Role of Dietary Methyl Sulfonyl Methane in Poultry
Abstract
Simple Summary
Abstract
1. Introduction
2. Oxidative Stress as a Factor Affecting Poultry Production
3. Synthetic and Natural Anti-Oxidants
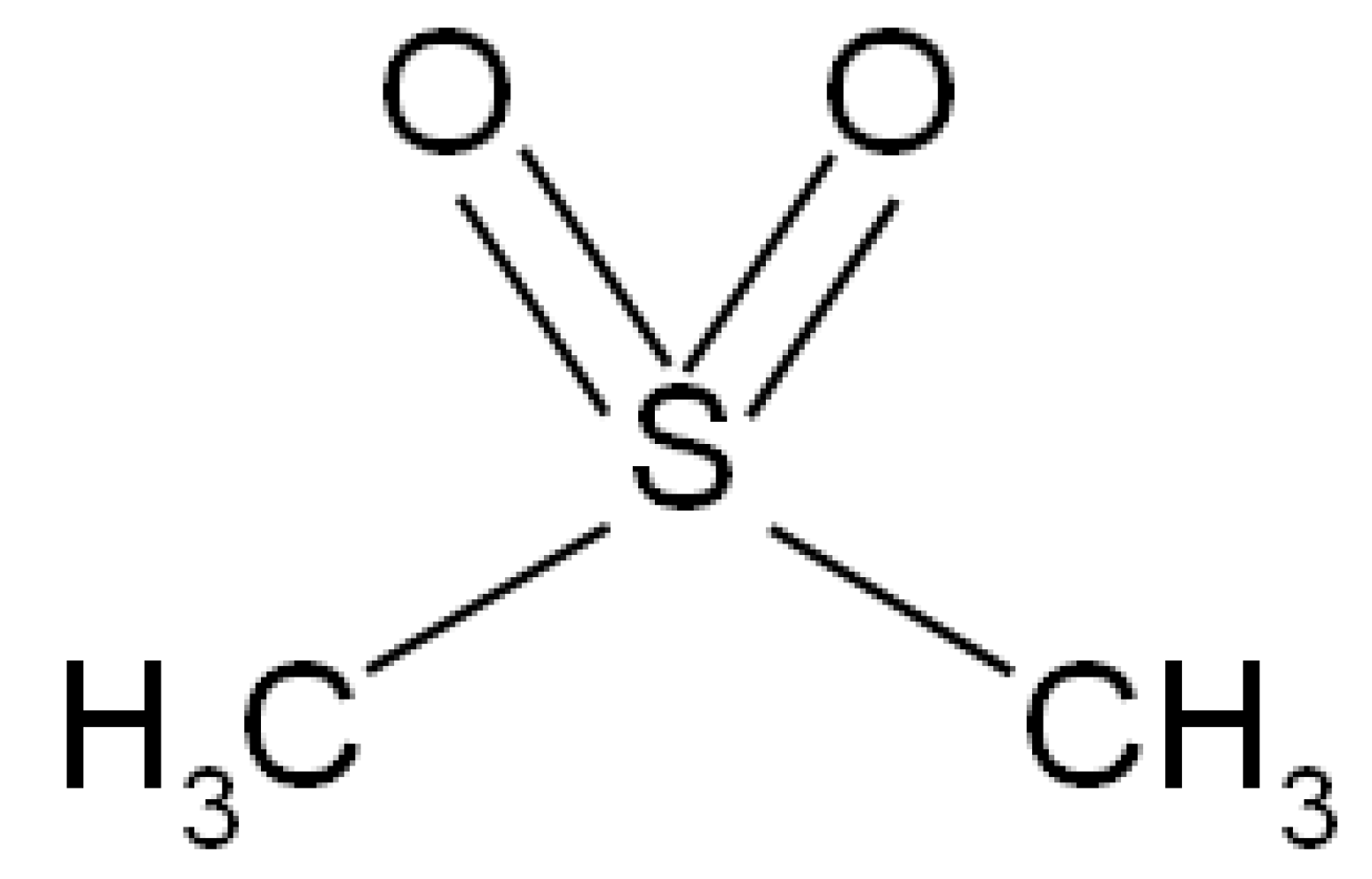
4. Methyl Sulfonyl Methane
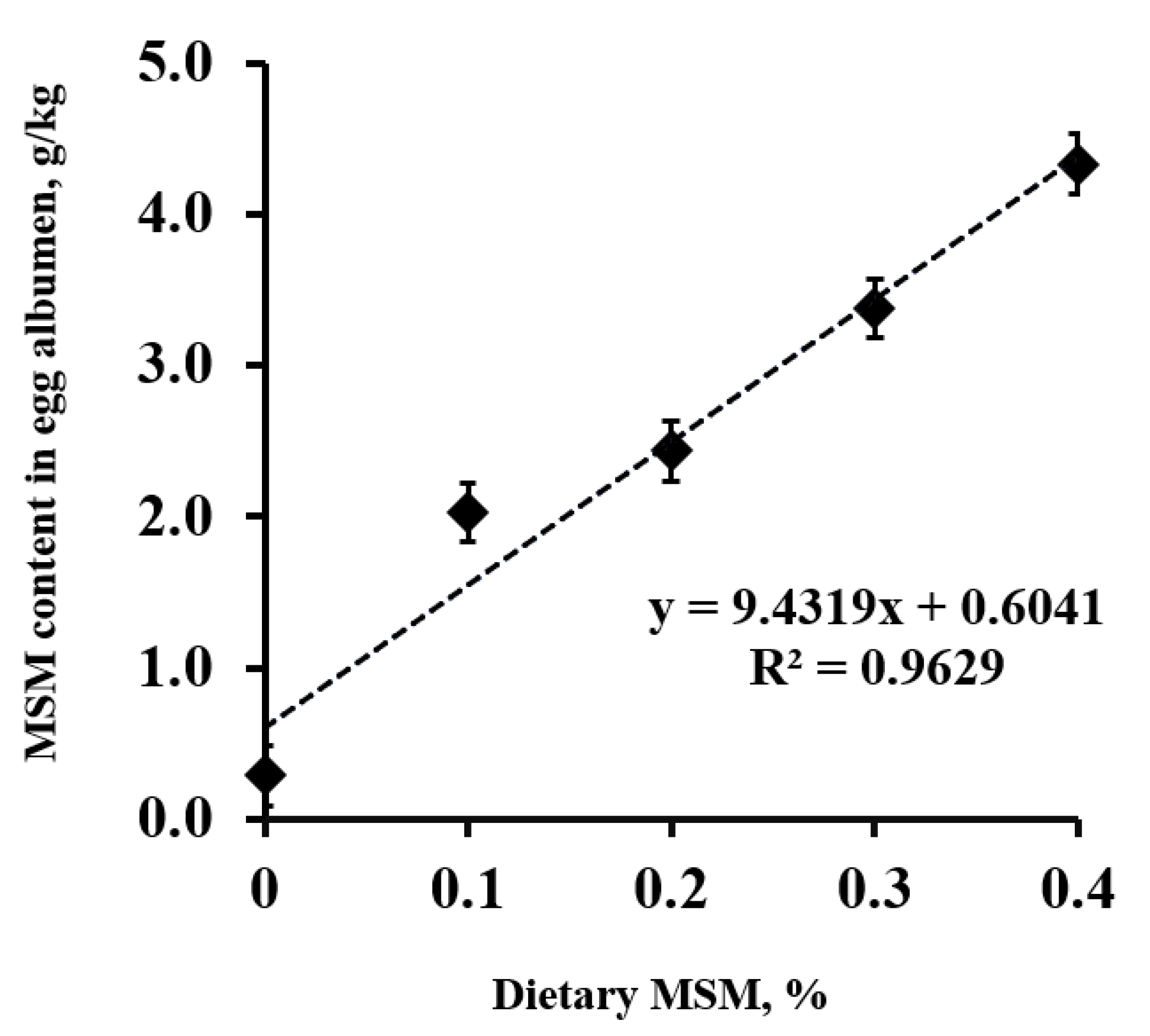
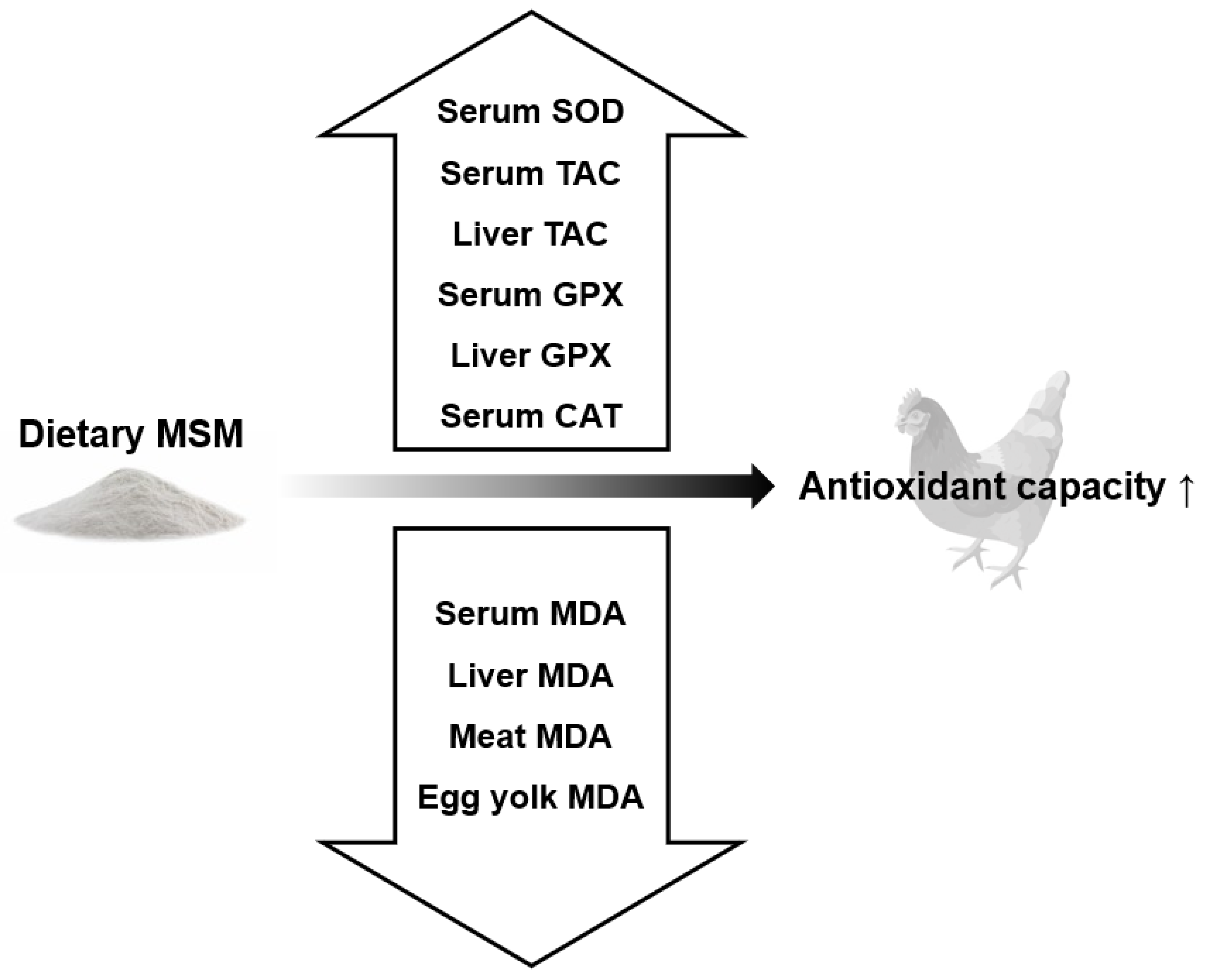
5. Effects of Dietary MSM on Biomarkers of Oxidative Stress and Anti-Oxidative Capacity
6. Effect of Dietary MSM on the Immune Response
7. Effect of Dietary MSM on Liver Function
8. Effect of Dietary MSM on Performance
9. Effect of Dietary MSM on Meat Quality
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, B.; Jha, R. Oxidative stress in the poultry gut: Potential challenges and interventions. Front. Vet. Sci. 2019, 6, 60. [Google Scholar] [CrossRef]
- Ali, A.; Ponnampalam, E.N.; Pushpakumara, G.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. Cinnamon: A natural feed additive for poultry health and production—A review. Animals 2021, 11, 2026. [Google Scholar] [CrossRef]
- Van Hieu, T.; Guntoro, B.; Qui, N.H.; Quyen, N.T.K.; Al Hafiz, F.A. The application of ascorbic acid as a therapeutic feed additive to boost immunity and antioxidant activity of poultry in heat stress environment. Vet. World 2022, 15, 685–693. [Google Scholar] [CrossRef]
- Lee, M.T.; Lin, W.C.; Lee, T.T. Potential crosstalk of oxidative stress and immune response in poultry through phytochemicals—A review. Asian-Australas. J. Anim. Sci. 2019, 32, 309–319. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant defence systems and oxidative stress in poultry biology: An update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef]
- Surai, P.F. Antioxidants in poultry nutrition and reproduction: An update. Antioxidants 2020, 9, 105. [Google Scholar]
- Surai, P.F.; Karadas, F.; Sparks, N.H. The importance of antioxidants in poultry. Zentralbl. Gynakol. 1997, 119, 1–5. [Google Scholar]
- Luna, A.; Lema-Alba, R.C.; Dambolena, J.S.; Zygadlo, J.A.; Labaque, M.C.; Marin, R.H. Thymol as natural antioxidant additive for poultry feed: Oxidative stability improvement. Poult. Sci. 2017, 96, 3214–3220. [Google Scholar] [CrossRef]
- Bacou, E.; Walk, C.; Rider, S.; Litta, G.; Perez-Calvo, E. Dietary oxidative distress: A review of nutritional challenges as models for poultry, swine and fish. Antioxidants 2021, 10, 525. [Google Scholar] [CrossRef]
- Smet, K.; Raes, K.; Huyghebaert, G.; Haak, L.; Arnouts, S.; De Smet, S. Lipid and protein oxidation of broiler meat as influenced by dietary natural antioxidant supplementation. Poult. Sci. 2008, 87, 1682–1688. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. The role of oxidative stress in the pathogenesis of multiple sclerosis: The need for effective antioxidant therapy. J. Neurol. 2004, 251, 261–268. [Google Scholar] [CrossRef]
