egg Yolk Lipids of Hens Is Low. Lipids 2021, 56, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Marono, S.; Loponte, R.; Lombardi, P.; Vassalotti, G.; Pero, M.E.; Russo, F.; Gasco, L.; Parisi, G.; Piccolo, G.; Nizza, S.; et al. Productive Performance and Blood Profiles of Laying Hens Fed Hermetia illucens Larvae Meal as Total Replacement of Soybean Meal from 24 to 45 Weeks of Age. Poult. Sci. 2017, 96, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Smulikowska, S.; Rutkowski, A. Recommended Allowances and Nutritive Value of Feedstuffs. In Poultry Feeding Standards, 5th ed.; The Kielanowski Institute of Animal Physiology and Nutrition PAS: Jabłonna, Poland, 2018; (In Polish). ISBN 978-83-951612-1-6. [Google Scholar]
- Janssen, W.M.M.A. European Table of Energy Values for Poultry Feedstuffs, 3rd ed.; Subcommittee Energy of the Working Group nr. 2 Nutrition of the European Federation of Branches of the World’s Poultry Science Association: Beekbergen, The Netherlands, 1989; ISBN 90-71463-00-0. [Google Scholar]
- Pikul, J.; Leszczynski, D.E.; Kummerow, F.A. Evaluation of Three Modified TBA Methods for Measuring Lipid Oxidation in Chicken Meat. J. Agric. Food Chem. 1989, 37, 1309–1313. [Google Scholar] [CrossRef]
- Mwaniki, Z.; Shoveller, A.K.; Huber, L.-A.; Kiarie, E.G. Complete Replacement of Soybean Meal with Defatted Black Soldier Fly Larvae Meal in Shaver White Hens Feeding Program (28–43 Wks of Age): Impact on Egg Production, Egg Quality, Organ Weight, and Apparent Retention of Components1. Poult. Sci. 2020, 99, 959–965. [Google Scholar] [CrossRef]
- March, B.E.; MacMILLAN, C. Linoleic Acid as a Mediator of Egg Size. Poult. Sci. 1990, 69, 634–639. [Google Scholar] [CrossRef]
- Al-Qazzaz, M.F.A.; Ismail, D.; Akit, H.; Idris, L.H. Effect of Using Insect Larvae Meal as a Complete Protein Source on Quality and Productivity Characteristics of Laying Hens. Rev. Bras. Zootec. 2016, 45, 518–523. [Google Scholar] [CrossRef]
- Cutrignelli, M.I.; Messina, M.; Tulli, F.; Randazzo, B.; Olivotto, I.; Gasco, L.; Loponte, R.; Bovera, F. Evaluation of an Insect Meal of the Black Soldier Fly (Hermetia illucens) as Soybean Substitute: Intestinal Morphometry, Enzymatic and Microbial Activity in Laying Hens. Res. Vet. Sci. 2018, 117, 209–215. [Google Scholar] [CrossRef]
