Home Range of the Caspian Whipsnake Dolichophis caspius (Gmelin, 1789) in a Threatened Peri-Urban Population
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
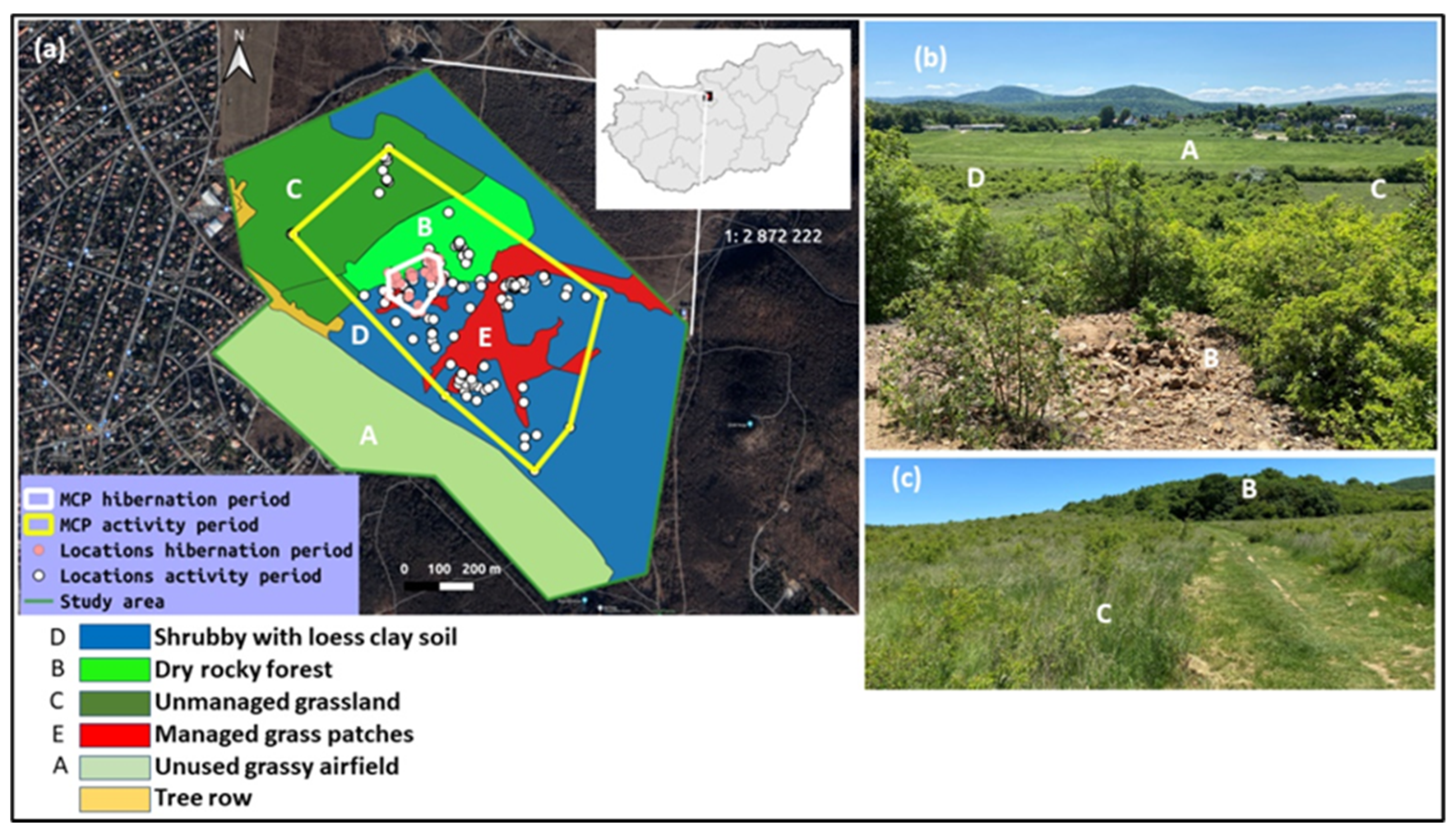
2.1. The Study Area
2.2. Handling the Snakes and Transmitter Implantation
2.3. Radiotelemetry
2.4. Data Analysis
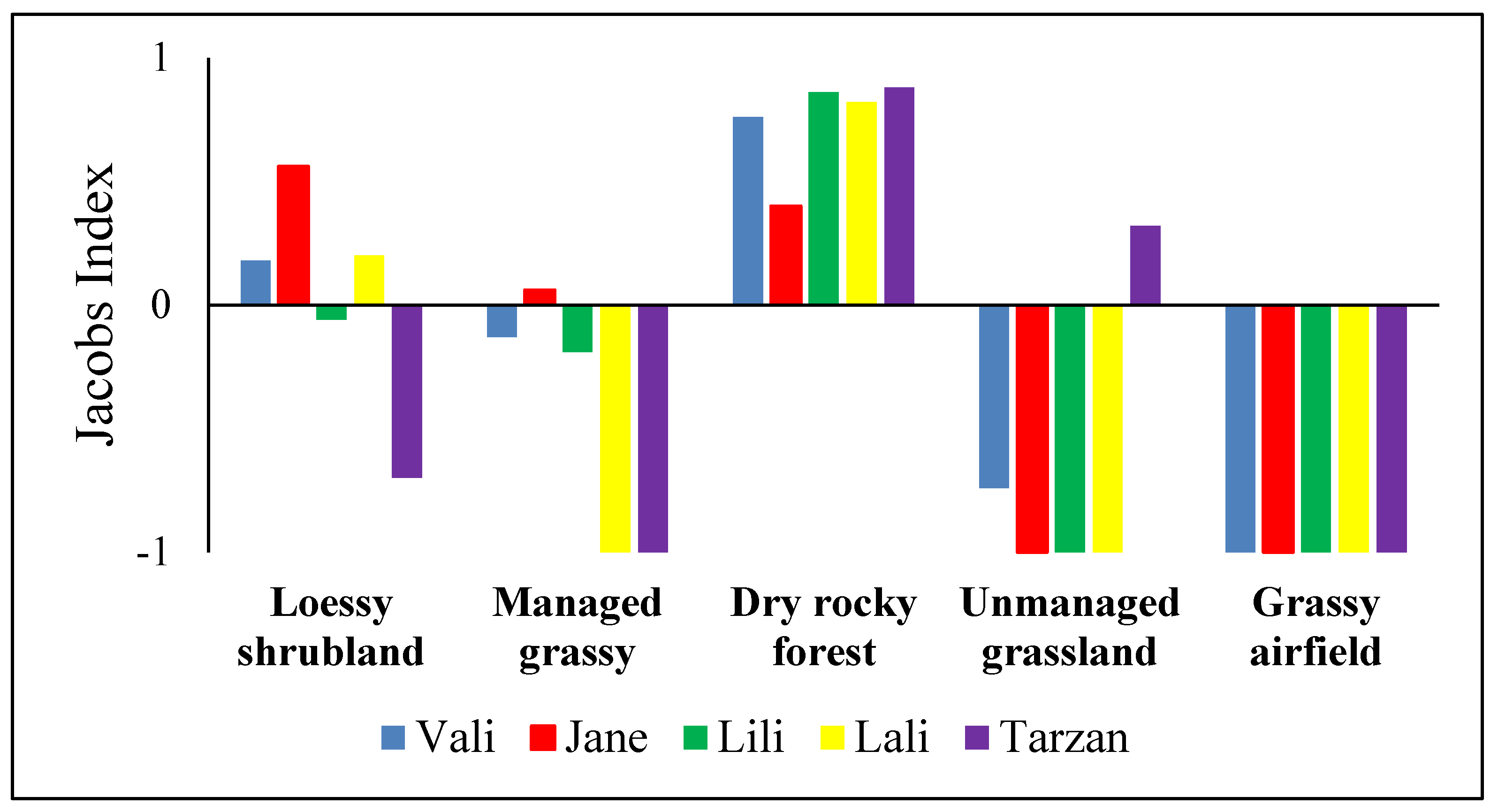
2.4.1. Habitat Availability and Use
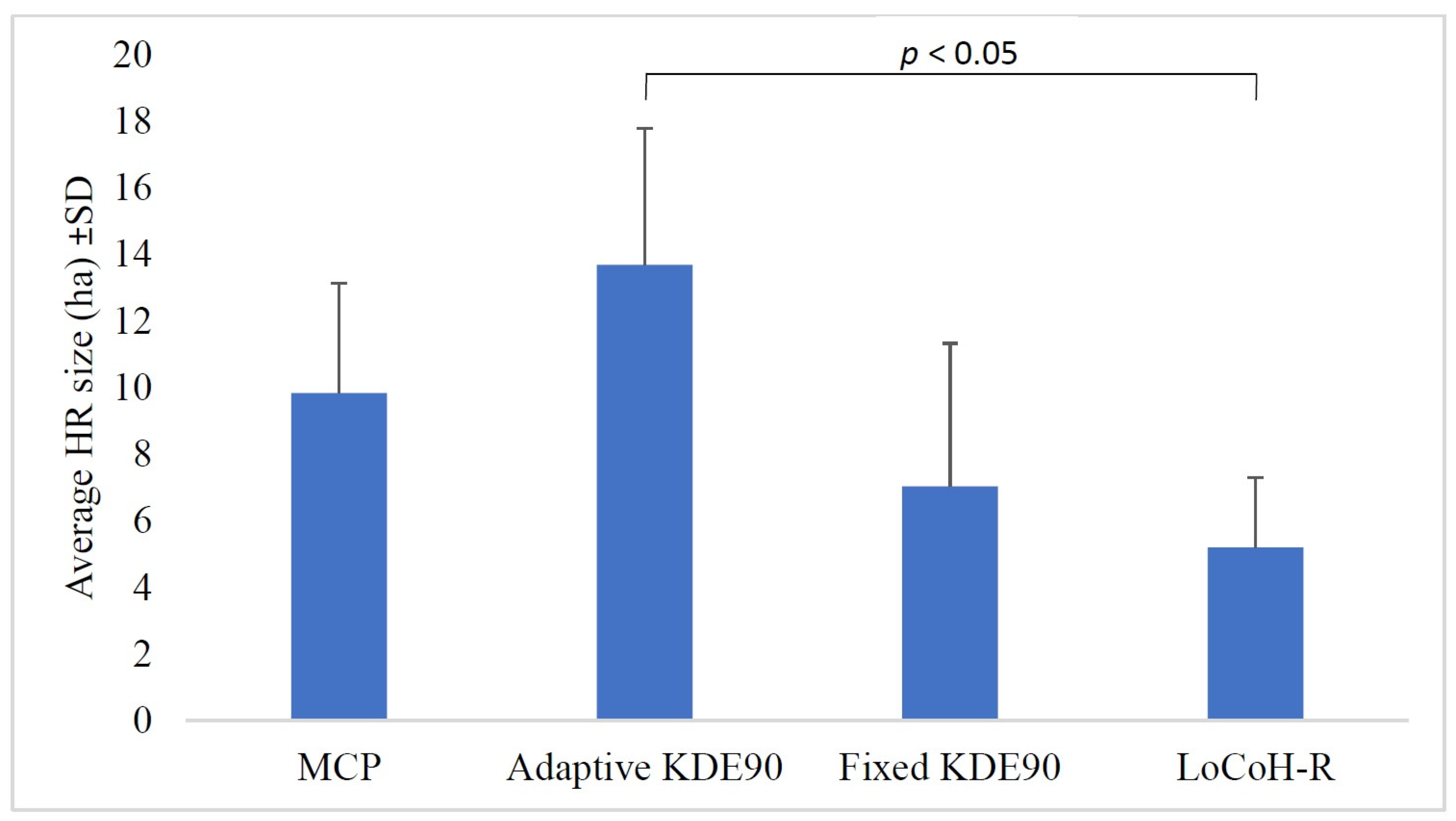
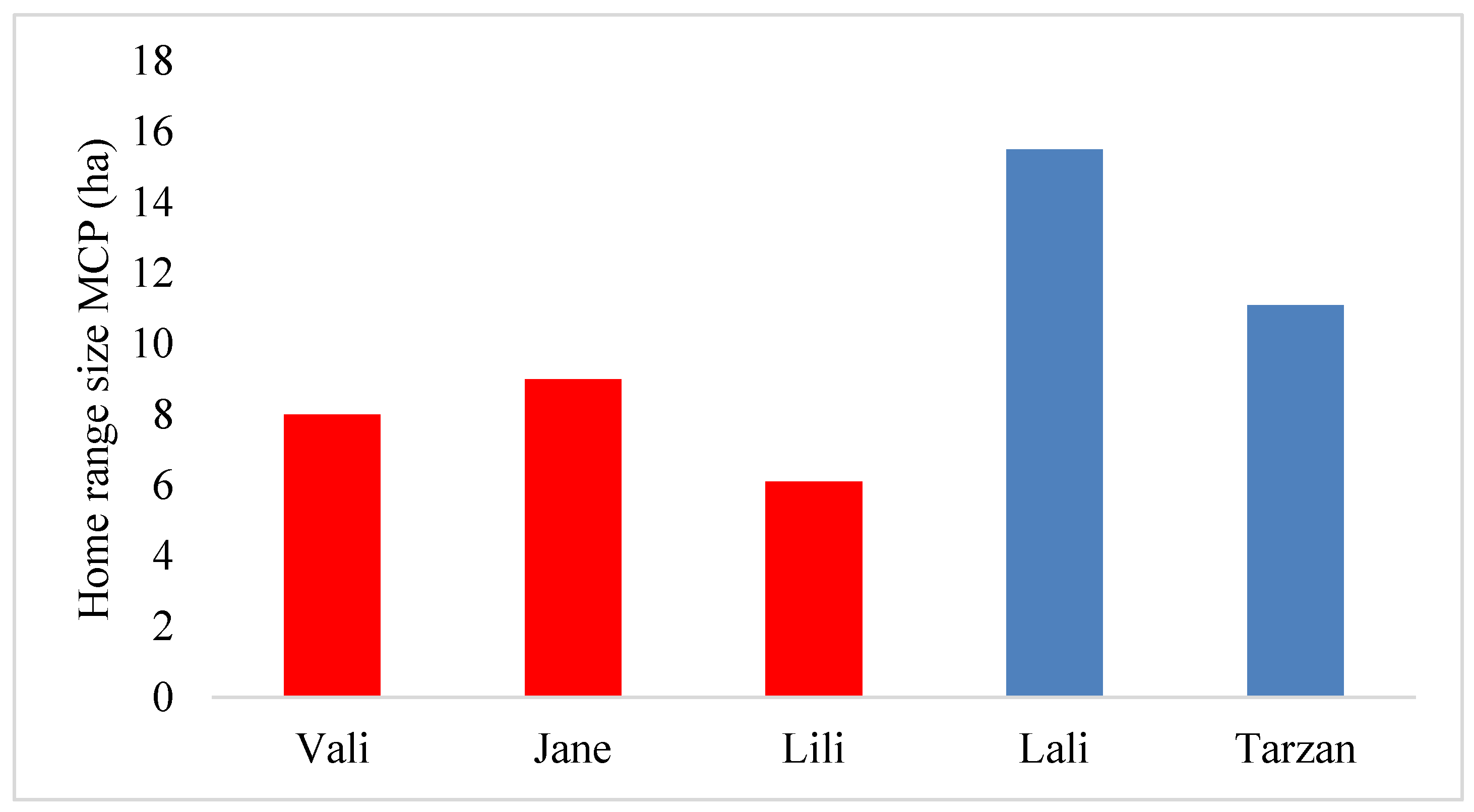
2.4.2. Calculating Home Range Size
2.4.3. Daily Movements
2.5. Statistical Analyses
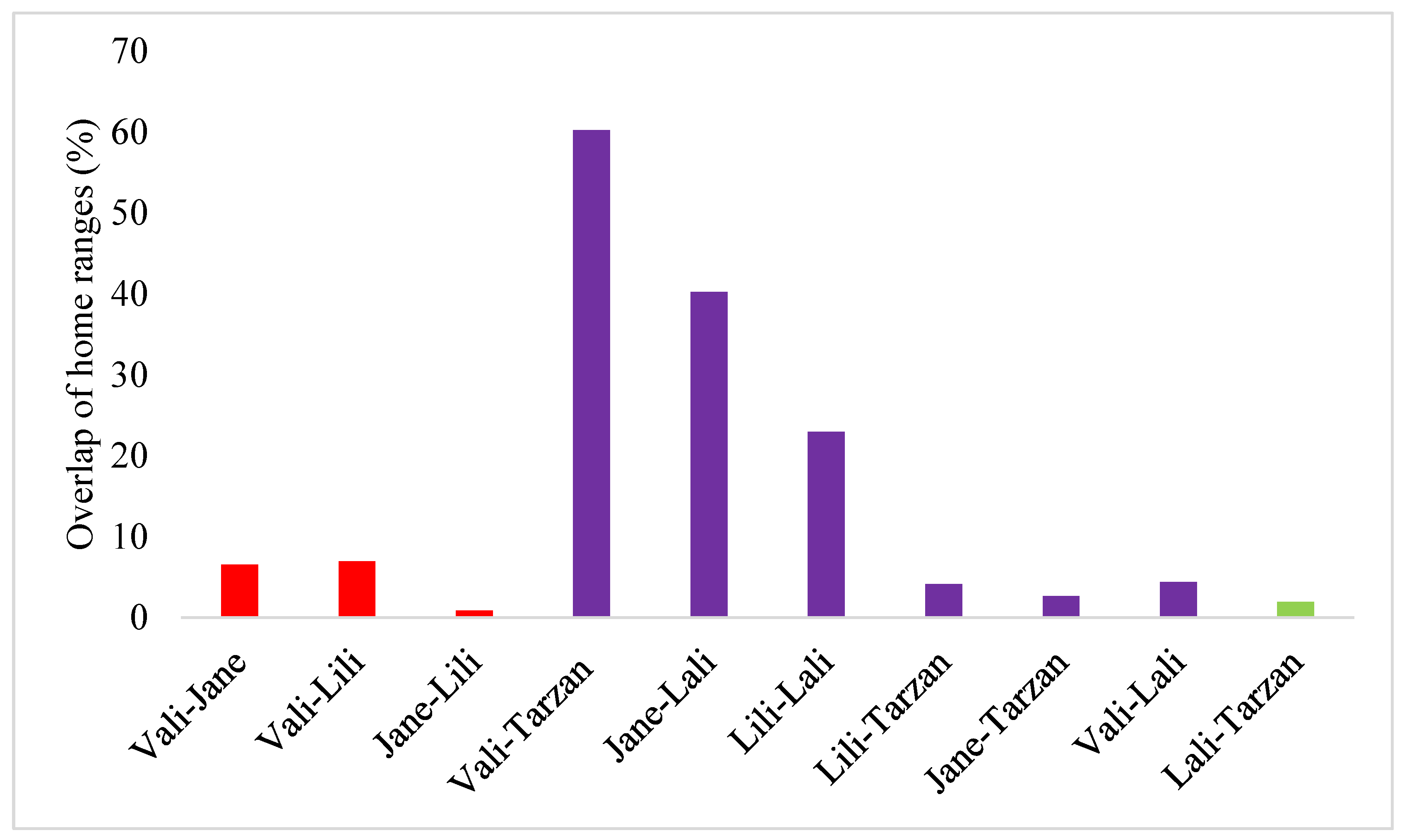
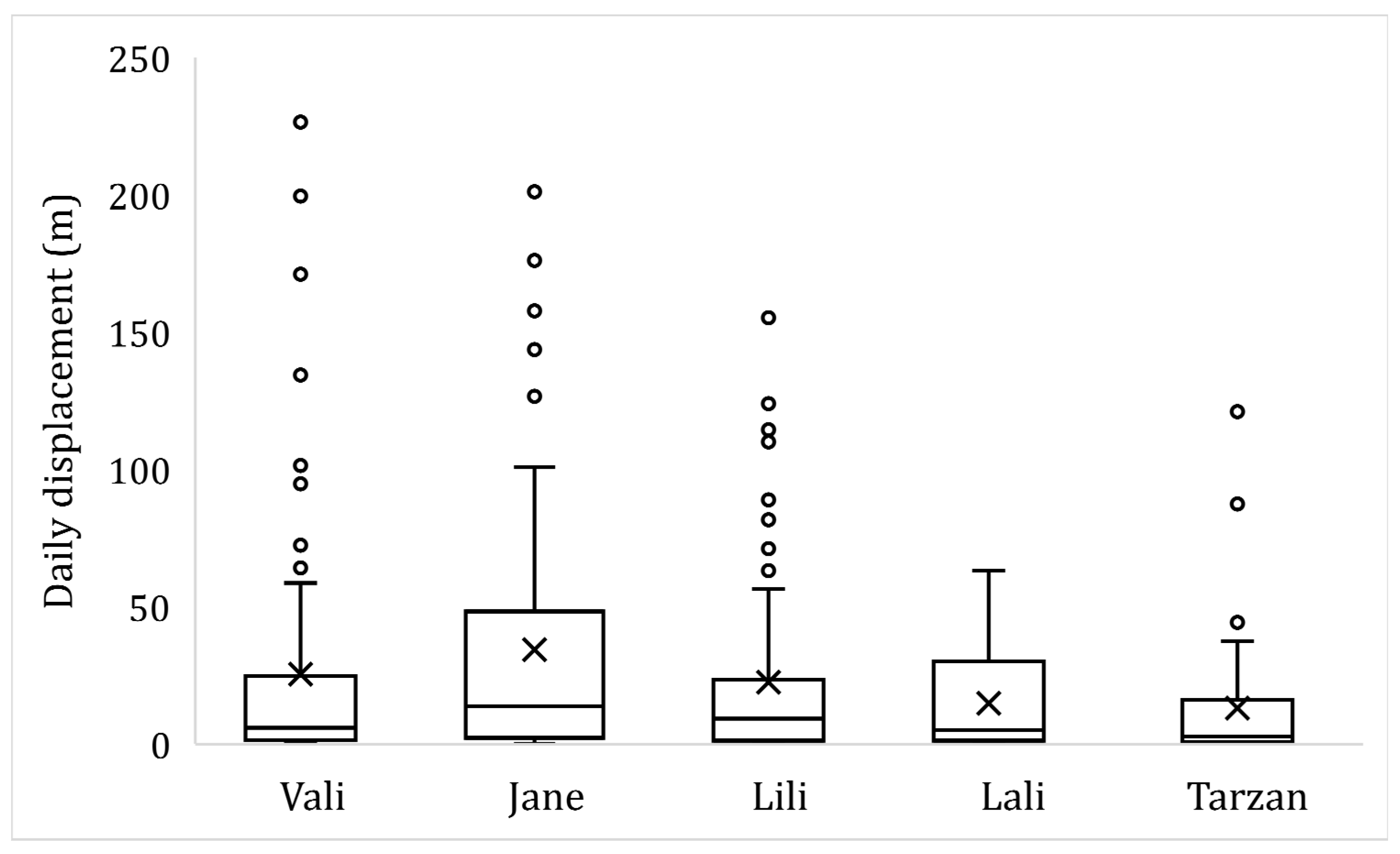
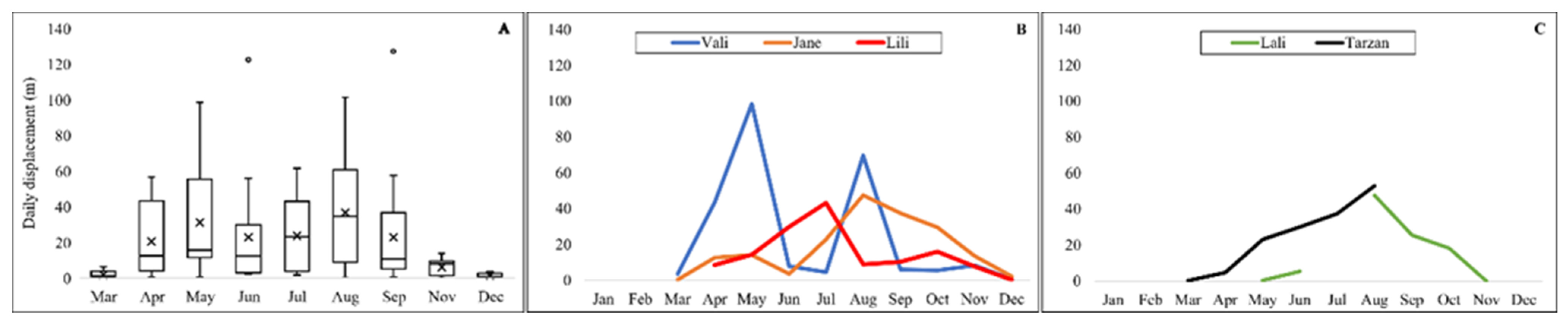
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitrovich, M.J.; Diffendorfer, J.E.; Fisher, R.N. Behavioral response of the coachwhip (Masticophis flagellum) to habitat fragment size and isolation in an urban landscape. J. Herpetol. 2009, 43, 646–656. [Google Scholar] [CrossRef]
- Hoss, S.K.; Guyer, C.; Smith, L.L.; Schuett, G.W. Multiscale influences of landscape composition and configuration on the spatial ecology of eastern diamond-backed rattlesnakes (Crotalus adamanteus). J. Herpetol. 2009, 44, 110–123. [Google Scholar] [CrossRef]
- Corey, B.; Doody, J. Anthropogenic influences on the spatial ecology of a semi-arid python. J. Zool. 2010, 281, 293–302. [Google Scholar] [CrossRef]
- Beale, M.; Poulin, S.; Ivanyi, C.; Blouin-Demers, G. Anthropogenic disturbance affects movement and increases concealment in Western diamondback rattlesnakes (Crotalus atrox). J. Herpetol. 2016, 50, 216–221. [Google Scholar] [CrossRef]
- Wolfe, A.K.; Bateman, P.W.; Fleming, P.A. Does urbanization influence the diet of a large snake? Curr. Zool. 2018, 64, 311–318. [Google Scholar] [CrossRef]
