miR-202-5p Inhibits Lipid Metabolism and Steroidogenesis of Goose Hierarchical Granulosa Cells by Targeting ACSL3
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Granulosa Cell Culture
2.3. Cell Transfection
2.4. RNA Extraction and qRT-PCR Analysis
2.5. Oil Red O Staining and Detection of Intracellular Lipids
2.6. Measurement of Intracellular Triglyceride and Cholesterol Concentrations
2.7. Determination of Progesterone and Estradiol Production
2.8. Prediction of MiR-202-5p Target Genes
2.9. Dual Luciferase Assays
2.10. Statistical Analysis
3. Results
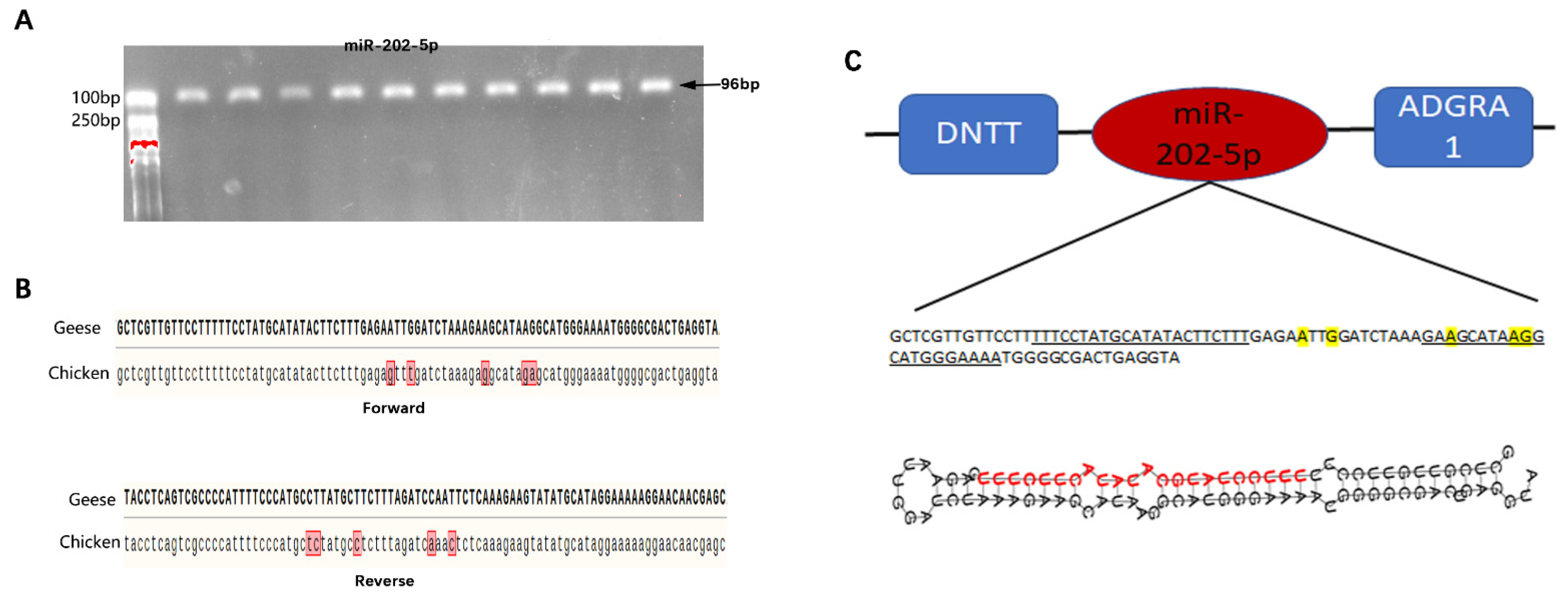
3.1. Identification and Characterization of the Goose miR-202-5p Precursor Sequence
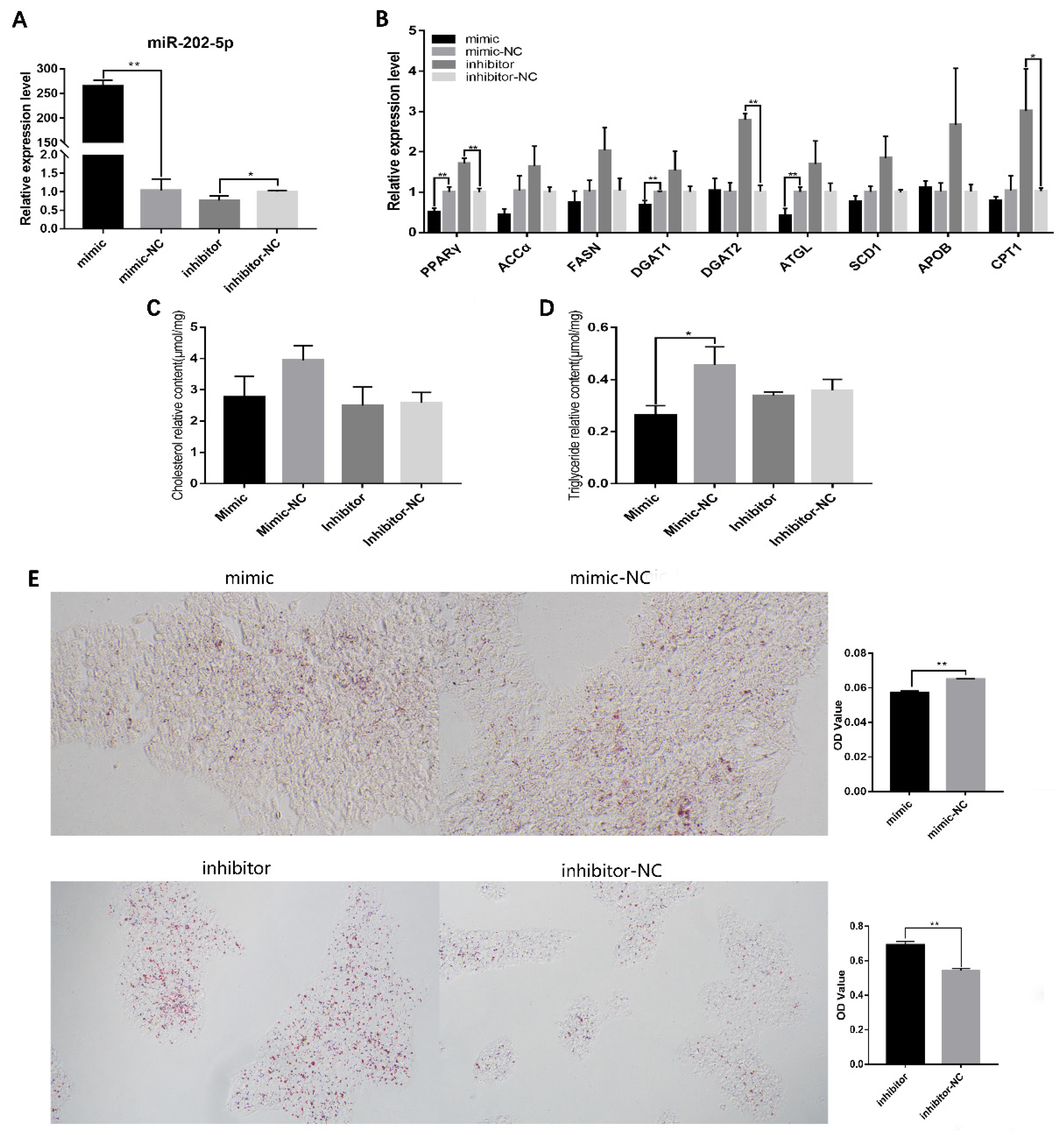
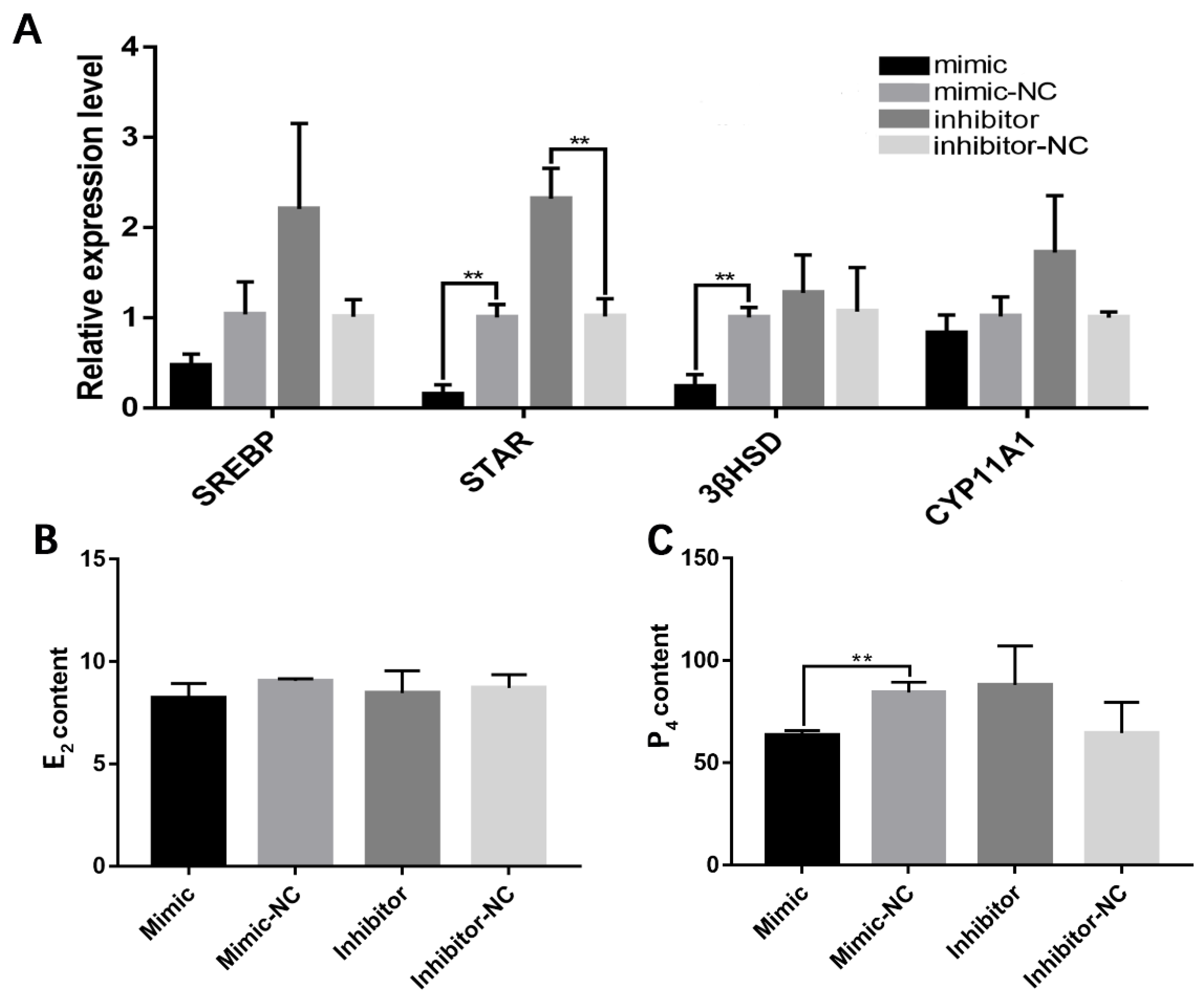
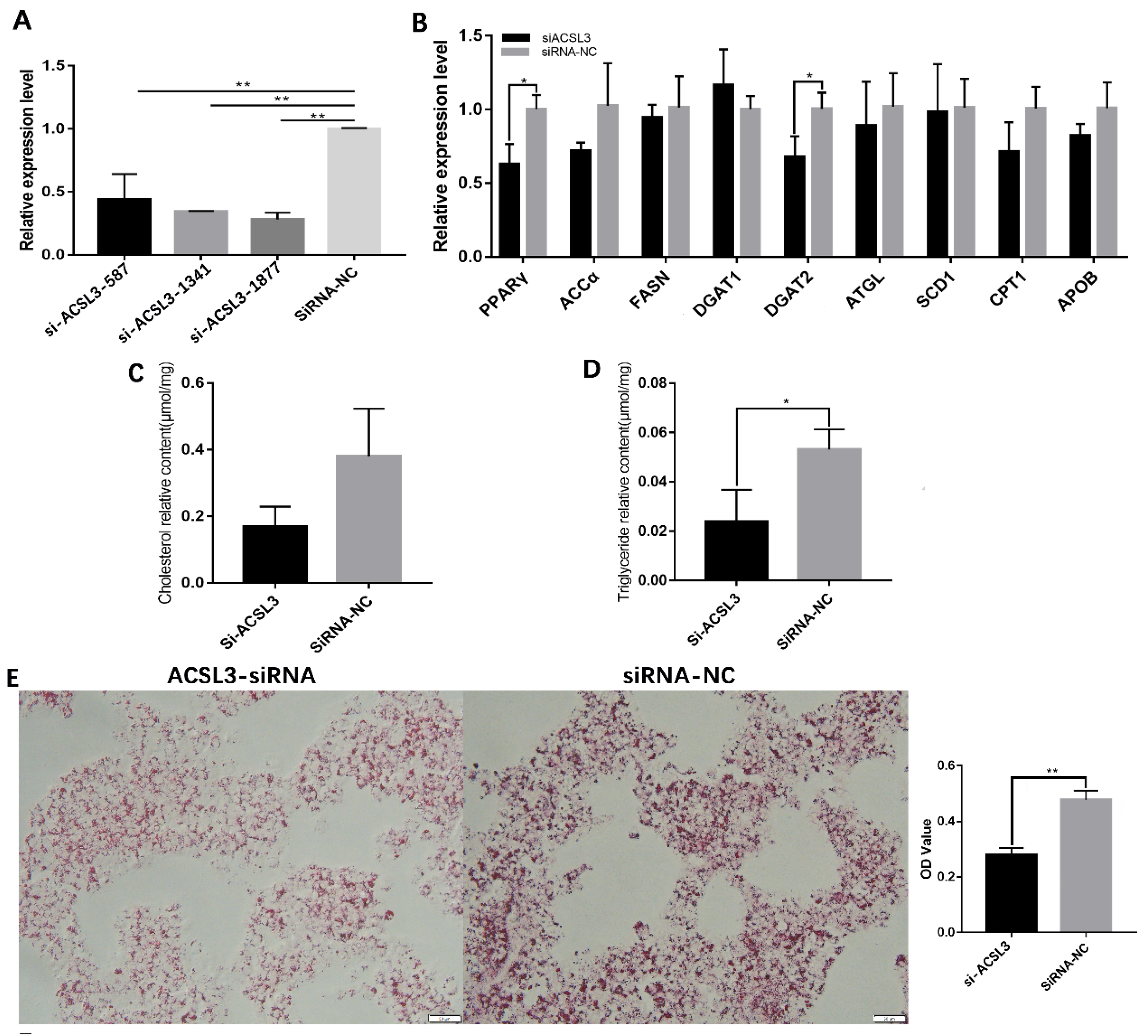
3.2. miR-202-5p Suppressed Lipid Deposition and Steroidogenesis of Goose hGCs
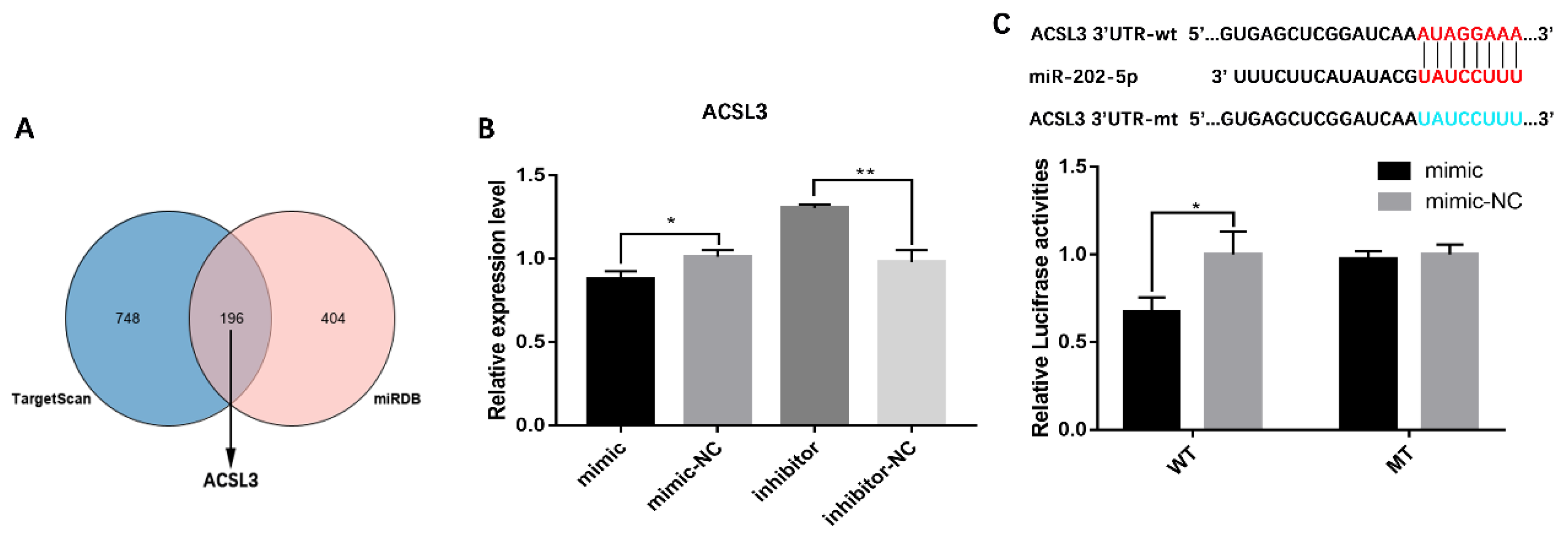
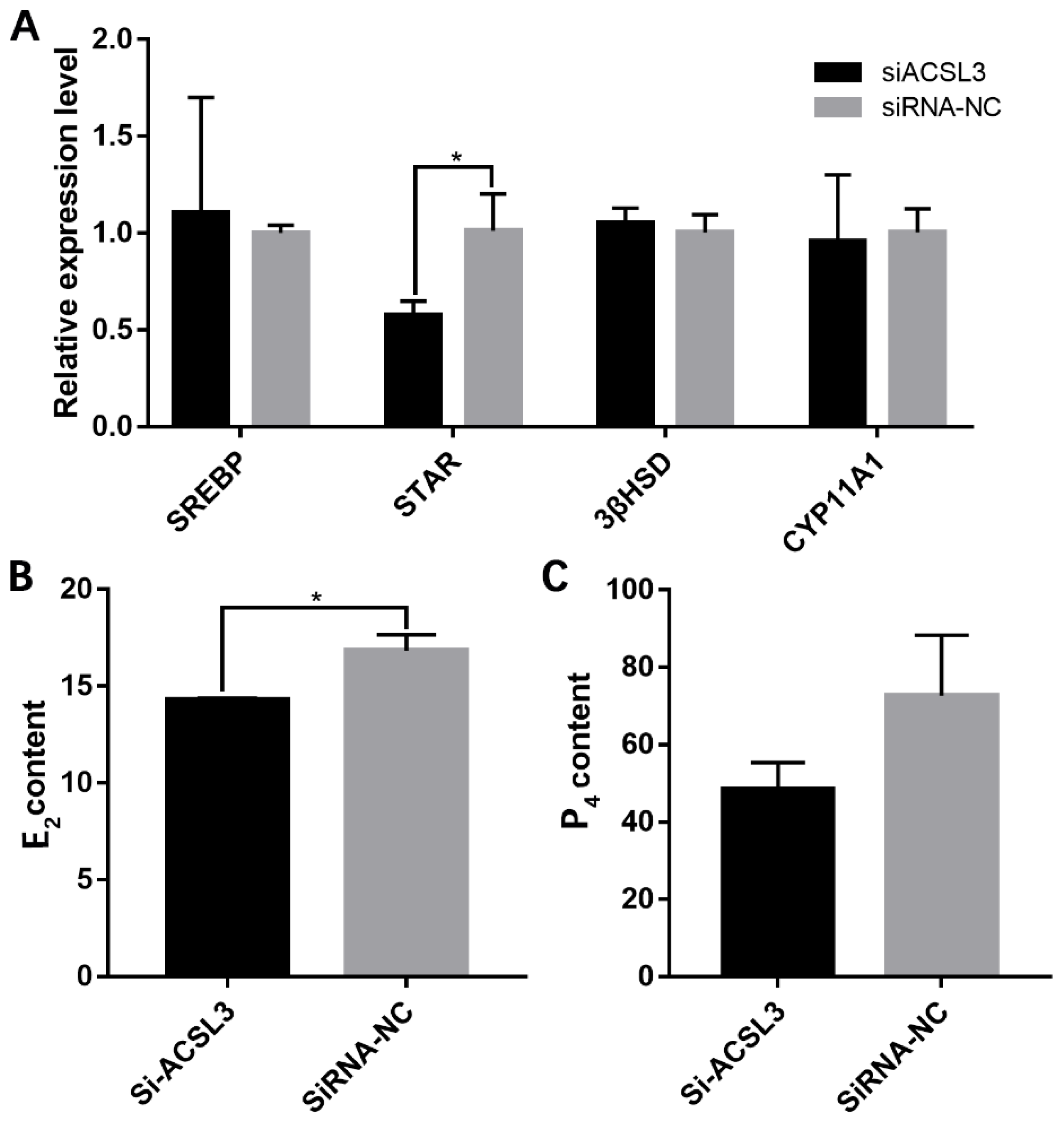
3.3. miR-202-5p Inhibits Lipid Deposition and Steroidogenesis by Targeting ACSL3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Zhang, W.; Li, Q.; Liu, J.; Zhang, T.; Zhou, T.; Li, L.; Wang, J.; Xu, H.; He, H. The comprehensive mechanisms underlying nonhierarchical follicular development in geese (Anser cygnoides). Anim. Reprod. Sci. 2015, 159, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Onagbesan, O.; Bruggeman, V.; Decuypere, E. Intra-ovarian growth factors regulating ovarian function in avian species: A review. Anim. Reprod. Sci. 2009, 111, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Orisaka, M.; Tajima, K.; Tsang, B.K.; Kotsuji, F. Oocyte-granulosa-theca cell interactions during preantral follicular development. J. Ovarian Res. 2009, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shi, X.; Shi, Y.; Wang, Z. The Signaling Pathways Involved in Ovarian Follicle Development. Front. Physiol. 2021, 12, 730196. [Google Scholar] [CrossRef]
