Simple Summary
Phytase activity is affected by extreme pressure and temperature during feed processing, resulting in variable residual activity and efficacy on the digestibility of phosphorus when applied to pelleted feed. The results of this study indicate that phytase from different sources has variable thermal stability when subjected to pelleting, but the dose does not influence the recovery rate of phytase. Furthermore, our findings suggest that the recovery rate of phytase decreased when the conditioning temperature increased from 75 to 85 °C. These findings may inform decision-making processes when applying phytase and pelleting to feed.
Abstract
Phytase activity can be impaired during pelleting because of extreme thermal conditions. This study investigated the effects of dose and source of phytase on phytase activity during the conditioning, pelleting, and cooling process. A split-plot design was used in two experiments, with five phytase doses (Exp. 1; 7560, 14310, 33830, 43590 and 61500 FTU/kg) or eight phytase sources (Exp. 2) as the main plot and steam conditioning temperatures (Exp. 1 and 2; 75 and 85 °C) as the subplot. Each treatment processed four batches, one batch per replicate. The results of Exp. 1 showed phytase dose in diets had no effect (p > 0.05) on the recovery rate of phytase activity after the conditioning, pelleting, or cooling process. The recovery rate of phytase activity in each process was higher (p < 0.05) at 75 °C than that at 85 °C for both Exp. 1 and 2. The phytase source significantly affected (p < 0.05) the recovery rate of phytase activity and had varied appearances of structure. In conclusion, the structure, phytase activity, and phytase recovery after steam conditioning–pelleting significantly varied across sources, but the stability of phytase was not affected by dose.
1. Introduction
Phytate (Inositol hexakisphosphate) is present in most cereal grains in the form of complexes with minerals and amino acids, and it impairs the bioavailability and nutritional value of the feed. Phytase can degrade phytate, increase the digestibility of phosphorus, minerals, and amino acids, and reduce excretion of inorganic phosphorus [1,2,3]. At present, a powdered phytase is treated as a regular ingredient, which is mixed with the main ingredients before the steam conditioning–pelleting process [3]. Typically, the feed mixture is steam conditioned to gelatinize starch and sterilize feed, before being processed into pellets [4]. The stability of phytase during the pelleting process is a major concern because phytase activity is negatively affected by pressure and high temperature [5]. Phytase must be biologically active when reaching the gastrointestinal tract to be able to act on the substrates. Therefore, understanding the loss of phytase activity during the steam conditioning–pelleting process and the variability of stability across sources is important to assess in order to ensure adequate digestible phosphorus in animal feeds [6,7].
Previous studies evaluating phytase stability assumed that the initial dietary phytase concentration in the unprocessed diet did not affect the recovery rate of phytase activity after processing, resulting in a wide variation in phytase activity (varied from 376 to 20,220 FTU/kg) [4,8,9,10]. However, the study of Boney and Moritz [11] revealed that the residual activity of phytase added to broiler diets at 6-fold the standard dose was about 6-fold the residual activity of phytase at the standard dose after steam conditioning–pelleting, but 4-fold greater than some other treatments. Another study showed that the recovery rate of phytase activity after 3 min in a water bath at 80 ℃ decreased when the initial activity of phytase decreased, which implied that phytase stability depends on the phytase dose [8]. These variable results warrant further investigations to clearly elucidate the effect of initial phytase dose on stability. Additionally, others report substantial differences in the stability of phytase across different sources and different conditions used in pelleting [12], further complicating interpretations of the previous findings.
Currently, few published studies evaluated the stability of commercially available phytase sources exposed to various temperatures during pelleting. It is hypothesized that differences in phytase doses may contribute to the variation in observed enzyme stability. Hence, this study systematically evaluated the effects of phytase dose on the recovery rate of phytase activity, to determine a suitable dose range to assess heat stability of phytase in corn–soybean meal diets. The thermal stability of eight phytases commercially available in China was then evaluated using steam conditioning–pelleting based on the above results.
2. Materials and Methods
2.1. Phytase Source
The phytase used in Exp. 1 was 6-phytase, which is derived from a liquid fermentation product of the phytase gene from Escherichia coli and expressed in Pichia pastoris. The enzyme was granularized via spray drying, and the labeled phytase activity was 10,000 FTU/g. In Exp. 2, 8 phytase products declared potency of 10,000 FTU/g and were collected from Chinese feed manufacturers: Premier Ⅰ (SunSon Industry Group Co., Ltd., Ningxia, China); Sunfize 10,000 (Wuhan Sunhy Biology Co., Ltd., Hubei, China); Microtech 10,000 Plus (Guangdong VTR Biotech Co., Ltd., Guangdong, China); Pecozyme Phytase (Challenge Group, Beijing, China); Winovazyme Phytase HT85 (Beijing Winovazyme Biotech Co., Ltd., Beijing, China); Kingphos (Qingdao Vland Biotech INC., Shandong, China); Natuphos (BASF Corporation, Florham Park, NJ, USA); Axtra Phy (Danisco Animal Nutrition, Marlborough, UK). These phytases were randomly numbered 1 to 8 for reference and included 6 powder samples and 2 pellet spheres.