- Marono, S.; Piccolo, G.; Loponte, R.; Di Meo, C.; Attia, Y.A.; Nizza, A.; Bovera, F. In Vitro Crude Protein Digestibility of Tenebrio molitor and Hermetia illucens Insect Meals and Its Correlation with Chemical Composition Traits. Ital. J. Anim. Sci. 2015, 14, 3889. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.-J.; Barroso, F.G.; Manzano-Agugliaro, F. Insect Meal as Renewable Source of Food for Animal Feeding: A Review. J. Clean. Prod. 2014, 65, 16–27. [Google Scholar] [CrossRef]
- Dabbou, S.; Gai, F.; Biasato, I.; Capucchio, M.T.; Biasibetti, E.; Dezzutto, D.; Meneguz, M.; Plachà, I.; Gasco, L.; Schiavone, A. Black Soldier Fly Defatted Meal as a Dietary Protein Source for Broiler Chickens: Effects on Growth Performance, Blood Traits, Gut Morphology and Histological Features. J. Anim. Sci. Biotechnol. 2018, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Gariglio, M.; Dabbou, S.; Crispo, M.; Biasato, I.; Gai, F.; Gasco, L.; Piacente, F.; Odetti, P.; Bergagna, S.; Plachà, I.; et al. Effects of the Dietary Inclusion of Partially Defatted Black Soldier Fly (Hermetia illucens) Meal on the Blood Chemistry and Tissue (Spleen, Liver, Thymus, and Bursa of Fabricius) Histology of Muscovy Ducks (Cairina moschata domestica). Animals 2019, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Shini, A.; Shini, S.; Bryden, W.L. Fatty Liver Haemorrhagic Syndrome Occurrence in Laying Hens: Impact of Production System. Avian Pathol. 2019, 48, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, L.; Coretti, L.; Dipineto, L.; Bovera, F.; Menna, F.; Chiariotti, L.; Nizza, A.; Lembo, F.; Fioretti, A. Insect-Based Diet, a Promising Nutritional Source, Modulates Gut Microbiota Composition and SCFAs Production in Laying Hens. Sci. Rep. 2017, 7, 16269. [Google Scholar] [CrossRef]
- Lutz, T.; Scharrer, E. Effect of Short-Chain Fatty Acids on Calcium Absorption by the Rat Colon. Exp. Physiol. 1991, 76, 615–618. [Google Scholar] [CrossRef]
- Donsbough, A.L.; Powell, S.; Waguespack, A.; Bidner, T.D.; Southern, L.L. Uric Acid, Urea, and Ammonia Concentrations in Serum and Uric Acid Concentration in Excreta as Indicators of Amino Acid Utilization in Diets for Broilers. Poult. Sci. 2010, 89, 287–294. [Google Scholar] [CrossRef]
