Changes in Head and Pelvic Movement Symmetry after Diagnostic Anaesthesia: Interactions between Subjective Judgement Categories and Commonly Applied Blocks
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Gait Analysis
2.3. Data Processing
2.4. Subjective Categorisation of Diagnostic Block Effects
2.5. Data Analysis
3. Results
3.1. Effect of “Type” and “Efficacy” on Changes in Movement Symmetry after Diagnostic Anaesthesia
3.1.1. Forelimb Diagnostic Anaesthesia
3.1.2. Hind Limb Diagnostic Anaesthesia
4. Discussion
4.1. Compensatory Movement Changes after Forelimb Diagnostic Anaesthesia
4.2. Compensatory Movement Changes after Hind Limb Diagnostic Anaesthesia
4.3. Effect of Forelimb Diagnostic Anaesthesia “Type”
4.4. Effect of Hind Limb Diagnostic Anaesthesia Type
4.5. Magnitude of Changes
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Adair, S.; Baus, M.; Bell, R.; Boero, M.; Bussy, C.; Cardenas, F.; Casey, T.; Castro, J.; Davis, W.; Erskine, M.; et al. Letter to the Editor: A Response to ‘What Is Lameness and What (or Who) Is the Gold Standard to Detect It?’. Equine Vet. J. 2019, 51, 270–272. [Google Scholar] [CrossRef]
- Arkell, M.; Archer, R.M.; Guitian, F.J.; May, S.A. Evidence of Bias Affecting the Interpretation of the Results of Local Anaesthetic Nerve Blocks When Assessing Lameness in Horses. Vet. Rec. 2006, 159, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Maliye, S.; Voute, L.; Lund, D.; Marshall, J.F. An Inertial Sensor-Based System Can Objectively Assess Diagnostic Anaesthesia of the Equine Foot. Equine Vet. J. 2013, 45, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Leelamankong, P.; Estrada, R.; Mählmann, K.; Rungsri, P.; Lischer, C. Agreement among Equine Veterinarians and between Equine Veterinarians and Inertial Sensor System during Clinical Examination of Hindlimb Lameness in Horses. Equine Vet. J. 