Simple Summary
We probed the effect of a probiotic complex on the immune response caused by Plesiomonas shigelloides infection in the southern catfish. Our study revealed the key genes for this inflammatory response, and the probiotic complex effectively inhibited the expression of key inflammatory factors caused by P. shigelloides, providing basic data for future artificial breeding of the southern catfish and the prevention and control of bacterial diseases caused by P. shigelloides.
Abstract
To explore whether a probiotic complex composed of Lactobacillus rhamnosus, Lactobacillus plantarum, and Lactobacillus casei can prevent or inhibit the inflammatory response caused by the invasion of Plesiomonas shigelloides in the southern catfish, we screened differentially expressed genes and enriched inflammation-related pathways among a control and three experimental groups and conducted analysis by transcriptome sequencing after a 21-day breeding experiment. Compared with those in the PS (Plesiomonas shigelloides) group, southern catfish in the L-PS (Lactobacillus-Plesiomonas shigelloides) group had no obvious haemorrhages or ulcerations. The results also showed that inflammation-related genes, such as mmp9, cxcr4, nfkbia, socs3, il-8, pigr, tlr5, and tnfr1, were significantly upregulated in the PS group compared with those in the L-PS groups. In addition, we verified six DEGs (mmp9, cxcr4, nfkbia, socs3, rbp2, and calr) and three proteins (CXCR4, NFKBIA, and CALR) by qRT-PCR and ELISA, respectively. Our results were consistent with the transcriptome data. Moreover, significantly downregulated genes (p < 0.05) were enriched in inflammation-related GO terms (lymphocyte chemotaxis and positive regulation of inflammatory response) and immune-related pathways (intestinal immune network for IgA production and IL-17 signalling pathway) in the L-PS vs. the PS group. Our results indicate that the infection of P. shigelloides can produce an inflammatory response, and probiotics could inhibit the inflammatory response caused by P. shigelloides to some extent.
1. Introduction
The goal of intensive aquaculture is to create the most suitable environment for the growth and development of aquatic organisms at high densities in small facilities by using advanced feeding management methods, achieving a high yield and efficiently breeding the organisms in a relatively short period of time by accelerating their growth rate to increase their production. However, the development of the aquaculture industry is restricted by animal diseases that are caused by the aquatic environment in high-density aquacultural facilities, which are more conducive to the reproduction, growth, and spread of bacteria. Therefore, bacterial diseases have seriously affected the economical profitability of the aquaculture industry [1].
The southern catfish (Silurus meridionalis) (Siluriformes, Siluridae) is an economically important species with a large size, high nutritional value [2], rapid growth [3,4,5], disease resistance [6], wide environmental adaptability [7], and euryphagous [8,9], and is widely cultured in China. Southern catfish can survive in sewage; however, despite being more disease-resistant than other fish, it is still unable to avoid the invasion of some bacterial diseases. At present, a set of pathogens have been isolated in southern catfish, such as Vibrio vermiculo [10], Proteus commoner [11], Aeromonas guinea [12], Aeromonas hydrophila [13], Edwardsiella ctalurid [14], Aeromonas viridans [15], Edwardsia [16], Columnar flex [17], Pseudomonas fluorescens [17], Pseudomonas albicans [17], Cronobacter sakazakii [6], and Aeromonas mild [18]. Plesimonas shigelloides, a man–fish zoonotic pathogenic that causes great losses in the aquaculture of many freshwater fish, was isolated from southern catfish in a pre-experiment. There are no studies on P. shigelloides in southern catfish thus far; therefore, a study into its pathogenic mechanisms is necessary.
To date, a large number of antibiotic drugs have been used to fight bacterial diseases in aquaculture, and the drug residues are causing harm to human health. Thus, it is urgent to find non-toxic alternatives that yield no side effects. The way probiotics antagonise pathogenic bacteria is mainly reflected in four aspects: competing with pathogenic bacteria for adhesion sites, copolymerising with pathogenic bacteria, producing antibacterial substances, and enhancing the body’s immunity. For example, Bifidobacterium breve has been shown to increase the production of anti-influenza IgG, inhibiting influenza virus infections [19]. Some clinical studies have shown that fermented milk containing Lactobacillus casei DN114001 can reduce the severity, duration, and incidence of acute diarrhoea in children [20,21,22]. A European trial showed that giving oral lactobacillus GG rehydration solution to children with acute diarrhoea resulted in a shorter duration of illness [23]. Therefore, we developed our study based on this idea.
L. rhamnosus is a probiotic with high acid and bile resistance, as well as strong adhesion, meaning it can effectively colonise human and animal intestines [24]. Studies have shown that intervention with L. rhamnosus can significantly (p < 0.05) reduce the proliferation of harmful bacteria, such as Shigella, in the intestines of mice with ulcerative colitis, as well as inhibit colonic inflammation [25]. L. casei and L. plantarum also exhibit strong probiotic properties, show strong resistance to acids and bile, and have strong antagonistic activity against Salmonella paratyphi in the gastrointestinal tract [26]. In addition, adding appropriate probiotics to fish feed can effectively enhance their immune system function and increase their resistance to various pathogenic infections [27,28]. For example, Oncorhynchus mykiss showed high resistance to pathogenic bacterial infection after feeding with different doses of L. rhamnosus for 51 days [29]. Considering that the three probiotics above can effectively colonise and survive in the intestinal tract, these three were selected to constitute our probiotic complex. In our study, we screened immune-related differentially expressed genes (DEGs) by transcriptome sequencing to explore whether probiotics can prevent or inhibit bacterial diseases caused by P. shigelloides. We verified six genes and three proteins by qRT-PCR and ELISA, respectively. Additionally, we also deeply analysed the inflammation-related pathways enriched by DEGs.
2. Materials and Methods
2.1. Experimental Bacterial Strain
We previously isolated P. shigelloides and Lactobacillus plantarum from diseased southern catfish and healthy carp, and purchased L. casei (BNCC134415) and Lactobacillus rhamnosus (BNCC134266) strains from the BeNa Culture Collection. For subsequent experiments, we cultured P. shigelloides in a tryptic soy broth (TSB) medium for 7 h (exponential stage) at 37 °C. We also cultured L. plantarum, L. casei, and L. rhamnosus in an MRS liquid medium for 24 h at 37 °C without oxygen. After collection and washing three times with sterilised phosphate-buffered saline (PBS, pH 7.4), we resuspended the bacteria in sterile saline water to obtain a concentration of 1.0 × 109 CFU/mL.
2.2. Animal and Experimental Design
We obtained healthy adult southern catfish, which were approximately 20 centimetres long, from a fish farm located in Hechuan, Chongqing, China. We kept the fish in freshwater tanks at 24–27 °C in a breeding room before acclimatising them to laboratory conditions for 8 weeks prior to the experiments.
First, we divided the experimental fish into four groups (each with 20 individuals): (1) blank control group (BC), (2) Lactobacillus group (L), (3) Lactobacillus + P. shigelloides group (L-PS), and (4) P. shigelloides group (PS). Feeding probiotic complex: L group and L-PS group were administered a mix of Lactobacillus (1.0 × 109 CFU/mL, 0.2 mL) by intragastric administration for 21 d, whereas the BC group and PS group were gavaged with normal saline (0.2 mL) to provide the same management stress. P. shigelloides infection test: After 21 d, the L-PS group and the PS group were injected intraperitoneally with P. shigelloides (1.0 × 109 CFU/mL, 0.2 mL), whereas the BC group and the L group were replaced by normal saline (0.2 mL) to provide the same management stress [30,31,32,33,34]. We collected samples 72 h after injecting P. shigelloides. We obtained spleens from each group (3 samples per group), which we immediately snap-froze in liquid nitrogen and stored at −80 °C for subsequent testing.
2.3. RNA Extraction, Library Construction, and Sequencing
We extracted the total RNA of the spleens using a mirVana™ miRNA ISOlation Kit (Ambion-1561) (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s instructions. We detected the purity (OD260/280), concentration, and absorption peak of the RNA by a Nano Drop 2000 (Thermo Fisher Scientific, Waltham, MA, USA), and then examined the quality of the RNA by 1% agarose gel electrophoresis.
We constructed the libraries using the TruSeq Stranded mRNA LT Sample Prep Kit (Illumia, San Diego, CA, USA) according to the manufacturer’s instructions. Then, sequencing was completed using the Illumina HiSeq X Ten platform by OE Biotech Co., Ltd. (Shanghai, China), which generated 150 bp paired-end reads.
2.4. Data Processing and Assembly
We processed the raw data (raw reads) using Trimmomatic [35] (parameters: LEADING: 3 TRAILING: 3 SLIDINGWINDOW: 4: 15 MINLEN: 50) to remove poly-N and low-quality reads, which provided clean reads. We mapped clean reads to the southern catfish reference genomes by hisat2 [36] (version: 2.2.1) (parameters: -x –ss –exon). We assembled and merged the transcripts by StringTie [37] (version: 1.3.0) (parameters: --merge -G).
2.5. Functional Annotation
We functionally annotated the transcripts by alignment of the transcripts with the NCBI nonredundant (NR), SwissProt, and clusters of orthologous groups for the eukaryotic complete genome (KOG) databases using Diamond. The threshold takes E-value < 1e-5 to find orthologs. We used the proteins with the highest hits to the transcripts to assign functional annotations.
2.6. Analysis of Differentially Expressed Genes (DEGs), Cluster Analysis, GO and KEGG Enrichment
We calculated the read counts and FPKM using featureCounts [38] (version: 2.0.0) (parameters: -p -a -t -g) and eXpress [39] (version: 1.5.1) (parameters: --rf-stranded). We identified DEGs using the R packages DESeq2 [40] (version: 1.38.2) (parameters: qvalue < 0.05, |log2FoldChange| > 1) and edgeR [41]. We performed GO enrichment and KEGG pathway enrichment analysis of the DEGs using R package clusterProfiler [42] (version: 4.6.0) based on the hypergeometric distribution. We used R packages ggplot2 [43] (version: 3.4.0) to draw the column and bubble diagrams of the significant enrichment terms.
2.7. Validation of DEGs by q-PCR
To test the RNA-Seq results, we selected six DEGs (mmp9, socs3, nfkbia, cxcr4, rbp2, and calr) for qRT-PCR analysis. The specific primers used are listed in Table 1, and we used β-actin and 18S rRNA as internal controls. We incubated reactions at 94 °C for 30 s, followed by 45 cycles of 94 °C for 5 s and 60 °C for 30 s. We performed melting curve analysis to verify the specific generation of the expected PCR product after the PCR cycles ended. We normalised the expression levels of mRNAs to β-actin, and we calculated 18S rRNA using the 2−ΔΔCt method [44].

Table 1.
Specific primers list.
2.8. Validation of DEGs by ELISA
We analysed three proteins (CXCR4, CALR, and NFKBIA) by enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s instructions (Enzyme-Free Biologics) to further verify the reliability of the transcriptome data. We drew the standard curve according to the concentration and absorbance values of the standard to obtain the corresponding calculation formula. Then, we entered the absorbance value of each sample into the formula to calculate the secretion of each protein.
