Pig Sedation and Anesthesia for Medical Research
Abstract
:Simple Summary
Abstract
1. Physical Examination
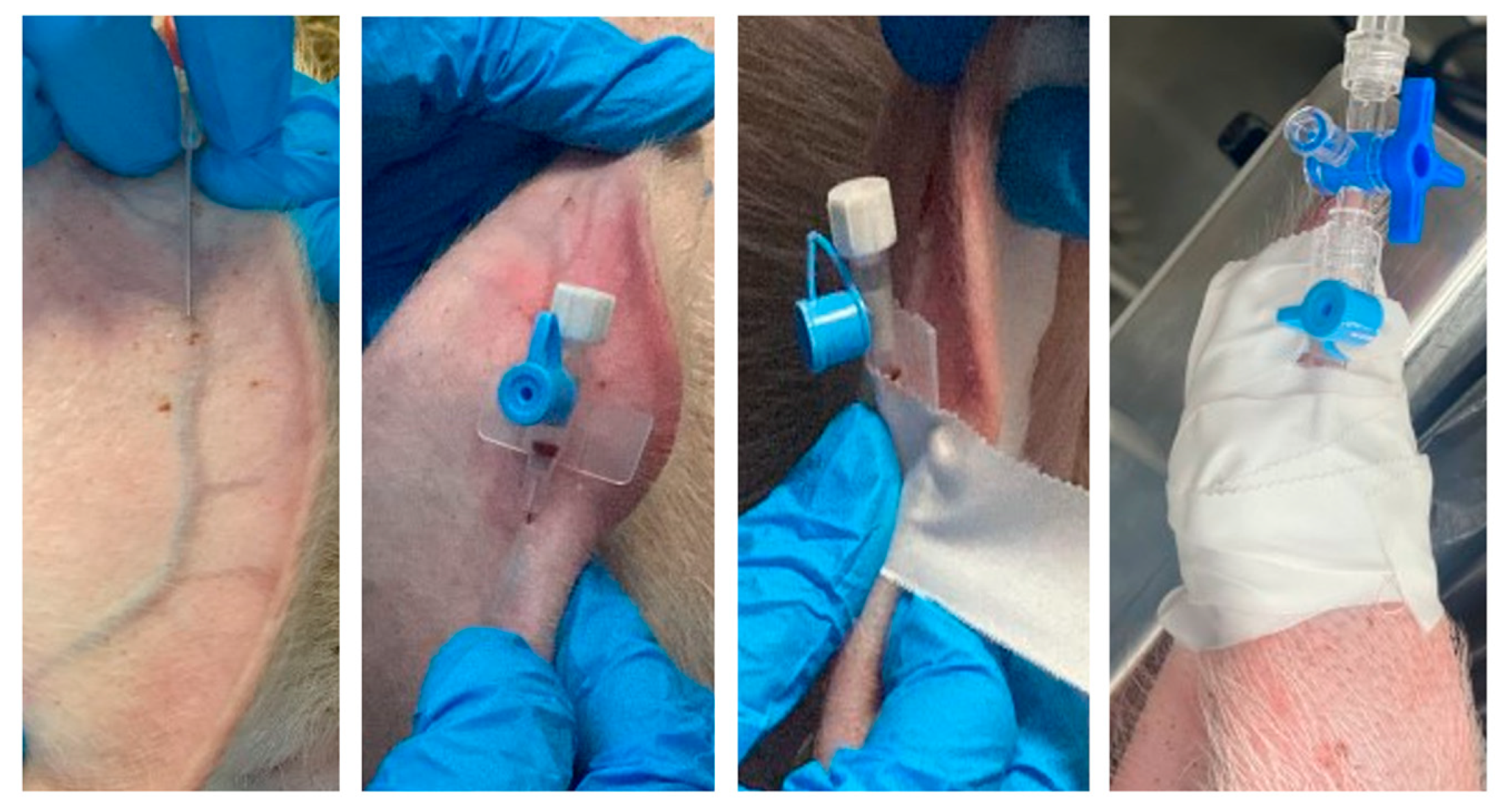
2. Recommendations for Injectable Administration
3. Sedation and Premedication
3.1. Butyrophenones
3.2. Phenothiazines
3.3. Benzodiazepines
3.4. Alpha-2 Adrenoreceptor Agonists
3.5. Dissociative Anesthetics
3.6. Opioids
3.7. Alfaxalone
3.8. Local Anesthetics
3.9. Neurokinin-1 (NK-1) Receptor Antagonists—Maropitant
3.10. Non-Depolarizing Neuromuscular Blocking Agents (NMBs)
4. Induction of Anesthesia
5. Endotracheal Intubation
6. Maintenance of Anesthesia
| Agent | Dose | Route | Considerations, References |
|---|---|---|---|
| Isoflurane | 1.6–1.9% MAC | ETT | [84] |
| Sevoflurane | 2.4–2.66% MAC | ETT | [85] |
| Propofol | 2–3 mg/kg, followed by 0.1–0.2 mg/kg/min | IV | [24] |
| Alfaxalone | 4.8 mg/kg/h | IV | [31] |
| Fentanyl | 50 µg/kg, followed by CRI 30–100 µg/kg/h. | IV | [23,46] |
| Alfaxalone Dexmedetomidine | 5.3 mg/kg/h alfaxalone 3.0 μg/kg/h dexmedetomidine | IV | [32] |
| Alfaxalone Dexmedetomidine Ketamine | 5 mg/kg/h alfaxalone 4 μg/kg/h dexmedetomidine 5 mg/kg/h ketamine | IV | [60] |
| Medetomidine Butorphanol Ketamine | 0.03–0.08 mg/kg medetomidine 0.2 mg/kg butorphanol 10 mg/kg ketamine | IM | Longer sedation than Xylazine-Butorphanol-Ketamine [48] |
| Xylazine Ketamine Midazolam | 2 mg/kg xylazine 0.25 mg/kg midazolam 10–20 mg/kg ketamine | IM | Immobilization in 2 min, effect for 50–90 min [8] |
| Tiletamine/Zolazepam Telazol® Xylazine | 4.4–6 mg/kg tiletamine/zolazepam 2–2.2 mg/kg xylazine | IM | Provides rapid sedation and can be used for sedation and induction [45,47] |
| Tiletamine/Zolazepam Telazol® Medetomidine | 5 mg/kg tiletamine/zolazepam 0.005 mg/kg medetomidine | IM | Provides rapid sedation and can be used for sedation and induction [45,47,56] |
| Guaifenesin Ketamine Xylazine “Triple drip” | 50 mg Guaifenesin 2 mg Ketamine 1 mg Xylazine CRI 2.2 mL/kg/h | IV | Recovery in 30–45 min, Guaifenesin- centrally acting muscle relaxant [23,47] |
| Flunixin Meglumine | 1–4 mg/kg q 24 h. | IV | managing postoperative pain [23] |
| Meloxicam | 0.4 mg/kg | IM | managing postoperative pain [8,22] |
| Carprofen | 1–4 mg/kg q 12 h. 2 mg/kg q 24 h. | IM, IV | managing postoperative pain [8] |
7. Perianesthetic Monitoring and Complications
8. Recovery
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flecknell, P. Laboratory Animal Anaesthesia; Academic Press: Cambridge, MA, USA, 2015; pp. 238–239. [Google Scholar]
- Sullivan, T.P.; Eaglstein, W.H.; Davis, S.C.; Mertz, P. The Pig as a Model for Human Wound Healing. Wound Repair Regen. 2001, 9, 66–76. [Google Scholar] [CrossRef]
- Kuzmuk, K.N.; Schook, L.B. Pigs as a Model for Biomedical Sciences. In The Genetics of the Pig; CABI: Wallingford, UK, 2011; pp. 426–444. [Google Scholar]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the Pig as a Human Biomedical Model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.C.; Sudarshan, C.D.; Khanna, R.; Roughan, J.V.; Flecknell, P.A.; Dark, J.H. A New Porcine Model of Reperfusion Injury after Lung Transplantation. Lab. Anim. 1999, 33, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.C.; Swindle, M.M. Preparation of swine for the laboratory. ILAR J. 2006, 47, 358–363. [Google Scholar] [CrossRef]
