Effects of Oregano Essential Oil on IgA+, IgG+, and IgM+ Cells in the Jejunum of Castrated Holstein Bulls
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Experimental Design and Feeding Management
2.3. Hematoxylin–Eosin (HE) Staining
2.4. Enzyme-Linked Immunosorbent Assay
2.5. Multiplex Immunofluorescence
2.6. Immunohistochemical Staining
2.7. Statistical Analysis
3. Results
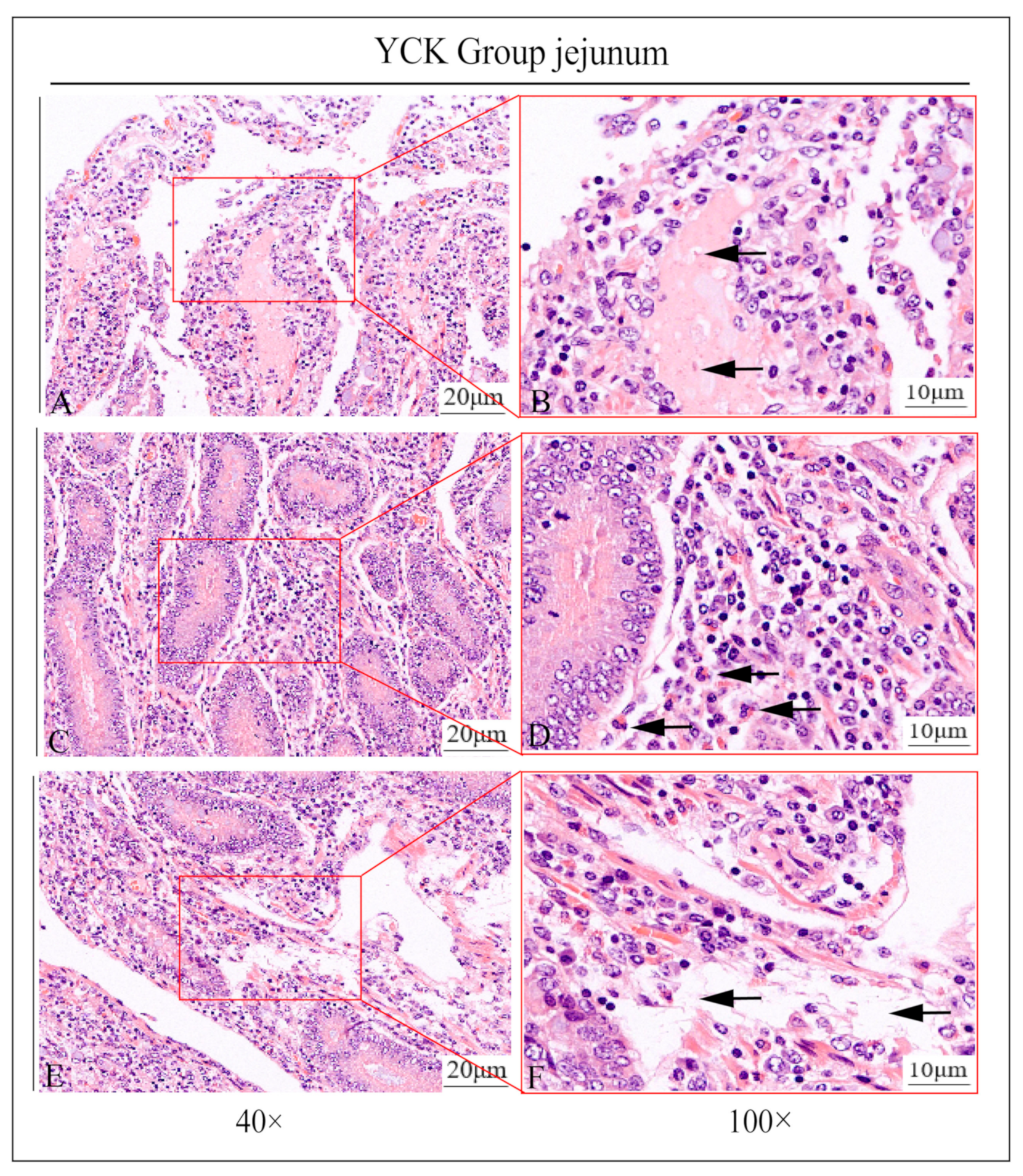
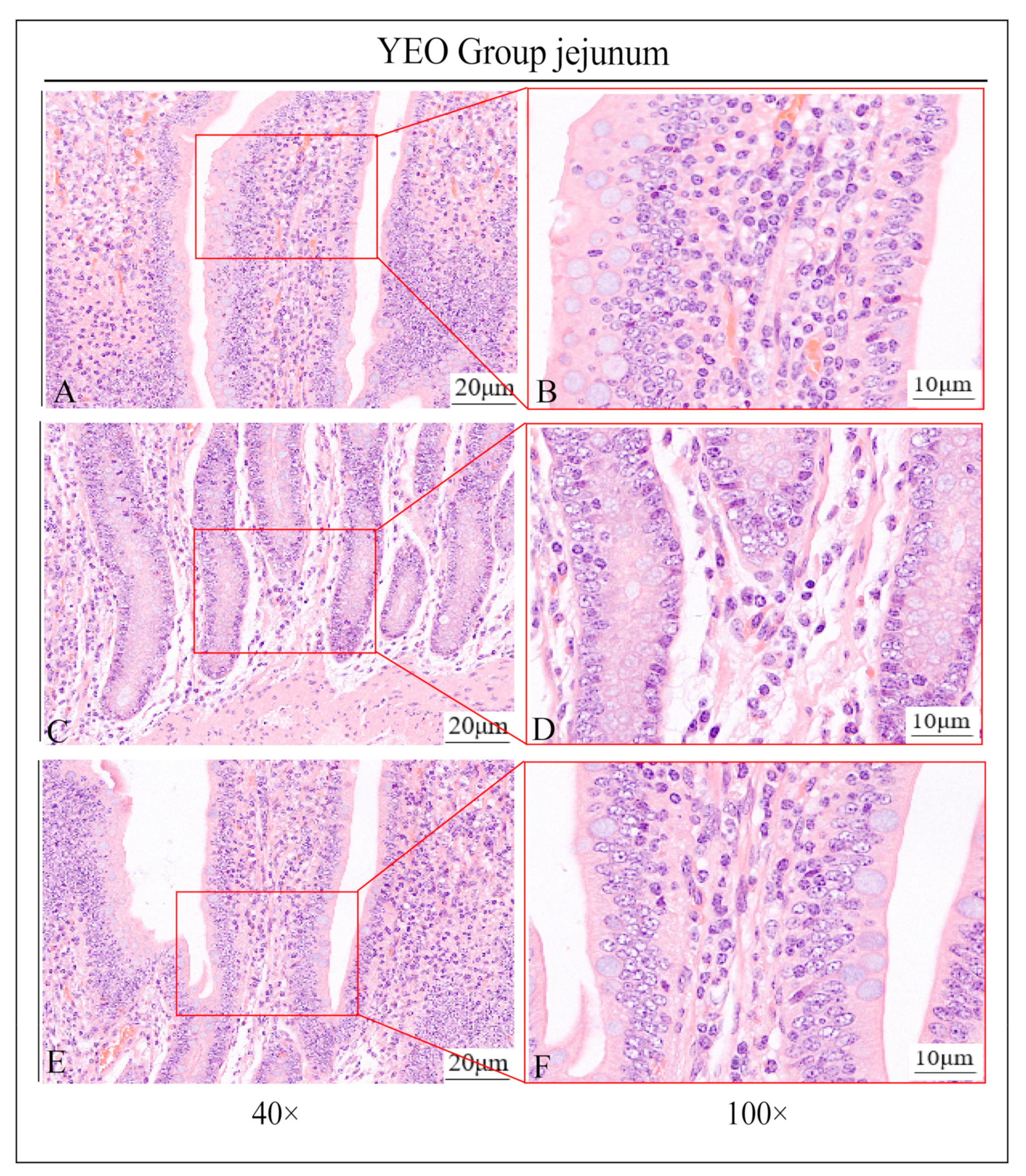
3.1. Pathologic Jejunum Changes in the YCK and YEO Groups
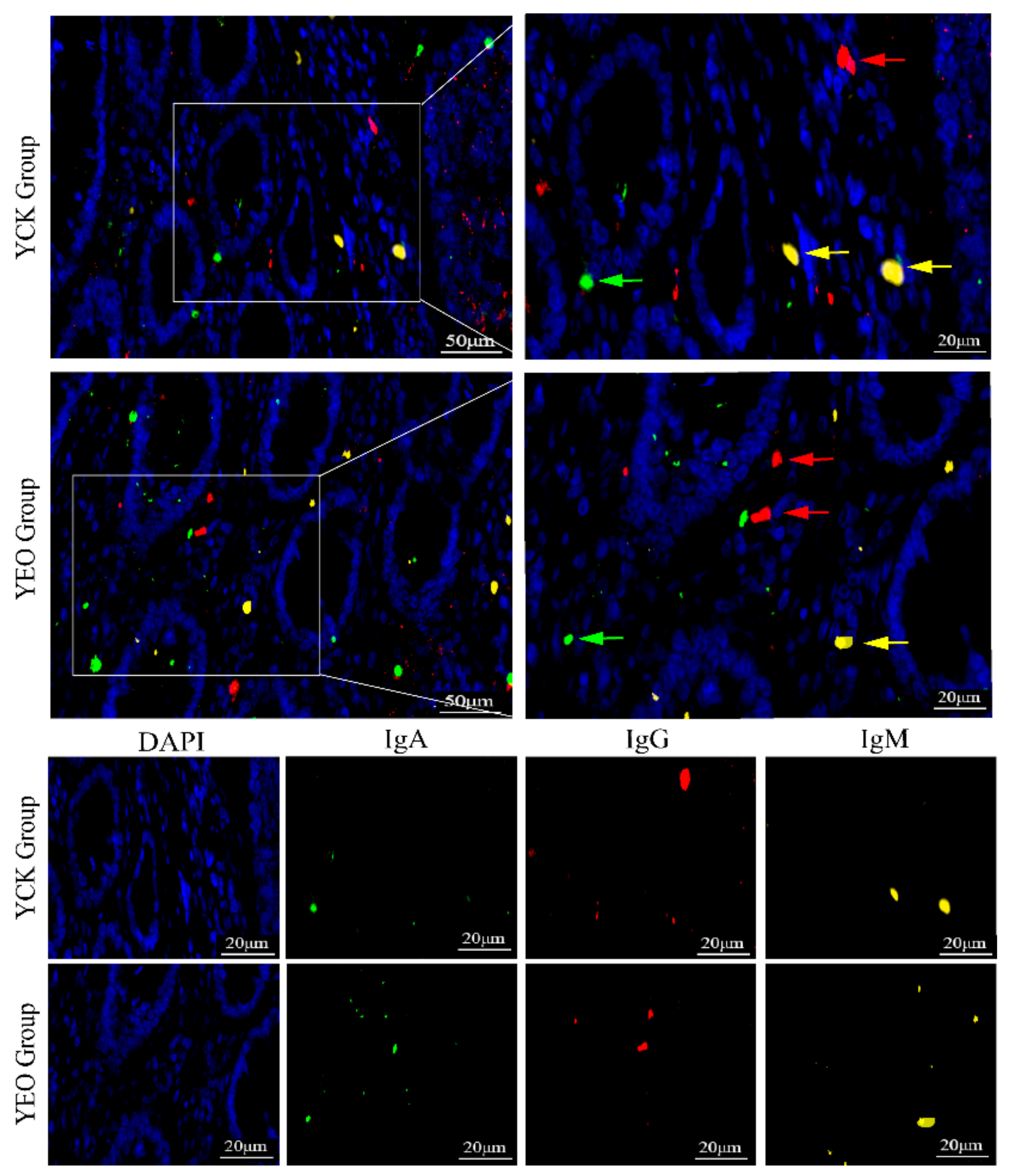
3.2. Expression of IgA, IgG, and IgM in the Jejunum
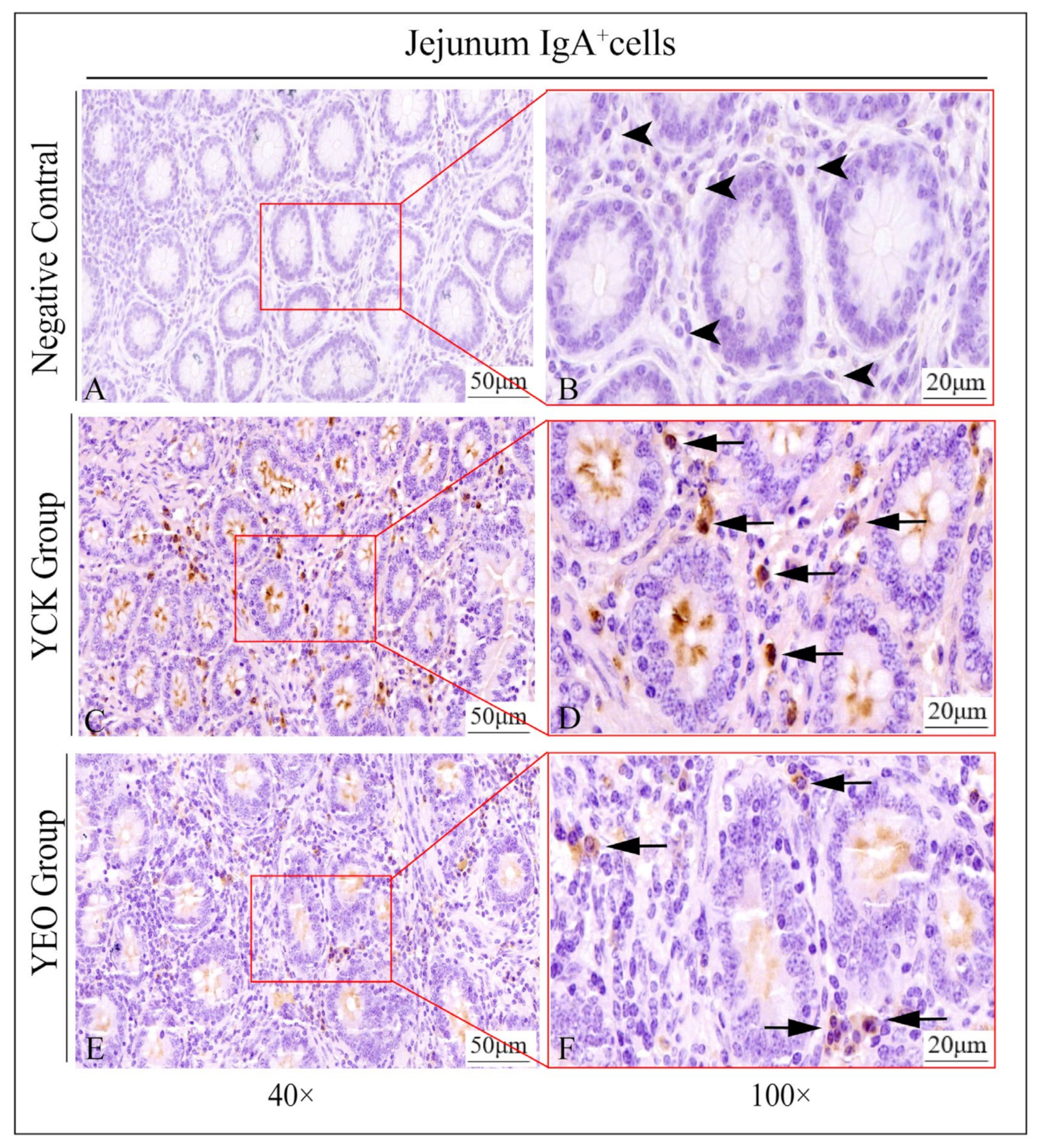
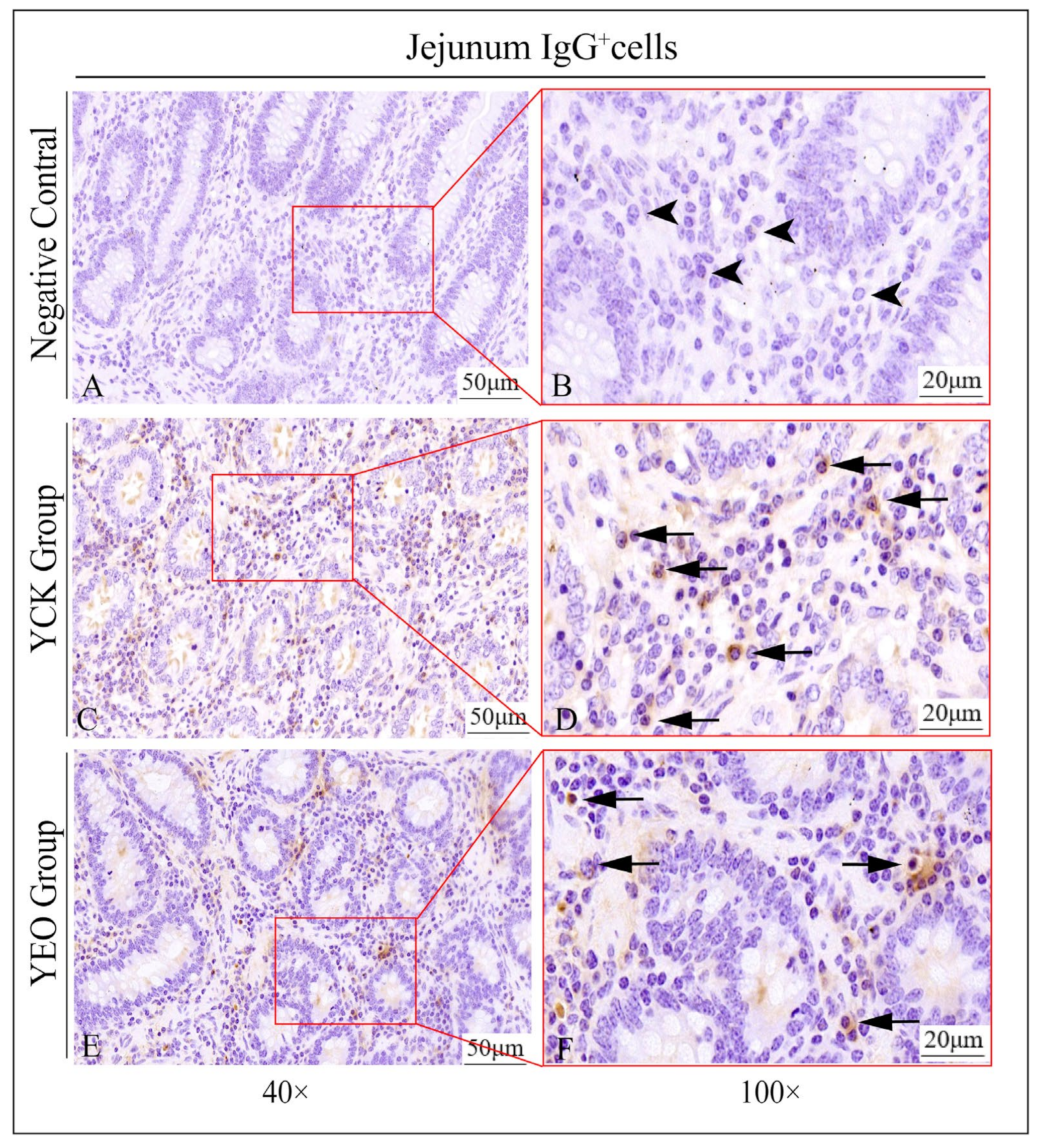
3.3. Distribution Pattern of IgA+, IgG+, and IgM+ Cells in the Jejunum
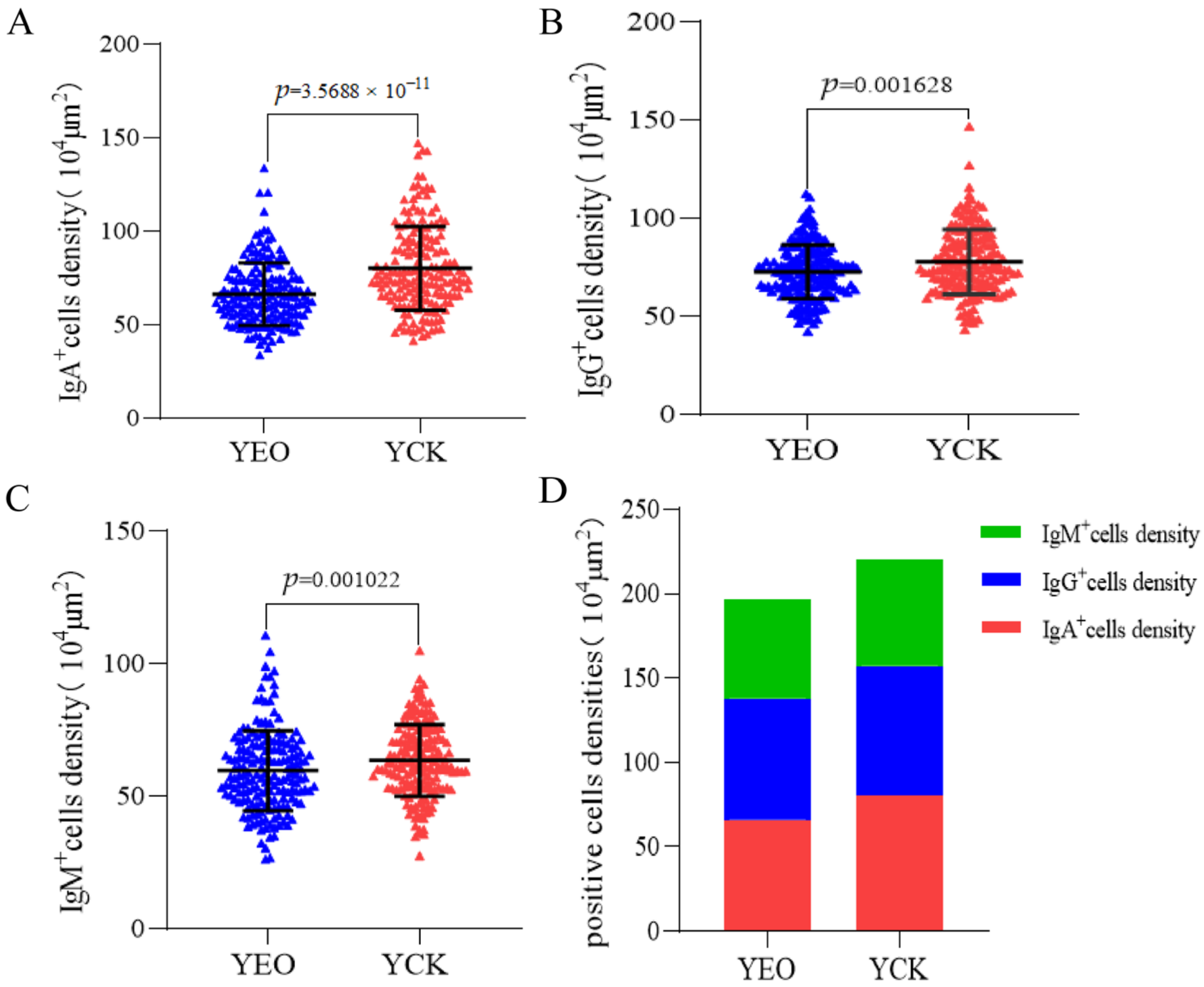
3.4. Number Changes of IgA+, IgG+, and IgM+ Cells in the Jejunum
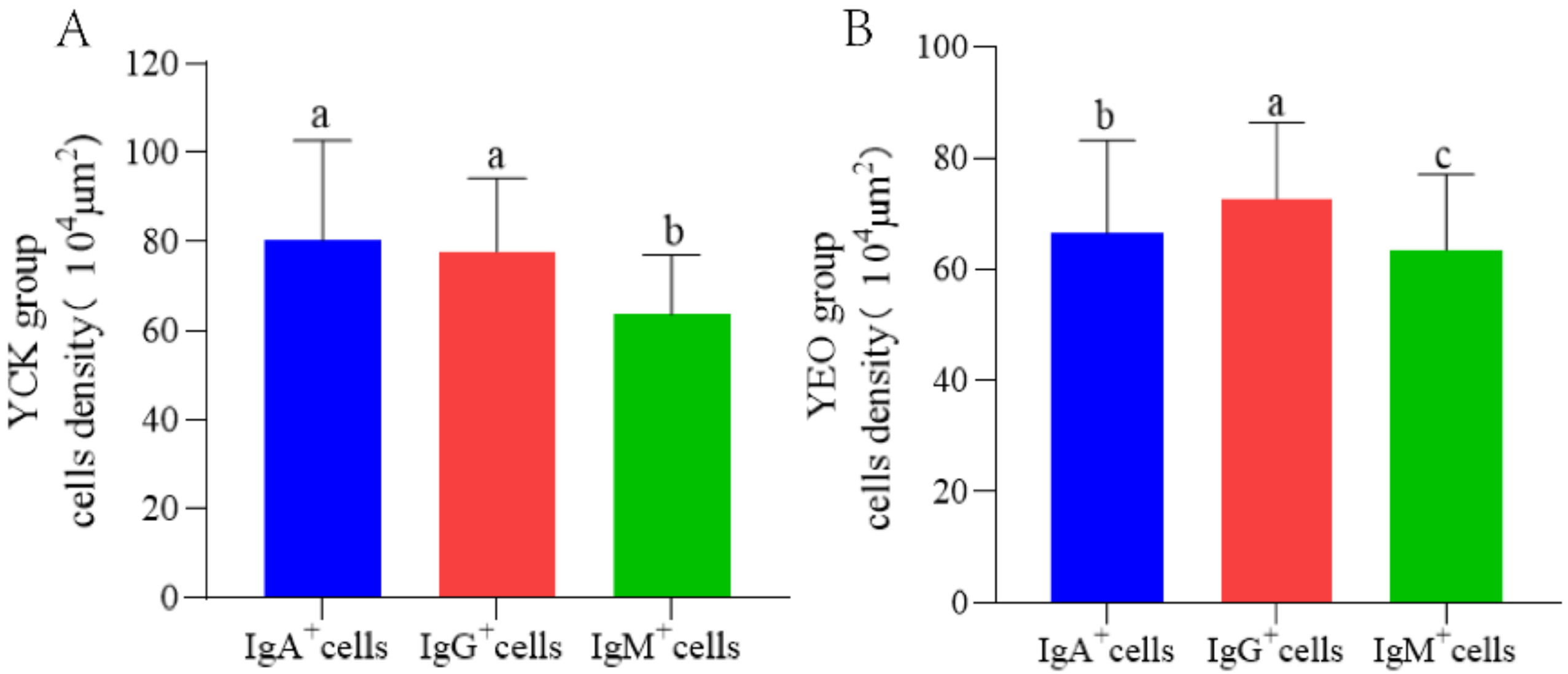
3.5. Differences between IgA+, IgG+, and IgM+ Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.P.; Ladinsky, M.S.; Yu, K.B.; Sanders, J.G.; Yoo, B.B.; Chou, W.C.; Conner, M.E.; Earl, A.M.; Knight, R.; Bjorkman, P.J.; et al. Gut microbiota utilize immunoglobulin a for mucosal colonization. Science 2018, 360, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Diversified IgA-bacteria interaction in gut homeostasis. Adv. Exp. Med. Biol. 2020, 1254, 105–116. [Google Scholar] [PubMed]
- Zhang, X.; Li, J.; Wang, Y.; Liu, M.; Liu, F.; Zhang, X.; Pei, L.; Wang, T.; Jiang, D.; Wang, X.; et al. A diagnostic model with IgM autoantibodies and carcinoembryonic antigen for early detection of lung adenocarcinoma. Front. Immunol. 2021, 12, 728853. [Google Scholar] [CrossRef] [PubMed]
- Prince, S.; Hollmen, M. Dimeric IgA specifically disables intracellular mutated oncodrivers. Immunity 2023, 56, 2461–2463. [Google Scholar] [CrossRef] [PubMed]
- Isho, B.; Florescu, A.; Wang, A.A.; Gommerman, J.L. Fantastic IgA plasma cells and where to find them. Immunol. Rev. 2021, 303, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Vujkovic-Cvijin, I.; Welles, H.C.; Ha, C.; Huq, L.; Mistry, S.; Brenchley, J.M.; Trinchieri, G.; Devkota, S.; Belkaid, Y. The systemic anti-microbiota igg repertoire can identify gut bacteria that translocate across gut barrier surfaces. Sci. Transl. Med. 2022, 14, l3927. [Google Scholar] [CrossRef]