3. Results
3.1. Clinical Symptoms
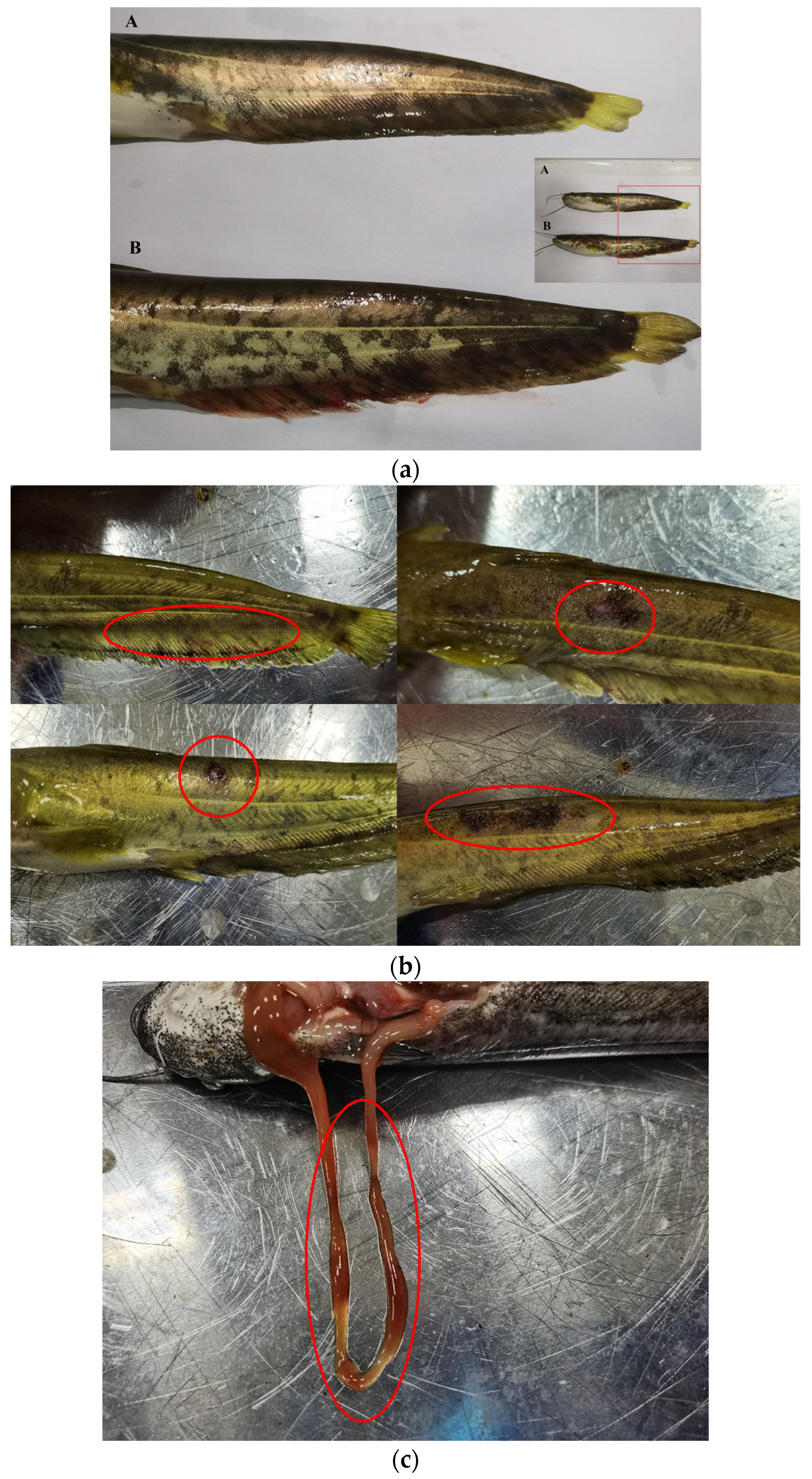
In our experiment, we observed body surface changes in the PS and L-PS groups 72 h after injection of P. shigelloides (Figure 1a). The most obvious symptoms in the PS group were bleeding of the caudal fins and anal fin, and the blackening, moulting, and ulceration of the skin surface; however, we did not find lesions in other parts (Figure 1b). After dissection, we found one case of intestinal redness and swelling; however, no lesions were found in the other individuals (Figure 1c). In the L-PS group, except for two individuals with hyperaemia of the anal fin base and one with redness and swelling at the injection site, no lesions were found in other individuals. The observations showed that the BC and L groups showed no body surface symptoms during the experimental period. The incidence table is as follows: southern catfish with caudal fin congestion; bleeding, ulceration, skin blackening, moulting, and other symptoms are counted as the disease (Table 2):
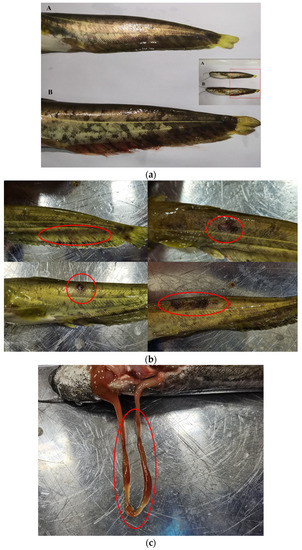
Figure 1.
Body surface symptoms of southern catfish infected with Plesiomonas shigelloides for 72 h. (a) Bleeding of anal fin of southern catfish infected with Plesiomonas shigelloides in PS vs. L-PS group. A: Southern catfish in L-PS group; B: southern catfish in PS groups. (b) Body surface symptoms of southern catfish. (c) Intestinal redness and swelling of southern catfish.

Table 2.
Incidence of southern catfish in each group.
3.2. Data Analysis of Transcriptome
From Illumina sequencing, a total of 79.37 Gb clean data were quality-filtered from 89.46 Gb of raw data (Table 3). The average length of merged transcripts was 1918 bp, and the N50 length was 32598 bp.

Table 3.
Summary of RNA-sequencing results and quality data output.
3.3. Differentially Expressed Genes (DEGs) Regulated by Exposure of P. shigelloides and Lactobacillus
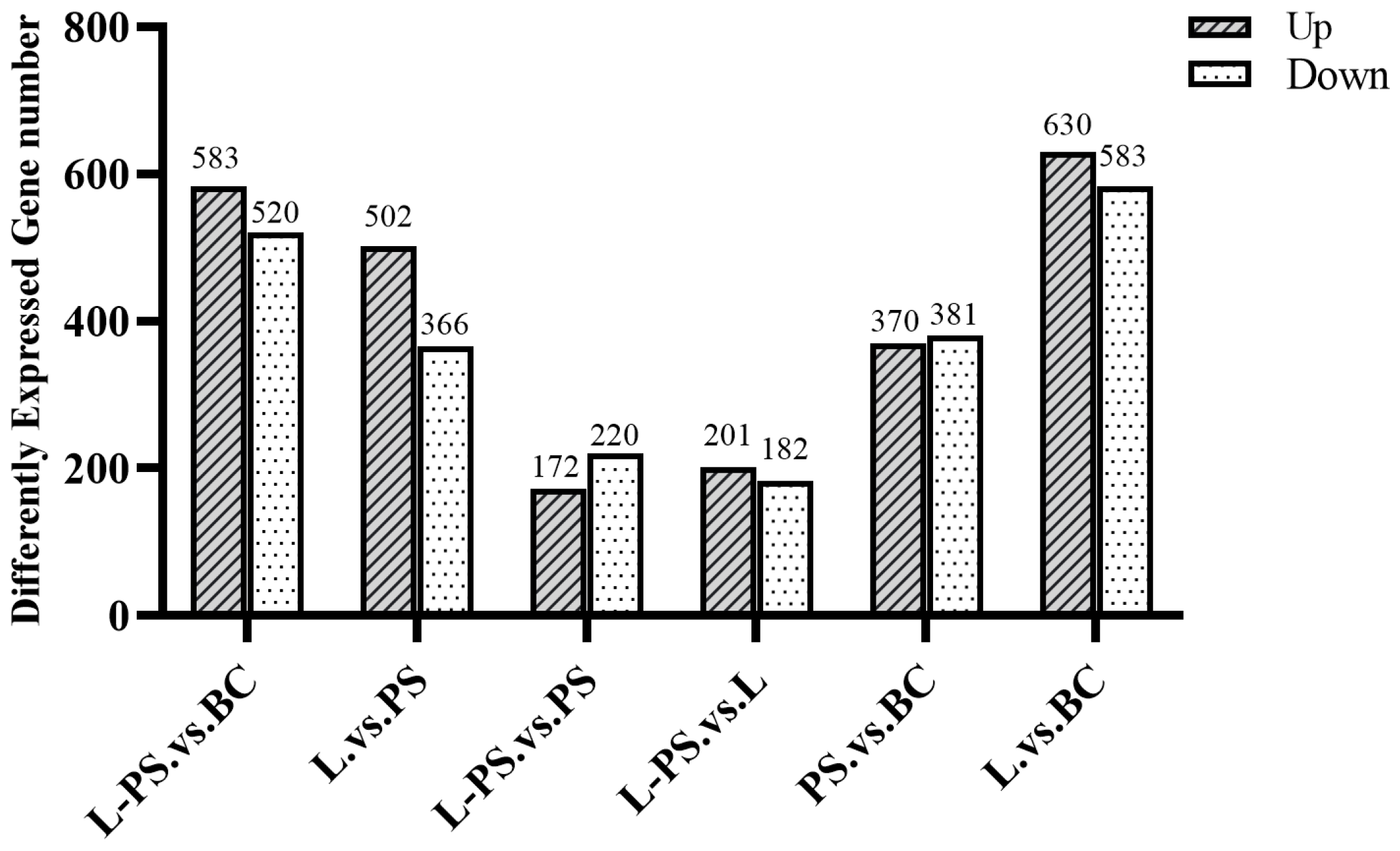
In total, 370 upregulated and 381 downregulated DEGs were detected in the PS group vs. the BC group, 630 upregulated and 583 downregulated DEGs were detected in the L group vs. the BC group, 583 upregulated and 520 downregulated DEGs were detected in the L-PS group vs. the BC group, and 172 upregulated and 220 downregulated DEGs were detected in the L-PS group vs. the PS group (Figure 2).
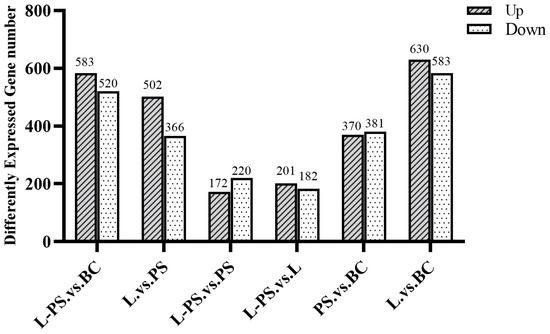
Figure 2.
DEG statistics for all groups.
3.4. Gene Ontology Enrichment Analysis for DEGs
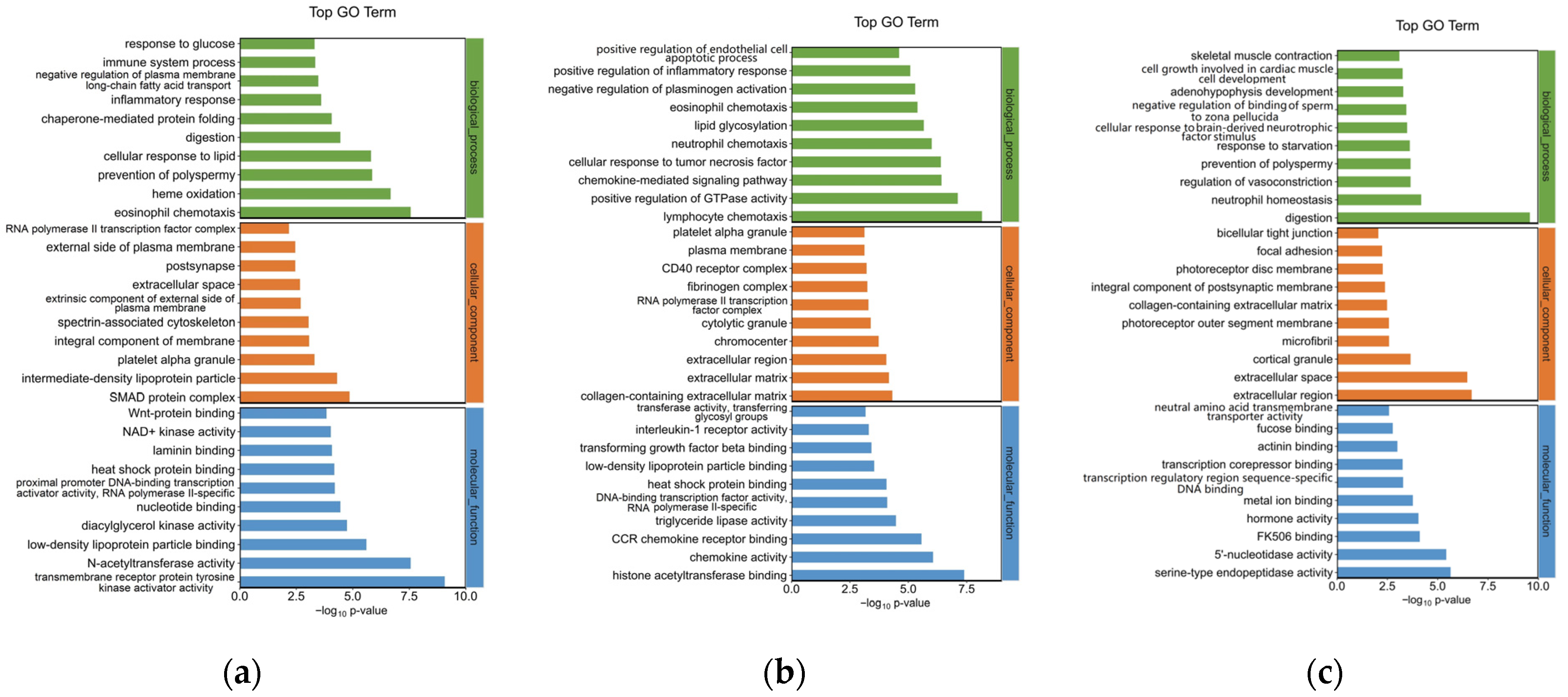
To explore the mechanism of the southern catfish’s immune response to P. shigelloides at the genetic level, 751 DEGs were processed for GO enrichment analysis between the BC and PS groups. The top 30 enriched GO terms of upregulated genes are presented in Figure 3a. The immune-related GO terms were significantly (p < 0.05) enriched in the spleens of southern catfish infected with P. shigelloides, such as the inflammatory response, immune system process, and platelet alpha granules.