- Grandin, T. Minimizing Stress in Pig Handling in the Research Lab. Lab Anim. 1986, 15, 15–20. [Google Scholar]
- Bradbury, A.G.; Clutton, R.E. Review of practices reported for preoperative food and water restriction of laboratory pigs (Sus scrofa). J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 35–40. [Google Scholar] [PubMed]
- Anderson, D.E.; Mulon, P.Y. Anesthesia and Surgical Procedures in Swine. In Diseases of Swine; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 171–196. [Google Scholar]
- DeRouchey, J.; Goodband, B.; Tokach, M.; Dritz, S.; Nelssen, J. Digestive System of the Pig: Anatomy and Function. N. Am. Vet. Commun. Conf. 2009, 23, 375–376. [Google Scholar]
- Lin, H. Perioperative Monitoring and Management of Complications. In Farm Animal Anesthesia: Cattle, Small Ruminants, Camelids, and Pigs; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 135–158. [Google Scholar]
- Costea, R.; Tudor, R.; Degan, A.; Girdan, G. Anesthesia Complications Related to Swine Experimental Invasive Surgical Procedures. Sci. Works. Ser. C Vet. Med. 2019, 65, 2065-1295. [Google Scholar]
- Portier, K.; Ida, K.K. The ASA Physical Status Classification: What Is the Evidence for Recommending Its Use in Veterinary Anesthesia?—A Systematic Review. Front. Vet. Sci. 2018, 5, 204. [Google Scholar] [CrossRef]
- Kaiser, G.M.; Heuer, M.M.; Frühauf, N.R.; Kühne, C.A.; Broelsch, C.E. General Handling and Anesthesia for Experimental Surgery in Pigs. J. Surg. Res. 2006, 130, 73–79. [Google Scholar] [CrossRef]
- Smith, A.C.; Ehler, W.J.; Swindle, M.M. Anesthesia and Analgesia in Swine. In Anesthesia and Analgesia in Laboratory Animals; Elsevier: Amsterdam, The Netherlands, 1997; pp. 313–336. [Google Scholar]
- Hedenqvist, P. Laboratory animal analgesia, anesthesia, and euthanasia. In Handbook of Laboratory Animal Science; CRC Press: Boca Raton, FL, USA, 2021; pp. 343–378. [Google Scholar]
- Xanthos, T.; Bassiakou, E.; Koudouna, E.; Tsirikos-Karapanos, N.; Lelovas, P.; Papadimitriou, D.; Dontas, I.; Papadimitriou, L. Baseline Hemodynamics in Anesthetized Landrace–Large White Swine: Reference Values for Research in Cardiac Arrest and Cardiopulmonary Resuscitation Models. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 21–25. [Google Scholar] [PubMed]
- Dyce, K.M.; Sack, W.O.; Wensing, C.J.G. Textbook of Veterinary Anatomy; Saunders Company: Philadelphia, PA, USA, 2002; pp. 400–401. [Google Scholar]
- Singh, B. Dyce, Sack, and Wensing’s Textbook of Veterinary Anatomy; Saunders: St. Louis, MI, USA, 2018. [Google Scholar]
- Erkert, R.S.; MacAllister, C.G. Use of a eutectic mixture of lidocaine 2.5% and prilocaine 2.5% as a local anesthetic in animals. J. Am. Vet. Med. Assoc. 2005, 226, 1990–1992. [Google Scholar] [CrossRef] [PubMed]
- Swindle, M.M. Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Hodgkinson, O. Practical Sedation and Anaesthesia in Pigs. Practice 2007, 29, 34–39. [Google Scholar] [CrossRef]
- Swindle, M.M.; Sistino, J.J.; Perioperative Care. Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques; CRC Press: Boca Raton, FL, USA, 2015; p. 39. [Google Scholar]
- Clarke, K.W.; Trim, C.M. Veterinary Anaesthesia E-Book; Elsevier Health Sciences: Oxford, UK, 2013. [Google Scholar]