2.2. Experimental Design
This study consisted of 2 experiments.
2.2.1. Exp. 1
A split-plot 5 × 2 factorial design was used to determine the effects of phytase doses and steam conditioning temperatures on the stability of phytase. Five dietary phytase doses (7560, 14,310, 33,830, 43,590, and 61,500 FTU/kg) were the main plot, and 2 conditioning temperatures (75 and 85 °C) were the subplot. To prevent residues of the higher phytase doses in the pelleting machine contaminating the lower dose pellets, repeated sample batches were produced each day for 5 days, from least to greatest dose. Four batches were produced daily at 08:00–9:30, 10:00–11:30, 13:00–14:30, and 15:00–16:30, resulting in four replicates per treatment for each dose.
2.2.2. Exp. 2
A split-plot 8 × 2 factorial design was used to determine the effects of phytase sources and steam conditioning temperatures on phytase stability. Eight phytase sources were the main plot and 2 conditioning temperatures (75 and 85 °C) were the subplot. A basal diet without phytase was used resulting in a 4 × 9 Youden square design with 4 batches of 9 diets (a basal diet and 8 experimental diets). The 8 experimental diets were formulated by adding 0.4% phytase from 8 sources in the basal diet. Taken production batches were blocked to give 4 replicates per diet (Table 1).

Table 1.
Batch of production and sequence of steam conditioning–pelleting (Exp. 2).
2.3. Experimental Diet
Experimental diets were composed of corn and soybean meal, limestone, dicalcium phosphate, phytase, sodium chloride, crystalline amino acids, and a vitamin and mineral premix (Table 2). Corn and soybean meal were ground and screened through a 2 mm sieve; the remaining ingredients were then mixed with ground corn and soybean meal step by step in equal proportion, and then put into the double-circle paddle mixer (FAMSUN SJHSO.2, Jiangsu Muyang Group Co., Ltd., Yangzhou, China), and mixed diets were pelleted through a pellet mill as described below.

Table 2.
Ingredient and calculated nutrient composition of experimental diet (as-fed basis, %).
2.4. Steam Conditioning–Pelleting Process and Sample Collection
The steam conditioning of homogenized diets was carried out using a double-shaft steam conditioner (FAMSUN SBTZ10, Jiangsu Muyang Group Co., Ltd., Yangzhou, China; conditioner cylinder = 1.2 m × 22 cm). The mixture was conditioned for 70 s at a constant temperature of approximately 75 or 85 °C. The length-to-diameter ratio of the pellet mill (FAMSUN SZLH180X25, Jiangsu Muyang Group Co., Ltd., China) was 6:1 with a 4 mm diameter hole and a production rate up to 100 kg/h. Two electronic temperature sensors located at the conditioner and the pellet mill discharge port logged actual temperature continuously. After pelleting, diets were cooled to room temperature using a pilot-scale forced air dryer.
The mixer, storage bin, and conditioner were thoroughly cleaned with high-pressure air to remove residual feed after each conditioning–pelleting session. When the conditioning temperature reached the target temperature (75 or 85 °C), the pellet mill operated constantly for 15 min. Samples were taken continuously for 10 min at the feeder sampling port before conditioning, after conditioning, after pelleting, and after cooling. One sample was collected every minute, and 10 subsamples were collected at each sampling site.
The steam conditioning–pelleting process was performed as follows: the main steam valve and compressed air valve were opened, diets were mixed in the mixer for 5 s, diets were transferred to the pelleting buffer bin, and the steam valve and drain valve sequentially opened and then closed after discharging cold water. The pellet mill, conditioner, and pellet feeder were computer controlled. The samples collected after each process were immediately refrigerated (4 °C) until analysis.
2.5. Determination of Feed and Phytases
The particle shape, color, and appearance of the phytase were observed using a stereomicroscope (Leica M205 FA, Wetzlar, Germany). The temperatures during the conditioning and pelleting process were automatically recorded using sensors at the discharging port of the conditioner and pelleting mill. Dry matter content of the feed samples was determined according to AOAC method 930.15. The phytase activity of concentrated phytase supplements and diets was determined using the colorimetric measurement of the released phosphate [13,14]. In both cases, the 1 FTU of phytase was defined as 1 μmol of inorganic phosphorus released from 5.0 mmol/L sodium phytate per minute at pH 5.50 and 37 °C.