- Estévez, M. Oxidative damage to poultry: From farm to fork. Poult. Sci. 2015, 94, 1368–1378. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I. Nutritional modulation of the antioxidant capacities in poultry: The case of selenium. Poult. Sci. 2019, 98, 4231–4239. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, G.; Wang, L.; Liu, S.; Tang, J. Effects of the Agaricus bisporus stem residue on performance, nutrients digestibility and antioxidant activity of laying hens and its effects on egg storage. Anim. Biosci. 2021, 34, 256–264. [Google Scholar] [CrossRef]
- Marãnon, G.; Mũoz-Escassi, B.; Manley, W.; García, C.; Cayado, P.; De La Muela, M.S.; Olábarri, B.; Len, R.; Vara, E. The effect of methyl sulphonyl methane supplementation on biomarkers of oxidative stress in sport horses following jumping exercise. Acta Vet. Scand. 2008, 50, 45. [Google Scholar]
- Panda, A.K.; Cherian, G. Role of vitamin E in counteracting oxidative stress in poultry. J. Poult. Sci. 2014, 51, 109–117. [Google Scholar] [CrossRef]
- Soleimani, A.F.; Zulkifli, I.; Omar, A.R.; Raha, A.R. Physiological responses of 3 chicken breeds to acute heat stress. Poult. Sci. 2011, 90, 1435–1440. [Google Scholar] [CrossRef]
- Abdul Rasheed, M.S.; Oelschlager, M.L.; Smith, B.N.; Bauer, L.L.; Whelan, R.A.; Dilger, R.N. Dietary methylsulfonylmethane supplementation and oxidative stress in broiler chickens. Poult. Sci. 2020, 99, 914–925. [Google Scholar]
- Delles, R.M.; Xiong, Y.L.; True, A.D.; Ao, T.; Dawson, K.A. Dietary antioxidant supplementation enhances lipid and protein oxidative stability of chicken broiler meat through promotion of antioxidant enzyme activity. Poult. Sci. 2014, 93, 1561–1570. [Google Scholar] [CrossRef]
- Eid, Y.; Ebeid, T.; Moawad, M.; El-Habbak, M. Reduction of dexamethasone-induced oxidative stress and lipid peroxidation in laying hens by dietary vitamin E supplementation. Emir. J. Food Agric. 2008, 20, 28–40. [Google Scholar] [CrossRef]
- Min, B.; Ahn, D.U. Mechanism of lipid peroxidation in meat and meat products—A review. Food Sci. Biotechnol. 2005, 14, 152–163. [Google Scholar]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Cortinas, L.; Barroeta, A.; Villaverde, C.; Galobart, J.; Guardiola, F.; Baucells, M.D. Influence of the dietary polyunsaturation level on chicken meat quality: Lipid oxidation. Poult. Sci. 2005, 84, 48–55. [Google Scholar] [CrossRef]
- Ren, Y.; Perez, T.I.; Zuidhof, M.J.; Renema, R.A.; Wu, J. Oxidative stability of omega-3 polyunsaturated fatty acids enriched eggs. J. Agric. Food Chem. 2013, 61, 11595–11602. [Google Scholar] [CrossRef]
- Estévez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef]
- Korzeniowska, M.; Króliczewska, B.; Kopeć, W. Effect of dietary selenium on protein and lipid oxidation and the antioxidative potential of selected chicken culinary parts during frozen storage. J. Chem. 2018, 2018, 3492456. [Google Scholar]
- Seifried, H.E.; Anderson, D.E.; Fisher, E.I.; Milner, J.A. A review of the interaction among dietary antioxidants and reactive oxygen species. J. Nutr. Biochem. 2007, 18, 567–579. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Wu, J. Hen egg as an antioxidant food commodity: A review. Nutrients 2015, 7, 8274–8293. [Google Scholar] [CrossRef]
