Effects of Caffeine and Glucose Supplementation at Birth on Piglet Pre-Weaning Growth, Thermoregulation, and Survival
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management
2.2. Experimental Design
2.3. Piglet Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campos, P.H.R.F.; Silva, B.; Donzele, J.; Oliveira, R.; Knol, E. Effects of sow nutrition during gestation on within-litter birth weight variation: A review. Animal 2012, 6, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Swinbourne, A.M.; Kind, K.L.; Flinn, T.; Kleemann, D.O.; van Wettere, W.H. Caffeine: A potential strategy to improve survival of neonatal pigs and sheep. Anim. Reprod. Sci. 2021, 226, 106700. [Google Scholar] [CrossRef] [PubMed]
- Condous, P.; Plush, K.; Tilbrook, A.; Van Wettere, W. Reducing sow confinement during farrowing and in early lactation increases piglet mortality. J. Anim. Sci. 2016, 94, 3022–3029. [Google Scholar] [CrossRef] [PubMed]
- KilBride, A.; Mendl, M.; Statham, P.; Held, S.; Harris, M.; Cooper, S.; Green, L.E. A cohort study of preweaning piglet mortality and farrowing accommodation on 112 commercial pig farms in England. Prev. Vet. Med. 2012, 104, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Randall, G. The relationship of arterial blood pH and pCO2 to the viability of the newborn piglet. Can. J. Comp. Med. 1971, 35, 141. [Google Scholar] [PubMed]
- Orozco-Gregorio, H.; Mota-Rojas, D.; Bonilla-Jaime, H.; Trujillo-Ortega, M.E.; Becerril-Herrera, M.; Hernández-González, R.; Villanueva-García, D. Effects of administration of caffeine on metabolic variables in neonatal pigs with peripartum asphyxia. Am. J. Vet. Res. 2010, 71, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Lodha, A.; Seshia, M.; McMillan, D.D.; Barrington, K.; Yang, J.; Lee, S.K.; Shah, P.S.; Canadian Neonatal Network. Association of early caffeine administration and neonatal outcomes in very preterm neonates. JAMA Pediatr. 