- Brotons, L.; Wolff, A.; Paulus, G.; Martin, J.-L. Effect of adjacent agricultural habitat on the distribution of passerines in natural grasslands. Biol. Conserv. 2005, 124, 407–414. [Google Scholar] [CrossRef]
- Ribeiro, R.; Santos, X.; Sillero, N.; Carretero, M.A.; Llorente, G.A. Biodiversity and Land uses at a regional scale: Is agriculture the biggest threat for reptile assemblages? Acta Oecologica 2009, 35, 327–334. [Google Scholar] [CrossRef]
- Deák, G.; Katona, K.; Biró, Z. Exploring the use of a carcass detection dog to assess mowing mortality in Hungary. J. Vertebr. Biol. 2021, 69, 20089.1. [Google Scholar] [CrossRef]
- Holt, C.A.; Fuller, R.J.; Dolman, P.M. Experimental evidence that deer browsing reduces habitat suitability for breeding Common Nightingales (Luscinia megarhynchos). Ibis 2010, 152, 335–346. [Google Scholar] [CrossRef]
- Ballouard, J.-M.; Kauffman, C.; Besnard, A.; Ausanneau, M.; Amiguet, M.; Billy, G.; Caron, S.; Fosseries, G.; Ferrari, T.; Mariani, V.; et al. Recent invaders in small Mediterranean islands: Wild boars impact snakes in Port-Cros National Park. Diversity 2021, 13, 498. [Google Scholar] [CrossRef]
- Subedi, S.C.; Walls, S.C.; Barichivich, W.J.; Boyles, R.; Ross, M.S.; Hogan, J.A.; Tupy, J.A. Future changes in habitat availability for two specialist snake species in the imperiled rocklands of South Florida, USA. Conserv. Sci. Pract. 2022, 4, e12802. [Google Scholar] [CrossRef]
- Mac Nally, R.; Brown, G.W. Reptiles and habitat fragmentation in the box-ironbark forests of central Victoria, Australia: Predictions, compositional change and faunal nestedness. Oecologia 2001, 128, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.N.; Krysko, K.L. Invasive and introduced reptiles and amphibians. In Current Therapy in Reptile Medicine and Surgery; Mader, I., Douglas, R., Drivers, S.J., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 304–309. [Google Scholar]