- Tu, J.; Chen, Y.; Li, Z.; Yang, H.; Chen, H.; Yu, Z. Long non-coding RNAs in ovarian granulosa cells. J. Ovarian Res. 2020, 13, 63. [Google Scholar] [CrossRef]
- Marei, W.F.A.; Van Raemdonck, G.; Baggerman, G.; Bols, P.E.J.; Leroy, J. Proteomic changes in oocytes after in vitro maturation in lipotoxic conditions are different from those in cumulus cells. Sci. Rep. 2019, 9, 3673. [Google Scholar] [CrossRef]
- Vireque, A.A.; Tata, A.; Belaz, K.R.; Grázia, J.G.; Santos, F.N.; Arnold, D.R.; Basso, A.C.; Eberlin, M.N.; Silva-de-Sá, M.F.; Ferriani, R.A.; et al. MALDI mass spectrometry reveals that cumulus cells modulate the lipid profile of in vitro-matured bovine oocytes. Syst. Biol. Reprod. Med. 2017, 63, 86–99. [Google Scholar] [CrossRef]
- Yuan, X.; Hu, S.; Li, L.; Han, C.; Liu, H.; He, H.; Xia, L.; Hu, J.; Hu, B.; Ran, M.; et al. Lipidomics profiling of goose granulosa cell model of stearoyl-CoA desaturase function identifies a pattern of lipid droplets associated with follicle development. Cell Biosci. 2021, 11, 95. [Google Scholar] [CrossRef]
- Liu, T.; Qu, J.; Tian, M.; Yang, R.; Song, X.; Li, R.; Yan, J.; Qiao, J. Lipid Metabolic Process Involved in Oocyte Maturation During Folliculogenesis. Front. Cell. Dev. Biol. 2022, 10, 806890. [Google Scholar] [CrossRef]
- Elis, S.; Desmarchais, A.; Maillard, V.; Uzbekova, S.; Monget, P.; Dupont, J. Cell proliferation and progesterone synthesis depend on lipid metabolism in bovine granulosa cells. Theriogenology 2015, 83, 840–853. [Google Scholar] [CrossRef]
