Comparison of the Microbiome-Metabolome Response to Copper Sulfate and Copper Glycinate in Growing Pigs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Experimental Design
2.2. Sample Collections and Preparations
2.3. Chemical Analysis of Diets and Feces
2.4. Measurements of Blood Physiological and Biochemical Indices
2.5. Pyrosequencing Analysis of Fecal Microbiome
2.6. Liquid Chromatograph–Mass Spectrometer (LC–MS) Metabolomics Analysis
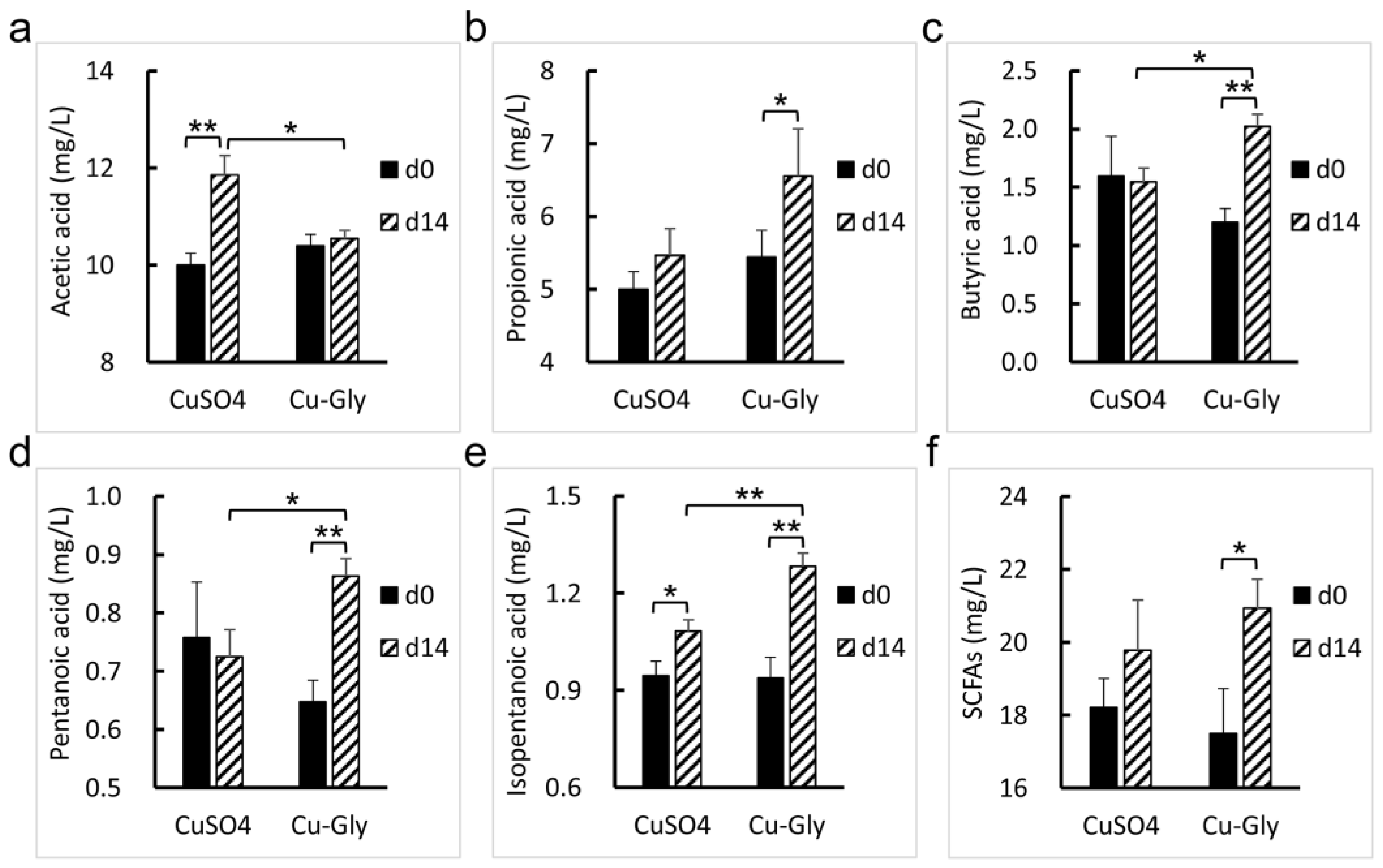
2.7. Determination of Short-Chain Fatty Acids (SCFAs)
2.8. Statistical Analysis
3. Results
3.1. Growth Performance, Digestibility, and Fecal Microelement Contents
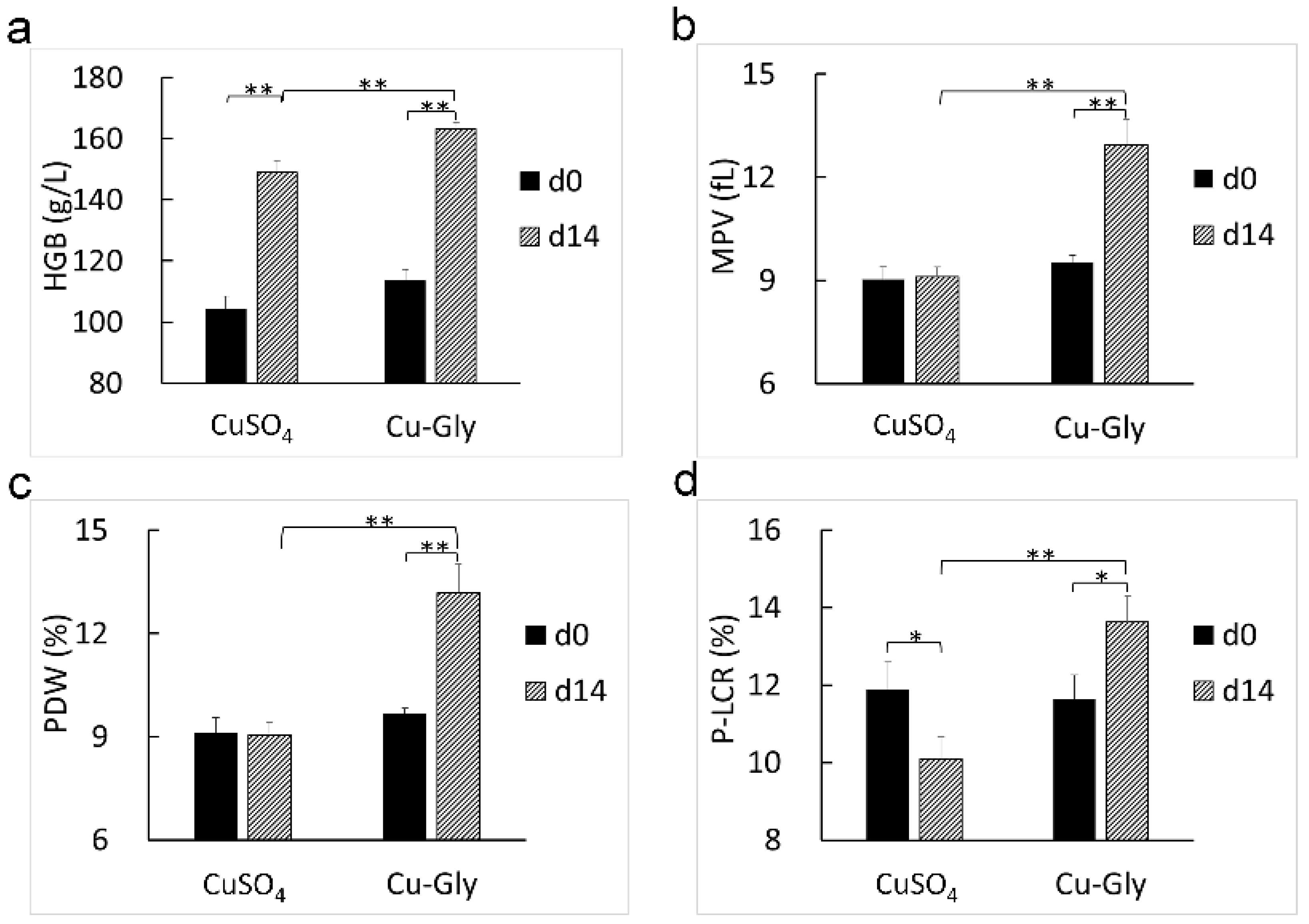
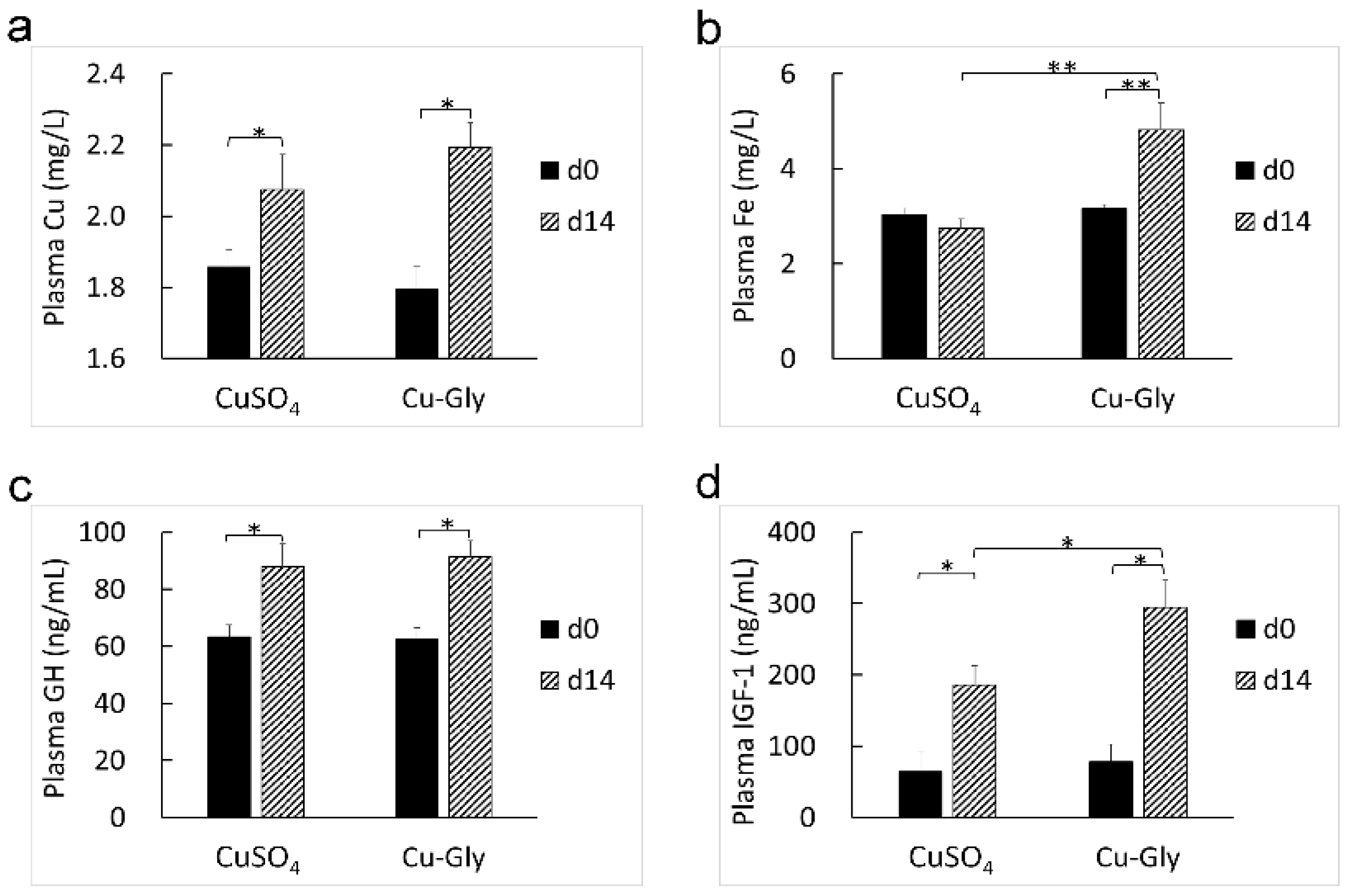
3.2. Hematology and Plasma Indicators
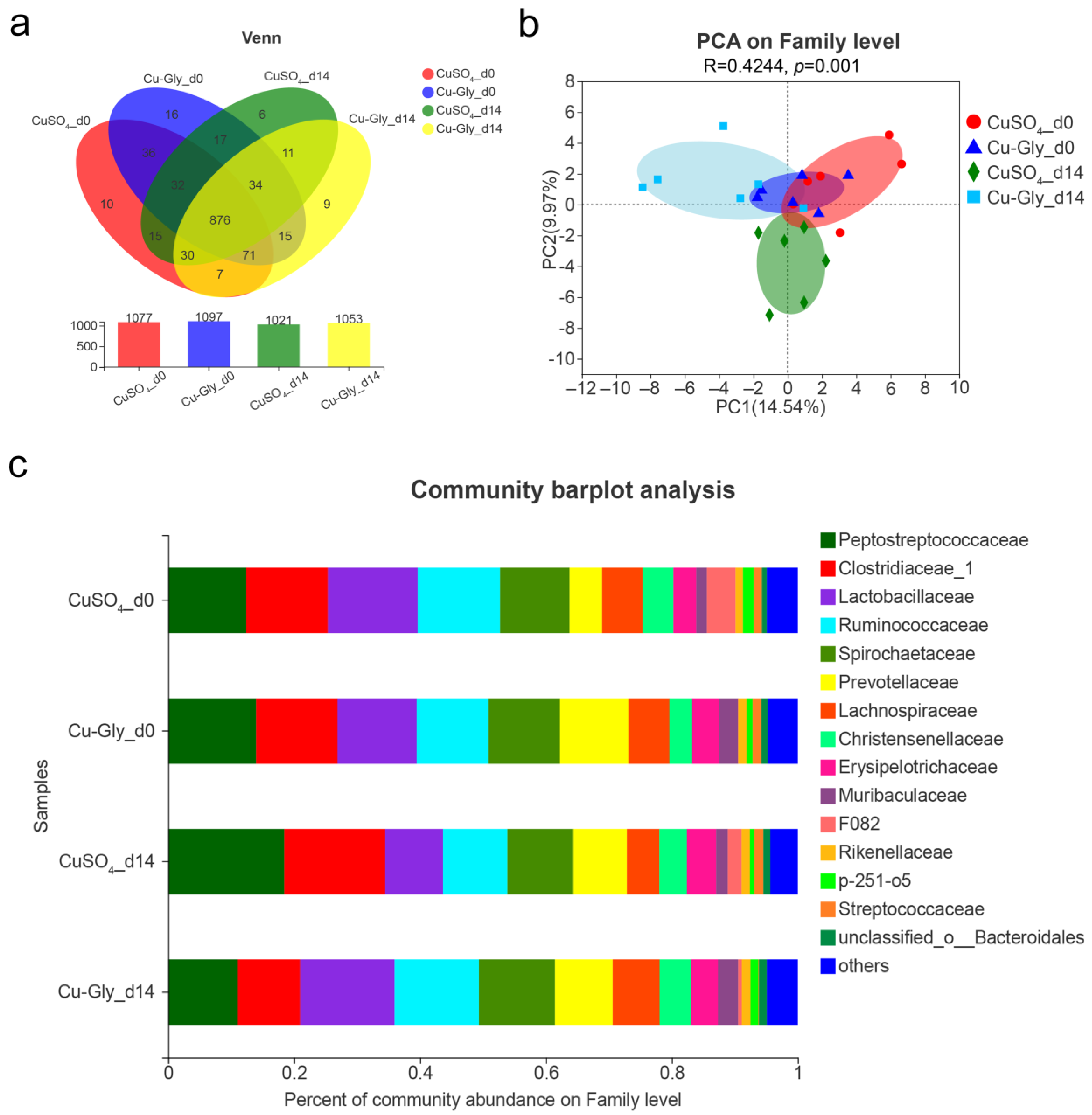
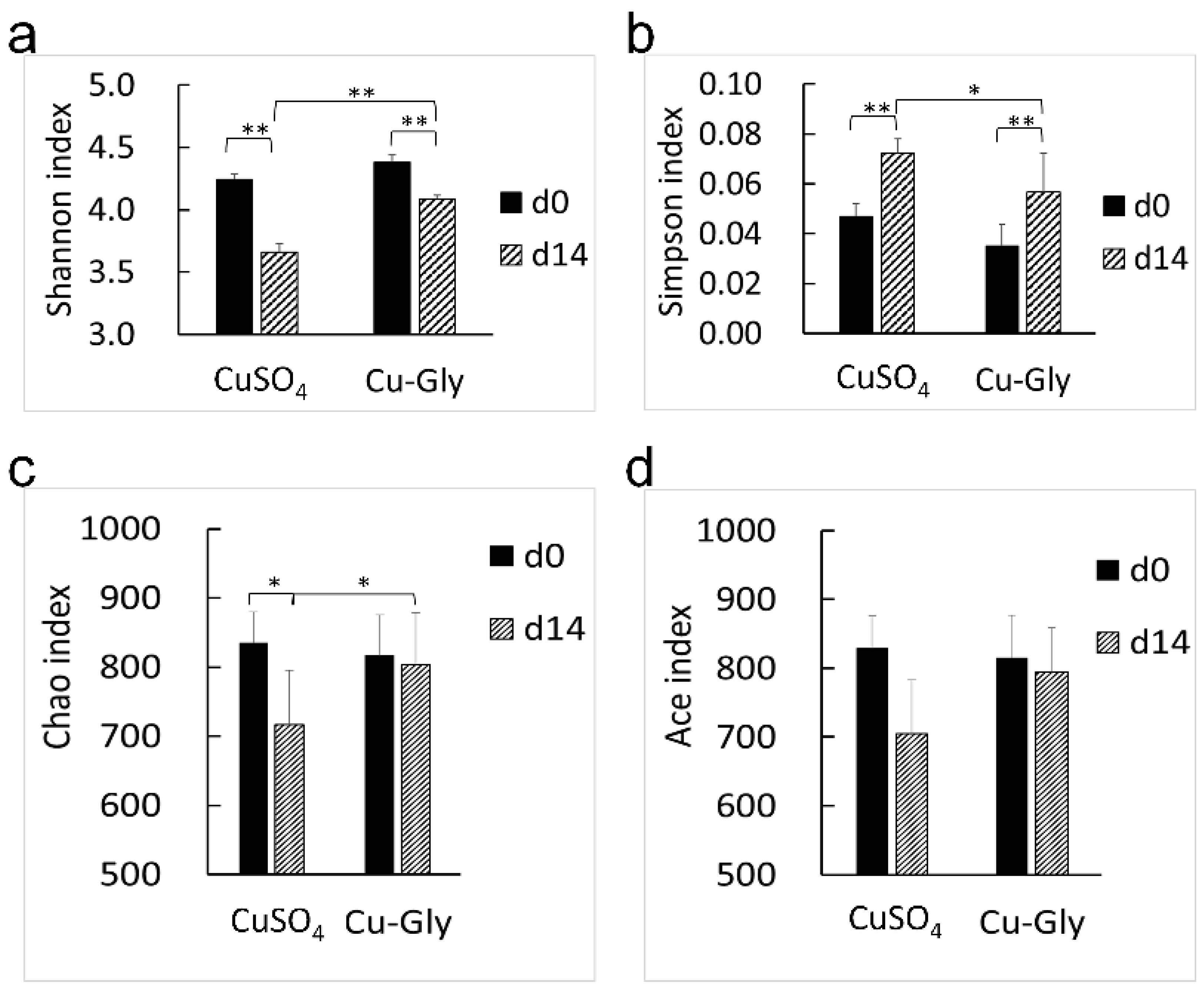
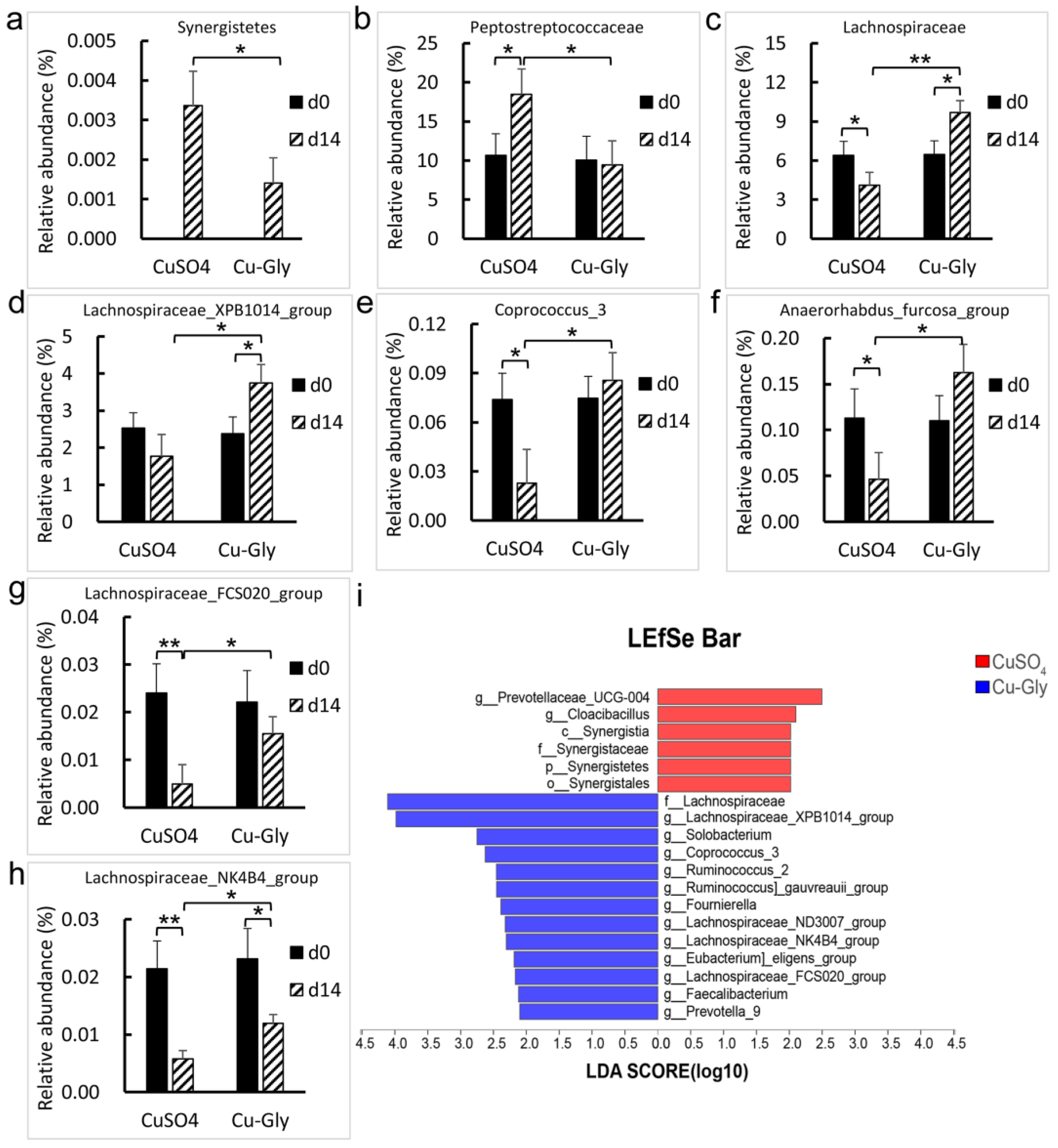
3.3. Fecal Microbiome
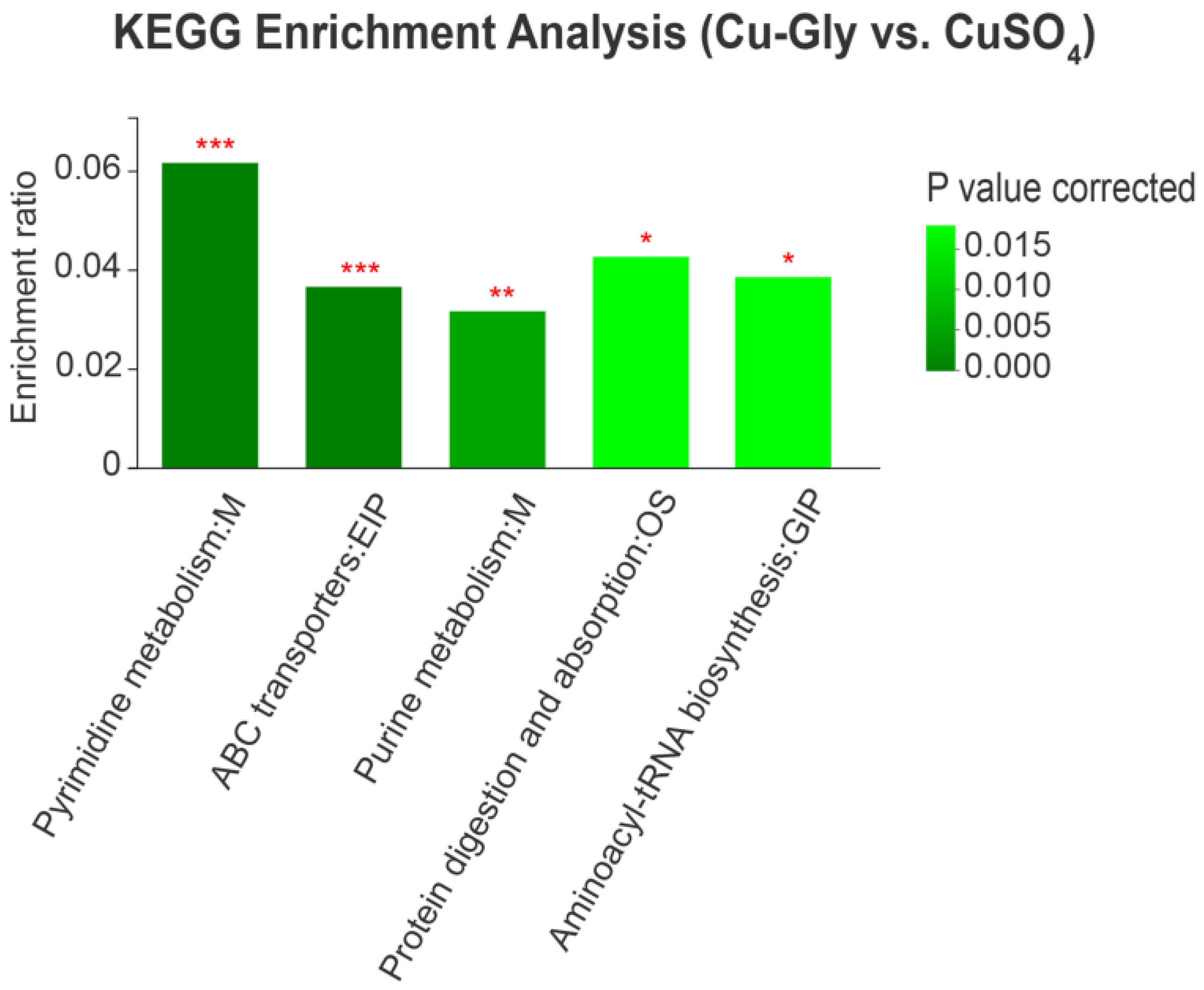
3.4. Metabolomic Profiles
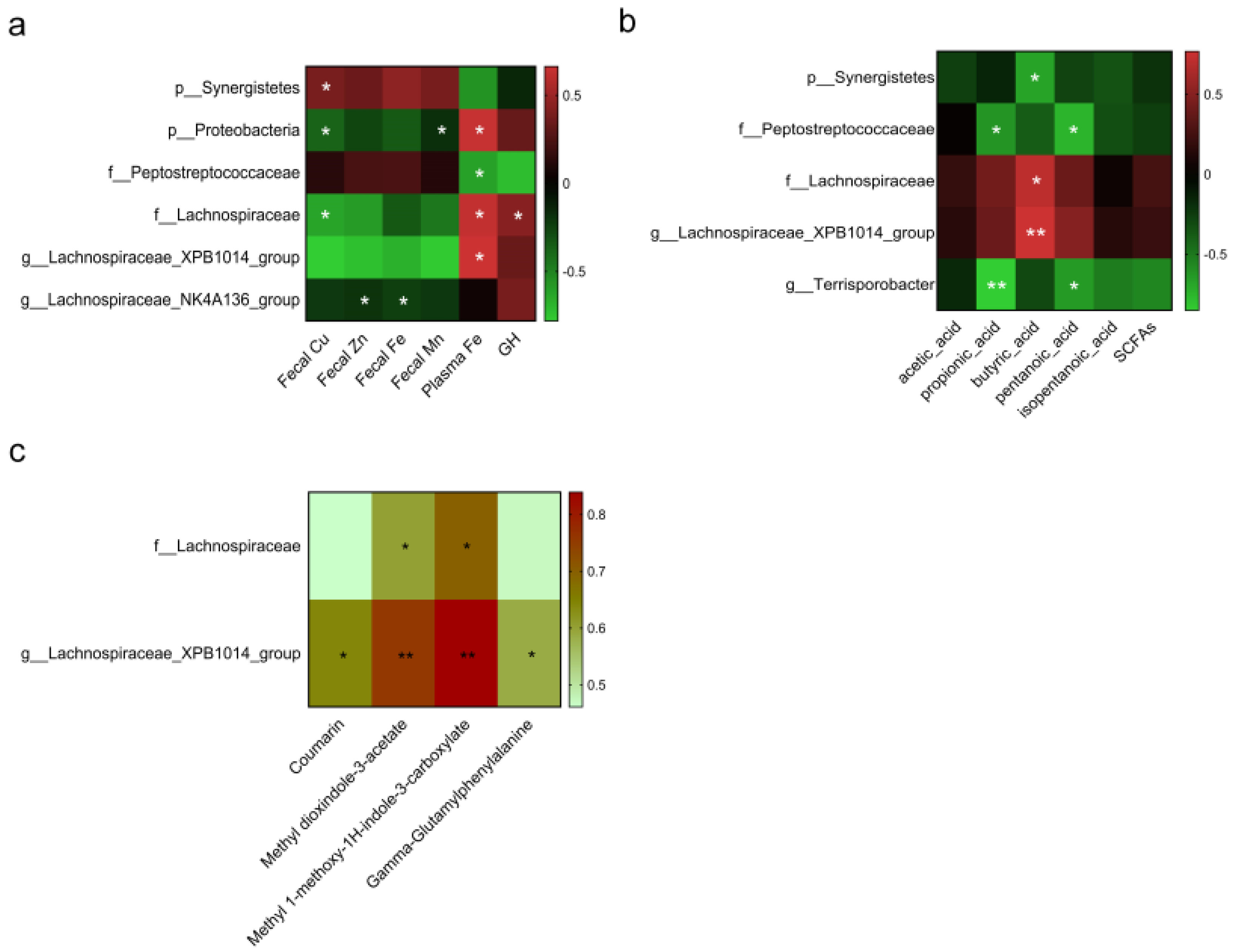
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jończy, A.; Mazgaj, R.; Starzyński, R.R.; Poznański, P.; Szudzik, M.; Smuda, E.; Kamyczek, M.; Lipiński, P. Relationship between dowregulation of copper-related genes and decreased ferroportin protein level in the duodenum of iron-deficient piglets. Nutrients 2020, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.G.; Dove, C.R. Effect of dietary copper and fat on nutrient utilization, digestive enzyme activities, and tissue mineral levels in weanling pigs. J. Anim. Sci. 1996, 74, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Namkung, H.; Gong, J.; Yu, H.; De Lange, C.F.M. Effect of pharmacological intakes of zinc and copper on growth performance, circulating cytokines and gut microbiota of newly weaned piglets challenged with coliform lipopolysaccharides. Can. J. Anim. Sci. 2006, 86, 511–522. [Google Scholar] [CrossRef]
- Coffey, R.D.; Cromwell, G.L.; Monegue, H.J. Efficacy of a copper-lysine complex as a growth promotant for weanling pigs. J. Anim. Sci. 1994, 72, 2880–2886. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Ma, W.Q.; Gu, Z.L.; Wang, Y.Z.; Liu, J.X. Effects of dietary copper (II) sulfate and copper proteinate on performance and blood indexes of copper status in growing pigs. Biol. Trace Elem. Res. 2007, 120, 171–178. [Google Scholar] [CrossRef]
- A Apgar, G.; Kornegay, E.T. Mineral balance of finishing pigs fed copper sulfate or a copper-lysine complex at growth-stimulating levels. J. Anim. Sci. 1996, 74, 1594–1600. [Google Scholar] [CrossRef]