- Fahrig, L. Effects of Habitat Fragmentation on Biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Dodd, C.K.; Barichivich, W.J. Movements of large snakes (Drymarchon, Masticophis) in North-Central Florida. Fla. Sci. 2007, 70, 83–94. [Google Scholar]
- Lepczyk, C.A.; Aronson, M.F.J.; Evans, K.L.; Goddard, M.A.; Lerman, S.B.; Macivor, J.S. Biodiversity in the City: Fundamental Questions for Understanding the Ecology of Urban Green Spaces for Biodiversity Conservation. BioScience 2017, 67, 799–807. [Google Scholar] [CrossRef]
- Todd, B.D.; Nowakowski, A.J. Ectothermy and the macroecology of home range scaling in snakes. Glob. Ecol. Biogeogr. 2021, 30, 262–276. [Google Scholar] [CrossRef]
- Bauder, J.M.; Breininger, D.R.; Bolt, M.R.; Legare, M.L.; Jenkins, C.L.; Rothermel, B.B.; McGarigal, K. The influence of sex and season on conspecific spatial overlap in a large, actively-foraging colubrid snake. PLoS ONE 2016, 11, e0160033. [Google Scholar] [CrossRef]
- Zappalorti, R.; Burger, J.; Peterson, F. Home Range Size and Distance Traveled from Hibernacula in Northern Pinesnakes in the New Jersey Pine Barrens. Herpetologica 2015, 71, 26–36. [Google Scholar] [CrossRef]
- Metcalf, M.F.; Gunnels, C.W., IV; Everham, E.M., III; Girimurugan, S.B.; Andreadis, P.; Herman, J.E. Movement of the Eastern Indigo snake (Drymarchon couperi) in Southern Florida, USA. Herpetol. Conserv. Biol. 2021, 16, 425–435. [Google Scholar]