- Uzbekova, S.; Elis, S.; Teixeira-Gomes, A.P.; Desmarchais, A.; Maillard, V.; Labas, V. MALDI Mass Spectrometry Imaging of Lipids and Gene Expression Reveals Differences in Fatty Acid Metabolism between Follicular Compartments in Porcine Ovaries. Biology 2015, 4, 216–236. [Google Scholar] [CrossRef] [PubMed]
- Tosca, L.; Chabrolle, C.; Uzbekova, S.; Dupont, J. Effects of metformin on bovine granulosa cells steroidogenesis: Possible involvement of adenosine 5′ monophosphate-activated protein kinase (AMPK). Biol. Reprod. 2007, 76, 368–378. [Google Scholar] [CrossRef]
- Wen, R.; Gan, X.; Hu, S.; Gao, S.; Deng, Y.; Qiu, J.; Sun, W.; Li, L.; Han, C.; Hu, J.; et al. Evidence for the existence of de novo lipogenesis in goose granulosa cells. Poult. Sci. 2019, 98, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Gan, X.; He, H.; Hu, S.; Deng, Y.; Chen, X.; Li, L.; Hu, J.; Li, L.; Wang, J. Dynamic characteristics of lipid metabolism in cultured granulosa cells from geese follicles at different developmental stages. Biosci. Rep. 2019, 39, bsr20192188. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.J.; Azhar, S.; Kraemer, F.B. Lipid droplets and steroidogenic cells. Exp. Cell Res. 2016, 340, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Zhan, X.; Li, J. MicroRNA-574 Impacts Granulosa Cell Estradiol Production via Targeting TIMP3 and ERK1/2 Signaling Pathway. Front. Endocrinol. 2022, 13, 852127. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, X.; Hu, S.; Yu, J. A brief review on the mechanisms of miRNA regulation. Genom. Proteom. Bioinform. 2009, 7, 147–154. [Google Scholar] [CrossRef]