2.6. Data Calculation and Statistical Analysis
All data were assessed with SAS 9.4 (SAS Inst. Inc., Cary, NC, USA). Differences among treatments in the measured conditioning temperature and dry matter content of diets were analyzed using the GLM model and Tukey’s multiple range test.
The recovery rate of phytase activity was defined as the ratio of the dietary phytase activity assessed after each processing phase to that assessed in raw (unprocessed) feed. According to the completely randomized split-plot design principle described by Kaps and Lamberson [15], PROC MIXED was used to analyze the differences in the recovery rate of phytase activity, with processing batch as the experimental unit. The statistical model of Exp. 1 assessed the dose of phytase, conditioning temperature, and their interaction as the main effect, and the random effect of block. The statistical model of Exp. 2 included the source of phytase, conditioning temperature, and their interaction as the main effects, and the random effect of block. Differences among treatments were compared using Tukey’s test. Only the main effects were discussed for responses in which the interaction was not significant, whereas the whole treatments were discussed for responses where the interaction was detected. For all analyses, p < 0.05 was considered significant.
3. Results
3.1. Effect of Phytase Dose on Recovery Rate of Phytase Activity (Exp. 1)
The actual steam conditioning temperature ranged from 75.1 to 75.3 °C or 84.6 to 85.0 °C when set at 75 or 85 °C, respectively, and there was no difference among treatments in Exp. 1 (Table 3). No difference was detected across treatments for dry matter content of the diets after conditioning, pelleting, or cooling when conditioned at 75 °C. However, the dry matter in the phytase dose of 61,500 FTU/kg was greater (p < 0.05) than that of 14,310 FTU/kg after the conditioning process when conditioned at 85 °C, but no other differences (p > 0.05) were detected among the treatments when conditioned at 85 °C. While statistically significant, this difference related to the small coefficient of variation within replicates and the SEM among treatments and is not practically significant.

Table 3.
The determined conditioning temperature and dietary dry matter content in steam conditioning–pelleting and cooling process for diets added different doses of phytase (Exp. 1).
No dose of interactive effect (p > 0.05) between phytase activity and conditioning temperature was found for the recovery rate of phytase activity after conditioning, pelleting, or cooling (Table 4). The dose of phytase in diets had no effect (p > 0.05) on the recovery rate of phytase activity after conditioning, pelleting, or cooling, whether conditioned at 75 or 85 °C. The recovery rate of phytase activity after conditioning, pelleting, and cooling was greater (p < 0.05) for diets conditioned at 75 vs. 85 °C. Consequently, the loss of phytase activity during the conditioning step was substantially greater at 85 °C than 75 °C (p < 0.05). However, the opposite result was observed during the pelleting step (Figure 1), but the difference is comparatively small in magnitude compared to the conditioning step.

Table 4.
Effect of dietary phytase dose and temperature on the recovery rate of phytase activity after steam conditioning–pelleting and cooling process (Exp. 1).
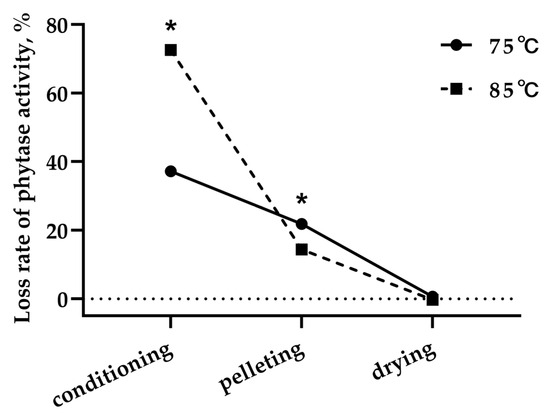
Figure 1.
Loss rate of phytase activity during the steam conditioning–pelleting and cooling process. * Significant differences between treatments.
3.2. Characteristics of Phytase from Different Sources (Exp. 2)
The eight phytase samples from different manufacturers differed in appearance under the microscope: two samples were spherical particles (Figure 2b,f) and six were powders (Figure 2). Among the six powder samples: one contained many spherical particles (Figure 2d), three contained a few spherical particles (Figure 2a,c,h), and two appeared as uniformly fine powder (Figure 2e,g). All eight phytases were yellow or pale yellow.