- Brito, J. Seasonal variation in movements, home range, and habitat use by male Vipera latastei in Northern Portugal. J. Herpetol. 2009, 37, 155–160. [Google Scholar] [CrossRef]
- Kovar, R.; Brabec, M.; Vita, R.; Vodicka, R.; Bogdan, V. Habitat use of the Aesculapian snake, Zamenis longissimus, at the northern extreme of its range in northwest Bohemia. Herpetol. Bulletin. 2016, 136, 1–9. [Google Scholar]
- Webb, J.K.; Shine, R. A field study of spatial ecology and movements of a threatened snake species, Hoplocephalus bungaroides. Biol. Conserv. 1997, 82, 203–217. [Google Scholar] [CrossRef]
- Breininger, D.; Bolt, R.; Legare, M.; Drese, J.; Stolen, E. Factors influencing home-range sizes of Eastern Indigo snakes in central Florida. J. Herpetol. 2011, 45, 484–490. [Google Scholar] [CrossRef]
- Jellen, B.C.; Shepard, D.B.; Dreslik, M.J.; Phillips, C.A. Male movement and body size affect mate acquisition in the eastern Massasauga (Sistrurus catenatus). J. Herpetol. 2007, 41, 451–457. [Google Scholar] [CrossRef]
- McEachern, M.A.; Adams, A.A.Y.; Klug, P.E.; Fitzgerald, L.A.; Reed, R.N. Brumation of Introduced Black and White Tegus, Tupinambis merianae (Squamata: Teiidae), in Southern Florida. Sena 2015, 14, 319–328. [Google Scholar] [CrossRef]
- Kreiner, G. The Snakes of Europe: All Species from West of the Caucasus Mountains; Chimaira: Frankfurt am Main, Germany, 2007. [Google Scholar]
- Sahlean, T.C.; Strugariu, A.; Zamfirescu, Ş.R.; Chişamera, G.; Stanciu, C.R.; Gavril, V.D.; Gherghel, I. Filling the Gaps: Updated Distribution of the Caspian Whip Snake (Dolichophis caspius, Reptilia: Colubridae) in Romania. Russ. J. Herpetol. 2019, 26, 305–308. [Google Scholar] [CrossRef]
- Nagy, Z.T.; Bellaagh, M.; Wink, M.; Paunovic, A.; Korsós, Z. Phylogeography of the Caspian whipsnake in Europe with emphasis on the westernmost populations. Amphib. Reptil. 2010, 31, 455–461. [Google Scholar]