- Luense, L.J.; Carletti, M.Z.; Christenson, L.K. Role of Dicer in female fertility. Trends Endocrinol. Metab. TEM 2009, 20, 265–272. [Google Scholar] [CrossRef]
- Guo, L.; Xu, H.; Li, Y.; Liu, H.; Zhao, J.; Lu, W.; Wang, J. Kisspeptin-10 Promotes Progesterone Synthesis in Bovine Ovarian Granulosa Cells via Downregulation of microRNA-1246. Genes 2022, 13, 298. [Google Scholar] [CrossRef]
- Liu, G.; Liu, S.; Xing, G.; Wang, F. lncRNA PVT1/MicroRNA-17-5p/PTEN Axis Regulates Secretion of E2 and P4, Proliferation, and Apoptosis of Ovarian Granulosa Cells in PCOS. Mol. Ther. Nucleic Acids 2020, 20, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Fulghesu, A.M.; Mikuš, M.; Watrowski, R.; D’Alterio, M.N.; Lin, L.T.; Shah, M.; Reyes-Muñoz, E.; Sathyapalan, T.; Angioni, S. The Translational Role of miRNA in Polycystic Ovary Syndrome: From Bench to Bedside-A Systematic Literature Review. Biomedicines 2022, 10, 1816. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, J.; Xiao, S.Y.; Han, X.Y.; Song, X.T.; Qi, M.Y.; Liu, G.S.; Yang, C.X.; Yao, Y.C. miRNA expression analysis of the sheep follicle during the prerecruitment, dominant, and mature stages of development under FSH stimulation. Theriogenology 2022, 181, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hu, S.; Wang, Y.; Deng, Y.; Yang, S.; Hu, J.; Li, L.; Wang, J. mRNA and miRNA Transcriptome Profiling of Granulosa and Theca Layers from Geese Ovarian Follicles Reveals the Crucial Pathways and Interaction Networks for Regulation of Follicle Selection. Front. Genet. 2019, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Chen, D.; Deng, Y.; Yuan, J.; Kang, B.; Qiu, J.; Sun, W.; Han, C.; Hu, J.; Li, L.; et al. Establishment of an in vitro culture model of theca cells from hierarchical follicles in ducks. Biosci. Rep. 2017, 37, bsr20160491. [Google Scholar] [CrossRef]