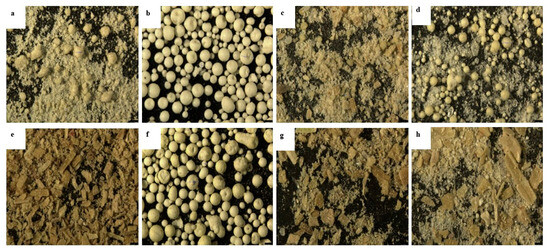
Figure 2.
Microscopic photos of phytase products (300×, Exp. 2). These photos of the phytase products were taken using a stereomicroscope (Leica M205 FA, Wetzlar, Germany) at a 300× of view. (a) Phytase 1; (b) Phytase 2; (c) Phytase 3; (d) Phytase 4; (e) Phytase 5; (f) Phytase 6; (g) Phytase 7; (h) Phytase 8.
The determined phytase activity showed that four of the phytases had measured activities greater than 12,000 FTU/g and two were less than 10,000 FTU/g (Table 5). The relative deviation between measured and calculated phytase activity was 7.02% or 5.11% in two sources, but other sources had comparatively less relative deviation. Dietary phytase activity was not different between phytases 2 and 3, but it was significantly higher (p < 0.05) than that of other phytases. Measured phytase activities between diets with phytases 1, 4 and 6 were not different (p > 0.05), but they were significantly greater (p < 0.05) than that with phytase 5. The measured phytase activity of diets with phytase 7 was significantly higher (p < 0.05) than that with phytase 5. The measured phytase activities were not different (p > 0.05) between diets added with phytases 5 and 8, but they were significantly higher (p < 0.05) than diets without phytase. A total of 4.2 FTU/g of activity was measured in the control diet without the addition of phytase.

Table 5.
Activity and appearance of phytase products and determined or calculated phytase activity in diets (Exp. 2).
3.3. Effect of Phytase Source on Recovery Rate of Phytase Activity (Exp. 2)
The measured conditioning temperatures of the eight diets with added phytase varied from 74.2 to 75.8 °C when set at 75 °C, and there was no difference (p > 0.05) among treatments (Table 6). The measured conditioning temperatures of the eight diets with added phytase varied from 85.0 to 85.4 °C when set at 85 °C, and there was also no difference (p > 0.05) among treatments.

Table 6.
Recovery rate of phytase activity after steam conditioning–pelleting and cooling process (Exp. 2).
No interactive effects (p > 0.05) were found between the phytase source and conditioning temperature on the recovery rate of phytase activity after conditioning, pelleting, or cooling (Table 6). The phytase source and conditioning temperature significantly affected (p < 0.05) the recovery rate of phytase activity in all processes. After the diets were steam conditioned, the recovery rate of phytase 2, 4, and 5 activities were higher (p < 0.05) than that of phytase 6 and 7, but there was no significant difference between the recovery rates of phytase 1, 3, and 8. After steam conditioning and pelleting, the recovery rate of phytase 5 activity was higher (p < 0.05) than that of phytase 2, 6, and 7, while there was no difference between the recovery rate of phytase 1, 3, 4 and 8. Subsequently, the pelleted diets were dried. The recovery rate of phytase 5 activity was higher (p < 0.05) than that of phytase 2, 6, and 7; but the recovery rate of phytase 1, 2, 3, 4, 6 and 7 did not significantly differ (p > 0.05). The recovery rate of phytase activity after conditioning, pelleting and cooling was greater (p < 0.05) for diets conditioned at 75 vs. 85 °C.
4. Discussion
4.1. Differences in Thermal Stability of Phytase at Different Doses
Phytase was recommended at the levels of 500 to 1000 FTU/kg in diets for pigs and poultry when it was introduced as a feed additive [16,17,18]. However, pig trials demonstrated the optimal phytase dose depended on diet composition [19]. For example, the optimal dose of phytase for corn, rapeseed meal, and soybean meal is 500 to 1000 FTU/kg, but that for sunflower meal and wheat is 2000 FTU/kg [19]. Generally, phytase doses greater than 1500 FTU/kg have been considered as supra dosage [20,21,22,23], but a dose of 1000 to 1500 FTU/kg is the current norm [24]. This shift is attributable to the reduced cost of phytase, increased cost of rock phosphate, and increasing use of the steam conditioning–pelleting technique. Previous studies showed that the addition of a super dosage (2000 to 4000 FTU/kg) in diets before feed pelleting improved growth performance, apparently due to the high loss of phytase activity during the steam conditioning–pelleting process. Over supplementing phytase ensures that the residual phytase activity in the pellets is sufficient to ensure complete phytate hydrolysis [22]. Nutritionists need solid data to adjust dietary phytase to account for the degradation of the enzyme during processing. If the percentage of residual phytase activity in pellets is similar across doses, a constant value could be used to adjust the inclusion of phytase in the feed to achieve a desired rate of phytase activity after processing. Walk et al. [20] reported the recovery rates of 92.8%, 97.8%, and 78.4% when adding 500, 1000, or 1500 FTU/kg phytase in broiler diets, respectively. However, the experimental results of Boney and Moritz [11] showed rates of phytase recovery after pelleting were similar when phytase was supplemented at either the standard or 6-fold the standard dose.