- Bellaagh, M.; Korsós, Z.; Szelényi, G. New occurrences of the Caspian whipsnake, Dolichophis caspius (Reptilia: Serpentes: Colubridae) along the river Danube in Hungary. Acta Zool. Bulg. 2008, 60, 213–217. [Google Scholar]
- Tóth, T. Data on the North Hungarian records of the Large Whip Snake Coluber caspius Gmelin, 1789. Herpetozoa 2002, 14, 163–167. [Google Scholar]
- Mahtani-Williams, S.; Fulton, W.; Desvars-Larrive, A.; Lado, S.; Elbers, J.P.; Halpern, B.; Herczeg, D.; Babocsay, G.; Laus, B.; Nagy, Z.T.; et al. Landscape genomics of a widely distributed snake, Dolichophis caspius (Gmelin, 1789) across Eastern Europe and Western Asia. Genes 2020, 11, 1218. [Google Scholar] [CrossRef]
- Babocsay, G.; Vági, B. Disappearing large whip snakes—Increasing citizen involvement in the Amphibian and Reptile Conservation Group of BirdLife Hungary. Természetvédelmi Közlemények 2012, 18, 34–44. [Google Scholar]
- Pásztor, L.; Laborczi, A.; Bakacsi, Z.; Szabó, J.; Illés, G. Compilation of a national soil-type map for Hungary by sequential classification methods. Geoderma 2018, 311, 93–108. [Google Scholar] [CrossRef]
- Horning, M.; Haulena, M.; Tuomi, P.A.; Mellish, J.-A.E.; Goertz, C.E.; Woodie, K.; Berngartt, R.K.; Johnson, S.; Shuert, C.R.; Walker, K.A.; et al. Best practice recommendations for the use of fully implanted telemetry devices in pinnipeds. Anim. Biotelemetry 2017, 5, 13. [Google Scholar] [CrossRef]
- Boyle, S.; Lourenço, W.; Silva, L.; Smith, A. Home Range Estimates Vary with Sample Size and Methods. Folia Primatol. 2009, 80, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Mohr, C.O. Table of equivalent populations of north american small mammals. Am. Midl. Nat. 1947, 37, 223–249. [Google Scholar] [CrossRef]