- Chen, X.; Huang, K.; Hu, S.; Lan, G.; Gan, X.; Gao, S.; Deng, Y.; Hu, J.; Li, L.; Hu, B.; et al. FASN-Mediated Lipid Metabolism Regulates Goose Granulosa Cells Apoptosis and Steroidogenesis. Front. Physiol. 2020, 11, 600. [Google Scholar] [CrossRef] [PubMed]
- Mourot, J.; Guy, G.; Lagarrigue, S.; Peiniau, P.; Hermier, D. Role of hepatic lipogenesis in the susceptibility to fatty liver in the goose (Anser anser). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2000, 126, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Wei, Y.; You, G.; Liu, W.; Amevor, F.K.; Zhang, Y.; He, H.; Ma, M.; Zhang, Y.; Li, D.; et al. Circular PPP1R13B RNA Promotes Chicken Skeletal Muscle Satellite Cell Proliferation and Differentiation via Targeting miR-9-5p. Animals 2021, 11, 2396. [Google Scholar] [CrossRef]
- Kassan, A.; Herms, A.; Fernández-Vidal, A.; Bosch, M.; Schieber, N.L.; Reddy, B.J.; Fajardo, A.; Gelabert-Baldrich, M.; Tebar, F.; Enrich, C.; et al. Acyl-CoA synthetase 3 promotes lipid droplet biogenesis in ER microdomains. J. Cell Biol. 2013, 203, 985–1001. [Google Scholar] [CrossRef]
- Sontakke, S.D.; Mohammed, B.T.; McNeilly, A.S.; Donadeu, F.X. Characterization of microRNAs differentially expressed during bovine follicle development. Reproduction 2014, 148, 271–283. [Google Scholar] [CrossRef]
- Ding, Q.; Jin, M.; Wang, Y.; Liu, J.; Kalds, P.; Wang, Y.; Yang, Y.; Wang, X.; Chen, Y. Transactivation of miR-202-5p by Steroidogenic Factor 1 (SF1) Induces Apoptosis in Goat Granulosa Cells by Targeting TGFβR2. Cells 2020, 9, 445. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.X.; Lee, S.; Taweechaipaisankul, A.; Kim, G.A.; Lee, B.C. Melatonin regulates lipid metabolism in porcine oocytes. J. Pineal Res. 2017, 62, e12388. [Google Scholar] [CrossRef] [PubMed]