- Costea, R. Anestezologie; Printech: Bucharest, Romania, 2017; pp. 127–131. [Google Scholar]
- Sogebi, E.A.; Makinde, O.A.; Cliff, A.I.; Koleoso, S.; Mshelbwala, F.; Olukunle, J.O. Multimodal Approach to Surgical Pain Management in Weaner Pigs: A Clinical Trial. Alex. J. Vet. Sci. 2021, 70, 151–157. [Google Scholar] [CrossRef]
- Bollen, P.J.; Hansen, A.K.; Alstrup, A.K.O. The Laboratory Swine; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Flôres, F.N.; Tavares, S.G.; de Moraes, A.N.; Oleskovicz, N.; Santos, L.C.P.; Minsky, V.; Keshen, E. Azaperone and its association with xylazine or dexmedetomidine in pigs. Ciência Rural 2009, 39, 1101–1107. [Google Scholar] [CrossRef]
- Nussbaumer, I.; Indermühle, N.; Zimmermann, W.; Leist, Y. Piglet Castration by Injection Anaesthesia: Experience with the Azaperone, Butorphanol and Ketamine Combination. SAT Schweiz. Arch. Für Tierheilkd. 2011, 153, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Short, C.E. Preanesthetic medications in ruminants and swine. Vet. Clin. N. Am. Food Anim. Pract. 1986, 2, 553–566. [Google Scholar] [CrossRef]
- Bigby, S.E.; Carter, J.E.; Bauquier, S.; Beths, T. The Use of Alfaxalone for Premedication, Induction and Maintenance of Anaesthesia in Pigs: A Pilot Study. Vet. Anaesth. Analg. 2017, 44, 905–909. [Google Scholar] [CrossRef]
- Kat, I.; Ahern, B.J.; Dhanani, J.; Whitten, G.; Cowling, N.; Goodwin, W. Long Duration Anaesthesia in Pigs with an Infusion of Alfaxalone and Dexmedetomidine. Vet. Med. Sci. 2022, 8, 2418–2421. [Google Scholar] [CrossRef]
- Thurmon, J.C.; Benson, G.J. Anesthesia in Ruminants and Swine. Curr. Vet. Ther. 1993, 3, 58–76. [Google Scholar]
- Nishimura, R.; Kim, H.; Matsunaga, S.; Sakaguchi, M.; Sasaki, N.; Tamura, H.; Takeuchi, A. Antagonism of Medetomidine Sedation by Atipamezole in Pigs. J. Vet. Med. Sci. 1992, 54, 1237–1240. [Google Scholar] [CrossRef]
- Gruen, M.E.; Sherman, B.L.; Papich, M.G. Drugs Affecting Animal Behavior; John Willey & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Lin, H. Injectable Anesthetics and Field Anesthesia. In Farm Animal Anesthesia; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 60–100. ISBN 978-1-119-67266-1. [Google Scholar]
- Golan, D.E.; Tashjian, A.H.; Armstrong, E.J. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Svoboda, M.; Fajt, Z.; Mruvčinská, M.; Vašek, J.; Blahová, J. The Effects of Buccal Administration of Azaperone on the Sedation Level and Biochemical Variables of Weaned Piglets. Acta Vet. Brno 2021, 90, 47–56. [Google Scholar] [CrossRef]
- Svoboda, M.; Blahova, J.; Jarkovsky, J.; Zacharda, A.; Hajkova, S.; Vanhara, J.; Vasek, J. Efficacy of the Intranasal Application of Azaperone for Sedation in Weaned Piglets. Vet. Med. 2023, 68, 145–151. [Google Scholar] [CrossRef]
- Swine, L.M. Veterinary Anesthesia and Analgesia: The Fifth Edition of Lumb and Jones; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 928–940. [Google Scholar]
- Moon, P.F.; Smith, L.J. General Anesthetic Techniques in Swine. Vet. Clin. N. Am. Food Anim. Pract. 1996, 12, 663–691. [Google Scholar] [CrossRef] [PubMed]
- McGrath, C.J.; Rempel, W.E.; Addis, P.B.; Crimi, A.J. Acepromazine and Droperidol Inhibition of Halothane-Induced Malignant Hyperthermia (Porcine Stress Syndrome) in Swine. Am. J. Vet. Res. 1981, 42, 195–198. [Google Scholar] [PubMed]