In Exp. 1, the required phytase dose was above 7500 FTU/kg, considering the loss of phytase activity and the detection limit calculated by the method stated in the determination of the phytase activity method [14]. Secondly, the reproducibility of phytase activity determination was unacceptable, and the relative standard deviations of repeatability and reproducibility were 2.2% to 10.6% and 5.4% to 15%, respectively [25]. For these reasons, the difference in recovery rate of phytase activity between different replicates of the same treatment was unacceptably high (the absolute different between two replicates can be up to 20%) [26]. Therefore, a high dose of phytase activity reduced the measurement error. This resulted in supra dosage of phytase added in diets (e.g., 10,000 FTU/kg) during the determination of residual phytase activity after steam conditioning and pelleting [27].
Timmons et al. [28] showed that the recovery rate was 64% to 80.0% and 69.5% to 81.0%, when two thermostable phytases were included at 0.5, 1 and 2 times the recommended dose and pelleted with a conditioning temperature of 93 °C. The statistical test showed that the dose had no effect on the recovery rate of phytase activity. In this experiment, the recovery rate of phytase activity varied numerically, but not significantly with doses after conditioning, pelleting, or cooling. This was similar to the results of Timmons et al. [28] and De Jong et al. [4], indicating that the phytase dose in diets did not affect the recovery rate of phytase activity.
4.2. Differences in Thermal Stability of Phytase from Different Sources
Genes for commercial phytases are mainly expressed in fungi, such as Aspergillus niger, and bacteria, such as E. coli, attributing to the variation in molecular weight, optimum pH, and optimum temperature of phytase from different sources [29]. The thermal stability of phytase can be improved via genetic modification, for example, by glycosylation [30]. Therefore, phytase from different genetic sources may differ significantly in terms of heat resistance. In addition, phytase is generally produced in a powdered or granulated form via spray drying and combining with an inert carrier, such as bran, or corn cob powder; but carriers are not uniform [29,31]. The problem with incorporating phytase into animal feed is the denaturation of the enzyme protein caused by moisture and high temperature during the steam conditioning–pelleting process [32]. Some progress has been made towards stabilizing phytase by screening potential encapsulation materials to minimize contact between the enzyme and water, improving its heat resistance during the conditioning process under low-moisture conditions [33,34]. Encapsulation further increases the diversity of phytase properties, in addition to variation from different phytase gene sources, as discussed above [31,35].
In Exp. 2, the phytase from eight different manufacturers showed apparent differences in physical characteristics likely from variation in the processing techniques. There were two spherical particle samples, possibly from spray drying. Four products consisted of a mixture of powder and particles (granulated phytase mixed with a carrier), and there were two fully granulated phytase products. However, it is not clear whether the pelleted phytases were coated with starch or treated with water-repellant coating materials to improve heat resistance. De Jong et al. [10] studied the phytase stability of the commercial phytases from four manufacturers (two powders and two coated phytase) and it varied greatly with storage time under different temperature conditions. The phytase activity loss rate of the coated product was similar to that of the powder samples. This result indicates that the coating did not improve the stability of the enzyme at an ambient temperature. In this experiment, the recovery rate of eight commercial phytase activities after steam conditioning–pelleting clearly stratified into high, medium, and low stability groups. The powdered product 5 had the highest recovery, but granulated product 2 was high in activity after steam conditioning and lost much of its activity after pelleting. The residual activity of particulate product 6 after conditioning–pelleting was the lowest. These results indicated that although pelleting or encapsulation protected phytase from the high temperature and humidity during conditioning, the high pressure, temperature, and shear force during extrusion appeared to disrupt the pellet structure and cause extensive enzyme denaturation. In summary, the encapsulation or pelleting treatments of phytase products from some manufacturers did not significantly improve the heat resistance of the enzymes.