- Janani, C.; Ranjitha Kumari, B.D. PPAR gamma gene—A review. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.H.; Carreiro, A.L.; Buhman, K.K. Dgat1 and Dgat2 regulate enterocyte triacylglycerol distribution and alter proteins associated with cytoplasmic lipid droplets in response to dietary fat. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2017, 1862, 600–614. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Louie, S.M.; Daniele, J.R.; Tran, Q.; Dillin, A.; Zoncu, R.; Nomura, D.K.; Olzmann, J.A. DGAT1-Dependent Lipid Droplet Biogenesis Protects Mitochondrial Function during Starvation-Induced Autophagy. Dev. Cell 2017, 42, 9.e25–21.e25. [Google Scholar] [CrossRef]
- Chitraju, C.; Walther, T.C.; Farese, R.V., Jr. The triglyceride synthesis enzymes DGAT1 and DGAT2 have distinct and overlapping functions in adipocytes. J. Lipid Res. 2019, 60, 1112–1120. [Google Scholar] [CrossRef]
- Johnson, A.L.; Woods, D.C. Dynamics of avian ovarian follicle development: Cellular mechanisms of granulosa cell differentiation. Gen. Comp. Endocrinol. 2009, 163, 12–17. [Google Scholar] [CrossRef]
- Kraemer, F.B.; Shen, W.J.; Azhar, S. SNAREs and cholesterol movement for steroidogenesis. Mol. Cell. Endocrinol. 2017, 441, 17–21. [Google Scholar] [CrossRef]
- Liu, Q.; Liang, Y.; Gao, N.; Gao, J.; Wang, Y.; Li, X.; Qin, J.; Xiang, Q.; Wu, X.; Chen, H.; et al. Regulation of lipid droplets via the PLCβ2-PKCα-ADRP pathway in granulosa cells exposed to cadmium. Environ. Pollut. 2020, 267, 115541. [Google Scholar] [CrossRef]