2015, 169, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Back, S.A.; Craig, A.; Ling, N.L.; Ren, J.; Akundi, R.S.; Riberio, I.; Rivkees, S.A. Protective effects of caffeine on chronic hypoxia-induced perinatal white matter injury. Ann. Neurol. 2006, 60, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Smith, A.L.; Rosenkrantz, T.S.; Fitch, R.H. Therapeutic effect of caffeine treatment immediately following neonatal hypoxic-ischemic injury on spatial memory in male rats. Brain Sci. 2013, 3, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Nowland, T.L.; Kind, K.; Hebart, M.; van Wettere, W. Caffeine supplementation at birth, but not 8 to 12 h post-birth, increased 24 h pre-weaning mortality in piglets. Animal 2010, 14, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Engelsmann, M.N.; Hansen, C.F.; Nielsen, M.N.; Kristensen, A.R.; Amdi, C. Glucose injections at birth, warmth and placing at a nurse sow improve the growth of IUGR piglets. Animals 2019, 9, 519. [Google Scholar] [CrossRef] [PubMed]
- Devillers, N.; Farmer, C.; Le Dividich, J.; and Prunier, A. Variability of colostrum yield and colostrum intake in pigs. Animal 2007, 1, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Herpin, P.; Le Dividich, J.; Hulin, J.C.; Filaut, M.; De Marco, F.; Bertin, R. Effects of the level of asphyxia during delivery on viability at birth and early postnatal vitality of newborn pigs. J. Anim. Sci. 1996, 74, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Spilsbury, M.; Ramirez-Necoechea, R.; Gonzlez-Lozano, M.; Mota-Rojas, D.; Trujillo-Ortega, M.E. Piglet survival in early lactation: A review. J. Anim. Vet. Adv. 2007, 6, 76–86. [Google Scholar]
- Hole, C.V.; Ayuso, M.; Aerts, P.; Prims, S.; Van Cruchten, S.; Van Ginneken, C. Glucose and glycogen levels in piglets that differ in birth weight and vitality. Heliyon 2019, 5, e02510. [Google Scholar]
- Hou, L.; Hu, C.Y.; Wang, C. Pig has no brown adipose tissue. FASEB J. 2018, 31, S1. [Google Scholar] [CrossRef]