- Mazurkiewicz, M. Poultry Diseases; Akademia Rolnicza we Wrocławiu: Wrocław, Poland, 2005; ISBN 83-89189-76-3. (In Polish) [Google Scholar]
- Seyedalmoosavi, M.M.; Mielenz, M.; Görs, S.; Wolf, P.; Daş, G.; Metges, C.C. Effects of Increasing Levels of Whole Black Soldier Fly (Hermetia illucens) Larvae in Broiler Rations on Acceptance, Nutrient and Energy Intakes and Utilization, and Growth Performance of Broilers. Poult. Sci. 2022, 101, 102202. [Google Scholar] [CrossRef]
- Bovera, F.; Piccolo, G.; Gasco, L.; Marono, S.; Loponte, R.; Vassalotti, G.; Mastellone, V.; Lombardi, P.; Attia, Y.A.; Nizza, A. Yellow Mealworm Larvae (Tenebrio molitor, L.) as a Possible Alternative to Soybean Meal in Broiler Diets. Br. Poult. Sci. 2015, 56, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Koide, S.S. Chitin-Chitosan: Properties, Benefits and Risks. Nutr. Res. 1998, 18, 1091–1101. [Google Scholar] [CrossRef]
- Akbarian, A.; Michiels, J.; Degroote, J.; Majdeddin, M.; Golian, A.; De Smet, S. Association between Heat Stress and Oxidative Stress in Poultry; Mitochondrial Dysfunction and Dietary Interventions with Phytochemicals. J. Anim. Sci. Biotechnol. 2016, 7, 37. [Google Scholar] [CrossRef]
- Lugata, J.K.; Ortega, A.D.S.V.; Szabó, C. The Role of Methionine Supplementation on Oxidative Stress and Antioxidant Status of Poultry-A Review. Agriculture 2022, 12, 1701. [Google Scholar] [CrossRef]
- Kozłowski, K.; Ognik, K.; Stępniowska, A.; Juśkiewicz, J.; Zduńczyk, Z.; Kierończyk, B.; Benzertiha, A.; Józefiak, D.; Jankowski, J. Growth Performance, Immune Status and Intestinal Fermentative Processes of Young Turkeys Fed Diet with Additive of Full Fat Meals from Tenebrio Molitor and Hermetia illucens. Anim. Feed Sci. Technol. 2021, 278, 114994. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Yao, Y.; Qu, X.; Chen, J.; Xie, K.; Wang, X.; Qi, Y.; Xiao, B.; He, C. Effects of Different Levels of Hermetia illucens Larvae Meal on Performance, Egg Quality, Yolk Fatty Acid Composition and Oxidative Status of Laying Hens. Ital. J. Anim. Sci. 2021, 20, 256–266. [Google Scholar] [CrossRef]