- Zendt, M.; Bustos, C.F.; Kelly, S.; Saturday, T.; Degrange, M.; Ginigeme, A.; Wu, L.; Callier, V.; Ortega-Villa, A.; Faust, M.; et al. Characterization of the antispike IgG immune response to COVID-19 vaccines in people with a wide variety of immunodeficiencies. Sci. Adv. 2023, 9, h3150. [Google Scholar] [CrossRef]
- St, G.R.; Bossard, E.L.; Corey, L.; Sholukh, A.M. Serum concentration of antigen-specific igg can substantially bias interpretation of antibody-dependent phagocytosis assay readout. iScience 2023, 26, 107527. [Google Scholar]
- Oskam, N.; Ooijevaar-De, H.P.; Derksen, N.; Kruithof, S.; de Taeye, S.W.; Vidarsson, G.; Reijm, S.; Kissel, T.; Toes, R.; Rispens, T. At critically low antigen densities, IgM hexamers outcompete both IgM pentamers and igg1 for human complement deposition and complement-dependent cytotoxicity. J. Immunol. 2022, 209, 16–25. [Google Scholar] [CrossRef]
- Zhu, W.; Li, J.; Wu, Z.; Li, H.; Zhang, Z.; Zhu, X.; Sun, M.; Dong, S. Dual blockages of a broad and potent neutralizing IgM antibody targeting GH loop of EV-As. Immunology 2023, 169, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Sharopov, F.S.; Kukaniev, M.A.; Setzer, W.N. Composition of the essential oil of Origanum tyttanthum from Tajikistan. Nat. Prod. Commun. 2011, 6, 1719–1722. [Google Scholar] [PubMed]
- Cheng, C.; Xia, M.; Zhang, X.; Wang, C.; Jiang, S.; Peng, J. Supplementing oregano essential oil in a reduced-protein diet improves growth performance and nutrient digestibility by modulating intestinal bacteria, intestinal morphology, and antioxidative capacity of growing-finishing pigs. Animals 2018, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wei, H.; Sun, H.; Ao, J.; Long, G.; Jiang, S.; Peng, J. Effects of dietary supplementation of oregano essential oil to sows on oxidative stress status, lactation feed intake of sows, and piglet performance. Biomed. Res. Int. 2015, 2015, 525218. [Google Scholar] [CrossRef]