- Lezama-García, K.; Mota-Rojas, D.; Martínez-Burnes, J.; Villanueva-García, D.; Domínguez-Oliva, A.; Gómez-Prado, J.; Mora-Medina, P.; Casas-Alvarado, A.; Olmos-Hernández, A.; Soto, P.; et al. Strategies for hypothermia compensation in altricial and precocial newborn mammals and their monitoring by infrared thermography. Vet. Sci. 2022, 9, 246. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-García, D.; Mota-Rojas, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Mora-Medina, P.; Salmerón, C.; Gómez, J.; Boscato, L.; Gutiérrez-Pérez, O.; Cruz, V.; et al. Hypothermia in newly born piglets: Mechanisms of thermoregulation and pathophysiology of death. J. Anim. Behav. Biometeorol. 2021, 9, 2101. [Google Scholar] [CrossRef]
- Villanueva-García, D.; Mota-Rojas, D.; Miranda-Cortés, A.; Ibarra-Ríos, D.; Casas-Alvarado, A.; Mora-Medina, P.; Martínez-Burnes, J.; Olmos-Hernández, A.; Hernández-Avalos, I. Caffeine: Cardiorespiratory effects and tissue protection in animal models. Exp. Anim. 2021, 70, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Tucker, B.S.; Petrovski, K.R.; Kirkwood, R.N. Neonatal piglet temperature changes: Effect of intraperitoneal warm saline injection. Animals 2022, 12, 1312. [Google Scholar] [CrossRef] [PubMed]
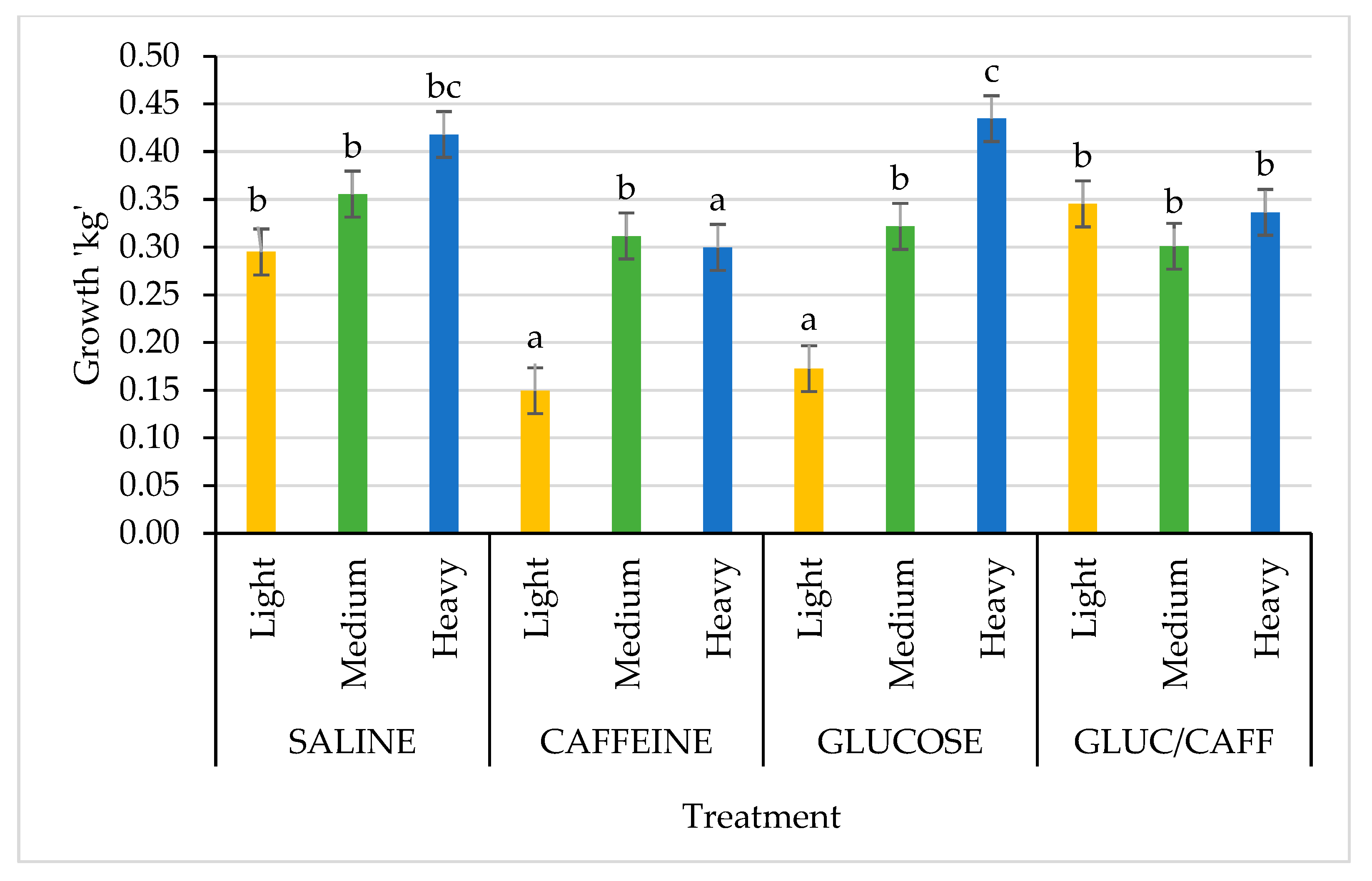
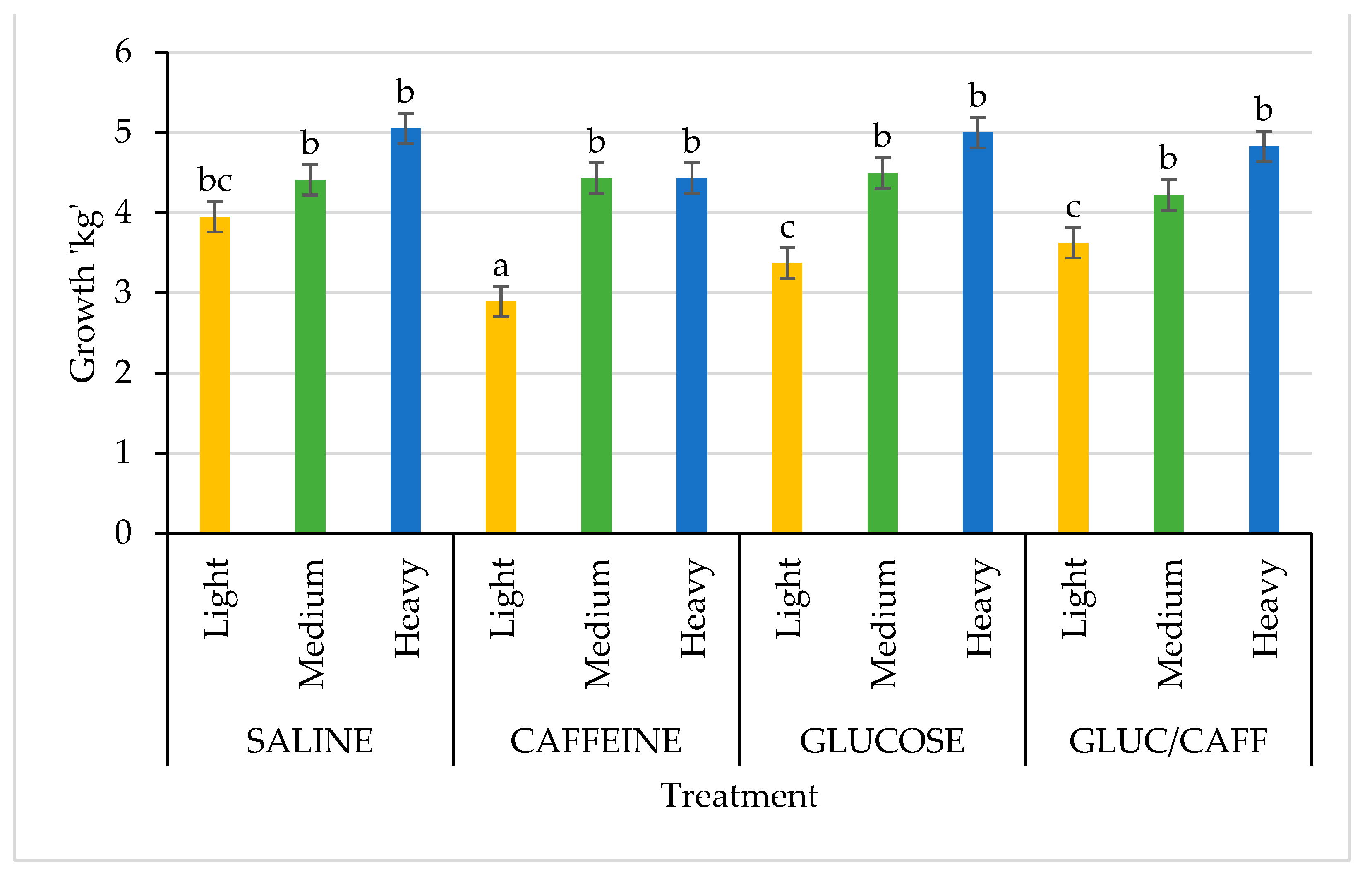
| Variables | n | SAL | CAFF | GLUC | CAFF-GLUC | p-Value |
|---|---|---|---|---|---|---|
| 4 h temperature (°C) | 218 | 36.33 ± 0.25 | 36.61 ± 0.26 | 36.49 ± 0.27 | 36.61 ± 0.25 | 0.705 |
| 24 h temperature (°C) # | 355 | 6.16 ± 0.10 | 6.15 ± 0.01 | 6.15 ± 0.01 | 6.14 ± 0.01 | 0.566 |
| Time to udder (min) * | 263 | 1.24 ± 0.07 | 1.16 ± 0.80 | 1.21 ± 0.08 | 1.21 ± 0.08 | 0.560 |
| Colostrum intake (g) * | 340 | 2.44 ± 0.30 | 2.48 ± 0.04 | 2.47 ± 0.03 | 2.48 ± 0.30 | 0.677 |
| Variables | n | SAL | CAFF | GLUC | CAFF-GLUC | p-Value |