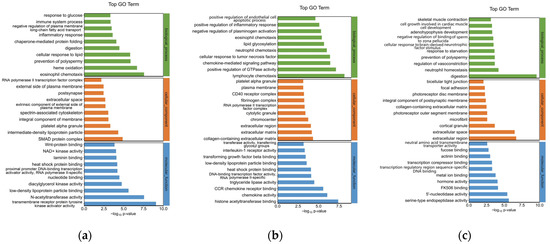
Figure 3.
GO functional analysis of differentially expressed genes in spleen tissues of southern catfish. (a) Top 30 enriched GO terms of upregulated DEGs between PS and BC groups. (b) Top 30 enriched GO terms of downregulated DEGs between L-PS and PS groups. (c) Top 30 enriched GO terms of downregulated DEGs between L and BC groups.
To explore the mechanism of Lactobacillus supplementation at the genetic level, 392 DEGs were processed for GO enrichment analysis between the L-PS and PS groups. Inflammation-related GO terms were significantly (p < 0.05) enriched, such as lymphocyte chemotaxis, positive regulation of inflammatory response, and interleukin-1 receptor activity. The top 30 enriched GO terms of downregulated DEGs are presented in Figure 3b. In the L group vs. the BC group, significantly upregulated genes (p < 0.05) were enriched in neutrophil homeostasis, regulation of vasoconstriction, and so on (Figure 3c).
3.5. KEGG Analysis for DEGs
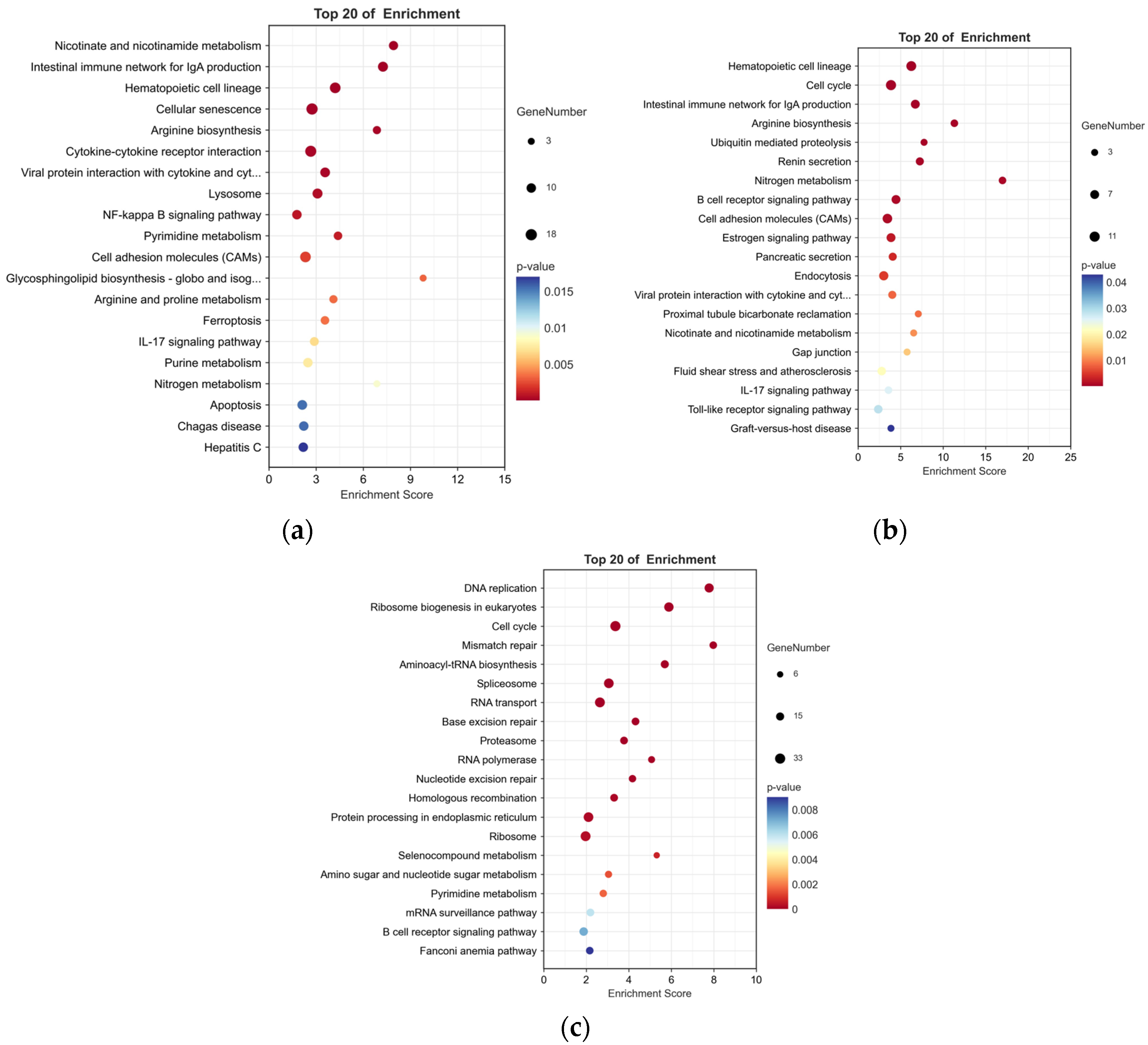
To examine the pathways affected by P. shigelloides, 751 DEGs were processed for KEGG pathway enrichment analysis between the PS and BC groups. Some immune-related pathways, such as the intestinal immune network for IgA production, IL-17 signalling pathway, and so on, were significantly (p < 0.05) enriched in upregulated DEGs (Figure 4a).
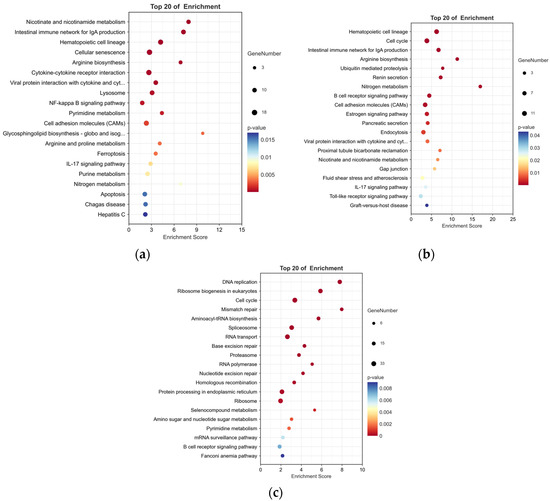
Figure 4.
KEGG highly significant enrichment pathways of DEGs between two pairwise comparisons. (a) Top 20 pathways enriched in upregulated DEGs between PS and BC groups. (b) Top 20 pathways enriched in downregulated DEGs between L-PS and PS groups. (c) Top 20 pathways enriched in downregulated DEGs between L and BC groups.
To explore the mechanism of Lactobacillus supplementation at the genetic level, 392 DEGs were processed for KEGG enrichment analysis between the L-PS and PS groups. In the L-PS vs. the PS group, significantly downregulated genes (p < 0.05) were also enriched in the intestinal immune network for IgA production and the IL-17 signalling pathway (Figure 4b). In the L group vs. the BC group, significantly downregulated genes (p < 0.05) were enriched in the B-cell receptor signalling pathway, which was the same as the results in the L-PS group vs. the PS group (Figure 4c).
3.6. Analysis of Immune-Related Pathways
3.6.1. IL-17 Signalling Pathway
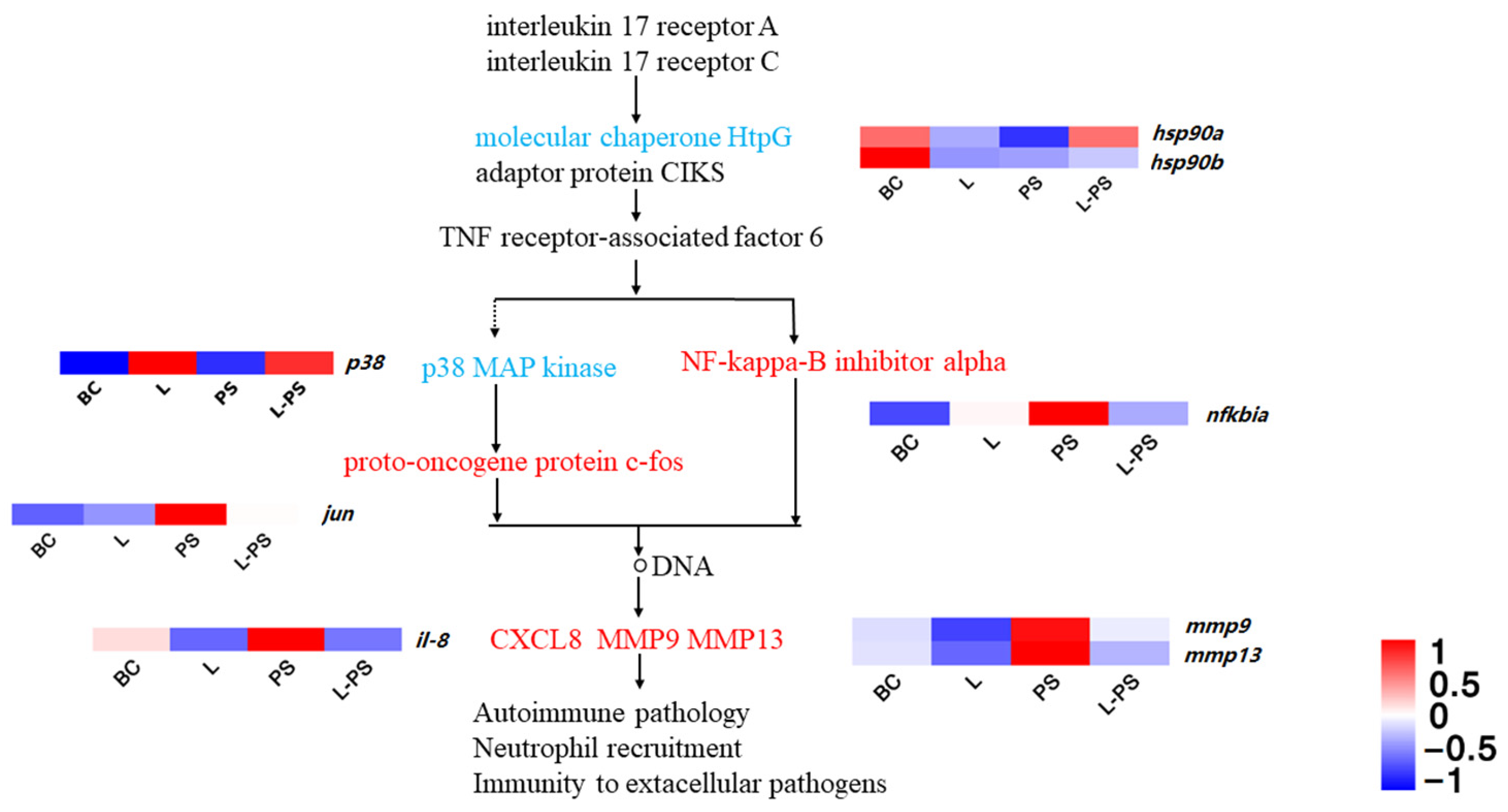
In the IL-17 signalling pathway, our results showed that the expression of proinflammatory factors within the range was increased, and the trend between groups, such as mmp9, il-8, jun, and mmp13, was significantly upregulated (p < 0.05) in the PS group vs. the BC group, and hsp90 was significantly downregulated (p < 0.05) in the PS group vs. the BC group. In the L-PS group vs. the PS group, mmp9 and mmp13 were significantly downregulated (p < 0.05), and hsp90 was significantly upregulated (p < 0.05), while il-8 expression was downregulated (p < 0.05). In the L group vs. the BC group, the expression of mmp9 and mmp13 was significantly downregulated (p < 0.05) (Figure 5).