- Hall, H.N.; Wilkinson, D.J.; Le Bon, M. Oregano essential oil improves piglet health and performance through maternal feeding and is associated with changes in the gut microbiota. Anim. Microbiome 2021, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wu, J.; Zhang, L.; Liu, L.; Casper, D.P.; Jiao, T.; Liu, T.; Wang, J.; Lang, X.; Song, S.; et al. Effects of oregano essential oil on the ruminal ph and microbial population of sheep. PLoS ONE 2019, 14, e217054. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Wang, L.; Zhou, X.; Wu, X.; Chen, D.; Yu, B.; Huang, Z.; Luo, Y.; Mao, X.; Zheng, P.; et al. Effects of essential oil on growth performance, digestibility, immunity, and intestinal health in broilers. Poult. Sci. 2021, 100, 101242. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Peng, Q.Y.; Liu, Y.R.; Ma, Q.G.; Zhang, J.Y.; Guo, Y.P.; Xue, Z.; Zhao, L.H. Effects of oregano essential oil as an antibiotic growth promoter alternative on growth performance, antioxidant status, and intestinal health of broilers. Poult. Sci. 2021, 100, 101163. [Google Scholar] [CrossRef]
- Ruan, D.; Fan, Q.; Fouad, A.M.; Sun, Y.; Huang, S.; Wu, A.; Lin, C.; Kuang, Z.; Zhang, C.; Jiang, S. Effects of dietary oregano essential oil supplementation on growth performance, intestinal antioxidative capacity, immunity, and intestinal microbiota in yellow-feathered chickens. J. Anim. Sci. 2021, 99, skab033. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, J.; Lei, Y.; Bai, Y.; Jia, L.; Li, Z.; Liu, T.; Xu, Y.; Sun, J.; Wang, Y.; et al. Oregano essential oils promote rumen digestive ability by modulating epithelial development and microbiota composition in beef cattle. Front. Nutr. 2021, 8, 722557. [Google Scholar] [CrossRef]
- He, P.; Lei, Y.; Zhang, K.; Zhang, R.; Bai, Y.; Li, Z.; Jia, L.; Shi, J.; Cheng, Q.; Ma, Y.; et al. Dietary oregano essential oil supplementation alters meat quality, oxidative stability, and fatty acid profiles of beef cattle. Meat Sci. 2023, 205, 109317. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Zhang, K.; Li, C.; Wu, J.; Davis, D.; Casper, D.; Jiang, H.; Jiao, T.; Wang, X.; Wang, J. Dietary supplementation with essential-oils-cobalt for improving growth performance, meat quality and skin cell capacity of goats. Sci. Rep. 2018, 8, 11634. [Google Scholar] [CrossRef] [PubMed]