2020, 52, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, A.; O’Leary, L.; Bolt, D.; Fiske-Jackson, A.; Berner, D.; Smith, R. Retrospective Analysis of Oblique and Straight Distal Sesamoidean Ligament Desmitis in 52 Horses. Equine Vet. J. 2021, 54, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Maliye, S.; Voute, L.C.; Marshall, J.F. Naturally-Occurring Forelimb Lameness in the Horse Results in Significant Compensatory Load Redistribution during Trotting. Vet. J. 2015, 204, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Maliye, S.; Marshall, J.F. Objective Assessment of the Compensatory Effect of Clinical Hind Limb Lameness in Horses: 37 Cases (2011–2014). Am. J. Vet. Res. 2016, 249, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Marunova, E.; Hoenecke, K.; Fiske-Jackson, A.; Smith, R.K.W.; Bolt, D.M.; Perrier, M.; Gerdes, C.; Hernlund, E.; Rhodin, M.; Pfau, T. Changes in Head, Withers, and Pelvis Movement Asymmetry in Lame Horses as a Function of Diagnostic Anesthesia Outcome, Surface and Direction. J. Equine Vet. Sci. 2022, 118, 104136. [Google Scholar] [CrossRef]
- Pfau, T.; Bolt, D.M.; Fiske-Jackson, A.; Gerdes, C.; Hoenecke, K.; Lynch, L.; Perrier, M.; Smith, R.K.W. Linear Discriminant Analysis for Investigating Differences in Upper Body Movement Symmetry in Horses before/after Diagnostic Analgesia in Relation to Expert Judgement. Animals 2022, 12, 762. [Google Scholar] [CrossRef]
- Rungsri, P.K.; Staecker, W.; Leelamankong, P.; Estrada, R.J.; Schulze, T.; Lischer, C.J. Use of Body-Mounted Inertial Sensors to Objectively Evaluate the Response to Perineural Analgesia of the Distal Limb and Intra-Articular Analgesia of the Distal Interphalangeal Joint in Horses with Forelimb Lameness. J. Equine Vet. Sci. 2014, 34, 972–977. [Google Scholar] [CrossRef]