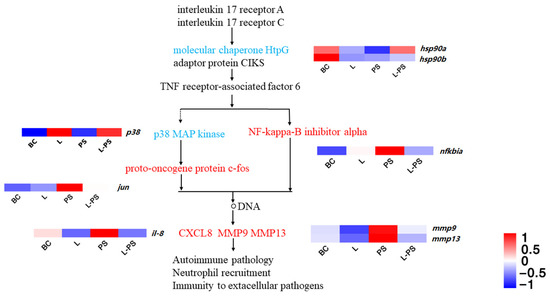
Figure 5.
IL-17 signalling pathway.
3.6.2. TNF Signalling Pathway
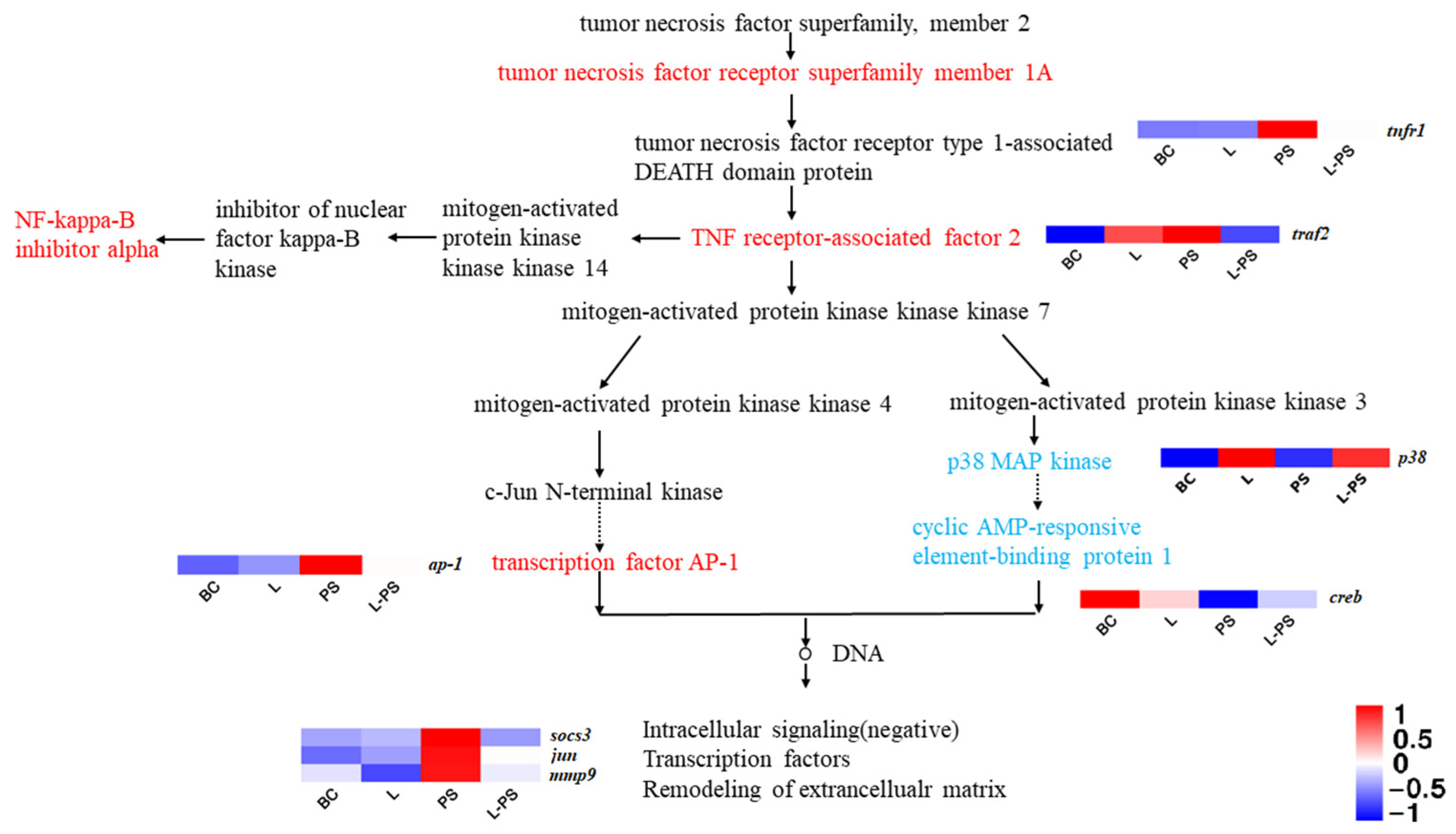
In the TNF signalling pathway, the results showed that the expression of socs3, tnfr1, traf2, and ap-1 was significantly upregulated (p < 0.05) in the PS group vs. the BC group; however, the expression of p38 and creb was significantly downregulated (p < 0.05). In the L-PS group vs. the PS group, the expression of socs3 and mmp9 was significantly downregulated (p < 0.05), while the expression of tnfr1, traf2, ap-1, and jun was downregulated (Figure 6). In the L group vs. the BC group, p38 expression was significantly upregulated (p < 0.05).
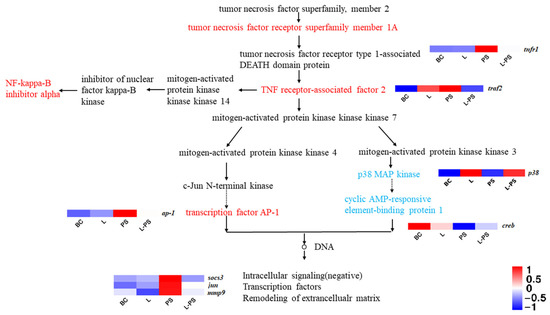
Figure 6.
TNF signalling pathway.
3.6.3. Toll-like Receptor Signalling Pathway
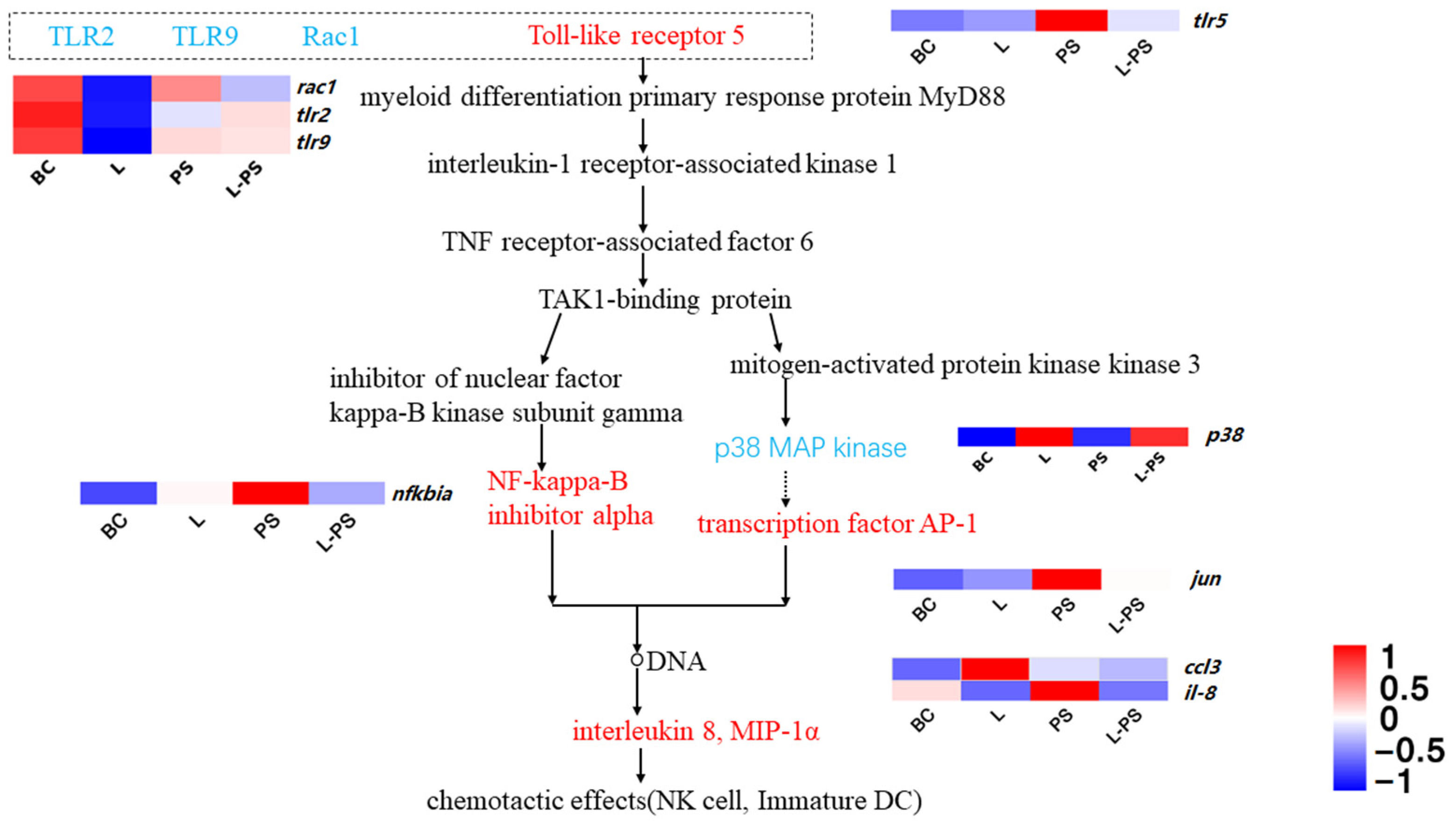
The results showed that several proinflammatory factors, such as tlr5, nfkbia, and ccl3, were significantly (p < 0.05) upregulated in the PS group vs. the BC group. In the L-PS group vs. the PS group, the expression of tlr5, jun, and nfkbia was downregulated; however, p38 expression was significantly (p < 0.05) upregulated. In the L group vs. the BC group, ccl3 expression was significantly (p < 0.05) upregulated, while the expression of rac1, tlr2, and tlr9 was significantly (p < 0.05) downregulated (Figure 7).
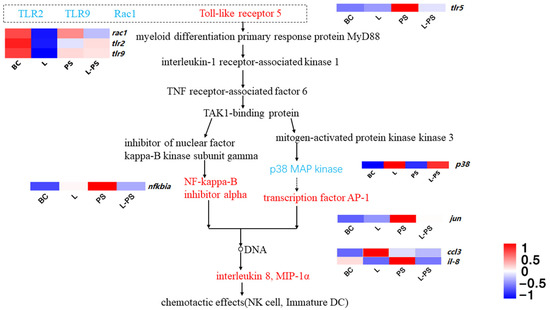
Figure 7.
Toll-like receptor signalling pathway.