- Xiao, N.; Zhao, Y.; Yao, Y.; Wu, N.; Xu, M.; Du, H.; Tu, Y. Biological Activities of Egg Yolk Lipids: A Review. J. Agric. Food Chem. 2020, 68, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Zotte, A.D.; Singh, Y.; Michiels, J.; Cullere, M. Black Soldier Fly (Hermetia illucens) as Dietary Source for Laying Quails: Live Performance, and Egg Physico-Chemical Quality, Sensory Profile and Storage Stability. Animals 2019, 9, 115. [Google Scholar] [CrossRef]
- Nogueira, C.M.; Zapata, J.F.F.; Fuentes, M.F.F.; Freitas, E.R.; Craveiro, A.A.; Aguiar, C.M. The Effect of Supplementing Layer Diets with Shark Cartilage or Chitosan on Egg Components and Yolk Lipids. Br. Poult. Sci. 2003, 44, 218–223. [Google Scholar] [CrossRef]
| Nutrient Composition [g/kg] | H. illucens Meal | Soybean Meal |
|---|---|---|
| Dry matter | 943.0 | 913.6 |
| Crude ash Crude fat | 93.7 116.2 | 59.4 23.2 |
| Crude protein | 480.4 | 451.7 |
| Crude fiber | 92.6 | 36.1 |
| Sodium Potassium Magnesium Calcium Zinc Manganese Copper Iron Phosphorus | 1.43 16.9 4.26 16.9 0.147 0.205 0.0117 0.350 12.2 | 0.045 24.7 2.85 2.25 0.0441 0.0256 0.0128 0.0819 6.35 |
| Aspartic acid | 38.55 | 54.01 |
| Threonine | 17.29 | 18.78 |
| Serine | 18.94 | 23.71 |
| Glutamic acid | 47.30 | 79.56 |
| Proline | 26.02 | 20.33 |
| Glycine | 25.20 | 19.35 |
| Alanine | 35.89 | 21.12 |
| Valine | 25.65 | 21.30 |
| Isoleucine | 34.77 | 21.27 |
| Leucine | 31.39 | 32.88 |
| Tyrosine | 29.18 | 18.49 |
| Phenylalanine | 19.26 | 22.98 |
| Histidine | 13.27 | 12.07 |
| Lysine | 28.97 | 29.12 |
| Arginine | 21.28 | 34.18 |
| Cysteine | 3.59 | 6.24 |
| Methionine | 6.72 | 6.15 |
| Tryptophan | 5.33 | 7.04 |
| Ingredient [g/kg] | HIM 0% | HIM 5% | HIM 10% | HIM 15% |
|---|---|---|---|---|
| H. illucens meal | 0 | 50 | 100 | 150 |
| Wheat | 362.55 | 342.1 | 349.78 | 354.22 |
| Maize | 220 | 250 | 250 | 250 |
| Soybean meal | 200 | 150 | 100 | 50 |
| Wheat bran | 70 | 65 | 61 | 60 |
| Rapeseed oil | 35 | 32 | 30 | 28 |
| Limestone | 91.8 | 91.5 | 91 | 91 |
| Monocalcium phosphate | 11 | 9.3 | 7.8 | 6 |
| Sodium chloride | 3 | 3 | 3 | 3 |
| DL-Methionine | 1.5 | 1.32 | 1.15 | 0.98 |
| L-Lysine hydrochloride | 0.15 | 0.78 | 1.3 | 1.8 |
| Vitamin–mineral premix * | 5 | 5 | 5 | 5 |
| Analyzed chemical composition (g/kg): | ||||
| Metabolizable energy (MJ/kg) 1 | 11.6 | 11.6 | 11.6 | 11.6 |