- Bell, R.P.; Reed, S.K.; Schoonover, M.J.; Whitfield, C.T.; Yonezawa, Y.; Maki, H.; Pai, P.F.; Keegan, K.G. Associations of Force Plate and Body-Mounted Inertial Sensor Measurements for Identification of Hind Limb Lameness in Horses. Am. J. Vet. Res. 2016, 77, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Keegan, K.G.; MacAllister, C.G.; Wilson, D.A.; Gedon, C.A.; Kramer, J.; Yonezawa, Y.; Maki, H.; Pai, P.F. Comparison of an Inertial Sensor System with a Stationary Force Plate for Evaluation of Horses with Bilateral Forelimb Lameness. Am. J. Vet. Res. 2012, 73, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Pfau, T.; Witte, T.H.; Wilson, A.M. A Method for Deriving Displacement Data during Cyclical Movement Using an Inertial Sensor. J. Exp. Biol. 2005, 208, 2503–2514. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.K.; Kramer, J.; Thombs, L.; Pitts, J.B.; Wilson, D.A.; Keegan, K.G. Comparison of Results for Body-Mounted Inertial Sensor Assessment with Final Lameness Determination in 1,224 Equids. J. Am. Vet. Med. Assoc. 2020, 256, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Pfau, T.; Stubbs, N.C.; Kaiser, L.J.; Brown, L.E.A.; Clayton, H.M. Effect of Trotting Speed and Circle Radius on Movement Symmetry in Horses during Lunging on a Soft Surface. Am. J. Vet. Res. 2012, 73, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Greve, L.; Pfau, T.; Dyson, S. Alterations in Body Lean Angle in Lame Horses before and after Diagnostic Analgesia in Straight Lines in Hand and on the Lunge. Vet. J. 2018, 239, 1–6. [Google Scholar] [CrossRef] [PubMed]
- McCracken, M.J.; Kramer, J.; Keegan, K.G.; Lopes, M.; Wilson, D.A.; Reed, S.K.; LaCarrubba, A.; Rasch, M. Comparison of an Inertial Sensor System of Lameness Quantification with Subjective Lameness Evaluation. Equine Vet. J. 2012, 44, 652–656. [Google Scholar] [CrossRef]