- Hayne, D.W. Calculation of size of home range. J. Mammal. 1949, 30, 1–18. [Google Scholar] [CrossRef]
- Getz, W.M.; Fortmann-Roe, S.; Cross, P.C.; Lyons, A.J.; Ryan, S.J.; Wilmers, C.C. LoCoH: Nonparameteric kernel methods for constructing home ranges and utilization distributions. PLoS ONE 2007, 2, e207. [Google Scholar] [CrossRef]
- Steiniger, S.; Hunter, A. OpenJUMP HoRAE—A free GIS and toolbox for home-range analysis. Wildl. Soc. Bull. 2012, 36, 600–608. [Google Scholar] [CrossRef]
- Kowalczyk, R.; Zalewski, A.; Jędrzejewska, B. Daily movement and territory use by badgers Meles meles in Białowieża Primeval Forest, Poland. Wildl. Biol. 2006, 12, 385–391. [Google Scholar] [CrossRef]
- Katona, K.; Kiss, M.; Bleier, N.; Székely, J.; Nyeste, M.; Kovács, V.; Terhes, A.; Fodor, Á.; Olajos, T.; Rasztovits, E.; et al. Ungulate browsing shapes climate change impacts on forest biodiversity in Hungary. Biodivers. Conserv. 2013, 22, 1167–1180. [Google Scholar] [CrossRef]
- Şahin, M.; Aybek, E. Jamovi: An Easy-to-Use Statistical Software for the Social Scientists. Int. J. Assess. Tools Educ. 2020, 6, 670–692. [Google Scholar] [CrossRef]
- Reading, C. Ranging behaviour and home range size of smooth snake inhabiting lowland heath in southern England. Herpetol. J. 2012, 22, 241–247. [Google Scholar]
- Bauder, J.M.; Breininger, D.R.; Bolt, M.R.; Legare, M.L.; Jenkins, C.L.; Rothermel, B.B.; McGarigal, K. Movement barriers, habitat heterogeneity or both? Testing hypothesized effects of landscape features on home range sizes in eastern indigo snakes. J. Zool. 2020, 311, 204–216. [Google Scholar] [CrossRef]