- Fellenberg, M.A.; Speisky, H. Antioxidants: Their effects on broiler oxidative stress and its meat oxidative stability. Worlds. Poult. Sci. J. 2006, 62, 53–70. [Google Scholar] [CrossRef]
- Al-Harthi, M.A. The effect of natural and synthetic antioxidants on performance, egg quality and blood constituents of laying hens grown under high ambient temperature. Ital. J. Anim. Sci. 2014, 13, 444–449. [Google Scholar] [CrossRef]
- Karre, L.; Lopez, K.; Getty, K.J.K. Natural antioxidants in meat and poultry products. Meat Sci. 2013, 94, 220–227. [Google Scholar]
- Pashtetsky, V.; Ostapchuk, P.; Il’Yazov, R.; Zubochenko, D.; Kuevda, T. Use of antioxidants in poultry farming (review). IOP Conf. Ser. Earth Environ. Sci. 2019, 341, 012042. [Google Scholar] [CrossRef]
- Fotina, A.A.; Fisinin, V.I.; Surai, P.F. Recent developments in usage of natural antioxidants to improve chicken meat production and quality. Bulg. J. Agric. Sci. 2013, 19, 889–896. [Google Scholar]
- Londok, J.J.M.R.; Manalu, W.; Wiryawan, K.G. Sumiati Growth performance, carcass characteristics and fatty acids profile of broilers supplemented with lauric acid and natural antioxidant from Areca vestiaria giseke. Pak. J. Nutr. 2017, 16, 719–730. [Google Scholar] [CrossRef]
- Surai, P.F. Effect of selenium and vitamin E content of the maternal diet on the antioxidant system of the yolk and the developing chick. Br. Poult. Sci. 2000, 41, 235–243. [Google Scholar]
- Vlaicu, P.A.; Panaite, T.D.; Turcu, R.P. Enriching laying hens eggs by feeding diets with different fatty acid composition and antioxidants. Sci. Rep. 2021, 11, 20707. [Google Scholar] [CrossRef]
- Butawan, M.; Benjamin, R.L.; Bloomer, R.J. Methylsulfonylmethane: Applications and safety of a novel dietary supplement. Nutrients 2017, 9, 290. [Google Scholar]
- Butawan, M.; Benjamin, R.L.; Bloomer, R.J. Methylsulfonylmethane as an antioxidant and its use in pathology. Pathology 2020, 27, 277–288. [Google Scholar] [CrossRef]
- Lawrence, R.M. Methylsulfonylmethane (MSM): A double-blind study of its use in degenerative arthritis. Int. J. Aesthetic Anti-Ageing Med. 1998, 1, 50. [Google Scholar]
- Jiao, Y.; Park, J.H.; Kim, Y.M.; Kim, I.H. Effects of dietary methyl sulfonyl methane (MSM) supplementation on growth performance, nutrient digestibility, meat quality, excreta microbiota, excreta gas emission, and blood profiles in broilers. Poult. Sci. 2017, 96, 2168–2175. [Google Scholar]
- Pearson, T.W.; Dawson, H.J.; Lackey, H.B. Natural occurring levels of dimethyl sulfoxide in selected fruits, vegetables, grains, and beverages. J. Agric. Food Chem. 1981, 29, 1089–1091. [Google Scholar] [CrossRef]
- Abdul Rasheed, M.S.; Oelschlager, M.L.; Smith, B.N.; Bauer, L.L.; Whelan, R.A.; Dilger, R.N. Toxicity and tissue distribution of methylsulfonylmethane following oral gavage in broilers. Poult. Sci. 2019, 98, 4972–4981. [Google Scholar] [CrossRef]