- Cardiff, R.D.; Miller, C.H.; Munn, R.J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb. Protoc. 2014, 2014, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Yao, W.; Pan, J.; Wang, B.; He, W.; Fan, X.; Wang, W.; Zhang, W. Moniezia benedeni infection restrain IgA+, IgG+, and IgM+ cells residence in sheep (Ovis aries) small intestine. Front. Vet. Sci. 2022, 9, 878467. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xie, W.; Zhou, S.; Ma, N.; Wang, Y.; Huang, J.; Shen, X.; Chang, G. A high-concentrate diet induces colonic inflammation and barrier damage in hu sheep. J. Dairy Sci. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Plaizier, J.C.; Danesh, M.M.; Derakhshani, H.; Golder, H.; Khafipour, E.; Kleen, J.L.; Lean, I.; Loor, J.; Penner, G.; Zebeli, Q. Review: Enhancing gastrointestinal health in dairy cows. Animal 2018, 12, s399–s418. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Reynolds, C.; Hollinger, K.; Pearce, S.C.; Gabler, N.K.; Baumgard, L.H.; Rhoads, R.P.; Selsby, J.T. Twelve hours of heat stress induces inflammatory signaling in porcine skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R1288–R1296. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.M.; Gomes, A.V.; Pinheiro, M.L.; Ribeiro, A.; Ferraz-De-Paula, V.; Astolfi-Ferreira, C.S.; Ferreira, A.J.; Palermo-Neto, J. Heat stress impairs performance and induces intestinal inflammation in broiler chickens infected with salmonella enteritidis. Avian Pathol. 2012, 41, 421–427. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.M.; Rodrigues, M.V.; Ribeiro, A.; Ferraz-De-Paula, V.; Pinheiro, M.L.; Sa, L.R.; Ferreira, A.J.; Palermo-Neto, J. Acute heat stress impairs performance parameters and induces mild intestinal enteritis in broiler chickens: Role of acute hypothalamic-pituitary-adrenal axis activation. J. Anim. Sci. 2012, 90, 1986–1994. [Google Scholar] [CrossRef]