3.6.4. Intestinal Immune Network for IgA Production
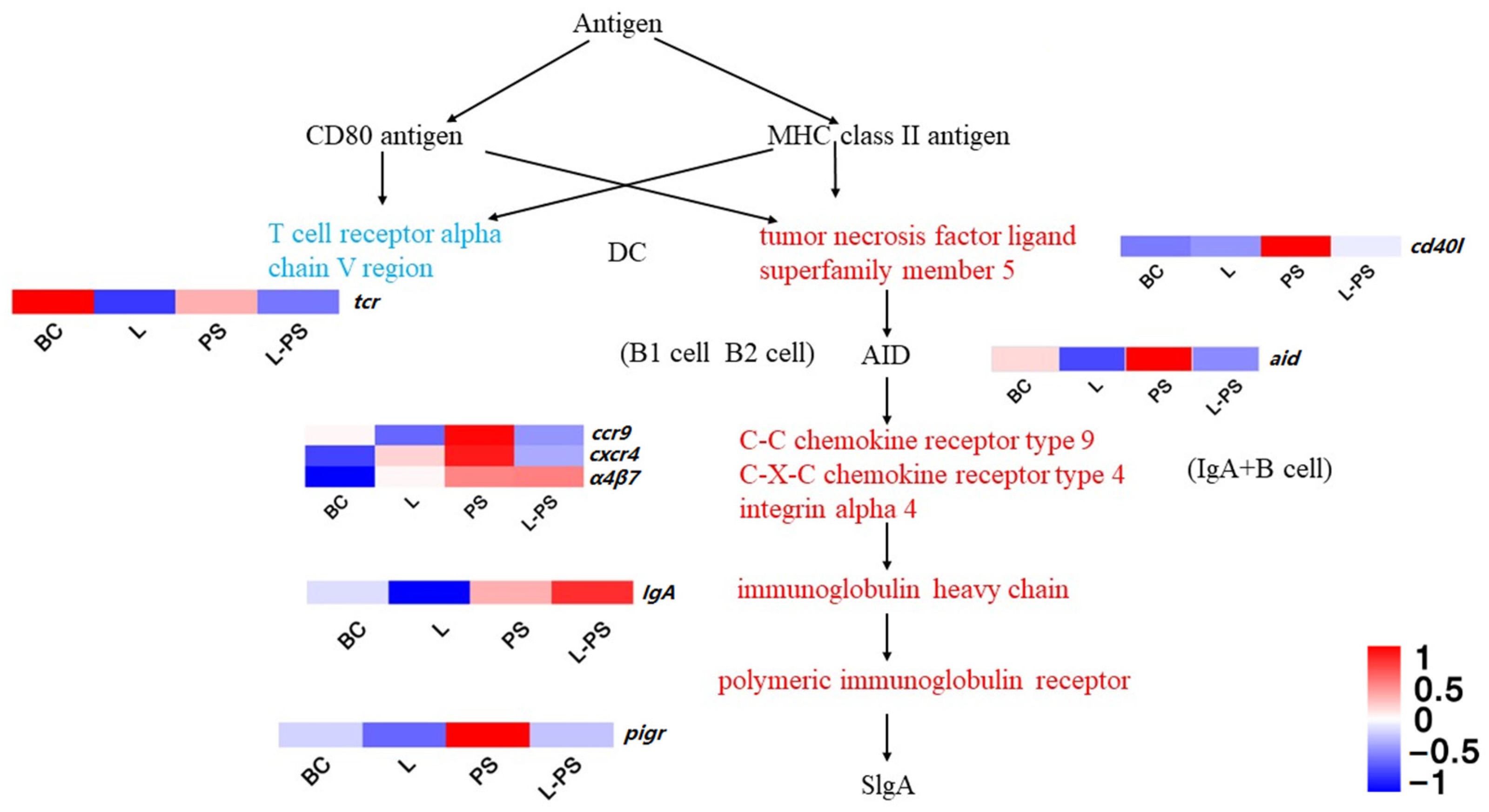
In this signalling pathway, the expression of cxcr4, pigr, ccr9, α4β7, and cd40l was significantly upregulated (p < 0.05) in the PS group vs. the BC group. In the L-PS group vs. the PS group, the expression of cxcr4, pigr, ccr9, tcr, and aid was significantly downregulated (p < 0.05) (Figure 8). In the L-PS group vs. the BC group, the expression of ccr9 and cxcr4 had no significant difference.
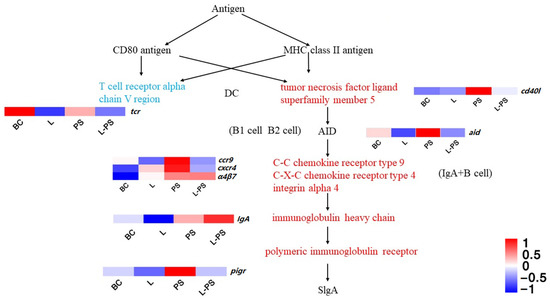
Figure 8.
Intestinal immune network for IgA production.
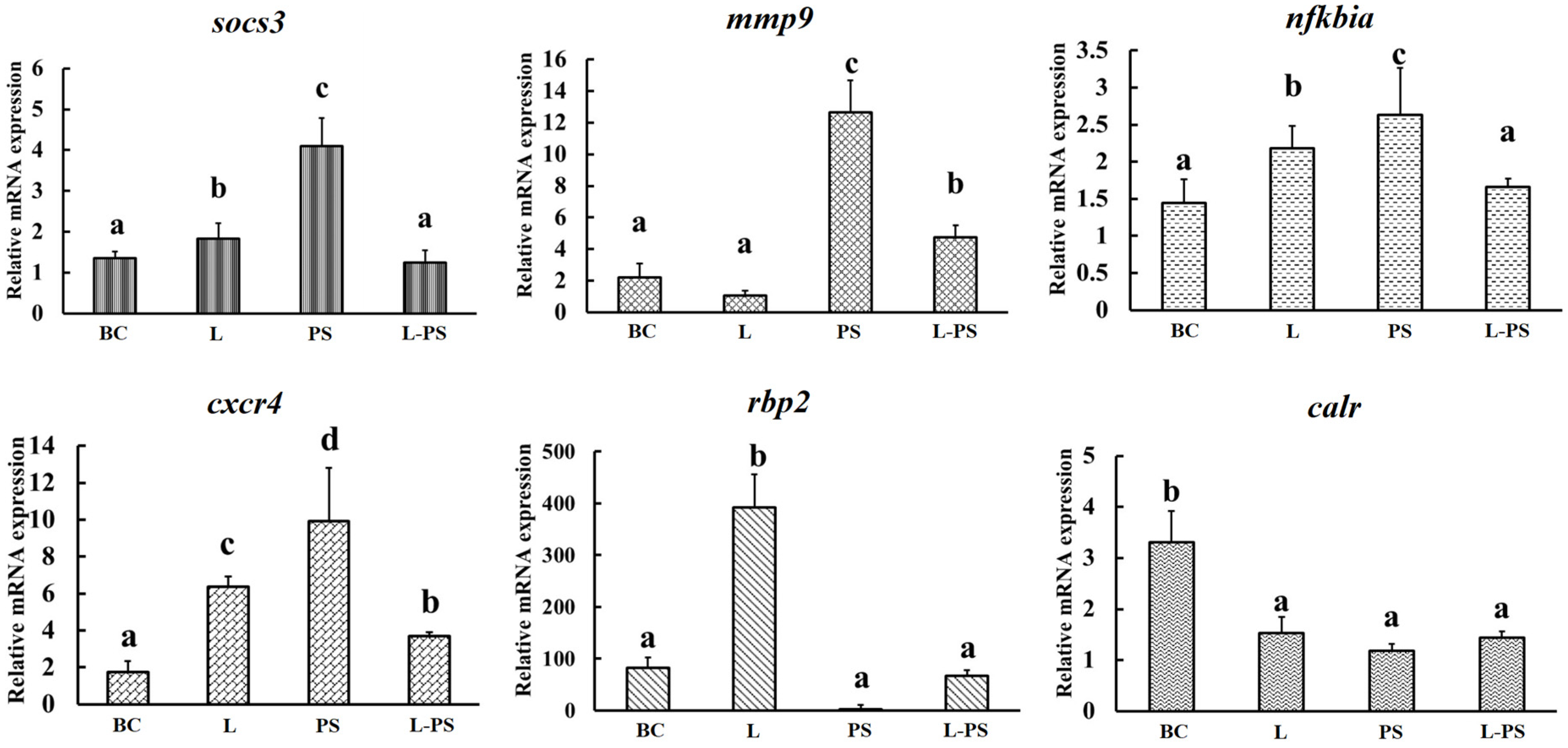
3.7. Validation of DEGs by qRT-PCR
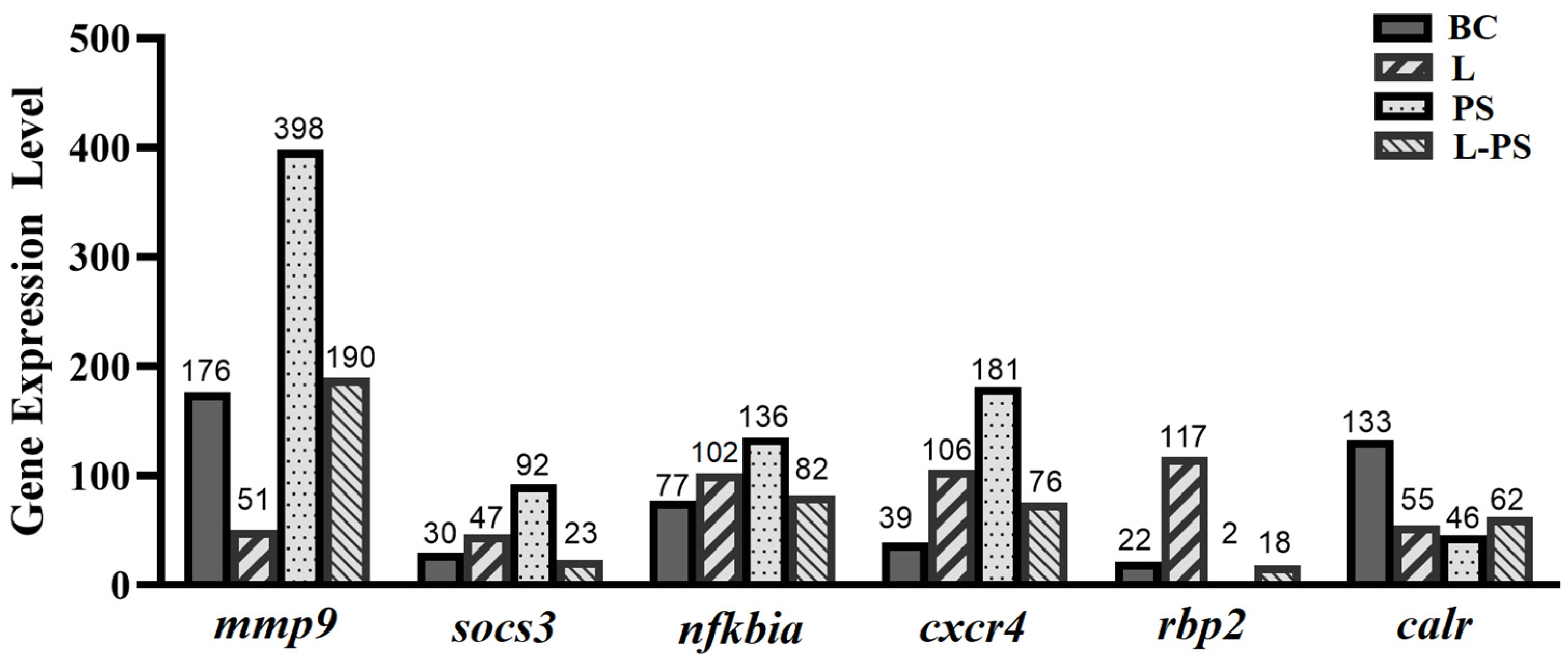
To validate the expression profiles of genes identified through RNA-Seq, we analysed the relative mRNA levels of the following six differentially expressed genes (mmp9, socs3, cxcr4, nfkbia, rbp2, and calr) by qRT-PCR (Figure 9). Our results showed that the qRT-PCR results and the results obtained through RNA-Seq were consistent (Figure 10).
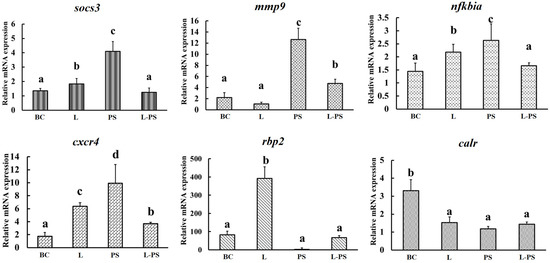
Figure 9.
Validation of DEGs by qRT-PCR. BC: blank control group; L: Lactobacillus group; PS: P. shigelloides group; L-PS: Lactobacillus + P. shigelloides group; different characters (a, b, c, d) show significant difference (p < 0.05).
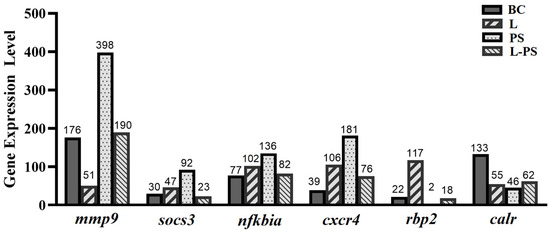
Figure 10.
Expression levels of six differentially expressed genes obtained through RNA-Seq.