| Crude protein | 166.0 | 166.5 | 169.3 | 169.3 |
| Calcium | 43.6 | 43.1 | 45.4 | 40.7 |
| Phosphorus | 6.10 | 6.42 | 6.17 | 5.86 |
| Aspartic acid | 13.94 | 14.32 | 12.64 | 14.91 |
| Threonine | 5.2 | 5.87 | 5.34 | 6.42 |
| Serine | 7.7 | 8.43 | 7.84 | 9.68 |
| Glutamic acid | 34.18 | 33.63 | 29.76 | 34.14 |
| Proline | 9.4 | 10.82 | 10.02 | 13.09 |
| Glycine | 6.98 | 7.17 | 6.92 | 8.39 |
| Alanine | 7.84 | 8.51 | 9.04 | 11.01 |
| Valine | 7.35 | 7.57 | 7.66 | 8.92 |
| Isoleucine | 6.46 | 6.33 | 6.52 | 7.48 |
| Leucine | 12.77 | 12.25 | 12.07 | 13.32 |
| Tyrosine | 5.92 | 6.53 | 7.03 | 7.65 |
| Phenylalanine | 8.46 | 7.95 | 7.87 | 8.69 |
| Histidine | 4.53 | 4.53 | 4.54 | 5.06 |
| Lysine | 7.75 | 8.02 | 7.96 | 9.31 |
| Arginine | 10.48 | 9.6 | 8.94 | 9.16 |
| Cysteine | 2.64 | 2.61 | 2.53 | 2.59 |
| Methionine | 4.52 | 4.32 | 4.36 | 4.41 |
| Tryptophan | 2.32 | 2.39 | 2.44 | 2.61 |
| Experimental Diets | ||||||
|---|---|---|---|---|---|---|
| Items | HIM 0% | HIM 5% | HIM 10% | HIM 15% | HIM | SBM |
| C8—caprylic acid | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| C10—capric acid | 0.00 | 0.15 | 0.27 | 0.40 | 1.61 | 0.00 |
| C12—lauric acid | 0.00 | 4.16 | 8.37 | 12.92 | 44.09 | 0.00 |
| C14—myristic acid | 0.08 | 1.25 | 2.19 | 2.95 | 9.48 | 0.15 |
| C16—palmitic acid | 10.31 | 10.67 | 10.77 | 11.12 | 14.81 | 17.55 |
| C16-1—palmitoleic acid | 0.22 | 0.59 | 0.89 | 1.11 | 3.19 | 0.35 |
| C18—stearic acid | 2.19 | 2.13 | 2.1 | 2.13 | 2.65 | 4.79 |
| C18-1—oleic acid | 50.14 | 45.53 | 42.81 | 39.66 | 14.77 | 14.46 |
| C18-2—linoleic acid | 28.74 | 27.55 | 25.03 | 23.05 | 7.7 | 47.27 |
| Gamma18-3—gamma-linolenic acid | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 |
| C20—arachidic acid | 0.4 | 0.36 | 0.33 | 0.3 | 0.08 | 0.19 |
| C18-3—linolenic acid | 7.55 | 7.31 | 6.98 | 6.1 | 1.5 | 14.88 |
| C20-4—arachidonic acid | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 |
| C22-1—erucic acid | 0.13 | 0.11 | 0.1 | 0.09 | 0.01 | 0.02 |
| EPA—eicosapentaenoic acid | 0.00 | 0.00 | 0.00 | 0.01 | 0.02 | 0.00 |
| DHA—docosahexaenoic acid | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 |
| SFA | 13.21 | 18.92 | 24.19 | 29.98 | 72.77 | 23.02 |
| UFA | 86.79 | 81.08 | 75.81 | 70.02 | 27.23 | 76.98 |