- Kim, Y.-B.; Lee, S.-H.; Kim, D.-H.; Lee, H.; Jeon, Y.; Lee, S.; Lee, K. Incorporation of dietary methyl sulfonyl methane into the egg albumens of laying hens. Antioxidants 2022, 11, 517. [Google Scholar]
- Kim, Y.-B.; Lee, S.-H.; Kim, D.-H.; Lee, H.-G.; Choi, Y.; Lee, S.-D.; Lee, K.-W. Effects of dietary organic and inorganic sulfur on laying performance, egg quality, ileal morphology, and antioxidant capacity in laying hens. Animals 2022, 12, 87. [Google Scholar]
- Sievert, S.M.; Kiene, R.P.; Schulz-Vogt, H.N. The sulfur cycle. Oceanography 2007, 20, 117–123. [Google Scholar] [CrossRef]
- Morton, J.I.; Siegel, B.V. Effects of oral dimethyl sulfoxide and dimethyl sulfone on murine autoimmune lymphoproliferative disease. Proc. Soc. Exp. Biol. Med. 1986, 183, 227–230. [Google Scholar] [CrossRef]
- Magnuson, B.A.; Appleton, J.; Ryan, B.; Matulka, R.A. Oral developmental toxicity study of methylsulfonylmethane in rats. Food Chem. Toxicol. 2007, 45, 977–984. [Google Scholar] [CrossRef]
- Horváth, K.; Noker, P.E.; Somfai-Relle, S.; Glávits, R.; Financsek, I.; Schauss, A.G. Toxicity of methylsulfonylmethane in rats. Food Chem. Toxicol. 2002, 40, 1459–1462. [Google Scholar] [CrossRef]
- Borzelleca, J.F.; Sipes, I.G.; Wallace, K.B. Dossier in Support of the Generally Recognized as Safe (Gras) Status of Optimsm (Methylsulfonylmethane; Msm) as a Food Ingredient; Food and Drug Administration: Vero Beach, FL, USA, 2007. [Google Scholar]
- Wong, T.; Bloomer, R.J.; Benjamin, R.L.; Buddington, R.K. Small intestinal absorption of methylsulfonylmethane (MSM) and accumulation of the sulfur moiety in selected tissues of mice. Nutrients 2018, 10, 19. [Google Scholar] [CrossRef]
- Otsuki, S.; Qian, W.; Ishihara, A.; Kabe, T. Elucidation of dimethylsulfone metabolism in rat using a 35S radioisotope tracer method. Nutr. Res. 2002, 22, 313–322. [Google Scholar] [CrossRef]
- Lin, A.; Nguy, C.H.; Shic, F.; Ross, B.D. Accumulation of methylsulfonylmethane in the human brain: Identification by multinuclear magnetic resonance spectroscopy. Toxicol. Lett. 2001, 123, 169–177. [Google Scholar]
- Magnuson, B.A.; Appleton, J.; Ames, G.B. Pharmacokinetics and distribution of [35S]methylsulfonylmethane following oral administration to rats. J. Agric. Food Chem. 2007, 55, 1033–1038. [Google Scholar] [CrossRef]
- Richmond, V.L. Incorporation of methylsulfonylmethane sulfur into guinea pig serum proteins. Life Sci. 1986, 39, 263–268. [Google Scholar]
- Parcell, S. Sulfur in human nutrition and applications in medicine. Altern. Med. Rev. 2002, 7, 22–44. [Google Scholar]
- Atmaca, G. Antioxidant effects of sulfur-containing amino acids. Yonsei Med. J. 2004, 45, 776–788. [Google Scholar] [CrossRef]