|---|---|---|---|---|---|---|
| Day 0 weight (kg) | 392 | 1.42 ± 0.02 | 1.40 ± 0.02 | 1.38 ± 0.02 | 1.42 ± 0.02 | 0.221 |
| Day 1 weight (kg) | 352 | 1.56 ± 0.02 | 1.51 ± 0.03 | 1.50 ± 0.02 | 1.53 ± 0.02 | 0.195 |
| Day 3 weight (kg) | 334 | 1.93 ± 0.03 a | 1.81 ± 0.03 b | 1.81 ± 0.03 b | 1.86 ± 0.03 b | 0.003 |
| Day 18 weight (kg) | 324 | 5.88 ± 0.15 | 5.60 ± 0.15 | 5.83 ± 0.15 | 5.67 ± 0.15 | 0.131 |
| Mortality | n | SAL | CAFF | GLUC | CAFF-GLUC | Total | p-Value |
|---|---|---|---|---|---|---|---|
| Day 0 to 1 | 28 | 8/101 (7.9%) | 6/98 (6.1%) | 5/100 (5%) | 9/98 (9.2%) | 28/397 (7.1%) | 0.667 |
| Day 1 to 3 | 30 | 7/93 (7.5%) | 6/92 (6.5%) | 11/95 (11.6%) | 6/89 (6.7%) | 30/369 (8.1%) | 0.551 |
| Day 3 to 18 | 11 | 3/86 (3.5%) | 6/86 (7%) | 1/84 (1.2%) | 1/83 (1.2%) | 11/339 (3.2%) | 0.109 |
| Total | 69 | 18/280 (6.4%) | 18/276 (6.5%) | 17/279 (6.1%) | 16/270 (5.9%) | 18.4% | 0.983 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarratt, L.; James, S.E.; Kirkwood, R.N.; Nowland, T.L. Effects of Caffeine and Glucose Supplementation at Birth on Piglet Pre-Weaning Growth, Thermoregulation, and Survival. Animals 2023, 13, 435. https://doi.org/10.3390/ani13030435
Jarratt L, James SE, Kirkwood RN, Nowland TL. Effects of Caffeine and Glucose Supplementation at Birth on Piglet Pre-Weaning Growth, Thermoregulation, and Survival. Animals. 2023; 13(3):435. https://doi.org/10.3390/ani13030435
Chicago/Turabian StyleJarratt, Lillie, Sarah E. James, Roy N. Kirkwood, and Tanya L. Nowland. 2023. "Effects of Caffeine and Glucose Supplementation at Birth on Piglet Pre-Weaning Growth, Thermoregulation, and Survival" Animals 13, no. 3: 435. https://doi.org/10.3390/ani13030435
APA StyleJarratt, L., James, S. E., Kirkwood, R. N., & Nowland, T. L. (2023). Effects of Caffeine and Glucose Supplementation at Birth on Piglet Pre-Weaning Growth, Thermoregulation, and Survival. Animals, 13(3), 435. https://doi.org/10.3390/ani13030435