- Monson, M.S.; Van Goor, A.G.; Ashwell, C.M.; Persia, M.E.; Rothschild, M.F.; Schmidt, C.J.; Lamont, S.J. Immunomodulatory effects of heat stress and lipopolysaccharide on the bursal transcriptome in two distinct chicken lines. BMC Genom. 2018, 19, 643. [Google Scholar] [CrossRef]
- Liu, C.; Chaudhry, M.T.; Zhao, D.; Lin, T.; Tian, Y.; Fu, J. Heat shock protein 70 protects the quail cecum against oxidant stress, inflammatory injury, and microbiota imbalance induced by cold stress. Poultry Sci. 2019, 98, 5432–5445. [Google Scholar] [CrossRef]
- Zoetendal, E.G.; Raes, J.; van den Bogert, B.; Arumugam, M.; Booijink, C.C.; Troost, F.J.; Bork, P.; Wels, M.; de Vos, W.M.; Kleerebezem, M. The human small intestinal microbiota is driven by rapid uptake and conversion of simple carbohydrates. ISME J. 2012, 6, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, H.; Mao, B.; Yang, Q.; Zhao, J.; Gu, Z.; Zhang, H.; Chen, Y.Q.; Chen, W. Microbial biogeography and core microbiota of the rat digestive tract. Sci. Rep. 2017, 8, 45840. [Google Scholar] [CrossRef]
- Blumberg, R.; Powrie, F. Microbiota, disease, and back to health: A metastable journey. Sci. Transl. Med. 2012, 4, 137r. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.; Whon, T.W.; Bae, J. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zheng, M.; Zhou, M.; Zhou, L.; Ge, X.; Pang, N.; Li, H.; Li, X.; Li, M.; Zhang, J.; et al. Lentinan supplementation protects the gut–liver axis and prevents steatohepatitis: The role of gut microbiota involved. Front. Nutr. 2022, 8, 803691. [Google Scholar] [CrossRef] [PubMed]
- Tokuhara, D.; Kurashima, Y.; Kamioka, M.; Nakayama, T.; Ernst, P.; Kiyono, H. A comprehensive understanding of the gut mucosal immune system in allergic inflammation. Allergol. Int. 2019, 68, 17–25. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Maintenance of gut homeostasis by the mucosal immune system. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2016, 92, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Ruytinx, P.; Vandormael, P.; Fraussen, J.; Pieters, Z.; Thonissen, S.; Hellings, N.; Stinissen, P.; Callebaut, I.; Penders, J.; Vanhove, K.; et al. Comprehensive antibody and cytokine profiling in hospitalized covid-19 patients in relation to clinical outcomes in a large belgian cohort. Sci. Rep. 2023, 13, 19322. [Google Scholar] [CrossRef]