- Kerr, B.J.; Weber, T.E.; Ziemer, C.J.; Spence, C.; Cotta, M.A.; Whitehead, T.R. Effect of dietary inorganic sulfur level on growth performance, fecal composition, and measures of inflammation and sulfate-reducing bacteria in the intestine of growing pigs. J. Anim. Sci. 2011, 89, 426–437. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. Sulfur containing amino acids and human disease. Biomed. Pharmacother. 2004, 58, 47–55. [Google Scholar] [CrossRef]
- Usha, P.R.; Naidu, M.U.R. Randomised, double-blind, parallel, placebo-controlled study of oral glucosamine, methylsulfonylmethane and their combination in osteoarthritis. Clin. Drug Investig. 2004, 24, 353–363. [Google Scholar] [CrossRef]
- Nakhostin-Roohi, B.; Barmaki, S.; Khoshkhahesh, F.; Bohlooli, S. Effect of chronic supplementation with methylsulfonylmethane on oxidative stress following acute exercise in untrained healthy men. J. Pharm. Pharmacol. 2011, 63, 1290–1294. [Google Scholar] [CrossRef]
- Dunn, J.D.; Alvarez, L.A.J.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, D.H.; Lim, H.; Baek, D.Y.; Shin, H.K.; Kim, J.K. The anti-inflammatory effects of methylsulfonylmethane on lipopolysaccharide-induced inflammatory responses in murine macrophages. Biol. Pharm. Bull. 2009, 32, 651–656. [Google Scholar]
- Hwang, J.W.; Cheong, S.H.; Kim, Y.S.; Lee, J.W.; You, B.I.; Moon, S.H.; Jeon, B.T.; Park, P.J. Effects of dietary supplementation of oriental herbal medicine residue and methyl sulfonyl methane on the growth performance and meat quality of ducks. Anim. Prod. Sci. 2017, 57, 948–957. [Google Scholar]
- Yan, H.L.; Cao, S.C.; Hu, Y.D.; Zhang, H.F.; Liu, J.B. Effects of methylsulfonylmethane on growth performance, immunity, antioxidant capacity, and meat quality in Pekin ducks. Poult. Sci. 2020, 99, 1069–1074. [Google Scholar]
- Amirshahrokhi, K.; Bohlooli, S. Effect of methylsulfonylmethane on paraquat-induced acute lung and liver injury in mice. Inflammation 2013, 36, 1111–1121. [Google Scholar]
- Kamel, R.; El Morsy, E.M. Hepatoprotective effect of methylsulfonylmethane against carbon tetrachloride-induced acute liver injury in rats. Arch. Pharm. Res. 2013, 36, 1140–1148. [Google Scholar]
- Nakhostin-Roohi, B.; Niknam, Z.; Vaezi, N.; Mohammadi, S.; Bohlooli, S. Effect of single dose administration of methylsulfonylmethane on oxidative stress following acute exhaustive exercise. Iran. J. Pharm. Res. 2013, 12, 845–853. [Google Scholar]
- Amirshahrokhi, K.; Bohlooli, S.; Chinifroush, M.M. The effect of methylsulfonylmethane on the experimental colitis in the rat. Toxicol. Appl. Pharmacol. 2011, 253, 197–202. [Google Scholar] [CrossRef]
- Bohlooli, S.; Mohammadi, S.; Amirshahrokhi, K.; Mirzanejad-Asl, H.; Yosefi, M.; Mohammadi-Nei, A.; Chinifroush, M.M. Effect of methylsulfonylmethane pretreatment on acetaminophen induced hepatotoxicity in rats. Iran. J. Basic Med. Sci. 2013, 16, 896–900. [Google Scholar]