- Silva-Opps, M.; Opps, S.B. Use of Telemetry Data to Investigate Home Range and Habitat Selection in Mammalian Carnivores. In Modern Telemetry; IntechOpen: London, UK, 2011. [Google Scholar]
- Gregory, T. Home Range Estimation. In The International Encyclopedia of Primatology; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 1–4. [Google Scholar]
- Frank, K.; Dudás, G. Body size and seasonal condition of Caspian Whip Snakes, Dolichophis caspius (Gmelin, 1789), in southwestern Hungary. Herpetozoa 2018, 30, 131–138. [Google Scholar]
- Bonnet, X.; Naulleau, G.; Shine, R. The dangers of leaving home: Dispersal and mortality in snakes. Biol. Conserv. 1999, 89, 39–50. [Google Scholar] [CrossRef]
- Charland, M.B. Movements and habitat use of gravid and nonegravid female garter snakes (Colubridae: Thamnophis). J. Zool. 2009, 236, 543–561. [Google Scholar] [CrossRef]
- Sahlean, T.; Strugariu, A.; Zamfirescu, Ş.; Gherghel, I. Data regarding habitat selection in the Caspian whipsnake (Dolichophis caspius Gmelin, 1789) towards the north-western limit of its distribution range (Romania). Int. Zool. Congr. Grigore Antipa Mus. 2016. [Google Scholar]
- Reading, C.J.; Jofré, G.M. Habitat selection and home range size of grasss snakes Natrix natrix in an agricultural landscape in southern England. Amphib. Reptil. 2009, 30, 379–388. [Google Scholar] [CrossRef]
- Dyugmedzhiev, A.; Slavchev, M.; Naumov, B. Emergence and dispersal of snakes after syntopic hibernation. Herpetozoa 2019, 32, 149–157. [Google Scholar] [CrossRef]
- Jackson, S.B. Home range size and habitat use of the Eastern Indigo Snake (Drymarchon couperi) at a disturbed agricultural site in south Florida. Master’s Thesis, Florida Gulf Coast University, Fort Myers, FL, USA, 2013; 88p. [Google Scholar]