- Hardeman, A.M.; Serra Bragança, F.M.; Swagemakers, J.H.; Weeren, P.R.; Roepstorff, L. Variation in Gait Parameters Used for Objective Lameness Assessment in Sound Horses at the Trot on the Straight Line and the Lunge. Equine Vet. J. 2019, 51, 831–839. [Google Scholar] [CrossRef]
- Keegan, K.G.; Kramer, J.; Yonezawa, Y.; Maki, H.; Pai, P.F.; Dent, E.V.; Kellerman, T.E.; Wilson, D.A.; Reed, S.K. Assessment of Repeatability of a Wireless Inertial Sensor-Based Lameness Evaluation System for Horses. Am. J. Vet. Res. 2011, 72, 1156–1163. [Google Scholar] [CrossRef]
- Keegan, K.G.; Wilson, D.A.; Kramer, J.; Reed, S.K.; Yonezawa, Y.; Maki, H.; Pai, P.F.; Lopes, M.A.F. Comparison of a Body-Mounted Inertial Sensor System-Based Method with Subjective Evaluation for Detection of Lameness in Horses. Am. J. Vet. Res. 2013, 74, 17–24. [Google Scholar] [CrossRef]
- Macaire, C.; Hanne-Poujade, S.; De Azevedo, E.; Denoix, J.-M.; Coudry, V.; Jacquet, S.; Bertoni, L.; Tallaj, A.; Audigié, F.; Hatrisse, C.; et al. Investigation of Thresholds for Asymmetry Indices to Represent the Visual Assessment of Single Limb Lameness by Expert Veterinarians on Horses Trotting in a Straight Line. Animals 2022, 12, 3498. [Google Scholar] [CrossRef]
- Parkes, R.S.V.; Weller, R.; Groth, A.M.; May, S.; Pfau, T. Evidence of the Development of ‘Domain-Restricted’ Expertise in the Recognition of Asymmetric Motion Characteristics of Hindlimb Lameness in the Horse. Equine Vet. J. 2009, 41, 112–117. [Google Scholar] [CrossRef]
- Bathe, A.; Judy, C.E.; Dyson, S.J. Letter to the Editor: Do We Have to Redefine Lameness in the Era of Quantitative Gait Analysis? Equine Vet. J. 2018, 50, 273. [Google Scholar] [CrossRef]
- Starke, S.D.; May, S.A. Expert Visual Assessment Strategies for Equine Lameness Examinations in a Straight Line and Circle: A Mixed Methods Study Using Eye Tracking. Vet. Rec. 2022, 191, e1684. [Google Scholar] [CrossRef]