- Kim, Y.B.; Lee, S.H.; Kim, D.H.; Lee, K.W. Effects of dietary methyl sulfonyl methane and selenium on laying performance, egg quality, gut health indicators, and antioxidant capacity of laying hens. Anim. Biosci. 2022, 35, 1566–1574. [Google Scholar]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Schwarz, B.C.; van den Hoven, R.; Schwendenwein, I. Diagnostic value of the neutrophil myeloperoxidase index in horses with systemic inflammation. Vet. J. 2012, 191, 72–78. [Google Scholar] [CrossRef]
- Lindblom, S.C.; Gabler, N.K.; Bobeck, E.A.; Kerr, B.J. Oil source and peroxidation status interactively affect growth performance and oxidative status in broilers from 4 to 25 d of age. Poult. Sci. 2019, 98, 1749–1761. [Google Scholar] [CrossRef]
- Lopes, A.R.; Trübenbach, K.; Teixeira, T.; Lopes, V.M.; Pires, V.; Baptista, M.; Repolho, T.; Calado, R.; Diniz, M.; Rosa, R. Oxidative stress in deep scattering layers: Heat shock response and antioxidant enzymes activities of myctophid fishes thriving in oxygen minimum zones. Deep. Res. Part I Oceanogr. Res. Pap. 2013, 82, 10–16. [Google Scholar] [CrossRef]
- Abdul Rasheed, M.S.; Tiwari, U.P.; Jespersen, J.C.; Bauer, L.L.; Dilger, R.N. Effects of methylsulfonylmethane and neutralizing anti–IL-10 antibody supplementation during a mild Eimeria challenge infection in broiler chickens. Poult. Sci. 2020, 99, 6559–6568. [Google Scholar] [CrossRef]
- Lim, C.I.; Choe, H.S.; Kang, C.; Lee, B.K.; Ryu, K.S. Effects of dietary organic sulfur on performance, egg quality and cell-mediated immune response of laying hens. Korean J. Poult. Sci. 2018, 45, 97–107. [Google Scholar]
- Gabay, C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006, 8 (Suppl. 2), S3. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, H.J.; Ha, H.L.; Park, Y.H.; Kwon, T.H.; Jung, M.R.; Moon, H.B.; Cho, E.S.; Son, H.Y.; Yu, D.Y. Methylsulfonylmethane suppresses hepatic tumor development through activation of apoptosis. World J. Hepatol. 2014, 6, 98. [Google Scholar] [CrossRef]
- Park, S.; Ahn, I.S.; Hong, S.M.; Kim, D.S.; Kwon, D.Y.; Yang, H.J. The effects of the supplementation of Opuntia humifusa water extracts and methyl sulfonyl methane on the laying productivity, egg quality and sensory characteristics. J. Korean Soc. Food Sci. Nutr. 2010, 39, 294–300. [Google Scholar] [CrossRef]
- Liu, H.-F.; Zhou, A.-G. Effects of plant extracts, cysteamine and methylsulfonylmethane on productive performance and slaughter characteristics in meat ducks. Nat. Prod. Res. Dev. 2008, 6880, 302–306. [Google Scholar]
- Cho, J.H.; Min, B.J.; Kwon, O.S.; Shon, K.S.; Jin, Y.G.; Kim, H.J.; Kim, I.H. Effects of MSM (methyl sulfonyl methane) supplementation on growth performance and digestibility of Ca and N in pigs. J. Korean Soc. Food Sci. Nutr. 2005, 34, 361–365. [Google Scholar]
- Joo, S.T.; Kim, G.D.; Hwang, Y.H.; Ryu, Y.C. Control of fresh meat quality through manipulation of muscle fiber characteristics. Meat Sci. 2013, 95, 828–836. [Google Scholar] [CrossRef]
- Ismail, I.; Joo, S.T. Poultry meat quality in relation to muscle growth and muscle fiber characteristics. Korean J. Food Sci. Anim. Resour. 2017, 37, 873–883. [Google Scholar] [CrossRef]