- Lacoste, L.; Bouquet, S.; Ingrand, P.; Caritez, J.C.; Carretier, M.; Debaene, B. Intranasal Midazolam in Piglets: Pharmacodynamics (0.2 vs. 0.4 mg/kg) and Pharmacokinetics (0.4 mg/kg) with Bioavailability Determination. Lab. Anim. 2000, 34, 29–35. [Google Scholar] [CrossRef]
- de Souza Dantas, L.M.; Crowell-Davis, S.L. Benzodiazepines. In Veterinary Psychopharmacology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 67–102. [Google Scholar]
- Lee, J.Y.; Kim, M.C. Anesthesia of Growing Pigs with Tiletamine-Zolazepam and Reversal with Flumazenil. J. Vet. Med. Sci. 2012, 74, 335–339. [Google Scholar] [CrossRef]
- Thurmon, J.C.; Smith, G.W. Swine. In Lumb and Jones’ Veterinary Anesthesia and Analgesia, 4th ed.; Tranquili, W.J., Thurmon, J.C., Grimm, K.A., Eds.; Blackwell Publishing: Ames, IA, USA, 2007; pp. 747–764. [Google Scholar]
- Riebold, T.; Geiser, D.; Goble, D.O. Anesthetic agents and ancillary drugs. In Large Animal Anesthesia; Iowa State University Press: Ames, IA, USA, 1995; pp. 11–64. [Google Scholar]
- Sakaguchi, M.; Nishimura, R.; Sasaki, N.; Ishiguro, T.; Tamura, H.; Takeuchi, A. Anesthesia Induced in Pigs by Use of a Combination of Medetomidine, Butorphanol, and Ketamine and Its Reversal by Administration of Atipamezole. Am. J. Vet. Res. 1996, 57, 529–534. [Google Scholar]
- Ugarte, C.E.; O’Flaherty, K. The use of a medetomidine, butorphanol and atropine combination to enable blood sampling in young pigs. N. Z. Vet. J. 2005, 53, 249–252. [Google Scholar] [CrossRef]
- Bettschart-Wolfensberger, R.; Stauffer, S.; Hässig, M.; Flaherty, D.; Ringer, S.K. Racemic Ketamine in Comparison to S-Ketamine in Combination with Azaperone and Butorphanol for Castration of Pigs. Schweiz. Arch. Tierheilkd. 2013, 155, 669–675. [Google Scholar] [CrossRef]
- Lester, P.A.; Moore, R.M.; Shuster, K.A.; Myers, D.D. Anesthesia and Analgesia. In The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents; Elsevier: Amsterdam, The Netherlands, 2012; pp. 33–56. [Google Scholar]
- Kumar, A.; Mann, H.J.; Remmel, R.P. Pharmacokinetics of Tiletamine and Zolazepam (Telazol®) in Anesthetized Pigs. J. Vet. Pharmacol. Ther. 2006, 29, 587–589. [Google Scholar] [CrossRef]
- Pavlovsky, V.H.; Corona, D.; Hug, P.J.; Kümmerlen, D.; Graage, R.; Bettschart-Wolfensberger, R. Butorphanol induces anxiety-like behaviour and distress in piglets. Schweiz. Arch. Für Tierheilkd. 2021, 163, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Viscardi, A.V.; Turner, P.V. Efficacy of buprenorphine for management of surgical castration pain in piglets. BMC Vet. Res. 2018, 14, 318. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.A.; Cooper, D.M.; Risdahl, J.M. Antinociceptive activity of and clinical experience with buprenorphine in swine. J. Am. Assoc. Lab. Anim. Sci. 2001, 40, 17–20. [Google Scholar]
- Lujan, S.O.; Habre, W.; Daali, Y.; Pan, Z.; Kronen, P.W. Plasma concentrations of transdermal fentanyl and buprenorphine in pigs (Sus scrofa domesticus). Vet. Anaesth. Analg. 2017, 44, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Malavasi, L.M.; Nyman, G.; Augustsson, H.; Jacobson, M.; Jensen-Waern, M. Effects of epidural morphine and transdermal fentanyl analgesia on physiology and behaviour after abdominal surgery in pigs. Lab. Anim. 2006, 40, 16–27. [Google Scholar] [CrossRef]