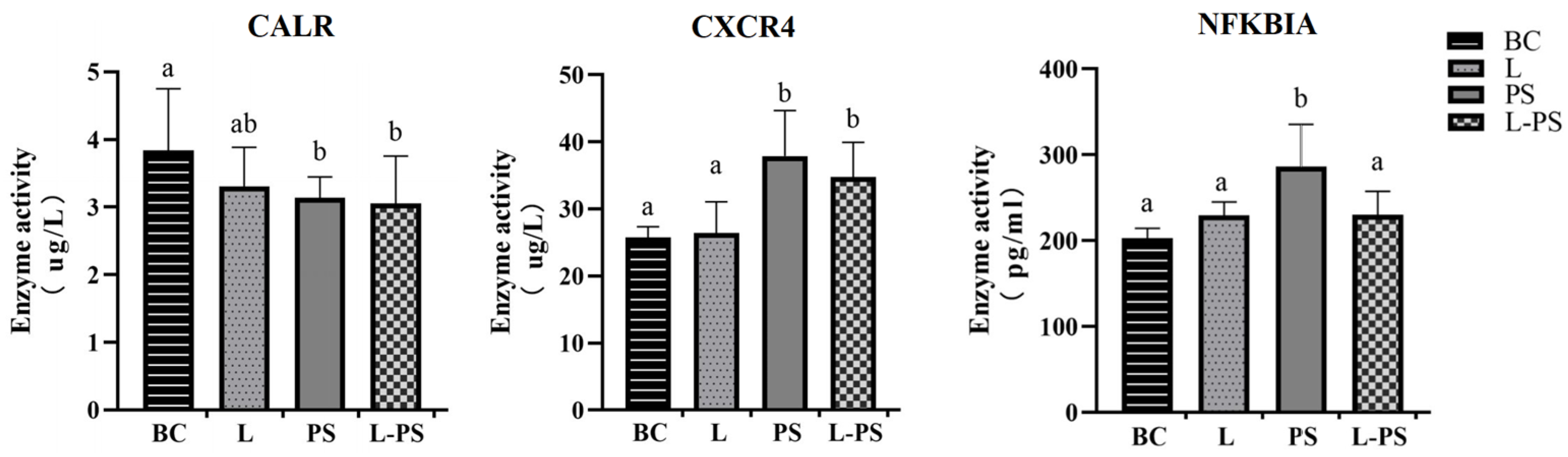
3.8. Validation of DEGs by ELISA
To further confirm the changes in the spleens, we used ELISA to detect the contents of CXCR4, CALR, and NFKBIA in the spleen tissue homogenate. The ELISA results showed that the concentrations of CXCR4 and NFKBIA proteins were significantly (p < 0.05) upregulated in the PS group compared with the other groups. The concentrations of the CALR protein were significantly (p < 0.05) downregulated between the PS and BC groups. The trends of the three proteins were consistent with the RNA expression and transcriptome data (Figure 11).
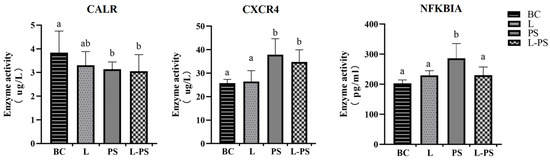
Figure 11.
Protein levels of CXCR4, NFKBIA, and CALR in four groups. Different characters (a, b, c, d) show significant difference (p < 0.05).
4. Discussion
The spleen, an important organ in a fish’s immune system, is considered a primordial secondary lymphoid organ where an adaptive immune response is produced [45]. Bacterial diseases are extremely harmful to animals in large-scale aquaculture facilities. Once an infection occurs, these diseases spread rapidly. At present, the most common treatment for bacterial diseases is the use of antibiotics. However, the long-term use of antibiotics will not only pollute the water body but also make the intestinal flora of the fish more resistant, making the treatment of fish diseases more difficult in the future. Therefore, it is of great significance to explore effective and green control methods.
4.1. Probiotic Complex Had Inhibitory Effects on Inflammatory Response Caused by P. shigelloides in Southern Catfish
4.1.1. IL-17 Signalling Pathway
IL-17 is a proinflammatory cytokine specifically secreted by helper T cells that can induce the secretion of early immune mediators [46,47]. Its family members, IL-17A and IL-17F, activate the downstream IK-Ba through IL-17R-Act1-TRAF6 and indirectly act on MAPKs to activate AP-1. A sustained inflammatory response can increase the expression of NF-κB, activate downstream chemokines and other effector genes of the NF-κB signalling pathway to increase the expression, and ultimately act on biological processes, such as neutrophil recruitment [48,49]. A high expression of matrix metalloproteinases can also indicate that the body is in a state of inflammation. Matrix metalloproteinase-9 (mmp9) is an important member of the MMP family, also known as gelatinase B [50]. A high expression of mmp9 under pathological conditions reshapes the extracellular matrix through excessive degradation, exposes relevant active sites, destroys the physiological barrier, and aggravates the inflammatory response [51]. Studies have confirmed that the upregulation of mmp9 is closely related to the body’s inflammatory response process [52,53,54]. In this study, the mmp9 gene was significantly (p < 0.05) expressed in the PS group, which proved that mmp9 may be involved in the pathogenesis of the inflammatory response caused by P. shigelloides. Combined with the comprehensive analysis of other groups, the expression of mmp9 in the L group and the L-PS group was significantly (p < 0.05) reduced, indicating that the probiotic complex had an inhibitory effect on the expression of this gene; therefore, it could inhibit the inflammatory response to a certain extent.
Neutrophils are the first line of immune defence and are phagocytes that play a crucial role in the early immune response of the host by clearing pathogens [55,56]. il-8 is a member of the α subfamily of chemokines, which acts on neutrophil chemotaxis, promotes T-cell chemotaxis and migration, and strengthens the immune response [57]. In our study, the reason for the significant expression of il-8 in the PS group may be due to the inflammatory response of the southern catfish infected with P. shigelloides, which activates macrophages and promotes the chemotaxis of neutrophils to the site of inflammation. Compared with the BC group, il-8 expression in the L group was significantly (p < 0.05) reduced, indicating that the expression of proinflammatory factors could be inhibited after treatment with the probiotic complex, thereby inhibiting the inflammatory response. Comparing the L-PS group and the PS group, we found that the expression of il-8 was significantly (p < 0.05) reduced, indicating that the feeding of probiotics may enhance the resistance of southern catfish to pathogenic invasion and that the probiotic complex can effectively inhibit the inflammatory response caused by the invasion of P. shigelloides.
4.1.2. TNF Signalling Pathway
The TNF (tumour necrosis factor), also known as TNFα, is a cytokine that can directly kill tumour cells and has no obvious cytotoxicity to normal cells. It is a cell signalling protein involved in systemic inflammation and a cytokine that constitutes the acute phase response [58]. The expression of the downstream gene socs3 in this pathway is also regulated to varying degrees. SOCS3 is one of the important members of the SOCS family proteins. socs3 has been certified to play a key role in immune response in aquatic animals, such as Cynoglossus semilaevis [59], Paralichthys olivaceus [60], and Ictalurus punctatus [61]. In our study, socs3 gene expression was significantly upregulated (p < 0.05) in the PS vs. BC group, indicating that P. shigelloides could induce the expression of socs3. Studies have also shown that socs3 expression can be induced by the lipopolysaccharides of Gram-negative bacteria [62]. In the L-PS vs. PS group, the expression of socs3 was significantly downregulated. We speculated that feeding of the probiotic complex had a protective effect on southern catfish.
The TNF is involved in the innate immune response through two receptors, TNFR1 and TNFR2. In this study, the significant expression of tnfr1 in the PS group showed that infection with P. shigelloides stimulated an immune response and activated the TNFR1 signalling pathway (TNFR1-TRADD-TRAF), which inhibits apoptosis and promotes inflammation. Because TNFα is associated with an acute inflammatory response, it may be the reason for the lack of a significant difference in tnfr1 expression between the L group and the BC group. Compared with the PS group, the expression of tnfr1 in the L-PS group was significantly (p < 0.05) decreased, indicating that long-term feeding of probiotics may effectively respond to the TNFα-mediated inflammatory pathway caused by the invasion of P. shigelloides and inhibit the inflammatory response to a certain extent.
4.1.3. Toll-like Receptor Signalling Pathway
The cytoplasmic TIR domain of TLR recruits signal adaptors MyD88, TIRAP, TRAM, and/or TRIF and activates various kinases (IRAK4, IRAK1, IRAK2, TBK1, and IKKε) and ubiquitin ligases (TRAF6 and pellino 1), thereby activating nuclear factor NF-κB and inducing the expression of inflammatory factors. nfkbia is the gene encoding the NF-κB complex inhibitory protein IκB [63]. There are related studies involving this gene in aquatic animals. nfkbia gene expression in zebrafish juveniles infected with Vibrio parahaemolyticus [64], Lampetra japonica stimulated by dsRNA virus mimetic [65], and Channa argus after infection with Americide nocardia [66] were significantly (p < 0.05) upregulated. In this study, nfkbia expression in the PS group was significantly (p < 0.05) upregulated compared with the BC group, consistent with the findings above, indicating that nfkbia may be involved in the early generation of the innate immune response to P. shigelloides infection in the southern catfish.
In the TLR family, TLR5, the receptor of flagellin, which is related to the pathogenicity of bacteria, has a defensive effect against the invasion of flagella [67]. In our study, the expression of tlr5 in the PS group was significantly (p < 0.05) upregulated, which may be due to the surface structure of bacteria inducing the body to produce a large number of TLR5 receptor proteins to resist bacterial invasion, indicating that P. shigelloides infection can cause TLR5-mediated innate immunity in the southern catfish. The expression of tlr5 in the L group was not significantly (p < 0.05) different from that in the BC group, which may be because probiotics have little effect on tlr5 regulation without pathogenic invasion. Compared with the PS group, the expression of tlr5 in the L-PS group was significantly (p < 0.05) reduced, and we hypothesised that this may have been due to the enhanced immunity and disease resistance of the southern catfish due to the probiotics. In addition, it is possible that the number of probiotics in the body is large and that the produced metabolites inhibit the growth of P. shigelloides. We performed a series of in vitro bacteriostatic experiments and showed that different probiotic metabolite concentrations can inhibit the growth of P. shigelloides to varying degrees.
4.1.4. Intestinal Immune Network for IgA Production
CXCR4 (chemokine receptor 4) is an inflammatory chemokine that acts by binding to its ligand SDF-1 (stromal cell-derived factor-1). The possible mechanism of the SDF-1/CXCR4 axis-mediated inflammatory response is chemotaxis of neutrophils and other inflammatory cells to the site of inflammation [68]. In our study, the expression of cxcr4 in the PS group was significantly (p < 0.05) increased, indicating that the invasion of P. shigelloides caused the southern catfish to be in an inflammatory state. In the L-PS vs. the PS group, the cxcr4 gene expression was significantly (p < 0.05) downregulated. Our results showed that the degree of inflammatory response in the L-PS group was lighter compared with the PS group. In addition, we detected the concentration of CXCR4 protein by ELISA, and the change trend was consistent with the change trend of cxcr4 expression.
pIgR (poly-Ig receptor) is involved in the innate immunity of the organism mucosa. pIgR mediates IgA transportation to neutralise extracellular pathogens in mammals [69,70]. To some extent, the expression level of pIgR in mucosal epithelium represents the expression level of IgA antibody in extra mucosal secretions [71]. In teleost fish, pIgR can transport IgM and IgT to mucosal secretions and exert its mucosal immune function [72,73]. Studies have also shown that IgT in fish can mediate the intestinal lumen through pIgR, which is considered a specific immunoglobulin involved in intestinal mucosal immunity, and its function is similar to mammalian IgA [74]. Studies have shown that the expression of pigr in the spleen of Scophthalmus maxima is significantly (p < 0.05) upregulated after infection by Vibrio anguillarum [75]. The expression of pigr in the spleen of zebrafish was also significantly (p < 0.05) upregulated after infection by Streptococcus iniae [76]. In our study, the expression of pigr was significantly (p < 0.05) upregulated in the PS group compared with the BC group, indicating that the invasion of P. shigelloides can lead to the activation of the intestinal immune network for IgA production, upregulating the downstream key gene pigr to promote inflammatory response. The expression of some transcripts of the pigr gene in the L vs. the BC group was downregulated, and pigr gene expression was significantly (p < 0.05) downregulated in the L-PS vs. the PS group. The above results show that the probiotic complex could inhibit the expression of pigr to relieve inflammation.
4.2. Other Related Key Genes
Calreticulin (CALR) protein can maintain intracellular calcium homeostasis and assist in protein folding, and plays a significant role in immune regulation, cell phagocytosis, cell proliferation, and apoptosis [77]. In our study, the expression of calr in the L, PS, and L-PS groups was significantly (p < 0.05) lower than the BC group; however, CALR protein concentration in the PS and L-PS groups was lower than that in BC and L groups, probably because the invasion of P. shigelloides could inhibit the function of CALR. RBP2 plays a key role in maintaining the normal DC response, which has functions in phagocytosis, processing, and presenting antigens [78]. In our study, we hypothesised that the downregulation of rbp2 in the PS group may be related to the mechanism of P. shigelloides invasion to evade immunity. MHCI cytotoxic T lymphocytes (CD8+) play a key role in the recognition of antigen peptide–MHC complexes [79]. In this study, mhc I expression was significantly (p < 0.05) downregulated in the PS group. We speculated that it was because P. shigelloides inhibited mhc I expression to evade the body’s immune system; however, this conjecture needs to be further verified. Arachidonate 5-lipoxygenase (Alox5) is a membrane-bound protein mainly expressed in polymorphonuclear leukocytes, peripheral blood monocytes, macrophages, and mast cells. It is highly expressed in many diseases [80,81,82]. Inhibition of alox5 expression attenuates lipopolysaccharide (LPS)-induced inflammation [83]. In our study, the expression of alox5 in the L and L-PS groups was significantly (p < 0.05) downregulated, which also showed that the probiotic complex could inhibit the expression of alox5 and block the production of inflammatory mediators (Supplementary Figure S1 [84,85,86]).