- Muhlisin; Kim, D.S.; Song, Y.R.; Kim, H.R.; Kwon, H.J.; An, B.K.; Kang, C.W.; Kim, H.K.; Lee, S.K. Comparison of meat characteristics between Korean native duck and imported commercial duck raised under identical rearing and feeding condition. Korean J. Food Sci. Anim. Resour. 2013, 33, 89–95. [Google Scholar] [CrossRef]
- Shon, J.; Chin, K.B. Effect of whey protein coating on quality attributes of low-fat, aerobically packaged sausage during refrigerated storage. J. Food Sci. 2008, 73, C469–C475. [Google Scholar] [CrossRef]
- Suryanti, U.; Bintoro, V.P.; Atmomarsono, U.; Pramono, Y.B.; Legowo, A.M. Antioxidant activity of indonesian endogenous duck meat marinated in ginger (Zingiber officinale Roscoe) extract. Int. J. Poult. Sci. 2014, 13, 102. [Google Scholar] [CrossRef]
- Fernández-López, J.; Zhi, N.; Aleson-Carbonell, L.; Pérez-Alvarez, J.A.; Kuri, V. Antioxidant and antibacterial activities of natural extracts: Application in beef meatballs. Meat Sci. 2005, 69, 371–380. [Google Scholar] [CrossRef]
- Yin, M.C.; Faustman, C. Influence of temperature, pH, and phospholipid composition upon the stability of myoglobin and phospholipid: A liposome model. J. Agric. Food Chem. 1993, 41, 853–857. [Google Scholar] [CrossRef]
- Fletcher, D.L. Broiler breast meat color variation, pH, and texture. Poult. Sci. 1999, 78, 1323–1327. [Google Scholar] [CrossRef]
- Sayre, R.N.; Kiernat, B.; Briskey, E.J. Processing characteristics of porcine muscle related to pH and temperature during rigor mortis development and to gross morphology 24 hr post-mortem. J. Food Sci. 1964, 29, 175–181. [Google Scholar] [CrossRef]
- Lonergan, E.H.; Zhang, W.; Lonergan, S.M. Biochemistry of postmortem muscle—Lessons on mechanisms of meat tenderization. Meat Sci. 2010, 86, 184–195. [Google Scholar] [CrossRef]
- Saedi, S.; Shokri, M.; Rhim, J.W. Antimicrobial activity of sulfur nanoparticles: Effect of preparation methods. Arab. J. Chem. 2020, 13, 6580–6588. [Google Scholar] [CrossRef]
| Species | Sample | Level of Inclusion | Results | Ref. |
|---|---|---|---|---|
| Pekin female ducklings | Serum | 0.3% | No effects on IgG; increased IL-2 by 11.4%; increased IL-6 by 15.4%; decreased IFN-γ by 14.1%; decreased TNF-α by 12.8% | [65] |
| Lohmann brown laying hens | Serum | 0.4% | Increased IL-2 by 58.2% | [77] |
| Species | Sample | Level of Inclusion | Results | Ref. |
|---|---|---|---|---|
| Ross 308 male broiler | Serum | 0.2% | ALT increased by 22%; No effects on AST | [41] |
| Ross 308 male broiler | Serum | 0.15% | AST decreased by 46.4%; CPK decreased by 48.7%; GLDH decreased by 54.9% | [43] |
| Cherry Valley male ducklings | Serum | 0.03% | AST and ALT remained unchanged | [64] |
| Lohmann brown laying hens | Serum | 0.4% | AST decreased by 10.6% | [77] |
| Adult male Swiss Wistar mice | Plasma | 0.05% | ALT decreased by 35.5%; ALP decreased by 62%; GGT decreased by 303.8% | [66] |
| Female Sprague-Dawley rats | Serum | 0.04% | ALT decreased by 46%; AST decreased by 23.5% | [67] |