- Keates, H. Induction of Anaesthesia in Pigs Using a New Alphaxalone Formulation. Vet. Rec. 2003, 153, 627–628. [Google Scholar] [CrossRef]
- Santos, M.; de Lis, B.T.B.; Tendillo, F.J. Effects of Intramuscular Dexmedetomidine in Combination with Ketamine or Alfaxalone in Swine. Vet. Anaesth. Analg. 2016, 43, 81–85. [Google Scholar] [CrossRef]
- Lervik, A.; Toverud, S.F.; Krontveit, R.; Haga, H.A. A Comparison of Respiratory Function in Pigs Anaesthetised by Propofol or Alfaxalone in Combination with Dexmedetomidine and Ketamine. Acta Vet. Scand. 2020, 62, 14. [Google Scholar] [CrossRef]
- Satas, S.; Johannessen, S.I.; Hoem, N.-O.; Haaland, K.; Sorensen, D.R.; Thoresen, M. Lidocaine Pharmacokinetics and Toxicity in Newborn Pigs. Anesth. Analg. 1997, 85, 306. [Google Scholar]
- Romera, A.; Cebollero, M.; Romero-Gómez, B.; Carricondo, F.; Zapatero, S.; García-Aldao, U.; Martín-Albo, L.; Ortega, J.; Vara, E.; Garutti, I.; et al. Effect of intravenous lidocaine on inflammatory and apoptotic response of ischemia-reperfusion injury in pigs undergoing lung resection surgery. BioMed Res. Int. 2021, 2021, 6630232. [Google Scholar] [CrossRef]
- Garutti, I.; Rancan, L.; Simón, C.; Cusati, G.; Sanchez-Pedrosa, G.; Moraga, F.; Olmedilla, L.; Lopez-Gil, M.T.; Vara, E. Intravenous lidocaine decreases tumor necrosis factor alpha expression both locally and systemically in pigs undergoing lung resection surgery. Anesth. Analg. 2014, 119, 815–828. [Google Scholar] [CrossRef]
- Smith, J.S.; Gebert, J.E.; Ebner, L.S.; Bennett, K.O.; Collins, R.J.; Hampton, C.E.; Kleine, S.A.; Mulon, P.-Y.; Smith, C.K.; Seddighi, R. Pharmacokinetics of Intramuscular Maropitant in Pigs (Sus scrofa domesticus). J. Vet. Pharmacol. Ther. 2023, 46, 158–164. [Google Scholar] [CrossRef]
- Hay Kraus, B.L. Spotlight on the perioperative use of maropitant citrate. Vet. Med. Res. Rep. 2017, 8, 41–51. [Google Scholar] [CrossRef]
- Bradbury, A.G.; Clutton, R.E. Are neuromuscular blocking agents being misused in laboratory pigs? Br. J. Anaesth. 2016, 116, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.; Kruhøffer, L.L.; Lykkesfeldt, J.; Kousholt, B.S. Comparison of the neuromuscular effects of two infusion rates of rocuronium in anesthetized pigs. Acta Vet. Scand. 2022, 64, 38. [Google Scholar] [CrossRef] [PubMed]
- Pehböck, D.; Dietrich, H.; Klima, G.; Paal, P.; Lindner, K.H.; Wenzel, V. Anesthesia in Swine. Anaesthesist 2015, 64, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Amornyotin, S. Ketofol: A Combination of Ketamine and Propofol. J. Anesth. Crit. Care Open Access 2014, 1, 00031. [Google Scholar]
- Chum, H.; Pacharinsak, C. Endotracheal Intubation in Swine. Lab. Anim. 2012, 41, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Mirra, A.; Spadavecchia, C.; Micieli, F. Intubation in Swine: What Recumbency to Choose? Animals 2022, 12, 2430. [Google Scholar] [CrossRef]
- Mirra, A.; Arnold, M.; Casoni, D.; Maidanskaia, E.G.; Casalta, L.G.G.; Levionnois, O. Fatal upper airway obstruction in a pig after general anaesthesia. Vet. Anaesth. Analg. 2022, 49, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Janiszewski, A.; Pasławski, R.; Skrzypczak, P.; Paslawska, U.; Szuba, A.; Nicpoń, J. The Use of a Plastic Guide Improves the Safety and Reduces the Duration of Endotracheal Intubation in the Pig. J. Vet. Med. Sci. 2014, 76, 1317–1320. [Google Scholar] [CrossRef] [PubMed]