- Gonzalez-Esquerra, R.; Araujo, R.B.; Haese, D.; Kill, J.L.; Cunha, A.; Monzani, P.S.; Lima, C.G. Effect of dietary copper sources on performance, gastric ghrelin-RNA expression, and growth hormone concentrations in serum in piglets. J. Anim. Sci. 2019, 97, 4242–4247. [Google Scholar] [CrossRef]
- Willing, B.P.; Van Kessel, A.G. Intestinal microbiota differentially affect brush border enzyme activity and gene expression in the neonatal gnotobiotic pig. J. Anim. Physiol. Anim. Nutr. 2009, 93, 586–595. [Google Scholar] [CrossRef]
- Heiss, C.N.; Olofsson, L.E. Gut microbiota-dependent modulation of energy metabolism. J. Innate Immun. 2018, 10, 163–171. [Google Scholar] [CrossRef]
- Ma, Y.; Han, X.; Fang, J.; Jiang, H. Role of dietary amino acids and microbial metabolites in the regulation of pig intestinal health. Anim. Nutr. 2022, 9, 1–6. [Google Scholar] [CrossRef]
- Zhang, F.; Zheng, W.; Xue, Y.; Yao, W. Suhuai suckling piglet hindgut microbiome-metabolome responses to different dietary copper levels. Appl. Microbiol. Biotechnol. 2019, 103, 853–868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, J.; Dong, Z.; Li, G.; Wang, J.; Li, Y.; Wan, D.; Yang, H.; Yin, Y. Effect of dietary copper on intestinal microbiota and antimicrobial resistance profiles of Escherichia coli in weaned piglets. Front. Microbiol. 2019, 10, 2808. [Google Scholar] [CrossRef] [PubMed]
- Crofts, A.A.; Poly, F.M.; Ewing, C.P.; Kuroiwa, J.M.; Rimmer, J.E.; Harro, C.; Sack, D.; Talaat, K.R.; Porter, C.K.; Gutierrez, R.L.; et al. Campylobacter jejuni transcriptional and genetic adaptation during human infection. Nat. Microbiol. 2018, 3, 494–502. [Google Scholar] [CrossRef]
- Becker, K.W.; Skaar, E.P. Metal limitation and toxicity at the interface between host and pathogen. FEMS Microbiol. Rev. 2014, 38, 1235–1249. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Li, R.; Piao, X.; Lin, G.; He, P. Different copper sources and levels affect growth performance, copper content, carcass characteristics, intestinal microorganism and metabolism of finishing pigs. Anim. Nutr. 2021, 8, 321–330. [Google Scholar] [CrossRef]
- Pan, X.; Gong, D.; Nguyen, D.N.; Zhang, X.; Hu, Q.; Lu, H.; Fredholm, M.; Sangild, P.T.; Gao, F. Early microbial colonization affects DNA methylation of genes related to intestinal immunity and metabolism in preterm pigs. DNA Res. 2018, 25, 287–296. [Google Scholar] [CrossRef]
- Heyer, C.M.E.; Schmucker, S.; Burbach, K.; Weiss, E.; Eklund, M.; Aumiller, T.; Capezzone, F.; Steuber, J.; Rodehutscord, M.; Hoelzle, L.E.; et al. Phytate degradation, intestinal microbiota, microbial metabolites and immune values are changed in growing pigs fed diets with varying calcium–phosphorus concentration and fermentable substrates. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1185–1197. [Google Scholar] [CrossRef]