| Name | Sex (F/M) | Body Weight (g) | Total Length (cm) | Date of First Localization | Date of Last Localization | Number of Localization Points | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Above Ground | Under- Ground | Activity Period | Hibernation Period | ||||||
| Vali | F | 464 | 148 | 22 May/2016 | 19 Jun/2019 | 92 | 37 | 55 | 62 | 30 |
| Jane | F | 266 | 120 | 22 May/2016 | 07 Jul/2017 | 68 | 29 | 39 | 60 | 8 |
| Lili | F | 398 | 143 | 02 Aug/2016 | 19 Oct/2018 | 78 | 42 | 36 | 64 | 14 |
| Lali | M | 778 | 159 | 01 Jun/2018 | 09 Aug/2019 | 27 | 12 | 15 | 18 | 9 |
| Tarzan | M | 1098 | 185 | 26 Aug/2016 | 06 Jul/2017 | 44 | 6 | 38 | 22 | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teffo, T.R.; Katona, K.; Babocsay, G.; Sós, E.; Halpern, B. Home Range of the Caspian Whipsnake Dolichophis caspius (Gmelin, 1789) in a Threatened Peri-Urban Population. Animals 2023, 13, 447. https://doi.org/10.3390/ani13030447
Teffo TR, Katona K, Babocsay G, Sós E, Halpern B. Home Range of the Caspian Whipsnake Dolichophis caspius (Gmelin, 1789) in a Threatened Peri-Urban Population. Animals. 2023; 13(3):447. https://doi.org/10.3390/ani13030447
Chicago/Turabian StyleTeffo, Thabang Rainett, Krisztián Katona, Gergely Babocsay, Endre Sós, and Bálint Halpern. 2023. "Home Range of the Caspian Whipsnake Dolichophis caspius (Gmelin, 1789) in a Threatened Peri-Urban Population" Animals 13, no. 3: 447. https://doi.org/10.3390/ani13030447
APA StyleTeffo, T. R., Katona, K., Babocsay, G., Sós, E., & Halpern, B. (2023). Home Range of the Caspian Whipsnake Dolichophis caspius (Gmelin, 1789) in a Threatened Peri-Urban Population. Animals, 13(3), 447. https://doi.org/10.3390/ani13030447