- Miller, W.L. StAR search—What we know about how the steroidogenic acute regulatory protein mediates mitochondrial cholesterol import. Mol. Endocrinol. 2007, 21, 589–601. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Eck, F.; Phuyal, S.; Smith, M.D.; Kaulich, M.; Wilkinson, S.; Farhan, H.; Behrends, C. ACSL3 is a novel GABARAPL2 interactor that links ufmylation and lipid droplet biogenesis. J. Cell Sci. 2020, 133, jcs243477. [Google Scholar] [CrossRef] [PubMed]
- Klasson, T.D.; LaGory, E.L.; Zhao, H.; Huynh, S.K.; Papandreou, I.; Moon, E.J.; Giaccia, A.J. ACSL3 regulates lipid droplet biogenesis and ferroptosis sensitivity in clear cell renal cell carcinoma. Cancer Metab. 2022, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Migita, T.; Takayama, K.I.; Urano, T.; Obinata, D.; Ikeda, K.; Soga, T.; Takahashi, S.; Inoue, S. ACSL3 promotes intratumoral steroidogenesis in prostate cancer cells. Cancer Sci. 2017, 108, 2011–2021. [Google Scholar] [CrossRef]
| Sense (5′–3′) | Anti-Sense (5′–3′) | |
|---|---|---|
| si-ACSL3-587 | GAGGUGACCACCAUCAUUATT | UAAUGAUGGUGGUCACCUCTT |
| si-ACSL3-1341 | CAGCAACCCAGCGAUUUAUTT | AUAAAUCGCUGGGUUGCUGTT |
| si-ACSL3-1877 | GCUCGAAAGAAAGGAUUUATT | UAAAUCCUUUCUUUCGAGCTT |
| Genes | Primers (5′–3′) | Tm (°C) | Size (bp) | |
|---|---|---|---|---|
| GAPDH | F: TTTCCCCACAGCCTTAGCA | R: GCCATCACAGCCACACAGA | 60 | 90 |
| PPARγ | F: CCTCCTTCCCCACCCTATT | R: CTTGTCCCCACACACACGA | 59 | 108 |
| ACCα | F: TGCCTCCGAGAACCCTAA | R: AAGACCACTGCCACTCCA | 56.6 | 163 |
| FASN | F: TGGGAGTAACACTGATGGC | R: TCCAGGCTTGATACCACA | 57 | 109 |
| DGAT1 | F: CCTGAGGAACTTGGACACG | R: CAGGGACTGGTGGAACTCG | 59 | 265 |
| DGAT2 | F: CGCCATCATCATCGTGGT | R: CGTGCCGTAGAGCCAGTTT | 60 | 113 |
| CPT1 | F: GTCTCCAAGGCTCCGACAA | R: GAAGACCCGAATGAAAGTA | 56 | 193 |