- Pajarillo, E.A.B.; Lee, E.; Kang, D.-K. Trace metals and animal health: Interplay of the gut microbiota with iron, manganese, zinc, and copper. Anim. Nutr. 2021, 7, 750–761. [Google Scholar] [CrossRef]
- Prawirodigdo, S.; Gannon, N.J.; Leury, B.J.; Dunshea, F.R. Acid-insoluble ash is a better indigestible marker than chromic oxide to measure apparent total tract digestibility in pigs. Anim. Nutr. 2021, 7, 64–71. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, N.; Zhao, H.; Yuan, H.; Xia, D.; Lei, H. The Microbiome–metabolome response in the colon of piglets under the status of weaning stress. Front. Microbiol. 2020, 11, 2055. [Google Scholar] [CrossRef]
- A Apgar, G.; Kornegay, E.T.; Lindemann, M.D.; Notter, D.R. Evaluation of copper sulfate and a copper lysine complex as growth promoters for weanling swine. J. Anim. Sci. 1995, 73, 2640–2646. [Google Scholar] [CrossRef] [PubMed]
- Veum, T.L.; Carlson, M.S.; Wu, C.W.; Bollinger, D.W.; Ellersieck, M.R. Copper proteinate in weanling pig diets for enhancing growth performance and reducing fecal copper excretion compared with copper sulfate1. J. Anim. Sci. 2004, 82, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Ju, D.; Teng, T.; Bai, G.; Fu, H.; Qiu, S.; Zhao, X.; Sun, Y.; Shi, B. The role of protein restriction and interaction with antibiotics in the regulation of compensatory growth in pigs: Growth performance, serum hormone concentrations, and messenger RNA levels in component tissues of the endocrine growth axis. Domest. Anim. Endocrinol. 2021, 74, 106524. [Google Scholar] [CrossRef] [PubMed]
- Louveau, I.; Bonneau, M. Effect of a growth hormone infusion on plasma insulin-like growth factor-I in Meishan and Large White pigs. Reprod. Nutr. Dev. 1996, 36, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, X.; Guo, Y.; Wang, Z.; Zhao, B.; Yin, Y.; Liu, G. Influence of Dietary Copper on Serum Growth-Related Hormone Levels and Growth Performance of Weanling Pigs. Biol. Trace Elem. Res. 2015, 172, 134–139. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Zhu, X.; Gao, Y.; Liu, Z.; Zhang, L.; Chen, H.; Shi, X.; Yang, L.; Liu, G. High Lever Dietary Copper Promote Ghrelin Gene Expression in the Fundic Gland of Growing Pigs. Biol. Trace Elem. Res. 2012, 150, 154–157. [Google Scholar] [CrossRef]
- Wang, S.; Yang, J.; Zhang, B.; Zhang, L.; Wu, K.; Yang, A.; Li, C.; Wang, Y.; Zhang, J.; Qi, D. Potential Link between Gut Microbiota and Deoxynivalenol-Induced Feed Refusal in Weaned Piglets. J. Agric. Food Chem. 2019, 67, 4976–4986. [Google Scholar] [CrossRef]