- Steinbacher, R.; Von Ritgen, S.; Moens, Y.P.S. Laryngeal Perforation during a Standard Intubation Procedure in a Pig. Lab. Anim. 2012, 46, 261–263. [Google Scholar] [CrossRef]
- Morath, U.; Skogmo, H.K.; Ranheim, B.; Levionnois, O.L. The use of bougie-guided insertion of a laryngeal mask airway device in neonatal piglets after unexpected complications. Vet. Rec. Case Rep. 2014, 2, e000040. [Google Scholar] [CrossRef]
- Beths, T. TIVA/TCI in Veterinary Practice. Total Intravenous Anesthesia and Target Controlled Infusions: A Comprehensive Global Anthology; Springer: Cham, Switzerland, 2017; pp. 589–618. [Google Scholar] [CrossRef]
- Costea, R.; Tanase, A.; Ioniță, L.; Copaescu, C.; Girjoaba, I.; Mocanu, J.; Drugociu, D.S. Inhalatory anaesthesia in pigs for laparascopic surgery. Lucr. Științifice Med. Vet. Univ. Științe Agric. Și Med. Vet. Ion Ionescu Brad Iași 2009, 52, 503–505. [Google Scholar]
- Rosenberg, H.; Pollock, N.; Schiemann, A.; Bulger, T.; Stowell, K. Malignant Hyperthermia: A Review. Orphanet J. Rare Dis. 2015, 10, 93. [Google Scholar] [CrossRef]
- Suckow, M.A.; Stevens, K.A.; Wilson, R.P. The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Organ Damage: A Current Perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Davis, B.L.; Brooks, T.A.; Coetzee, J.F. The Physiological and Behavioral Response of Pigs Castrated with and without Anesthesia or Analgesia. J. Anim. Sci. 2012, 90, 2211–2221. [Google Scholar] [CrossRef]
- Holman, S.D.; Gierbolini-Norat, E.M.; Lukasik, S.L.; Campbell-Malone, R.; Ding, P.; German, R.Z. Duration of Action of Bupivacaine Hydrochloride Used for Palatal Sensory Nerve Block in Infant Pigs. J. Vet. Dent. 2014, 31, 92–95. [Google Scholar] [CrossRef]
- Costea, R.; Degan, A.; Tudor, R. Crystalloids/Colloids Ratio for Fluid Resuscitation during Anesthesia. Sci. Works. Ser. C Vet. Med. 2017, 63, 65–66. [Google Scholar]
- Malavasi, L.M.; Jensen-Waern, M.; Augustsson, H.; Nyman, G. Changes in Minimal Alveolar Concentration of Isoflurane Following Treatment with Medetomidine and Tiletamine/Zolazepam, Epidural Morphine or Systemic Buprenorphine in Pigs. Lab. Anim. 2008, 42, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Allaouchiche, B.; Duflo, F.; Tournadre, J.-P.; Chassard, D. Influence of Sepsis on Sevoflurane (SEV) Minimum Alveolar Concentration (MAC) in a Swine Model. Eur. J. Anaesthesiol. EJA 2000, 17, 57. [Google Scholar] [CrossRef]
- Mirra, A.; Gamez Maidanskaia, E.; Carmo, L.P.; Levionnois, O.; Spadavecchia, C. How is depth of anaesthesia assessed in experimental pigs? A scoping review. PLoS ONE 2023, 18, e0283511. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, A.; Moll, X.; García, F.; Andaluz, A. Neuromuscular block monitoring after the administration of 1 mg/kg intravenous cisatracurium in the anaesthetized pig. J. Vet. Pharmacol. Ther. 2019, 42, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Seddighi, R. Miniature Companion Pig Sedation and Anesthesia. Vet. Clin. Exot. Anim. Pract. 2022, 25, 297–319. [Google Scholar] [CrossRef]