- Selhorst, P.; van Ierssel, S.H.; Michiels, J.; Mariën, J.; Bartholomeeusen, K.; Dirinck, E.; Vandamme, S.; Jansens, H.; Ariën, K.K. Symptomatic severe acute respiratory syndrome coronavirus 2 reinfection of a healthcare worker in a Belgian nosocomial outbreak despite primary neutralizing antibody response. Clin. Infect. Dis. 2021, 73, e2985–e2991. [Google Scholar] [CrossRef]
- Takato, K.; Woan-Yu, L.; Jin-Gyu, C.; Gagandeep, S.; Arjun, R.; Stephen, T.Y.; Marissa, M.; Meghan, B.D.; Guilhermina, C.; Zhen, Z.; et al. Fungal microbiota sustains lasting immune activation of neutrophils and their progenitors in severe COVID-19. Nat. Immunol. 2023, 24, 1879–1889. [Google Scholar]
- Yoshihara, T.; Shimada, K.; Fukao, K.; Sai, E.; Sato-Okabayashi, Y.; Matsumori, R.; Shiozawa, T.; Alshahi, H.; Miyazaki, T.; Tada, N.; et al. Omega 3 polyunsaturated fatty acids suppress the development of aortic aneurysms through the inhibition of macrophage-mediated inflammation. Circ. J. 2015, 79, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, M.E.; Hammer, S.S.; Mccollum, G.W.; Penn, J.S. Epoxygenated fatty acids inhibit retinal vascular inflammation. Sci. Rep. 2016, 6, 39211. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, A.; Ramakrishna, B.S.; Shaji, R.V.; Kumar, N.S.; Pulimood, A.; Patra, S. Amelioration of dextran sulfate colitis by butyrate: Role of heat shock protein 70 and NF-kappab. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G177–G184. [Google Scholar] [CrossRef] [PubMed]
- Strandmark, J.; Steinfelder, S.; Berek, C.; Kühl, A.A.; Rausch, S.; Hartmann, S. Eosinophils are required to suppress Th2 responses in Peyer’s patches during intestinal infection by nematodes. Mucosal Immunol. 