| MUFA | 50.49 | 46.23 | 43.8 | 40.86 | 17.96 | 14.83 |
| PUFA | 36.3 | 34.86 | 32.01 | 29.16 | 9.28 | 62.15 |
| PUFA-6 | 28.74 | 27.55 | 25.03 | 23.05 | 7.74 | 47.27 |
| PUFA-3 | 7.56 | 7.31 | 6.98 | 6.1 | 1.54 | 14.88 |
| DFA | 88.98 | 83.22 | 77.91 | 72.15 | 29.88 | 81.77 |
| UFA/SFA | 6.57 | 4.29 | 3.13 | 2.34 | 0.37 | 3.34 |
| MUFA/SFA | 3.82 | 2.44 | 1.81 | 1.36 | 0.25 | 0.64 |
| PUFA/SFA | 2.75 | 1.84 | 1.32 | 0.97 | 0.13 | 2.7 |
| PUFA 6/3 | 3.8 | 3.77 | 3.58 | 3.78 | 5.04 | 3.18 |
| Items | HIM 0% | HIM 5% | HIM 10% | HIM 15% | SEM | p-Value |
|---|---|---|---|---|---|---|
| Laying rate [%] | 98.5 | 97.4 | 97.2 | 98.9 | 0.284 | 0.085 |
| Daily mass of eggs [g per hen] | 57.5 | 58.1 | 57.6 | 57.6 | 0.288 | 0.89 |
| Egg weight [g] | 58.4 | 59.7 | 59.3 | 58.2 | 0.278 | 0.197 |
| Daily feed intake [g per hen] | 122 | 117 | 121 | 119 | 1.11 | 0.190 |
| FCR per one egg [g] | 124 | 120 | 125 | 121 | 1.14 | 0.127 |
| FCR per g of egg [g] | 2.12 | 2.02 | 2.11 | 2.07 | 0.0232 | 0.159 |
| Items | HIM 0% | HIM 5% | HIM 10% | HIM 15% | SEM | p-Value |
|---|---|---|---|---|---|---|
| Gizzard [%] | 1.15 | 1.13 | 1.12 | 1.07 | 0.022 | 0.688 |
| Liver [%] | 1.32 | 1.34 | 1.28 | 1.30 | 0.024 | 0.844 |
| Pancreas [%] | 0.17 | 0.16 | 0.17 | 0.16 | 0.004 | 0.545 |
| Spleen [%] | 0.082 | 0.076 | 0.071 | 0.083 | 0.002 | 0.084 |
| Heart [%] | 0.31 | 0.31 | 0.29 | 0.32 | 0.004 | 0.250 |
| Abdominal fat [%] | 3.67 | 4.44 | 4.75 | 4.01 | 0.175 | 0.137 |
| Items | HIM 0% | HIM 5% | HIM 10% | HIM 15% | SEM | p-Value |
|---|---|---|---|---|---|---|
| Tp [g/dL] | 5.22 | 5.4 | 5.64 | 5.5 | 0.101 | 0.329 |
| Alb [g/dL] | 2.36 | 2.46 | 2.46 | 2.44 | 0.027 | 0.516 |
| TG [mg/dL] | 1123 | 1264 | 1318 | 1276 | 29.6 | 0.103 |
| TC [mg/dL] | 129.9 | 153.4 | 161.9 | 151.9 | 5.63 | 0.156 |
| HDL [mg/dL] | 46.6 | 54.8 | 55 | 56.8 | 1.46 | 0.058 |
| LDL [mg/dL] | 44.2 | 43 | 43.7 | 45.6 | 1.17 | 0.881 |
| UA [mg/dL] | 3.99 c | 5.79 a | 5.47 ab | 4.68 bc | 0.185 | 0.001 |
| Alt [U/L] | 2.91 | 4.53 | 3.6 | 4.35 | 0.325 | 0.205 |
| Ast [U/L] | 225 | 198 | 199 | 207 | 4.19 | 0.069 |
| LDH [U/L] | 1775 | 1692 | 1831 | 1512 | 74.9 | 0.258 |
| Alp [U/L] | 578 | 462 | 601 | 632 | 32.1 | 0.203 |
| Amylase [U/L] | 379 | 390 | 374 | 334 | 11.4 | 0.332 |