- Lin, H. Comparative Anesthesia and Analgesia of Ruminants and Swine. In Veterinary Anesthesia and Analgesia: The Fifth Edition of Lumb and Jones; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 743–753. [Google Scholar]
- Stadlbauer, K.H.; Wagner-Berger, H.G.; Raedler, C.; Voelckel, W.G.; Wenzel, V.; Krismer, A.C.; Klima, G.; Rheinberger, K.; Nussbaumer, W.; Pressmar, D. Vasopressin, but Not Fluid Resuscitation, Enhances Survival in a Liver Trauma Model with Uncontrolled and Otherwise Lethal Hemorrhagic Shock in Pigs. J. Am. Soc. Anesthesiol. 2003, 98, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Swindle, M.M.; Smith, A.C. Best Practices for Performing Experimental Surgery in Swine. J. Investig. Surg. 2013, 26, 63–71. [Google Scholar] [CrossRef]
- Tanwar, P.; Naagar, M.; Malik, G.; Alam, M.S.; Singh, T.; Singh, O.; Maity, M.K. A Review on Malignant Hyperthermia: Epidemiology, Etiology, Risk Factors, Diagnosis, Clinical Management and Treatment Modalities. World J. Biol. Pharm. Health Sci. 2023, 13, 138–161. [Google Scholar] [CrossRef]
- O’Brien, P.J.; Shen, H.; Cory, C.R.; Zhang, X. Use of a DNA-Based Test for the Mutation Associated with Porcine Stress Syndrome (Malignant Hyperthermia) in 10,000 Breeding Swine. J. Am. Vet. Med. Assoc. 1993, 203, 842–851. [Google Scholar]
- El-Hayek, R.; Parness, J.; Valdivia, H.H.; Coronado, R.; Hogan, K. Dantrolene and Azumolene Inhibit [3H] PN200-110 Binding to Porcine Skeletal Muscle Dihydropyridine Receptors. Biochem. Biophys. Res. Commun. 1992, 187, 894–900. [Google Scholar] [CrossRef]
- Do Carmo, P.L.; Zapata-Sudo, G.; Trachez, M.M.; Antunes, F.; Guimarães, S.E.F.; Debom, R.; Rizzi, M.D.R.; Sudo, R.T. Intravenous Administration of Azumolene to Reverse Malignant Hyperthermia in Swine. J. Vet. Intern. Med. 2010, 24, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Schütte, J.K.; Becker, S.; Burmester, S.; Starosse, A.; Lenz, D.; Kröner, L.; Wappler, F.; Gerbershagen, M.U. Comparison of the Therapeutic Effectiveness of a Dantrolene Sodium Solution and a Novel Nanocrystalline Suspension of Dantrolene Sodium in Malignant Hyperthermia Normal and Susceptible Pigs. Eur. J. Anaesthesiol. EJA 2011, 28, 256–264. [Google Scholar] [CrossRef]
- Wu, X.; Kochanek, P.M.; Cochran, K.; Nozari, A.; Henchir, J.; Stezoski, S.W.; Wagner, R.; Wisniewski, S.; Tisherman, S.A. Mild Hypothermia Improves Survival after Prolonged, Traumatic Hemorrhagic Shock in Pigs. J. Trauma Acute Care Surg. 2005, 59, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Couto, M.; Cates, C. Laboratory Guidelines for Animal Care. In Vertebrate Embryogenesis: Embryological, Cellular, and Genetic Methods; Springer Science+Business Media, LLC: Berlin/Heidelberg, Germany, 2019; pp. 407–430. [Google Scholar] [CrossRef]
- Hubrecht, R.C.; Carter, E. The 3Rs and Humane Experimental Technique: Implementing Change. Animals 2019, 9, 754. [Google Scholar] [CrossRef] [PubMed]

| Agent | Dose | Route | Considerations, References |
|---|---|---|---|
| Azaperone | 1–8 mg/kg (2–5 mg/kg mean) | IM | 20 min to effect, sedative [21] |
| Acepromazine | 0.03–1.1 mg/kg | IM, IV | tranquilizer [21,22] |
| Alfaxalone | 5 mg/kg | IM | sedation [23,24] |
| Diazepam | 0.2–1 mg/kg | IV | mild sedative [21,24] |
| Midazolam | 0.1–0.5 mg/kg | IM, IV | sedation [21,24] |