- Zeng, H.; Huang, C.; Lin, S.; Zheng, M.; Chen, C.; Zheng, B.; Zhang, Y. Lotus Seed Resistant Starch Regulates Gut Microbiota and Increases Short-Chain Fatty Acids Production and Mineral Absorption in Mice. J. Agric. Food Chem. 2017, 65, 9217–9225. [Google Scholar] [CrossRef]
- Dębski, B. Supplementation of pigs diet with zinc and copper as alternative to conventional antimicrobials. Pol. J. Veter.-Sci. 2016, 19, 917–924. [Google Scholar] [CrossRef]
- Villagómez-Estrada, S.; Pérez, J.F.; Darwich, L.; Vidal, A.; Van Kuijk, S.; Duran, D.A.M.; Solà-Oriol, D. Effects of copper and zinc sources and inclusion levels of copper on weanling pig performance and intestinal microbiota. J. Anim. Sci. 2020, 98, skaa117. [Google Scholar] [CrossRef]
- Dostal, A.; Lacroix, C.; Bircher, L.; Pham, V.T.; Follador, R.; Zimmermann, M.B.; Chassard, C. Iron Modulates Butyrate Production by a Child Gut Microbiota In Vitro. Mbio 2015, 6, e01453-15. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.M.; Egli, I.M.; Aeberli, I.; Hurrell, R.F.; Meile, L. Phytic acid degrading lactic acid bacteria in tef-injera fermentation. Int. J. Food Microbiol. 2014, 190, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Torre, M.; Rodriguez, A.R.; Saura-Calixto, F. Effects of dietary fiber and phytic acid on mineral availability. Crit. Rev. Food Sci. Nutr. 1991, 30, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Dersjant-Li, Y.; Villca, B.; Sewalt, V.; de Kreij, A.; Marchal, L.; Velayudhan, D.E.; Sorg, R.A.; Christensen, T.; Mejldal, R.; Nikolaev, I.; et al. Functionality of a next generation biosynthetic bacterial 6-phytase in enhancing phosphorus availability to weaned piglets fed a corn-soybean meal-based diet without added inorganic phosphate. Anim. Nutr. 2020, 6, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Bikker, P.; Van Diepen, J.T.M.; Binnendijk, G.P.; Jongbloed, A.W. Phytase inclusion in pig diets improves zinc status but its effect on copper availability is inconsistent1. J. Anim. Sci. 2012, 90 (Suppl. S4), 197–199. [Google Scholar] [CrossRef]
- Jalanka, J.; Cheng, J.; Hiippala, K.; Ritari, J.; Salojärvi, J.; Ruuska, T.; Kalliomäki, M.; Satokari, R. Colonic Mucosal Microbiota and Association of Bacterial Taxa with the Expression of Host Antimicrobial Peptides in Pediatric Ulcerative Colitis. Int. J. Mol. Sci. 2020, 21, 6044. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, H.; Ma, G.; Sun, Y.; Li, Y.; Zhang, Y. Digestibility, lactation performance, plasma metabolites, ruminal fermentation, and bacterial communities in Holstein cows fed a fermented corn gluten-wheat bran mixture as a substitute for soybean meal. J. Dairy Sci. 2021, 104, 2866–2880. [Google Scholar] [CrossRef]