2017, 10, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Gribonika, I.; Eliasson, D.G.; Chandode, R.K.; Schön, K.; Strömberg, A.; Bemark, M.; Lycke, N.Y. Class-switch recombination to IgA in the Peyer’s patches requires natural thymus-derived Tregs and appears to be antigen independent. Mucosal Immunol. 2019, 12, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chou, N.; Yu, Y.; Shih, H.; Lin, H.; Lee, C.; Chang, M. Phrf1 promotes the class switch recombination of IgA in CH12F3-2A cells. PLoS ONE 2023, 18, e285159. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Meza, J.M.; Jarillo-Luna, R.A.; Rivera-Aguilar, V.; Miliar-García, A.; Campos-Rodríguez, R. Cytokine profile of NALT during acute stress and its possible effect on IgA secretion. Immunol. Lett. 2017, 188, 68–78. [Google Scholar] [CrossRef]
- Kuret, T.; Lakota, K.; Žigon, P.; Ogrič, M.; Sodin-Aemrl, S.; Čučnik, S.; Tomšič, M.; Hočevar, A. Insight into inflammatory cell and cytokine profiles in adult IgA vasculitis. Clin. Rheumatol. 2019, 38, 331–338. [Google Scholar] [CrossRef]
- Bagheri, Y.; Babaha, F.; Falak, R.; Yazdani, R.; Azizi, G.; Sadri, M.; Abolhassani, H.; Shekarabi, M.; Aghamohammadi, A. Il-10 induces tgf-βsecretion, tgf-βreceptor ii upregulation, and iga secretion in b cells. Eur. Cytokine Netw. 2019, 30, 107–113. [Google Scholar] [CrossRef]


| Items% | Months | ||||
|---|---|---|---|---|---|
| I | II | III | IV | V~IX | |
| Ingredients | |||||
| Corn silage | 45.00 | 40.00 | 30.00 | 25.00 | 20.00 |
| Whole cottonseed | 0.00 | 0.00 | 0.00 | 0.00 | 10.78 |
| Flattened corn | 14.84 | 10.00 | 8.00 | 5.00 | 10.00 |
| Corn | 25.17 | 41.36 | 51.06 | 59.93 | 54.36 |
| Soybean meal | 8.21 | 3.20 | 0.00 | 2.00 | 0.00 |
| Rapeseed meal | 0.00 | 0.54 | 5.17 | 3.57 | 0.00 |
| Cottonseed meal | 0.00 | 2.00 | 2.00 | 1.00 | 1.00 |
| Pea protein powder | 3.49 | 0.00 | 0.00 | 0.00 | 0.00 |
| Fatty acid calcium | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 |
| CaHPO4 | 1.12 | 0.80 | 1.10 | 1.28 | 0.65 |
| Na Cl | 0.62 | 0.30 | 0.47 | 0.50 | 0.47 |
| NaHCO3 | 1.11 | 1.00 | 1.28 | 1.00 | 1.15 |
| MgO | 0.00 | 0.19 | 0.28 | 0.16 | 0.13 |
| Premix 1 | 0.44 | 0.61 | 0.64 | 0.56 | 0.46 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutrition levels 2 | |||||