| Transgenic male mice | Plasma | 0.01% | ALT and AST decreased | [79] |
| Male Sprague-Dawley rats | Serum | 0.01% | ALT and AST decreased | [70] |
| Species | House | Level of Inclusion | Experiment Period | Results | Ref. |
|---|---|---|---|---|---|
| Ross 308 male broiler | Battery cages | 0.05% | 1 to 21 D of age | No effects on BW and BWG | [19] |
| Ross 308 male broiler | Battery cages | 0.15% | 1 to 21 D of age | No effects on BWG, FI, and FCR | [43] |
| Ross 308 male broiler | Battery cages | 0.2% | 1 to 29 D of age | BWG increased by 2.8%, and FCR decreased by 2.6% compared to the control group for the whole period of the trial (days 1–29) | [41] |
| Cherry Valley male ducklings | Pens | 0.03% | 21 to 62 D of age | No effects on BWG, FI, and FCR | [64] |
| Pekin female ducklings | Battery cages | 0.3% | 1 to 42 D of age | No effects on BWG, FI, and FCR (days 1–21); BWG increased by 3.3% compared to the control group for the whole period of the trial (days 1–42) | [65] |
| Lohmann Brown laying hen | Battery cages | 0.4% | 31-wk-old (experiment lasted 24 weeks) | No effects on FI, FCR, and egg weight; egg production increased by 3.7% compared to the control group (weeks 17–24) | [77] |
| Lohmann Brown laying hen | Battery cages | 0.1% | 35-wk-old (experiment lasted 5 weeks) | No effects on egg production, egg weight, and FCR | [80] |
| Lohmann Brown-Lite laying hen | Battery cages | 0.2% | 73-wk-old (experiment lasted 12 weeks) | No effects on FI, FCR, and egg production | [71] |
| Lohmann Brown-Lite laying hen | Battery cages | 0.1–0.4% | 73-wk-old (experiment lasted 12 weeks) | No effects on FI, FCR, egg production, egg weight, and egg mass | [44] |
| Species | Sample | Level of Inclusion | Collection Day (for the Feeding Period) | Results | Ref. |
|---|---|---|---|---|---|
| Ross 308 male broiler | Breast meat | 0.2% | Day 29 | No effect on pH; redness increased by 3.1%; drip loss decreased by 4.1% | [41] |
| Cherry Valley male ducklings | Breast meat | 0.03% | Day 42 | No effects on the cooking loss and pH; PML decreased by 15.7%; WHC increased by 5.3%; redness increased by 9.5% | [64] |
| Pekin female ducklings | Breast meat | 0.3% | Day 42 | pH increased by 2.5%; WHC increased by 10.4%; redness increased by 38.4%; drip loss decreased by 5.1% | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-B.; Lee, K.-W. Role of Dietary Methyl Sulfonyl Methane in Poultry. Animals 2023, 13, 351. https://doi.org/10.3390/ani13030351
Kim Y-B, Lee K-W. Role of Dietary Methyl Sulfonyl Methane in Poultry. Animals. 2023; 13(3):351. https://doi.org/10.3390/ani13030351
Chicago/Turabian StyleKim, Yoo-Bhin, and Kyung-Woo Lee. 2023. "Role of Dietary Methyl Sulfonyl Methane in Poultry" Animals 13, no. 3: 351. https://doi.org/10.3390/ani13030351
APA StyleKim, Y.-B., & Lee, K.-W. (2023). Role of Dietary Methyl Sulfonyl Methane in Poultry. Animals, 13(3), 351. https://doi.org/10.3390/ani13030351