- Okamoto, T.; Morino, K.; Ugi, S.; Nakagawa, F.; Lemecha, M.; Ida, S.; Ohashi, N.; Sato, D.; Fujita, Y.; Maegawa, H. Microbiome potentiates endurance exercise through intestinal acetate production. Am. J. Physiol. Metab. 2019, 316, E956–E966. [Google Scholar] [CrossRef]
- Looft, T.; Levine, U.Y.; Stanton, T.B. Cloacibacillus porcorum sp. nov., a mucin-degrading bacterium from the swine intestinal tract and emended description of the genus Cloacibacillus. Int. J. Syst. Evol. Microbiol. 2013, 63, 1960–1966. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.; Wang, Y.; Liu, C.; Zhang, L.; Zhu, S.; Liu, S.; Duan, L. Beneficial effect of butyrate-producing Lachnospiraceae on stress-induced visceral hypersensitivity in rats. J. Gastroenterol. Hepatol. 2019, 34, 1368–1376. [Google Scholar] [CrossRef]
- Kang, S.; Khan, S.; Webb, R.; Denman, S.; McSweeney, C. Characterization and survey in cattle of a rumen Pyrimadobacter sp. which degrades the plant toxin fluoroacetate. FEMS Microbiol. Ecol. 2020, 96, fiaa077. [Google Scholar] [CrossRef]
- Aoyagi, T.; Inaba, T.; Aizawa, H.; Mayumi, D.; Sakata, S.; Charfi, A.; Suh, C.; Lee, J.H.; Sato, Y.; Ogata, A.; et al. Unexpected diversity of acetate degraders in anaerobic membrane bioreactor treating organic solid waste revealed by high-sensitivity stable isotope probing. Water Res. 2020, 176, 115750. [Google Scholar] [CrossRef]
- Misiukiewicz, A.; Gao, M.; Filipiak, W.; Cieslak, A.; Patra, A.; Szumacher-Strabel, M. Review: Methanogens and methane production in the digestive systems of nonruminant farm animals. Animal 2021, 15, 100060. [Google Scholar] [CrossRef]
- Huang, S.-M.; Wu, Z.-H.; Li, T.-T.; Liu, C.; Han, D.-D.; Tao, S.-Y.; Pi, Y.; Li, N.; Wang, J.-J. Perturbation of the lipid metabolism and intestinal inflammation in growing pigs with low birth weight is associated with the alterations of gut microbiota. Sci. Total Environ. 2020, 719, 137382. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J.-H.; Yu, T.; Chen, Q.-K. Effects of berberine and metformin on intestinal inflammation and gut microbiome composition in db/db mice. Biomed. Pharmacother. 2019, 118, 109131. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.-J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, Q.; Tang, C.; Li, Y.; Zhang, K.; Li, F.; Zhang, J. Butyrate Mitigates Weanling Piglets From Lipopolysaccharide-Induced Colitis by Regulating Microbiota and Energy Metabolism of the Gut–Liver Axis. Front. Microbiol. 2020, 11, 588666. [Google Scholar] [CrossRef]
