Rapid Visual Detection of African Swine Fever Virus with a CRISPR/Cas12a Lateral Flow Strip Based on Structural Protein Gene D117L
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Viruses
2.2. Cloning of LbCas12a Gene and Protein Expression and Purification
2.3. Detection of Endonuclease Activity of the Purified LbCas12a Protein
2.4. Design of ASFV Structural Protein p17 Gene (D117L) crRNA and Its Mediated CRISPR/Cas12a Reaction
2.5. Recombinant Polymerase Amplification (RPA)
2.6. Establishment of RPA-CRISPR/Cas12a-Lateral Flow Strip (LFS) Detection Method
2.7. Sensitivity and Specificity of D117L-RPA-Cas12a-LFS Detection Method
2.8. Clinical Sample Processing and Nucleic Acid Extraction
3. Results
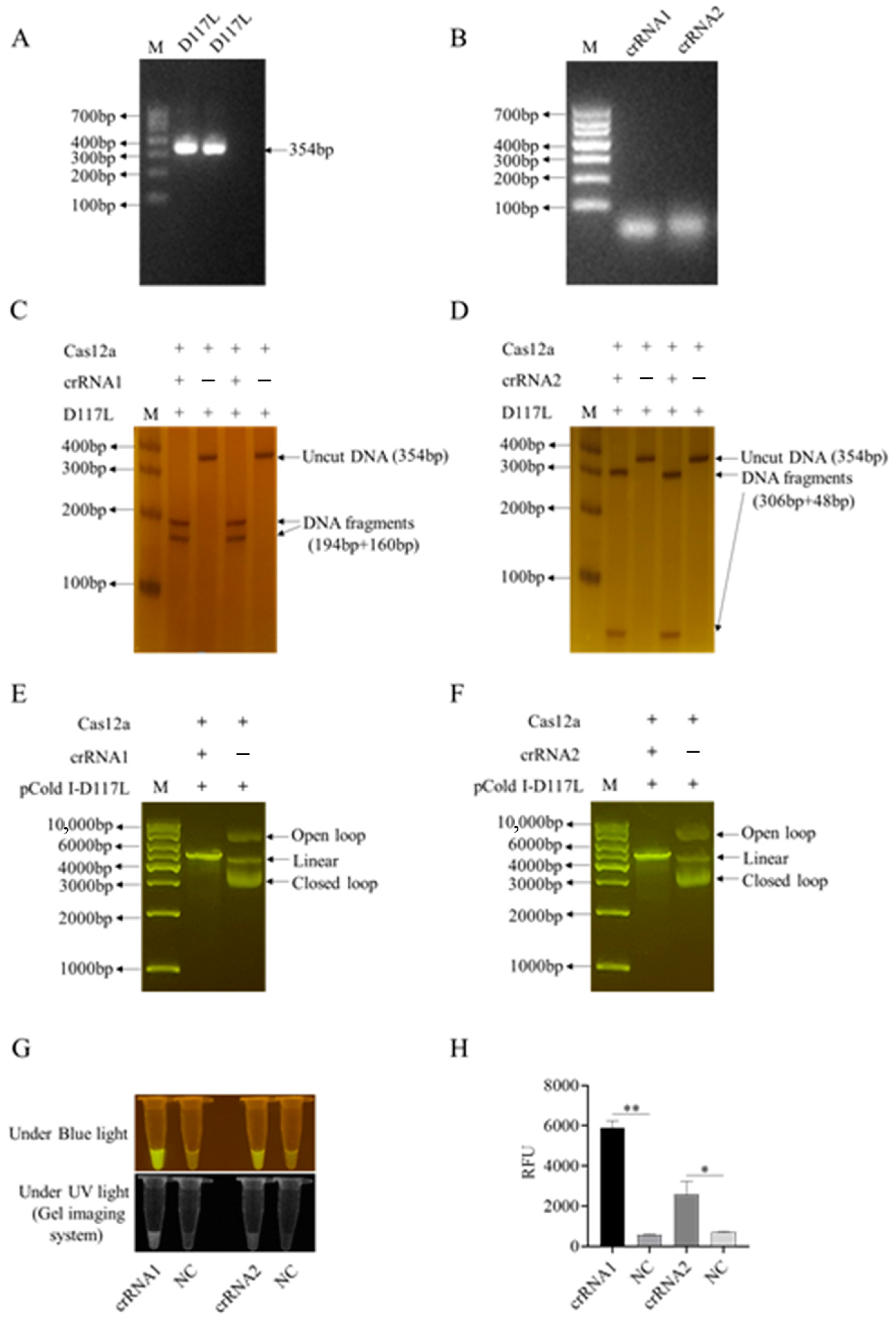
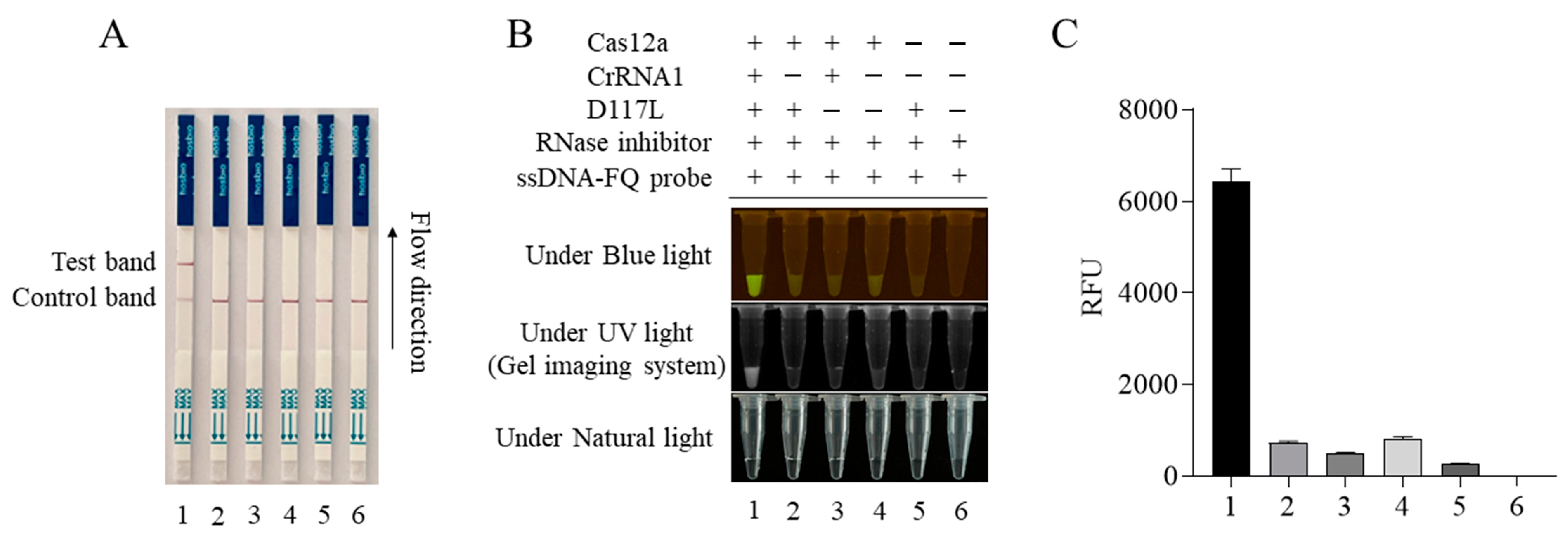
3.1. CRISPR/Cas12a Reaction Mediated by ASFV Structural Protein Gene D117L crRNA1 and Its Optimization