4.3. Loss of Phytase Activity during Conditioning, Pelleting, and Cooling Process
The recovery rate of phytase activity after steam conditioning and pelleting in different studies varied greatly from 22.3% to 96.0% [9,28,34]. Firstly, there are differences in the conditions of conditioning and pelleting, such as the conditioning time (range = 10 to 90 s), steam pressure, type of conditioner, and pellet mill die specifications [33]. These conditions affected parameters related to activity loss, such as the conditioning time under high temperature and the moisture change during the pelleting process. Secondly, there are differences in the source of phytase products. Different genes of phytase and post-treatment processes further increase the variation in products from different manufacturers [9,10]. The above studies clearly indicate that phytase has a high sensitivity to steam conditioning and pelleting. In this experiment, when phytases were conditioned at 75 °C and 85 °C, the recovery rates of phytase activity after conditioning were 54.1% to 71.7% and 17.3% to 68.7%, respectively. Increasing the conditioning temperature significantly decreased the recovery rate of phytase activity, and the difference was independent of phytase dose and source. The difference in recovery rate of activity with temperature was caused by greater heat denaturation of phytase at the higher temperature. Most enzyme proteins will be denatured at 60 to 70 °C [32]. The interaction between water molecules and enzyme proteins through non-covalent van der Waals bonds affects enzyme stability during the conditioning–pelleting process [36,37]. Interestingly, the phytases with the highest or the lowest recovery rate of activity after conditioning at 75 °C had the same high or low activity after conditioning at 85 °C. De Jong et al. [4] reported that the recovery rates of four phytase activities after steam conditioning at 75 °C and 85 °C were 21.4% to 78.2% and 3.5% to 43.3%, respectively, and also showed the phytases with a high or low recovery rate of activity after conditioning at 75 °C remained high or low after conditioning at 85 °C. The above results demonstrated substantial differences in heat stability across phytase sources which is exacerbated by elevated temperature.
In Exp. 2, the average recovery of phytase activity was 62.8% and 49.7%, after steam conditioning at 75 °C and 85 °C, respectively, and 36.3% and 26.4% after pelleting. The loss of phytase activity during pelleting was equivalent to 72.6% and 46.1% of the loss during conditioning at 75 °C and 85 °C, respectively, while the loss of phytase activity during cooling was minor (less than 5%). This effect relates the diet temperature rising 3 to 15 °C after pelleting with a greater temperature difference when conditioning at a lower temperature [33,34,37]. During cooling, the hot particle samples were quickly transferred to an air-forced drier for cooling, and the temperature of the samples could be reduced to room temperature within 5 min, and the moisture content was less than 14%. The simultaneous reduction in temperature and moisture in this process is beneficial to the preservation of phytase activity [10], and the cooling process does not significantly impair the phytase activity. Consequently, future work should focus on stability during conditioning and pelleting, with less emphasis on cooling.
5. Conclusions
The phytase dose added to animal diets did not affect the recovery rate of phytase activity in either conditioning–pelleting processes. This means that the dose of a particular phytase required to achieve a desired recovery rate of activity after conditioning–pelleting can be determined with reasonable accuracy using a single dose. However, the recovery rate of eight commercial phytase activities varied greatly after the conditioning–pelleting process, so it is critical for nutritionists to know specific expectations for expected recovery. The recovery rate of phytase activity after conditioning and pelleting at 75 °C was higher than that at 85 °C, because of the decreased enzyme inactivation at the lower temperature.