| ATGL | F: TCGCAACCTCTACCGCCTCT | R: TCCGCACAAGCCTCCATAAGA | 60 | 300 |
| APOB | F: CTCAAGCCAACGAAGAAG | R: AAGCAAGTCAAGGCAAAA | 56 | 153 |
| SCD1 | F: GCCATCGGTCCTACAAAGC | R: AGCCAATGTGGGAGAAGAAA | 60 | 180 |
| SREBP | F: CGAGTACATCCGCTTCCTGC | R: TGAGGGACTTGCTCTTCTGC | 60 | 92 |
| STAR | F: AGAATCTTGACCTCTTTGACGCTG | R: GAGACGGTGGTGGATAACGGA | 60 | 87 |
| 3βHSD | F: GACCTGGGGTTTGGAATTGAG | R: TAGGAGAAGGTGAATGGGGTGT | 60 | 170 |
| CYP11A1 | F: AGGGAGAAGTTGGGTGTCTACGA | R: CGTAGGGCTTGTTGCGGTAGT | 60 | 89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ran, M.; Hu, S.; Ouyang, Q.; Xie, H.; Zhang, X.; Lin, Y.; Li, X.; Hu, J.; Li, L.; He, H.; et al. miR-202-5p Inhibits Lipid Metabolism and Steroidogenesis of Goose Hierarchical Granulosa Cells by Targeting ACSL3. Animals 2023, 13, 325. https://doi.org/10.3390/ani13030325
Ran M, Hu S, Ouyang Q, Xie H, Zhang X, Lin Y, Li X, Hu J, Li L, He H, et al. miR-202-5p Inhibits Lipid Metabolism and Steroidogenesis of Goose Hierarchical Granulosa Cells by Targeting ACSL3. Animals. 2023; 13(3):325. https://doi.org/10.3390/ani13030325
Chicago/Turabian StyleRan, Mingxia, Shenqiang Hu, Qingyuan Ouyang, Hengli Xie, Xi Zhang, Yueyue Lin, Xuejian Li, Jiwei Hu, Liang Li, Hua He, and et al. 2023. "miR-202-5p Inhibits Lipid Metabolism and Steroidogenesis of Goose Hierarchical Granulosa Cells by Targeting ACSL3" Animals 13, no. 3: 325. https://doi.org/10.3390/ani13030325
APA StyleRan, M., Hu, S., Ouyang, Q., Xie, H., Zhang, X., Lin, Y., Li, X., Hu, J., Li, L., He, H., Liu, H., & Wang, J. (2023). miR-202-5p Inhibits Lipid Metabolism and Steroidogenesis of Goose Hierarchical Granulosa Cells by Targeting ACSL3. Animals, 13(3), 325. https://doi.org/10.3390/ani13030325