- Salvi, P.S.; Cowles, R.A. Butyrate and the Intestinal Epithelium: Modulation of Proliferation and Inflammation in Homeostasis and Disease. Cells 2021, 10, 1775. [Google Scholar] [CrossRef]
- He, B.; Li, T.; Wang, W.; Gao, H.; Bai, Y.; Zhang, S.; Zang, J.; Li, D.; Wang, J. Metabolic characteristics and nutrient utilization in high-feed-efficiency pigs selected using different feed conversion ratio models. Sci. China Life Sci. 2019, 62, 959–970. [Google Scholar] [CrossRef]
- Takii, T.; Hayashi, M.; Hiroma, H.; Chiba, T.; Kawashima, S.; Zhang, H.L.; Nagatsu, A.; Sakakibara, J.; Onozaki, K. Serotonin Derivative, N-(p-Coumaroyl)serotonin, Isolated from Safflower (Carthamus tinctorius L.) Oil Cake Augments the Proliferation of Normal Human and Mouse Fibroblasts in Synergy with Basic Fibroblast Growth Factor (bFGF) or Epidermal Growth Factor (EGF). J. Biochem. 1999, 125, 910–915. [Google Scholar] [CrossRef]
- Takii, T.; Kawashima, S.; Chiba, T.; Hayashi, H.; Hayashi, M.; Hiroma, H.; Kimura, H.; Inukai, Y.; Shibata, Y.; Nagatsu, A.; et al. Multiple mechanisms involved in the inhibition of proinflammatory cytokine production from human monocytes by N-(p-coumaroyl)serotonin and its derivatives. Int. Immunopharmacol. 2003, 3, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Paruchuri, S.; Tashimo, H.; Feng, C.; Maekawa, A.; Xing, W.; Jiang, Y.; Kanaoka, Y.; Conley, P.; Boyce, J.A. Leukotriene E4–induced pulmonary inflammation is mediated by the P2Y12 receptor. J. Exp. Med. 2009, 206, 2543–2555. [Google Scholar] [CrossRef] [PubMed]
- Hof, M.I.; Schnyder, M.; Hartnack, S.; Stanke-Labesque, F.; Luckschander, N.; Burgener, I. Urinary Leukotriene E4 Concentrations as a Potential Marker of Inflammation in Dogs with Inflammatory Bowel Disease. J. Veter.-Intern. Med. 2012, 26, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Postler, T.S.; Ghosh, S. Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab. 2017, 26, 110–130. [Google Scholar] [CrossRef]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Zheng, H.; Ji, H.; Fan, K.; Xu, H.; Huang, Y.; Zheng, Y.; Xu, Q.; Li, C.; Zhao, L.; Li, Y.; et al. Targeting Gut Microbiota and Host Metabolism with Dendrobium officinale Dietary Fiber to Prevent Obesity and Improve Glucose Homeostasis in Diet-Induced Obese Mice. Mol. Nutr. Food Res. 2022, 66, e2100772. [Google Scholar] [CrossRef] [PubMed]
- Nazeam, J.A.; Alshareef, W.; Helmy, M.W.; El-Haddad, A.E. Bioassay-guided isolation of potential bioactive constituents from pomegranate agrifood by-product. Food Chem. 2020, 326, 126993. [Google Scholar] [CrossRef]