- Serra Bragança, F.M.; Hernlund, E.; Thomsen, M.H.; Waldern, N.M.; Rhodin, M.; Byström, A.; Van Weeren, P.R.; Weishaupt, M.A. Adaptation Strategies of Horses with Induced Forelimb Lameness Walking on a Treadmill. Equine Vet. J. 2021, 53, 600–611. [Google Scholar] [CrossRef]
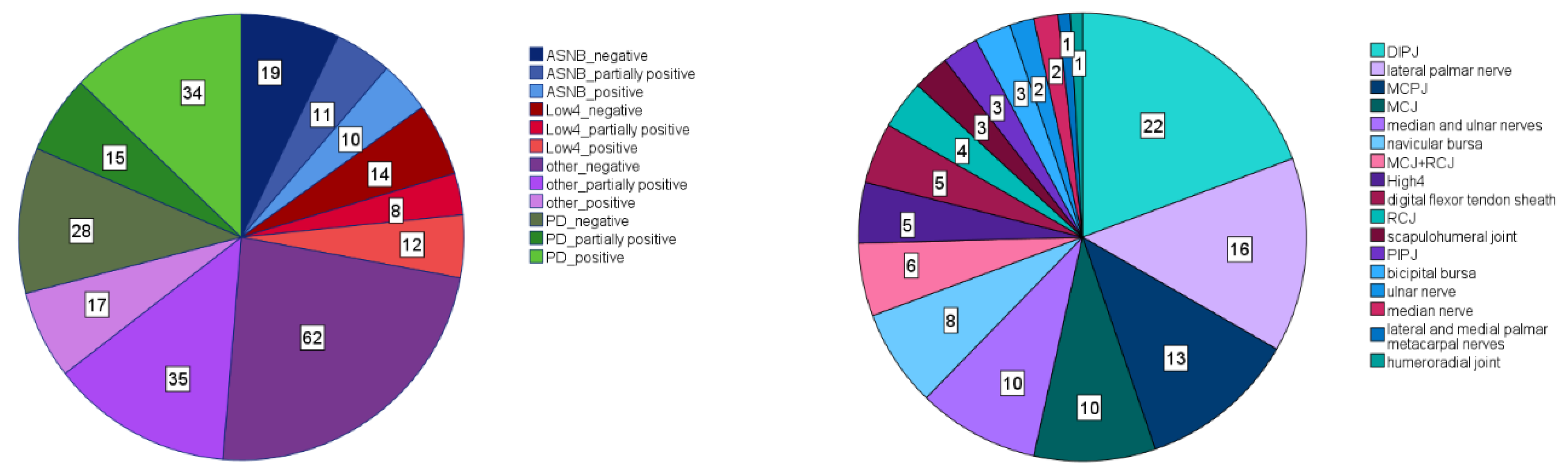

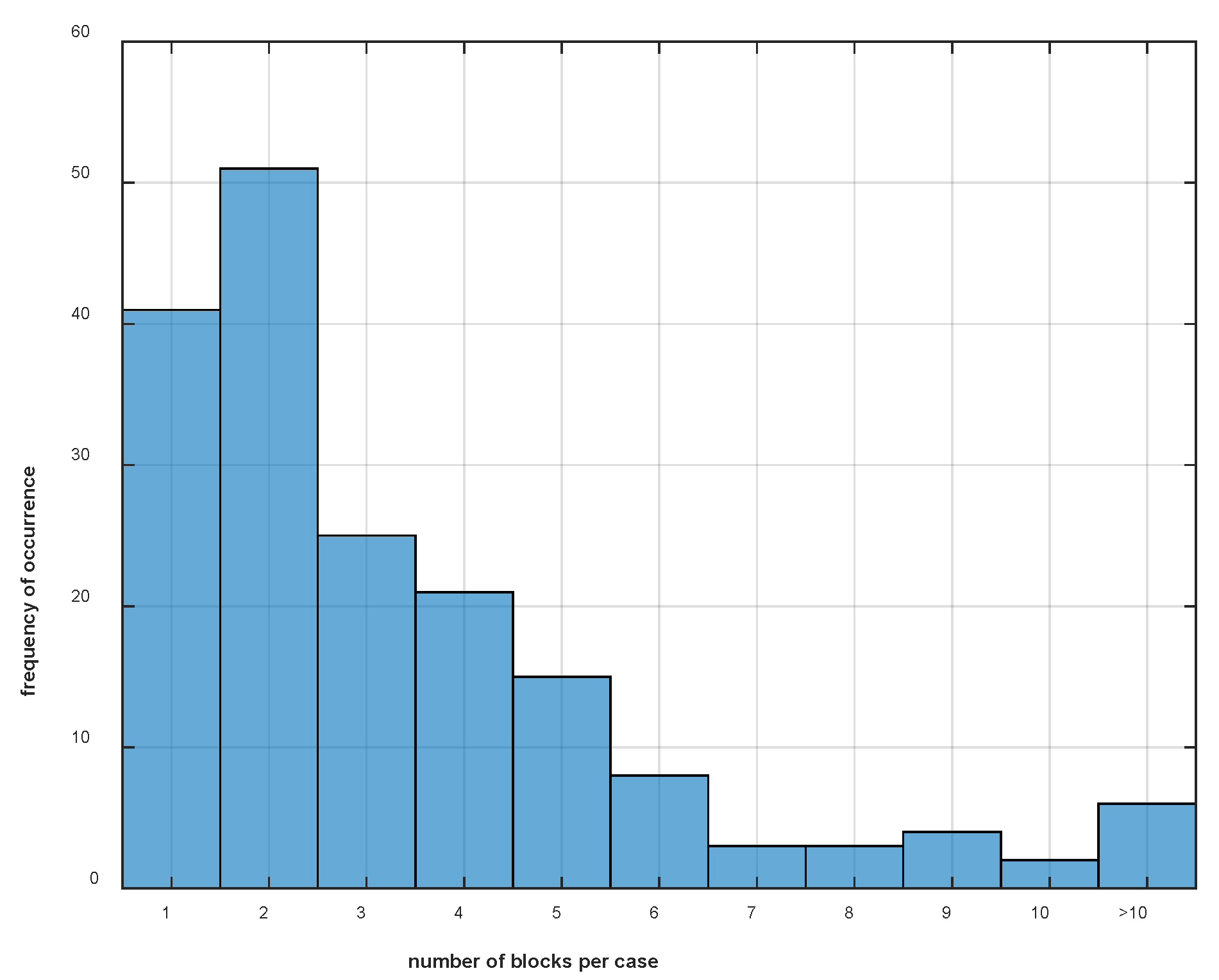
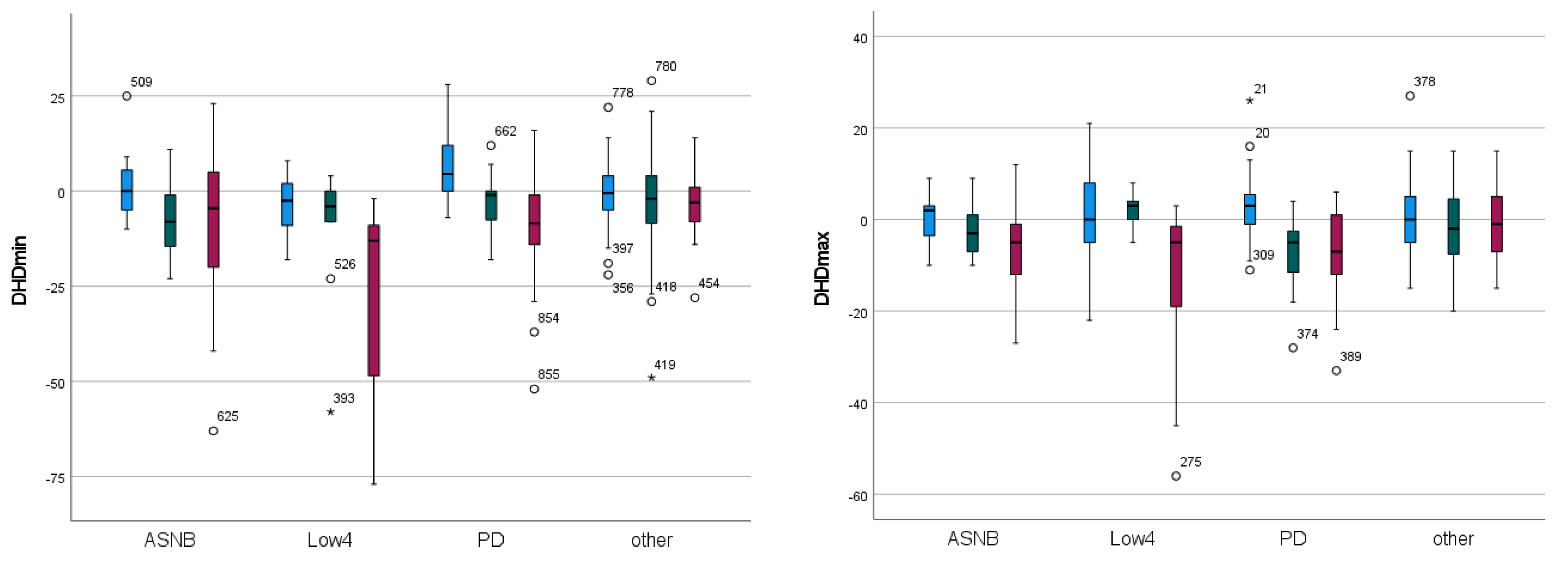
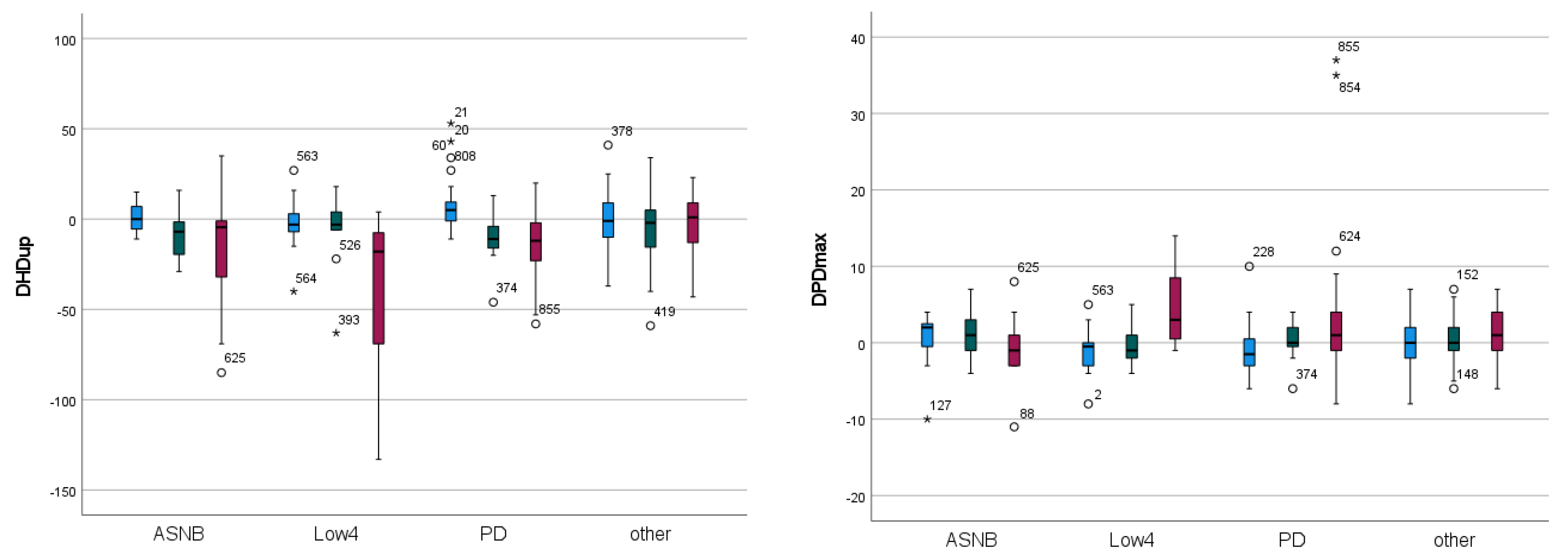

| Forelimb | Significance | “Efficacy” EMM | “Type” EMM | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Param. | Efficacy | Block Type | 2-Way | Neg. | Part. Pos. | Pos. | ASNB | Low4 | PD | Other |
| DHDmin | <0.001 | <0.001 | 0.023 | 1.498 | −6.536 | −12.160 | −5.223 | −13.369 | −2.272 | −2.068 |
| DHDmax | <0.001 | 0.121 | 0.001 | 0.895 | −2.355 | −7.298 | −3.080 | −3.627 | −4.120 | −0.850 |
| DHDup | <0.001 | 0.007 | 0.004 | 1.978 | −8.430 | −18.552 | −8.306 | −16.077 | −6.401 | −2.554 |
| DPDmin | 0.907 | 0.813 | 0.603 | 0.610 | 0.819 | 0.885 | 0.783 | 1.187 | 0.630 | 0.485 |
| DPDmax | 0.031 | 0.668 | 0.003 | −0.554 | 0.533 | 1.489 | 0.245 | 0.849 | 0.773 | 0.090 |
| DPDup | 0.187 | 0.601 | 0.260 | 0.295 | 1.474 | 2.435 | 1.864 | 1.860 | 1.352 | 0.529 |
| DHHD | 0.153 | 0.418 | 0.560 | 0.268 | −0.443 | 2.659 | −0.492 | 1.720 | 1.839 | 0.245 |
| Hind Limb | Significance | Block Efficacy EMM | Block Type EMM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Param. | Efficacy | Block Type | 2-Way | Neg. | Part. Pos. | Pos. | ASNB | DBLPN | Low 6 | MTPJ | TMTJ | Other |
| DHDmin | <0.001 | 0.090 | 0.208 | 0.750 | −4.930 | −2.329 | −1.804 | −2.978 | −2.667 | −5.253 | 0.177 | −0.492 |
| DHDmax | 0.093 | 0.304 | 0.067 | 1.149 | −0.611 | 1.209 | 1.290 | −0.729 | −0.410 | 2.108 | 0.875 | 0.361 |
| DHDup | 0.001 | 0.446 | 0.100 | 1.776 | −5.187 | −0.532 | −0.263 | −2.846 | −2.744 | −2.723 | 1.206 | −0.515 |