Author Contributions
Conceptualization, F.Z., W.Z. and J.X.; methodology, H.Z. and Q.Z.; validation, R.S.; formal analysis, Y.W.; investigation, H.Z. and R.S.; data curation, F.Z.; writing—original draft preparation, Y.W.; writing—review and editing, F.Z. and J.X.; visualization, H.Z.; supervision, Q.Z. and W.Z.; project administration, Y.W.; funding acquisition, J.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Key Research and Development Program of China (2021YFD1300300) (Beijing, China) and a fund of King Techina Technology Co., Ltd. (2021110002001444) (Hangzhou, China).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare that there is no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- She, Y.; Sparks, J.C.; Stein, H.H. Effects of increasing concentrations of an Escherichia coli phytase on the apparent ileal digestibility of amino acids and the apparent total tract digestibility of energy and nutrients in corn-soybean meal diets fed to growing pigs. J. Anim. Sci. 2018, 96, 2804–2816. [Google Scholar] [CrossRef]
- Babatunde, O.; Cowieson, A.; Wilson, J.; Adeola, O. The impact of age and feeding length on phytase efficacy during the starter phase of broiler chickens. Poult. Sci. 2019, 98, 6742–6750. [Google Scholar] [CrossRef] [PubMed]
- Bedford, M.R.; Apajalahti, J.H. The role of feed enzymes in maintaining poultry intestinal health. J. Sci. Food Agric. 2022, 102, 1759–1770. [Google Scholar] [CrossRef]
- De Jong, J.; Woodworth, J.; DeRouchey, J.; Goodband, R.; Tokach, M.; Dritz, S.; Stark, C.; Jones, C. Stability of four commercial phytase products under increasing thermal conditioning temperatures. Transl. Anim. Sci. 2017, 1, 255–260. [Google Scholar] [CrossRef]
- Abdollahi, M.; Ravindran, V.; Svihus, B. Pelleting of broiler diets: An overview with emphasis on pellet quality and nutritional value. Anim. Feed Sci. Technol. 2013, 179, 1–23. [Google Scholar] [CrossRef]
- Evans, C.E.; Saensukjaroenphon, M.; Gebhardt, J.T.; Stark, C.R.; Paulk, C.B. Effects of conditioning temperature and pellet mill die speed on pellet quality and relative stabilities of phytase and xylanase. Transl. Anim. Sci. 2021, 5, txab043. [Google Scholar] [CrossRef]
- Greiner, R. Limitations of an in vitro model of the poultry digestive tract on the evaluation of the catalytic performance of phytases. J. Sci. Food Agric. 2021, 101, 2519–2524. [Google Scholar] [CrossRef] [PubMed]
- Brinch-Pedersen, H.; Hatzack, F.; Stöger, E.; Arcalis, E.; Pontopidan, K.; Holm, P.B. Heat-stable phytases in transgenic wheat (Triticum aestivum L.): Deposition pattern, thermostability, and phytate hydrolysis. J. Agric. Food Chem. 2006, 54, 4624–4632. [Google Scholar] [CrossRef] [PubMed]
- Slominski, B.; Davie, T.; Nyachoti, M.; Jones, O. Heat stability of endogenous and microbial phytase during feed pelleting. Livest. Sci. 2007, 109, 244–246. [Google Scholar] [CrossRef]
- De Jong, J.; DeRouchey, J.M.; Tokach, M.D.; Dritz, S.S.; Goodband, R.D.; Woodworth, J.C.; Jones, C.K.; Stark, C.R. Stability of commercial phytase sources under different environmental conditions. J. Anim. Sci. 2016, 94, 4259–4266. [Google Scholar] [CrossRef][Green Version]
- Boney, J.; Moritz, J. Phytase dose effects in practically formulated diets that vary in ingredient composition on feed manufacturing and broiler performance. J. Appl. Poult. Res. 2017, 26, 273–285. [Google Scholar] [CrossRef]
- Thomas, M.; van der Poel, A. Fundamental factors in feed manufacturing: Towards a unifying conditioning/pelleting framework. Anim. Feed Sci. Technol. 2020, 268, 114612. [Google Scholar] [CrossRef]
- Engelen, A.J.; Van Der Heeft, F.C.; Randsdorp, P.H.; Smtt, E.L. Simple and rapid determination of phytase activity. J. AOAC Int. 1994, 77, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Engelen, A.J.; van der Heeft, F.C.; Randsdorp, P.H.; Somers, W.A.; Schaefer, J.; van der Vat, B.J. Determination of phytase activity in feed by a colorimetric enzymatic method: Collaborative interlaboratory study. J. AOAC Int. 2001, 84, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Kaps, M.; Lamberson, W.R. Biostatistics for Animal Science; CABI: Oxfordshire, UK, 2017. [Google Scholar]
- Poulsen, H.; Blaabjerg, K.; Feuerstein, D. Comparison of different levels and sources of microbial phytases. Livest. Sci. 2007, 109, 255–257. [Google Scholar] [CrossRef]
- Singh, P. Significance of phytic acid and supplemental phytase in chicken nutrition: A review. World’s Poult. Sci. J. 2008, 64, 553–580. [Google Scholar] [CrossRef]