| Crude Protein | 11.94 | 10.95 | 10.80 | 10.60 | 9.80 |
| Total digestible nutrients | 72.10 | 76.54 | 77.51 | 78.74 | 80.52 |
| Net energy maintenance/(MJ/kg) | 7.44 | 7.97 | 8.14 | 8.31 | 8.53 |
| Net weight gain/(MJ/kg) | 4.92 | 5.31 | 5.47 | 5.61 | 5.80 |
| Ca | 0.61 | 0.50 | 0.59 | 0.63 | 0.37 |
| P | 0.31 | 0.33 | 0.36 | 0.35 | 0.34 |
| Items | YEO | YCK | p Value | Decrease Rate |
|---|---|---|---|---|
| IgA(pg/mg) | 132.12 ± 38.64 | 222.98 ± 27.61 | 0.002693 | 40.75% |
| IgG(pg/mg) | 591.95 ± 146.78 | 854.90 ± 101.13 | 0.008138 | 30.76% |
| IgM(pg/mg) | 182.30 ± 33.48 | 371.04 ± 83.18 | 0.004848 | 50.87% |
| Items | YEO | YCK | p Value | Decrease Rate |
|---|---|---|---|---|
| IgA+ cells/104 μm2 | 66.49 ± 16.67 | 80.18 ± 22.48 | 3.5688 × 10−11 | 17.07% |
| IgG+ cells/104 μm2 | 72.80 ± 13.68 | 77.80 ± 16.47 | 0.001628 | 6.44% |
| IgM+ cells/104 μm2 | 59.63 ± 15.06 | 63.54 ± 13.57 | 0.001022 | 6.15% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Zhang, W.; Wang, B.; Shi, J.; He, P.; Jia, L.; Huang, Y.; Xu, M.; Ma, Y.; Cheng, Q.; et al. Effects of Oregano Essential Oil on IgA+, IgG+, and IgM+ Cells in the Jejunum of Castrated Holstein Bulls. Animals 2023, 13, 3766. https://doi.org/10.3390/ani13243766
Liu Q, Zhang W, Wang B, Shi J, He P, Jia L, Huang Y, Xu M, Ma Y, Cheng Q, et al. Effects of Oregano Essential Oil on IgA+, IgG+, and IgM+ Cells in the Jejunum of Castrated Holstein Bulls. Animals. 2023; 13(24):3766. https://doi.org/10.3390/ani13243766
Chicago/Turabian StyleLiu, Qiyan, Wangdong Zhang, Baoshan Wang, Jinping Shi, Pengjia He, Li Jia, Yongliang Huang, Meiling Xu, Yue Ma, Qiang Cheng, and et al. 2023. "Effects of Oregano Essential Oil on IgA+, IgG+, and IgM+ Cells in the Jejunum of Castrated Holstein Bulls" Animals 13, no. 24: 3766. https://doi.org/10.3390/ani13243766
APA StyleLiu, Q., Zhang, W., Wang, B., Shi, J., He, P., Jia, L., Huang, Y., Xu, M., Ma, Y., Cheng, Q., & Lei, Z. (2023). Effects of Oregano Essential Oil on IgA+, IgG+, and IgM+ Cells in the Jejunum of Castrated Holstein Bulls. Animals, 13(24), 3766. https://doi.org/10.3390/ani13243766