5. Conclusions
In conclusion, in our experiment, we found that P. shigelloides can cause hyperaemia and the festering of fins, while southern catfish fed a probiotic complex were free of related inflammation after the infection of P. shigelloides. Moreover, significantly downregulated genes (p < 0.05) were enriched in inflammation-related GO terms (lymphocyte chemotaxis and positive regulation of inflammatory response) and immune-related pathways (intestinal immune network for IgA production and IL-17 signalling pathway) in the L-PS vs. the PS group. Hence, probiotics could inhibit the inflammatory response caused by P. shigelloides to some extent. Therefore, probiotics are promising alternatives to antibiotics to prevent bacterial diseases in the aquaculture industry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani13030449/s1, Figure S1: Arachidonic acid metabolism.
Author Contributions
Experimental design and methodology, J.Q.; sample collection and validation, X.L.; formal software analysis, R.W and D.J.; writing—original draft preparation, R.W.; writing—review and editing, R.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Funder: Ranran Dong, Funding number 31802270) and the Science and Technology Fund of Guizhou Province (Funder: Ranran Dong, Funding numbers 20191112, 2020-1Y100, and 2018-5781).
Institutional Review Board Statement
The animal study protocol was approved by the Experimental Animal Ethics Committee of Guizhou University (protocol code EAE-GZU-2022-P007 and date of approval 10 June 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in the National Genomics Data Center (Nucleic Acids Res 2022), China National Center for Bioinformation/Beijing Institute of Genomics, and the Chinese Academy of Sciences (GSA: CRA008891) and are publicly accessible at https://ngdc.cncb.ac.cn/gsa, accessed on 15 November 2022.
Acknowledgments
OE Biotech Co., Ltd. (Shanghai, China) provided high-throughput sequencing service and support. The authors express their most sincere gratitude to these individuals for their help.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Santos, R.A.; Oliva-Teles, A.; Pousão-Ferreira, P.; Jerusik, R.; Saavedra, M.J.; Enes, P.; Serra, C.R. Isolation and Characterization of Fish-Gut Bacillus spp. As Source of Natural Antimicrobial Compounds to Fight Aquaculture Bacterial Diseases. Mar. Biotechnol. 2021, 23, 276–293. [Google Scholar] [CrossRef]
- Liu, C.; Mai, K.; Zhang, W.; Chen, Q.; Leng, Y. Studies on the nutrition of two species of catfish, Silurus meridionalis Chen and S. asotus Linnaeus. I. Effects of dietary protein and lipid on growth performance and feed utilization. Aquaculture 2013, 404–405, 71–76. [Google Scholar] [CrossRef]
- Li, X.M.; Liu, L.; Yuan, J.M.; Xiao, Y.Y.; Fu, S.J.; Zhang, Y.G. The effect of aerobic exercise and starvation on growth performance and postprandial metabolic response in Juvenile southern catfish (Silurus meridionalis). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 193, 36–44. [Google Scholar] [CrossRef]
- Liu, W.; Qiu, H.; Yan, Y.; Xie, X. Acute Cd Toxicity, Metal Accumulation, and Ion Loss in Southern Catfish (Silurus meridionalis Chen). Toxics 2021, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.J.; Pang, X.; Cao, Z.D.; Peng, J.L.; Yan, G. The effects of fasting on the metabolic interaction between digestion and locomotion in Juvenile southern catfish (Silurus meridionalis). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 158, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.D.; Huang, Y.H.; Liang, J.Z.; Long, S.; Niu, Z.W.; Fan, H.L.; Huang, J.; Zeng, G.Z. Isolation, identification and drug sensitivity test of pathogenic bacteria from Silurus meridionalis infected with tail-rotted disease. J. South. Agric. 2015, 46, 1720–1725. [Google Scholar]
- Luo, Y.; Xie, X. Effects of temperature on the specific dynamic action of the southern catfish, Silurus meridionalis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008, 149, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Ai, Q.; Xie, X. Effects of replacement of fish meal by soybean meal and supplementation of methionine in fish meal/soybean meal-based diets on growth performance of the southern catfish Silurus meridionalis. J. World Aquac. Soc. 2005, 36, 498–507. [Google Scholar] [CrossRef]
- Fu, S.J.; Xie, X.J.; Cao, Z.D. Effect of dietary composition on specific dynamic action in southern catfish Silurus meridionalis Chen. Aquac. Res. 2005, 36, 1384–1390. [Google Scholar] [CrossRef]
- Li, Y.W.; Zhu, W.L.; Gu, J.R.; Wu, C.F.; Wu, J.; Xie, X.L.; Xu, H. Isolation of bdellovibrio infacting Silurus meridionalis pathogens and its biological characteristics. Freshw. Fish 2006, 36, 9–12. [Google Scholar]
- Cao, H.J.; Li, Y.W.; Lei, Y.; Wu, J.; Xu, H.; Zhang, T.; Zhang, X.J. Isolation, identification, phylogenetic analysis and related properties of a pathogen in Silurus meridionalis Chen. Acta Microbiol. Sin. 2007, 47, 1–6. [Google Scholar]
- Ji, L.L.; Wang, K.Y.; Xiao, D.; Yang, T. Isolation and identification of pathogenic bacteria causing ulcer disease of Silurus meriordinalis Chen. Freshw. Fish 2008, 38, 68–72. [Google Scholar]
- Chen, Z.; Cheng, K.; Zhou, X.; Zhang, Q. Pathogenic bacterium identification and histopathology of septicemia of Juvenile southern catfish, Silurus meridionalis. J. Fish Sci. China 2011, 18, 360–370. [Google Scholar]
- Li, C.W. Lsolation and Characterization of Edwardsiella ctalurid in South Catfish and Pathology and Immunohistochemistry after Experimental Infection; Sichuan Agricultural University: Chengdu, China, 2012. [Google Scholar]
- Jiang, L.P.; Liang, Q.L.; Li, Y.L.; She, R.; Wu, Z.W.; Qi, Z.L.; Zhang, J.L. Identification of pathogens in an outbreak deaths of southern catfish (Silurus meridionalis Chen) Juveniles. In Proceedings of the 2013 Annual Conference of the Chinese Fisheries Society, Sichuan, China. 2013, p. 117. Available online: https://en.cnki.com.cn/Article_en/CJFDTotal-DSYY201403010.htm (accessed on 23 October 2022).
- Guo, X. Isolation and Identification of Edwardsiellain South Catfish and Establishment of the Duplex PCR for Detection of Edwardsiella; Sichuan Agricultural University: Chengdu, China, 2014. [Google Scholar]
- Lin, C.Y.; Jin, B.Q.; Fan, Z.Z. A description of common diseases of southern largemouth catfish farmed in Tianjin. J. Hydroecology 2004, 24, 74–75. [Google Scholar]
- Wei, M.L.; Li, S.M.; Liang, J.Z. Isolation and identification and susceptibility test of pathogenic mild aeromonas in southern catfish. Guangxi, J. Anim. Husb. Vet. Med. 2020, 36, 195–198. [Google Scholar]
- Yasui, H.; Kiyoshima, J.; Hori, T.; Shida, K. Protection against influenza virus infection of mice fed Bifidobacterium breve YIT4064. Clin. Diagn. Lab. Immunol. 1999, 6, 186–192. [Google Scholar] [CrossRef]
- Agarwal, K.N.; Bhasin, S.K. Feasibility studies to control acute diarrhoea in children by feeding fermented milk preparations Actimel and Indian Dahi. Eur. J. Clin. Nutr. 2002, 56, S56–S59. [Google Scholar] [CrossRef]
- Pedone, C.A.; Arnaud, C.C.; Postaire, E.R.; Bouley, C.F.; Reinert, P. Multicentric study of the effect of milk fermented by Lactobacillus casei on the incidence of diarrhoea. Int. J. Clin. Pract. 2000, 54, 568–571. [Google Scholar]
- Pedone, C.A.; Bernabeu, A.O.; Postaire, E.R.; Bouley, C.F.; Reinert, P. The effect of supplementation with milk fermented by Lactobacillus casei (strain DN-114 001) on acute diarrhoea in children attending day care centres. Int. J. Clin. Pract. 1999, 53, 179–184. [Google Scholar]
- Szajewska, H.; Skórka, A.; Ruszczyński, M.; Gieruszczak-Białek, D. Meta-analysis: Lactobacillus GG for treating acute diarrhoea in children. Aliment. Pharmacol. Ther. 2007, 25, 871–881. [Google Scholar] [CrossRef]
- Segers, M.E.; Lebeer, S. Towards a better understanding of Lactobacillus rhamnosus GG—Host interactions. Microb. Cell Fact. 2014, 13, S7. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Zhang, X.; Hao, H.; Liu, Q.; Zhou, Z.; Liang, X.; Liu, T.; Gong, P.; Zhang, L.; Zhai, Z.; et al. Lactobacillus rhamnosus GG derived extracellular vesicles modulate gut microbiota and attenuate inflammatory in DSS-induced colitis mice. Nutrients 2021, 13, 3319. [Google Scholar] [CrossRef] [PubMed]
- Divyashree, S.; Anjali, P.G.; Somashekaraiah, R.; Sreenivasa, M.Y. Probiotic properties of Lactobacillus casei–MYSRD 108 and Lactobacillus plantarum-MYSRD 71 with potential antimicrobial activity against Salmonella paratyphi. Biotechnol. Rep. 2021, 32, e00672. [Google Scholar] [CrossRef] [PubMed]
- Rengpipat, S.; Rueangruklikhit, T.; Piyatiratitivorakul, S. Evaluations of lactic acid bacteria as probiotics for Juvenile seabass Lates calcarifer. Aquac. Res. 2008, 39, 134–143. [Google Scholar] [CrossRef]
- Lee, J.S.; Cheng, H.; Damte, D.; Lee, S.J.; Kim, J.C.; Rhee, M.H.; Suh, J.W.; Park, S.C. Effects of dietary supplementation of Lactobacillus pentosus PL11 on the growth performance, immune and antioxidant systems of Japanese eel Anguilla japonica challenged with Edwardsiella tarda. Fish Shellfish. Immunol. 2013, 34, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Nikoskelainen, S.; Ouwehand, A.C.; Bylund, G.; Salminen, S.; Lilius, E.M. Immune enhancement in rainbow trout (Oncorhynchus mykiss) by potential probiotic bacteria (Lactobacillus rhamnosus). Fish Shellfish. Immunol. 2003, 15, 443–452. [Google Scholar] [CrossRef]
- Lin, H.L.; Shiu, Y.L.; Chiu, C.S.; Huang, S.L.; Liu, C.H. Screening probiotic candidates for a mixture of probiotics to enhance the growth performance, immunity, and disease resistance of Asian seabass, Lates calcarifer (Bloch), against Aeromonas hydrophila. Fish Shellfish. Immunol. 2017, 60, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Beck, B.R.; Kim, D.; Jeon, J.; Lee, S.M.; Kim, H.K.; Kim, O.J.; Lee, J.I.; Suh, B.S.; Do, H.K.; Lee, K.H.; et al. The effects of combined dietary probiotics Lactococcus lactis BFE920 and Lactobacillus plantarum FGL0001 on innate immunity and disease resistance in olive flounder (Paralichthys olivaceus). Fish Shellfish. Immunol. 2015, 42, 177–183. [Google Scholar] [CrossRef]
- Feng, J.G.; Cai, Z.L.; Chen, Y.Y.; Chang, X.L.; Liu, H.F.; Liu, Y.J.; Zhang, J.X. Effects of an exopolysaccharide from Lactococcus lactis Q-9 on innate immune response, antioxidant activity, and disease resistance against Aeromonas hydrophila in Cyprinus carpio. J. Fish. China 2020, 44, 1477–1487. [Google Scholar] [CrossRef]
- Park, I.; Lee, Y.; Goo, D.; Zimmerman, N.P.; Smith, A.H.; Rehberger, T.; Lillehoj, H.S. The effects of dietary Bacillus subtilis supplementation, as an alternative to antibiotics, on growth performance, intestinal immunity, and epithelial barrier integrity in broiler chickens infected with Eimeria maxima. Poult. Sci. 2020, 99, 725–733. [Google Scholar] [CrossRef]