| Lipase [U/L] | 9.21 | 9.52 | 9.53 | 9.23 | 0.075 | 0.262 |
| Ca [mg/dL] | 25.0 b | 27.2 a | 27.4 a | 26.9 a | 0.319 | 0.028 |
| P [mg/dL] | 5.14 | 5.92 | 6.12 | 5.75 | 0.148 | 0.080 |
| Mg [mg/dL] | 3.41 | 3.77 | 3.71 | 3.66 | 0.061 | 0.182 |
| Fe [mcg/dL] | 566 | 579 | 599 | 631 | 12.4 | 0.278 |
| Items | HIM 0% | HIM 5% | HIM 10% | HIM 15% | SEM | p-Value |
|---|---|---|---|---|---|---|
| SOD [ng/mL] | 15.4 | 14.9 | 13.3 | 16.4 | 17.0 | 0.759 |
| CAT [ng/mL] | 78.4 | 59.0 | 50.5 | 68.3 | 5.32 | 0.286 |
| GPx [ng/mL] | 10.8 | 8.68 | 7.12 | 10.5 | 0.64 | 0.136 |
| MDA [nmol/mL] | 47.2 | 39.1 | 33.3 | 38.4 | 2.70 | 0.520 |
| Items | HIM 0% | HIM 5% | HIM 10% | HIM 15% | SEM | p-Value |
|---|---|---|---|---|---|---|
| MDA [mg/kg liver] | 1.05 | 1.03 | 0.98 | 1.3 | 0.026 | 0.981 |
| Fat content [%] | 18.1 | 19.6 | 19.8 | 19.5 | 0.380 | 0.340 |
| Items | HIM 0% | HIM 5% | HIM 10% | HIM 15% | SEM | p-Value |
|---|---|---|---|---|---|---|
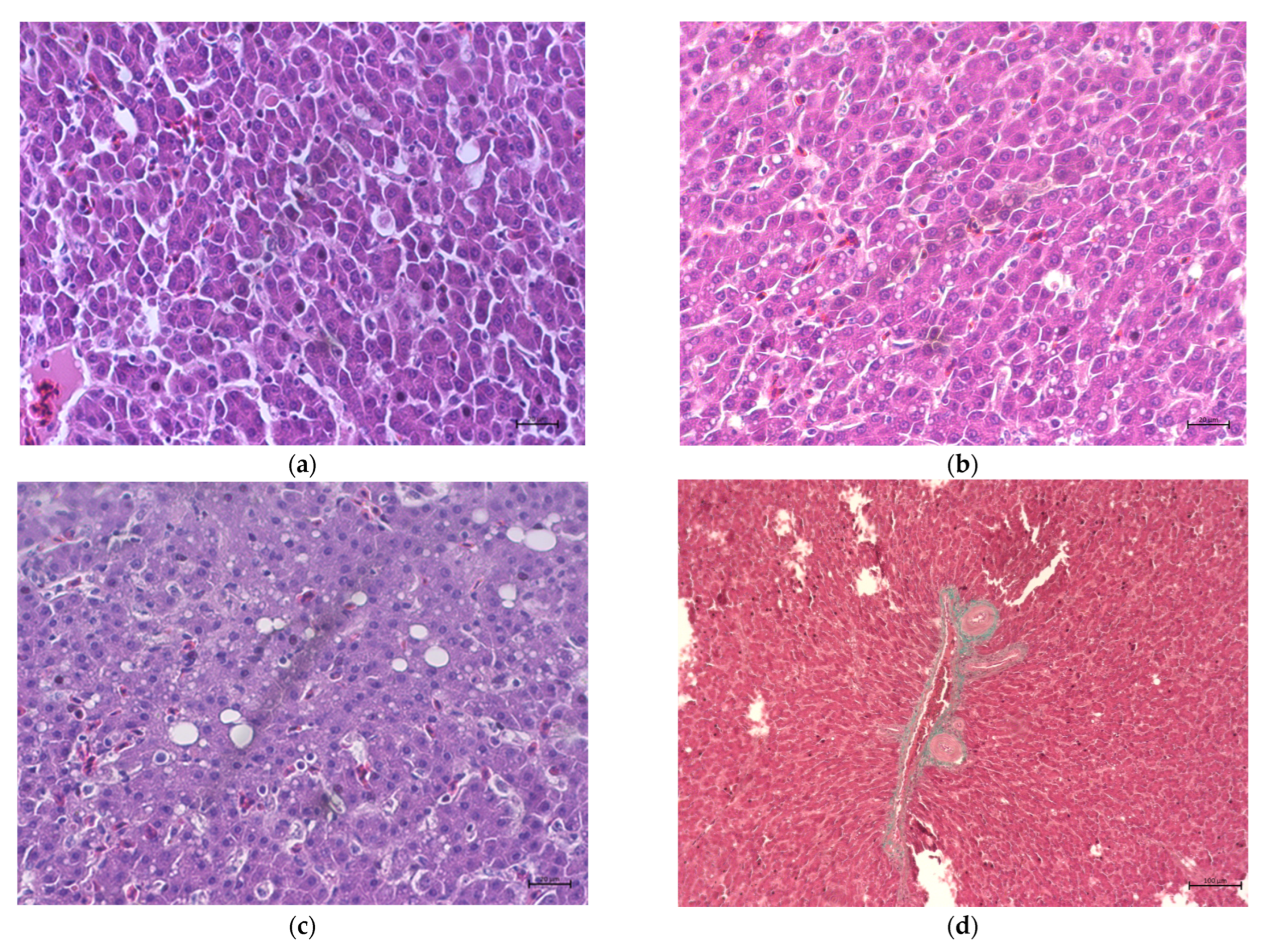
| Liver steatosis scoring [points] | 1.50 | 1.625 | 2.00 | 1.375 | 0.114 | 0.320 |
| Liver fibrosis | nd | nd | nd | nd | ||
| Number of hepatocytes [H/0.1 mm2] | 2199 a | 2014 ab | 1841 b | 1872 b | 44.1 | 0.009 |
| Items | HIM 0% | HIM 5% | HIM 10% | HIM 15% | SEM | p-Value |
|---|---|---|---|---|---|---|
| Total cholesterol [mg/g yolk] | 13.5 a | 12.7 b | 12.8 b | 12.5 b | 0.115 | 0.020 |
| MDA [g/kg yolk] | 0.65 ab | 0.66 a | 0.57 b | 0.62 ab | 0.012 | 0.036 |
| Fatty acid [g/100 g of total fatty acids] | ||||||
| C12—lauric acid | 0.00417 c | 0.0267 bc | 0.0483 ab | 0.08 a | 0.0044 | 0.000 |
| C14—myristic acid | 0.357 c | 0.558 bc | 0.723 ab | 1.03 a | 0.0377 | 0.000 |
| C16—palmitic acid | 24.4 | 25.0 | 25.2 | 25.1 | 0.113 | 0.058 |
| C16-1—palmitoleic acid | 3.63 | 4.01 | 3.90 | 4.08 | 0.073 | 0.145 |
| C18—stearic acid | 8.49 | 8.47 | 8.68 | 8.55 | 0.080 | 0.796 |
| C18-1—oleic acid | 46.7 | 46.7 | 46.8 | 45.4 | 0.218 | 0.150 |
| C18-2—linoleic acid | 11.8 a | 11.0 ab | 10.7 b | 11.7 a | 0.156 | 0.028 |
| Gamma18-3—gamma-linolenic acid | 0.0658 a | 0.0533 ab | 0.0500 b | 0.0542 ab | 0.001 | 0.007 |
| C20—arachidic acid | 0.0492 a | 0.0450 ab | 0.0325 bc | 0.0283 c | 0.0017 | 0.000 |
| C18-3—linolenic acid | 0.710 a | 0.608 b | 0.588 b | 0.67 ab | 0.0153 | 0.012 |