- Woyengo, T.; Nyachoti, C. Supplementation of phytase and carbohydrases to diets for poultry. Can. J. Anim. Sci. 2011, 91, 177–192. [Google Scholar] [CrossRef]
- Almeida, F.N.; Vazquez-Añón, M.; Escobar, J. Dose-dependent effects of a microbial phytase on phosphorus digestibility of common feedstuffs in pigs. Asian-Australas. J. Anim. Sci. 2017, 30, 985. [Google Scholar] [CrossRef]
- Walk, C.; Bedford, M.; Santos, T.; Paiva, D.; Bradley, J.; Wladecki, H.; Honaker, C.; McElroy, A. Extra-phosphoric effects of superdoses of a novel microbial phytase. Poult. Sci. 2013, 92, 719–725. [Google Scholar] [CrossRef]
- Walk, C.; Santos, T.; Bedford, M. Influence of superdoses of a novel microbial phytase on growth performance, tibia ash, and gizzard phytate and inositol in young broilers. Poult. Sci. 2014, 93, 1172–1177. [Google Scholar] [CrossRef]
- Guggenbuhl, P.; Calvo, E.; Fru, F. Effects of dietary doses of three phytases on performance in pigs. J. Anim. Sci. 2016, 94, 286–288. [Google Scholar] [CrossRef]
- Beeson, L.; Walk, C.; Bedford, M.; Olukosi, O. Hydrolysis of phytate to its lower esters can influence the growth performance and nutrient utilization of broilers with regular or super doses of phytase. Poult. Sci. 2017, 96, 2243–2253. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; Kim, B.G. Supplemental phytase increases phosphorus digestibility in pigs regardless of phytase source or feed pelleting. Anim. Feed Sci. Technol. 2021, 276, 114901. [Google Scholar] [CrossRef]
- Gizzi, G.; Thyregod, P.; von Holst, C.; Bertin, G.; Vogel, K.; Faurschou-Isaksen, M.; Betz, R.; Murphy, R.; Andersen, B.B. Determination of phytase activity in feed: Interlaboratory study. J. AOAC Int. 2008, 91, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Loop, S.; Lilly, K.; Shires, L.; Gehring, C.; Beaman, K.; Persia, M.; Moritz, J. The phytase analytical activity of pelleted diets may not adequately describe efficacy in the bird. J. Appl. Poult. Res. 2012, 21, 492–501. [Google Scholar] [CrossRef]
- Fan, L.; He, Z.; Ao, X.; Sun, W.; Xiao, X.; Zeng, F.; Wang, Y.; He, J. Effects of residual superdoses of phytase on growth performance, tibia mineralization, and relative organ weight in ducks fed phosphorus-deficient diets. Poult. Sci. 2019, 98, 3926–3936. [Google Scholar] [CrossRef]
- Timmons, J.; Angel, R.; Harter-Dennis, J.; Saylor, W.; Ward, N. Evaluation of heat-stable phytases in pelleted diets fed to broilers from day zero to thirty-five during the summer months. J. Appl. Poult. Res. 2008, 17, 482–489. [Google Scholar] [CrossRef]
- Singh, B.; Satyanarayana, T. Fungal phytases: Characteristics and amelioration of nutritional quality and growth of non-ruminants. J. Anim. Physiol. Anim. Nutr. 2015, 99, 646–660. [Google Scholar] [CrossRef]
- Han, Y.; Lei, X.G. Role of Glycosylation in the Functional Expression of anAspergillus nigerPhytase (phyA) inPichia pastoris. Arch. Biochem. Biophys. 1999, 364, 83–90. [Google Scholar] [CrossRef]
- Singh, N.; Kuhar, S.; Priya, K.; Jaryal, R.; Yadav, R. Phytase: The feed enzyme, an overview. In Advances in Animal Biotechnology and its Applications; Springer: Singapore, 2018; pp. 269–327. [Google Scholar]
- Svihus, B.; Zimonja, O. Chemical alterations with nutritional consequences due to pelleting animal feeds: A review. Anim. Prod. Sci. 2011, 51, 590–596. [Google Scholar] [CrossRef]
- Bedford, M.; Partridge, G. Thermostability of feed enzymes and their practical application in the feed mill. In Enzymes in Farm Animal Nutrition; Bedford, M.R., Partridge, G.G., Eds.; CABI: Oxfordshire, UK, 2010; p. 368. [Google Scholar]
- Amerah, A.; Gilbert, C.; Simmins, P.; Ravindran, V. Influence of feed processing on the efficacy of exogenous enzymes in broiler diets. World’s Poult. Sci. J. 2011, 67, 29–46. [Google Scholar] [CrossRef]
- Vasudevan, U.M.; Jaiswal, A.K.; Krishna, S.; Pandey, A. Thermostable phytase in feed and fuel industries. Bioresour. Technol. 2019, 278, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Zhao, G.; Ning, Y. Interactions between plant proteins/enzymes and other food components, and their effects on food quality. Crit. Rev. Food Sci. Nutr. 2017, 57, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Homan, V.; Boney, J.; Moritz, J. Effects of steam conditioning temperatures on commercial phytases and subsequent broiler performance and tibia mineralization. Appl. Anim. Sci. 2019, 35, 298–303. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).