- Luchini, A.C.; Rodrigues-Orsi, P.; Cestari, S.H.; Seito, L.N.; Witaicenis, A.; Pellizzon, C.H.; Di Stasi, L.C. Intestinal Anti-inflammatory Activity of Coumarin and 4-Hydroxycoumarin in the Trinitrobenzenesulphonic Acid Model of Rat Colitis. Biol. Pharm. Bull. 2008, 31, 1343–1350. [Google Scholar] [CrossRef]
- Witaicenis, A.; Seito, L.N.; Chagas, A.D.S.; de Almeida, L.D.; Luchini, A.C.; Rodrigues-Orsi, P.; Cestari, S.H.; Di Stasi, L.C. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine 2014, 21, 240–246. [Google Scholar] [CrossRef]
- Ji, J.; Ge, X.; Chen, Y.; Zhu, B.; Wu, Q.; Zhang, J.; Shan, J.; Cheng, H.; Shi, L. Daphnetin ameliorates experimental colitis by modulating microbiota composition and Treg/Th 17 balance. FASEB J. 2019, 33, 9308–9322. [Google Scholar] [CrossRef]
| Ingredient | Content (%) | Nutrient Composition | |
|---|---|---|---|
| Corn | 65.30 | Metabolic energy (MJ/kg) | 12.21 |
| Soybean meal | 18.40 | Crude protein 4 (%) | 16.21 |
| Wheat bran | 12.20 | Calcium (%) | 0.71 |
| Limestone | 1.12 | Total phosphorus (%) | 0.55 |
| Dicalcium phosphate | 0.85 | Lysine (%) | 1.12 |
| Salt | 0.30 | Methionine + cysteine (%) | 0.65 |
| Phytase 2 | 0.01 | Threonine (%) | 0.72 |
| L-Lysine hydrochloride | 0.50 | Tryptophan (%) | 0.18 |
| DL-Methionine | 0.16 | ||
| Threonine | 0.16 | ||
| Premix 3 | 1.00 | ||
| Item | CuSO4 | Cu-Gly | SEM | p-Value |
|---|---|---|---|---|
| Initial BW (kg) | 24.78 | 24.92 | 2.96 | 0.707 |
| Final BW (kg) | 31.86 | 32.72 | 3.36 | 0.509 |
| ADG (g/d) | 505.43 | 557.43 | 57.08 | 0.227 |
| ADFI (g/d) | 1348.29 | 1483.57 | 101.07 | 0.039 |
| Feed efficiency (feed/gain) | 2.67 | 2.66 | 0.629 | 0.258 |
| ATTD 1 of DM (%) | 90.44 | 90.45 | 0.25 | 0.949 |
| ATTD of CP (%) | 83.91 | 84.72 | 1.75 | 0.656 |
| ATTD of Cu (%) | 45.17 | 56.49 | 5.03 | 0.048 |
| ATTD of Zn (%) | 26.11 | 27.55 | 4.01 | 0.727 |
| ATTD of Fe (%) | 52.62 | 67.49 | 5.38 | 0.021 |
| ATTD of Mn (%) | 49.46 | 61.57 | 5.41 | 0.048 |
| Fecal content of DM (%) | 25.37 | 26.18 | 1.09 | 0.475 |
| Fecal content of CP (%) | 47.70 | 48.09 | 1.11 | 0.730 |
| Fecal content of Cu (mg/kg) | 522.04 | 484.28 | 14.04 | 0.023 |
| Fecal content of Zn (mg/kg) | 1062.75 | 979.68 | 65.50 | 0.270 |
| Fecal content of Fe (mg/kg) | 1805.68 | 1120.97 | 230.41 | 0.016 |
| Fecal content of Mn (mg/kg) | 1047.37 | 885.47 | 67.47 | 0.040 |
| Metabolite | HMDB ID | CuSO4 vs. Baseline | Cu-Gly vs. Baseline | Cu-Gly vs. CuSO4 | |||
|---|---|---|---|---|---|---|---|
| FC 1 | p-Value | FC | p-Value | FC | p-Value | ||
| 3-hydroxytridecanoic acid | HMDB0061655 | 3.697 | 0.034 | 3.528 | 0.009 | - | - |
| N-Acetylmuramate | HMDB0060493 | 2.188 | 0.002 | 2.075 | 0.003 | - | - |