| Xylazine | 1–2 mg/kg | IM, IV | pigs are the least sensitive to xylazine [11] |
| Medetomidine | 0.03–0.08 mg/kg | IM, IV | sedation and muscle relaxation [11,22] |
| Ketamine | 2–30 mg/kg | IM, IV | poor muscle relaxation and analgesia [21,24,25] |
| Buprenorphine | 0.01–0.05 mg/kg q 8–12 h. | IM, SC | significant respiratory depression [14,26] |
| Butorphanol | 0.1–0.3 mg/kg q 4–6 h. | IM, IV | analgesia, short duration [21,25] |
| Tiletamine/Zolazepam Telazol® | 2–8.8 mg/kg | IM, IV | sedation or anesthesia for minor surgery, 20–30 min, reversed with flumazenil 0.08 mg/kg [23] |
| Naloxone | 0.5–2 mg/kg | IV | [21] |
| Glycopyrrolate | 0.005–0.01 mg/kg | IM, IV | correct bradycardia, decrease salivation [9,15] |
| Atropine | 0.02–0.04 mg/kg | IM, IV | correct bradycardia, decrease salivation [9,26] |
| Combinations | |||
| Azaperone Midazolam | 4 mg/kg azaperone | IM | [27,28] |
| 1 mg/kg midazolam | |||
| Azaperone Xylazine | 2 mg/kg azaperone | IM | [27,28] |
| 2 mg/kg xylazine | |||
| Azaperone Butorphanol Ketamine | 5 mg azaperone, | IM | [28,29] |
| 0.2 mg butorphanol | |||
| 15 mg ketamine | |||
| Azaperone Xylazine Ketamine | 6 mg/kg azaperone | IM | [28,30] |
| 2 mg/kg xylazine | |||
| 15 mg/kg ketamine | |||
| Azaperone Midazolam Ketamine | 2 mg/kg azaperone | IM | [21,28] |
| 0.3 mg/kg midazolam | |||
| 15 mg/kg ketamine | |||
| Acepromazine Ketamine | 1.1 mg/kg acepromazine | IM | [21] |
| 33 mg/kg ketamine | |||
| Alfaxalone Butorphanol Medetomidine | 4 mg/kg alfaxalone | IM | [31] |
| 0.4 mg/kg butorphanol | |||
| 40 μg/kg medetomidine | |||
| Dexmedetomidine Ketamine Methadone | 10 μg/kg dexmedetomidine | IM | Premedication, facilitate intubation [32] |
| 10 mg/kg ketamine | |||
| 0.25–0.4 mg/kg methadone | |||
| Xylazine Ketamine | 1–2 mg/kg xylazine | IM | Premedication, short-term anesthesia [12,33] |
| 10–20 mg/kg ketamine | |||
| Medetomidine Ketamine | 0.04–08 mg/kg medetomidine | IV, IM | Premedication, short-term anesthesia [34] |
| 10 mg/kg ketamine | |||
| 10 mg/kg ketamine |
| Agent | Dose | Route | Considerations, References |
|---|---|---|---|
| Propofol | 2–5 mg/kg | IV | [37,68] |
| Propofol Fentanyl | 2 mg/kg 5 µg/kg | IV | allows intubation [14,46] |
| Dexmedetomidine Propofol | 20–40 µg/kg dexmedetomidine 2–4 mg/kg propofol | [46] | |
| Propofol Ketamine | 1–1.5 mg/kg propofol 0.5–1 mg/kg ketamine | IV | sedation, induction, no respiratory depression, good recovery [68,69] |
| Alfaxalone | 0.6–1.1 mg/kg | IV, IM | [46] |
| Etomidate | 2–4 mg/kg | IV | provides cardiovascular stability [46,69] |
| Thiopental | 10–20 mg/kg | IV | apnea, prolonged recovery [9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costea, R.; Ene, I.; Pavel, R. Pig Sedation and Anesthesia for Medical Research. Animals 2023, 13, 3807. https://doi.org/10.3390/ani13243807
Costea R, Ene I, Pavel R. Pig Sedation and Anesthesia for Medical Research. Animals. 2023; 13(24):3807. https://doi.org/10.3390/ani13243807
Chicago/Turabian StyleCostea, Ruxandra, Ioana Ene, and Ruxandra Pavel. 2023. "Pig Sedation and Anesthesia for Medical Research" Animals 13, no. 24: 3807. https://doi.org/10.3390/ani13243807
APA StyleCostea, R., Ene, I., & Pavel, R. (2023). Pig Sedation and Anesthesia for Medical Research. Animals, 13(24), 3807. https://doi.org/10.3390/ani13243807