| C20-4—arachidonic acid | 2.23 a | 2.07 b | 1.97 bc | 1.89 c | 0.0266 | 0.000 |
| C22-1—erucic acid | 0.0142 a | 0.0050 b | 0 b | 0 b | 0.0010 | 0.000 |
| EPA—eicosapentaenoic acid | 0.0100 | 0.0100 | 0.00917 | 0.0108 | 0.0003 | 0.271 |
| DHA—docosahexaenoic acid | 1.53 a | 1.46 ab | 1.36 bc | 1.33 bc | 0.0213 | 0.000 |
| SFA | 33.3 b | 34.1 ab | 34.7 a | 34.8 a | 0.128 | 0.000 |
| UFA | 66.7 a | 65.9 ab | 65.3 b | 65.2 b | 0.128 | 0.000 |
| MUFA | 50.3 | 50.7 | 50.7 | 49.5 | 0.188 | 0.110 |
| PUFA | 16.3 a | 15.2 b | 14.6 b | 15.6 ab | 0.192 | 0.013 |
| PUFA-6 | 14.0 a | 13.1 bc | 12.7 c | 13.6 ab | 0.166 | 0.016 |
| PUFA-3 | 2.25 a | 2.08 ab | 1.95 b | 2.01 b | 0.031 | 0.004 |
| DFA | 75.2 a | 74.4 b | 74.0 bc | 73.7 c | 0.134 | 0.000 |
| UFA/SFA | 2.00 a | 1.94 ab | 1.89 b | 1.87 b | 0.011 | 0.000 |
| PUFA 6/3 | 6.25 b | 6.35 b | 6.5 b | 6.76 a | 0.052 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawisza, P.; Szymczyk, B.; Arczewska-Włosek, A.; Szczepanik, K. Effects of Partial Replacement of Soybean Meal with Defatted Hermetia illucens Meal in the Diet of Laying Hens on Performance, Dietary Egg Quality, and Serum Biochemical and Redox Indices. Animals 2023, 13, 527. https://doi.org/10.3390/ani13030527
Zawisza P, Szymczyk B, Arczewska-Włosek A, Szczepanik K. Effects of Partial Replacement of Soybean Meal with Defatted Hermetia illucens Meal in the Diet of Laying Hens on Performance, Dietary Egg Quality, and Serum Biochemical and Redox Indices. Animals. 2023; 13(3):527. https://doi.org/10.3390/ani13030527
Chicago/Turabian StyleZawisza, Patrycja, Beata Szymczyk, Anna Arczewska-Włosek, and Kinga Szczepanik. 2023. "Effects of Partial Replacement of Soybean Meal with Defatted Hermetia illucens Meal in the Diet of Laying Hens on Performance, Dietary Egg Quality, and Serum Biochemical and Redox Indices" Animals 13, no. 3: 527. https://doi.org/10.3390/ani13030527
APA StyleZawisza, P., Szymczyk, B., Arczewska-Włosek, A., & Szczepanik, K. (2023). Effects of Partial Replacement of Soybean Meal with Defatted Hermetia illucens Meal in the Diet of Laying Hens on Performance, Dietary Egg Quality, and Serum Biochemical and Redox Indices. Animals, 13(3), 527. https://doi.org/10.3390/ani13030527