| DPDmin | <0.001 | 0.024 | 0.020 | 0.230 | −3.360 | −2.840 | 0.537 | −2.559 | −2.821 | −3.326 | −1.385 | −2.387 |
| DPDmax | <0.001 | 0.034 | 0.168 | −0.662 | −3.742 | −4.082 | −3.088 | −3.335 | −3.274 | −2.095 | −1.461 | −3.720 |
| DPDup | <0.001 | 0.003 | 0.019 | −0.421 | −7.516 | −7.134 | −2.466 | −6.588 | −6.479 | −5.498 | −2.594 | −6.515 |
| DHHD | 0.001 | 0.442 | 0.787 | 0.301 | −5.270 | −3.296 | −2.749 | −4.687 | −2.317 | −3.818 | −0.868 | −2.092 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfau, T.; Clark, K.S.; Bolt, D.M.; Lai, J.S.; Perrier, M.; Rhodes, J.B.; Smith, R.K.; Fiske-Jackson, A. Changes in Head and Pelvic Movement Symmetry after Diagnostic Anaesthesia: Interactions between Subjective Judgement Categories and Commonly Applied Blocks. Animals 2023, 13, 3769. https://doi.org/10.3390/ani13243769
Pfau T, Clark KS, Bolt DM, Lai JS, Perrier M, Rhodes JB, Smith RK, Fiske-Jackson A. Changes in Head and Pelvic Movement Symmetry after Diagnostic Anaesthesia: Interactions between Subjective Judgement Categories and Commonly Applied Blocks. Animals. 2023; 13(24):3769. https://doi.org/10.3390/ani13243769
Chicago/Turabian StylePfau, Thilo, Kaitlyn Sophia Clark, David M. Bolt, Jaclyn Samantha Lai, Melanie Perrier, Jessica Bryce Rhodes, Roger K. Smith, and Andrew Fiske-Jackson. 2023. "Changes in Head and Pelvic Movement Symmetry after Diagnostic Anaesthesia: Interactions between Subjective Judgement Categories and Commonly Applied Blocks" Animals 13, no. 24: 3769. https://doi.org/10.3390/ani13243769
APA StylePfau, T., Clark, K. S., Bolt, D. M., Lai, J. S., Perrier, M., Rhodes, J. B., Smith, R. K., & Fiske-Jackson, A. (2023). Changes in Head and Pelvic Movement Symmetry after Diagnostic Anaesthesia: Interactions between Subjective Judgement Categories and Commonly Applied Blocks. Animals, 13(24), 3769. https://doi.org/10.3390/ani13243769