- Memon, F.U.; Yang, Y.; Leghari, I.H.; Lv, F.; Soliman, A.M.; Zhang, W.; Si, H. Transcriptome Analysis Revealed Ameliorative Effects of Bacillus Based Probiotic on Immunity, Gut Barrier System, and Metabolism of Chicken under an Experimentally Induced Eimeria tenella Infection. Genes 2021, 12, 536. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Biotechnol. 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Roberts, A.; Trapnell, C.; Donaghey, J.; Rinn, J.L.; Pachter, L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011, 12, R22. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Robinson, M.D.; Smyth, G.K. Moderated statistical tests for assessing differences in tag abundance. Bioinformatics 2007, 2, 2881–2887. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. Clusterprofiler: An r package for comparing biological themes among gene clusters. Omics-A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Flajnik, M.F. A cold-blooded view of adaptive immunity. Nat. Rev. Immunol. 2018, 18, 438–453. [Google Scholar] [CrossRef] [PubMed]
- Miossec, P.; Kolls, J.K. Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Drug Discov. 2012, 11, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Ritzmann, F.; Lunding, L.P.; Bals, R.; Wegmann, M.; Beisswenger, C. IL-17 Cytokines and Chronic Lung Diseases. Cells 2022, 11, 2132. [Google Scholar] [CrossRef]
- Ruiz de Morales, J.M.G.; Puig, L.; Daudén, E.; Cañete, J.D.; Pablos, J.L.; Martín, A.O.; Juanatey, C.G.; Adán, A.; Montalbán, X.; Borruel, N.; et al. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: An updated review of the evidence focusing in controversies. Autoimmun. Rev. 2020, 19, 102429. [Google Scholar] [CrossRef]
- Amatya, N.; Garg, A.V.; Gaffen, S.L. IL-17 Signaling: The Yin and the Yang. Trends Immunol. 2017, 38, 310–322. [Google Scholar] [CrossRef]
- Vandooren, J.; Van Den Steen, P.E.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): The next decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [CrossRef]
- Parks, W.C.; Wilson, C.L.; López-Boado, Y.S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 2004, 4, 617–629. [Google Scholar] [CrossRef]
- Wu, L.; Wang, X.; He, X.; Li, Q.; Hua, Q.; Liu, R.; Qiu, Z. MMP9 Expression Correlates with Cisplatin Resistance in Small Cell Lung Cancer Patients. Front. Pharmacol. 2022, 13, 868203. [Google Scholar] [CrossRef]
- Xu, T.; Gao, S.; Liu, J.; Huang, Y.; Chen, K.; Zhang, X. MMP9 and IGFBP1 Regulate Tumor Immune and Drive Tumor Progression in Clear Cell Renal Cell Carcinoma. J. Cancer 2021, 12, 2243–2257. [Google Scholar] [CrossRef]
- Xu, L.; Cai, Z.; Yang, F.; Chen, M. Activation-induced upregulation of MMP9 in mast cells is a positive feedback mediator for mast cell activation. Mol. Med. Rep. 2017, 15, 1759–1764. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zeng, H.; Gao, C. The role of neutrophil extracellular traps in central nervous system diseases and prospects for clinical application. Oxidative Med. Cell. Longev. 2021, 2021, 9931742. [Google Scholar] [CrossRef] [PubMed]
- Nakabo, S.; Romo-Tena, J.; Kaplan, M.J. Neutrophils as drivers of immune dysregulation in autoimmune diseases with skin manifestations. J. Investig. Dermatol. 2022, 142, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Amoras, E.; De Brito, W.B.; Queiroz, M.A.F.; Conde, S.; Vallinoto, I.M.V.C.; Ishak, R.; Vallinoto, A.C.R. The genetic profile and serum level of IL-8 are associated with chronic hepatitis B and C virus infection. Biomolecules 2021, 11, 1664. [Google Scholar] [CrossRef]
- Horiuchi, T.; Mitoma, H.; Harashima, S.; Tsukamoto, H.; Shimoda, T. Transmembrane TNF-alpha: Structure, function and interaction with anti-TNF agents. Rheumatology 2010, 49, 1215–1228. [Google Scholar] [CrossRef]
- Hao, L.X.; Sun, L. Comparative analysis of the expression patterns of eight suppressors of cytokine signaling in tongue sole, Cynoglossus semilaevis. Fish Shellfish. Immunol. 2016, 55, 595–601. [Google Scholar] [CrossRef]
- Thanasaksiri, K.; Hirono, I.; Kondo, H. Identification and expression analysis of suppressors of cytokine signaling (SOCS) of Japanese flounder Paralichthys olivaceus. Fish Shellfish. Immunol. 2016, 58, 145–152. [Google Scholar] [CrossRef]
- Yao, J.; Mu, W.; Liu, S.; Zhang, J.; Wen, H.; Liu, Z. Identification, phylogeny and expression analysis of suppressors of cytokine signaling in channel catfish. Mol. Immunol. 2015, 64, 276–284. [Google Scholar] [CrossRef]
- Stoiber, D.; Stockinger, S.; Steinlein, P.; Kovarik, J.; Decker, T. Listeria monocytogenes modulates macrophage cytokine responses through STAT serine phosphorylation and the induction of suppressor of cytokine signaling 3. J. Immunol. 2001, 166, 466–472. [Google Scholar] [CrossRef]
- Kolesnichenko, M.; Mikuda, N.; Höpken, U.E.; Kärgel, E.; Uyar, B.; Tufan, A.B.; Milanovic, M.; Sun, W.; Krahn, I.; Schleich, K.; et al. Transcriptional repression of NFKBIA triggers constitutive IKK- and proteasome-independent p65/RelA activation in senescence. EMBO J. 2021, 40, e104296. [Google Scholar] [CrossRef]
- Ji, C. Transcriptome Analysis of Notch1a in Innate Immune Response in Zebrafish (Danio rerio) Larvae Challaged by Vibrio parahaemolyticus; Shanghai Ocean University: Shanghai, China, 2017. [Google Scholar]
- Song, T. Molecular Characteristics of RIG-I and MAVS of the Lamprey and their Signaling Pathways in Antiviral Immunity; Liaoning Normal University: Dalian, China, 2018. [Google Scholar]
- Tan, K.A. Transcriptomic Analysis and Determination of Immune-Related Genes in Channa Argus after Challenge with Nocardia Seriolae. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2017. [Google Scholar]
- Chen, J.; Qiao, Y.; Chen, G.; Chang, C.; Dong, H.; Tang, B.; Cheng, X.; Liu, X.; Hua, Z. Salmonella flagella confer anti-tumor immunological effect via activating Flagellin/TLR5 signalling within tumor microenvironment. Acta Pharm. Sin. B 2021, 11, 3165–3177. [Google Scholar] [CrossRef] [PubMed]
- Kantele, J.M.; Kurk, S.; Jutila, M.A. Effects of continuous exposure to stromal cell-derived factor-1 alpha on T cell rolling and tight adhesion to monolayers of activated endothelial cells. J. Immunol. 2000, 164, 5035–5040. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, K.M.; Nilsson, H.; Nyström, J.; Lindgren, D.; Leandersson, K.; Swärd, K.; Johansson, M.E. Localization and Regulation of Polymeric Ig Receptor in Healthy and Diseased Human Kidney. Am. J. Pathol. 2019, 189, 1933–1944. [Google Scholar] [CrossRef] [PubMed]
- Maruthachalam, B.V.; Zwolak, A.; Lin-Schmidt, X.; Keough, E.; Tamot, N.; Venkataramani, S.; Geist, B.; Singh, S.; Ganesan, R. Discovery and characterization of single-domain antibodies for polymeric Ig receptor-mediated mucosal delivery of biologics. MAbs 2020, 12, 1708030. [Google Scholar] [CrossRef] [PubMed]
- Ohlmeier, S.; Mazur, W.; Linja-Aho, A.; Louhelainen, N.; Rönty, M.; Toljamo, T.; Bergmann, U.; Kinnula, V.L. Sputum proteomics identifies elevated PIGR levels in smokers and mild-to-moderate COPD. J. Proteome Res. 2012, 11, 599–608. [Google Scholar] [CrossRef]
- Hamuro, K.; Suetake, H.; Saha, N.R.; Kikuchi, K.; Suzuki, Y. A teleost polymeric Ig receptor exhibiting two Ig-like domains transports tetrameric IgM into the skin. J. Immunol. 2007, 178, 5682–5689. [Google Scholar] [CrossRef]
- Rombout, J.H.; van der Tuin, S.J.; Yang, G.; Schopman, N.; Mroczek, A.; Hermsen, T.; Taverne-Thiele, J.J. Expression of the polymeric Immunoglobulin Receptor (pIgR) in mucosal tissues of common carp (Cyprinus carpio L.). Fish Shellfish. Immunol. 2008, 24, 620–628. [Google Scholar] [CrossRef]
- Zhang, Y.A.; Salinas, I.; Li, J.; Parra, D.; Bjork, S.; Xu, Z.; LaPatra, S.E.; Bartholomew, J.; Sunyer, J.O. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010, 11, 827–835. [Google Scholar] [CrossRef]
- Ding, B.J.; Sheng, X.Y.; Tang, X.Q.; Xing, J.; Zhan, W.B. Molecular cloning and expression analysis of the plgR gene in Scophthalmus. J. Fish. Sci. China 2013, 20, 792–801. [Google Scholar]
- Kortum, A.N.; Rodriguez-Nunez, I.; Yang, J.; Shim, J.; Runft, D.; O’Driscoll, M.L.; Haire, R.N.; Cannon, J.P.; Turner, P.M.; Litman, R.T.; et al. Differential expression and ligand binding indicate alternative functions for zebrafish polymeric immunoglobulin receptor (pIgR) and a family of pIgR-like (PIGRL) proteins. Immunogenetics 2014, 66, 267–279. [Google Scholar] [CrossRef]
- Gold, L.I.; Eggleton, P.; Sweetwyne, M.T.; Van Duyn, L.B.; Greives, M.R.; Naylor, S.M.; Michalak, M.; Murphy-Ullrich, J.E. Calreticulin: Non-endoplasmic reticulum functions in physiology and disease. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 665–683. [Google Scholar] [CrossRef] [PubMed]
- McDonald, K.G.; Leach, M.R.; Brooke, K.W.M.; Wang, C.; Wheeler, L.W.; Hanly, E.K.; Rowley, C.W.; Levin, M.S.; Wagner, M.; Li, E.; et al. Epithelial expression of the cytosolic retinoid chaperone cellular retinol binding protein II is essential for in vivo imprinting of local gut dendritic cells by lumenal retinoids. Am. J. Pathol. 2012, 180, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Jaratlerdsiri, W.; Deakin, J.; Godinez, R.M.; Shan, X.; Peterson, D.G.; Marthey, S.; Lyons, E.; McCarthy, F.M.; Isberg, S.R.; Higgins, D.P.; et al. Comparative genome analyses reveal distinct structure in the saltwater crocodile MHC. PLoS ONE 2014, 9, e114631. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, D.; Wang, P.; Wu, W.; Fang, Q. Alox-5 as a potent therapeutic target on overcoming tki-resistance in chronic myeloid leukemia with t315i mutation in bcr-abl. Blood 2015, 126, 4835. [Google Scholar] [CrossRef]
- Connor, A.E.; Baumgartner, R.N.; Baumgartner, K.B.; Pinkston, C.M.; Boone, S.D.; John, E.M.; Torres-Mejía, G.; Hines, L.M.; Giuliano, A.R.; Wolff, R.K.; et al. Associations between ALOX, COX, and CRP polymorphisms and breast cancer among Hispanic and non-Hispanic white women: The breast cancer health disparities study. Mol. Carcinog. 2015, 54, 1541–1553. [Google Scholar] [CrossRef]
- Sun, Q.Y.; Zhou, H.H.; Mao, X.Y. Emerging Roles of 5-Lipoxygenase Phosphorylation in Inflammation and Cell Death. Oxidative Med. Cell. Longev. 2019, 2019, 2749173. [Google Scholar] [CrossRef]
- Lopes, D.E.M.; Jabr, C.L.; Decani, N.N.; Saraiva, A.C.; de Aquino, S.G.; Medeiros, A.I.; Rossa Junior, C. Inhibition of 5-lipoxygenase attenuates inflammation and BONE resorption in lipopolysaccharide-induced periodontal disease. J. Periodontol. 2017, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. Kegg: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. A Publ. Protein Soc. 2019, 28, 1947–1951. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).