| 5-[(E)-2-(3,5-dihydroxyphenyl)ethenyl]-2-methoxyphenyl oxidanesulfonic acid | HMDB0134587 | 1.699 | 0.005 | 1.593 | 0.012 | - | - |
| Physagulin F | HMDB0039631 | 1.527 | 0.007 | 1.418 | 0.031 | - | - |
| Grevilline B | HMDB0033240 | 1.498 | 0.014 | - | - | - | - |
| 7-hydroxy-1-oxo-1H-isochromene-3-carbaldehyde | HMDB0128617 | 1.456 | 0.043 | - | - | - | - |
| 3-hydroxypristanic acid | HMDB0061651 | 1.417 | 0.036 | - | - | - | - |
| Ricinoleic acid | HMDB0034297 | 1.390 | 0.004 | - | - | - | - |
| Alpha-Carboxy-delta-decalactone | HMDB0030985 | 1.388 | 0.013 | 1.410 | 0.014 | - | - |
| 3,4,5-trihydroxy-6-(2-hydroxy-1,2-diphenylethoxy)oxane-2-carboxylic acid | HMDB0135201 | 1.371 | 0.013 | - | - | - | - |
| 5-Pentyltetrahydro-2-oxo-3-furancarboxylic acid | HMDB0030989 | 1.369 | 0.033 | 1.413 | 0.026 | - | - |
| 23-Hydroxyphysalolactone | HMDB0031388 | 1.355 | 0.015 | - | - | - | - |
| Pristanoylglycine | HMDB0013303 | 1.350 | 0.002 | - | - | - | - |
| Trans-Grandmarin | HMDB0039030 | 1.347 | 0.016 | - | - | - | - |
| Tyrosyl-Serine | HMDB0029114 | 1.326 | 0.020 | - | - | - | - |
| Zizybeoside I | HMDB0034954 | 0.752 | 0.017 | - | - | - | - |
| Prolyl-Arginine | HMDB0029011 | - | - | 2.052 | 0.021 | - | - |
| Xi-Linalool 3-[rhamnosyl-(1->6)-glucoside] | HMDB0030422 | - | - | 1.360 | 0.001 | - | - |
| Coutaric acid | HMDB0029225 | - | - | 1.343 | 0.000 | - | - |
| Nb-p-Coumaroyltryptamine (CT) | HMDB0041518 | - | - | 1.474 | 0.031 | 1.388 | 0.036 |
| Coumarin | HMDB0001218 | - | - | 1.421 | 0.004 | 1.440 | 0.024 |
| 20-Oxo-leukotriene E4 | HMDB0012642 | - | - | 0.713 | 0.019 | 0.757 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, H.; Du, Q.; Lu, N.; Jiang, X.; Li, M.; Xia, D.; Long, K. Comparison of the Microbiome-Metabolome Response to Copper Sulfate and Copper Glycinate in Growing Pigs. Animals 2023, 13, 345. https://doi.org/10.3390/ani13030345
Lei H, Du Q, Lu N, Jiang X, Li M, Xia D, Long K. Comparison of the Microbiome-Metabolome Response to Copper Sulfate and Copper Glycinate in Growing Pigs. Animals. 2023; 13(3):345. https://doi.org/10.3390/ani13030345
Chicago/Turabian StyleLei, Hulong, Qian Du, Naisheng Lu, Xueyuan Jiang, Mingzhou Li, Dong Xia, and Keren Long. 2023. "Comparison of the Microbiome-Metabolome Response to Copper Sulfate and Copper Glycinate in Growing Pigs" Animals 13, no. 3: 345. https://doi.org/10.3390/ani13030345
APA StyleLei, H., Du, Q., Lu, N., Jiang, X., Li, M., Xia, D., & Long, K. (2023). Comparison of the Microbiome-Metabolome Response to Copper Sulfate and Copper Glycinate in Growing Pigs. Animals, 13(3), 345. https://doi.org/10.3390/ani13030345