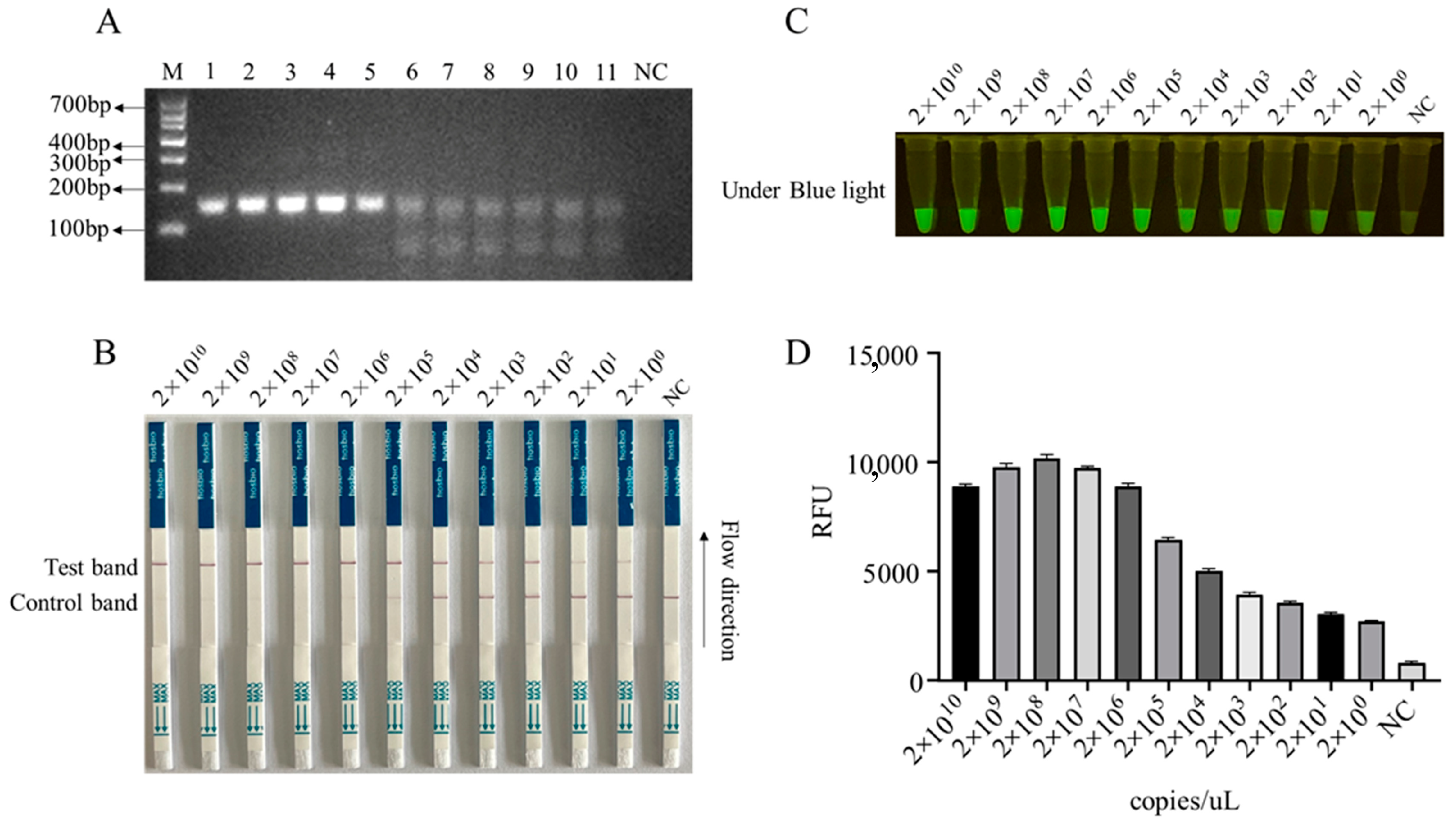
3.2. The Detection Sensitivity of the CRISPR/Cas12a Reaction
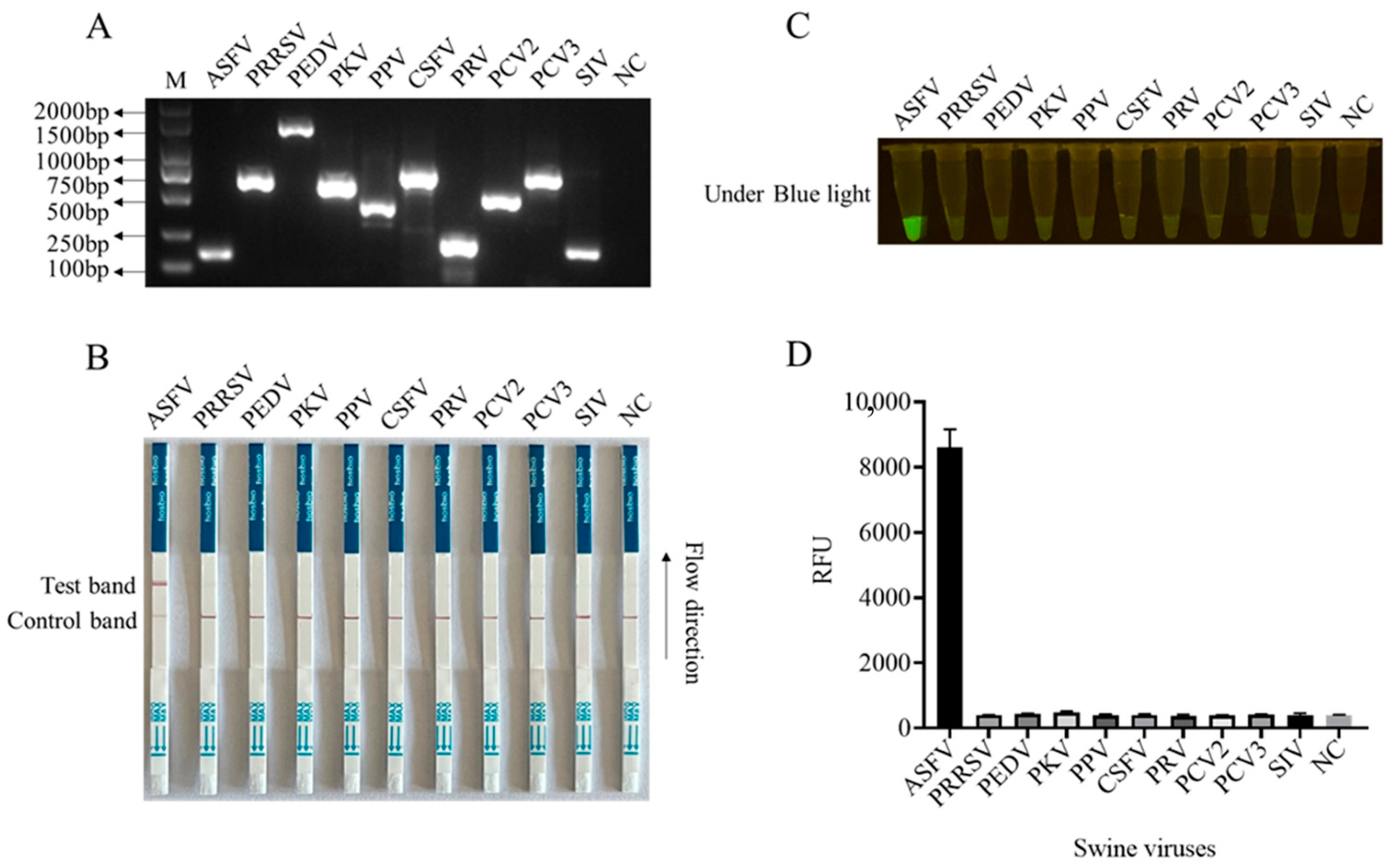
3.3. Establishment of CRISPR/Cas12a Mediated Lateral Flow Strip (LFS) Method, Its Sensitivity, and Its Specificity
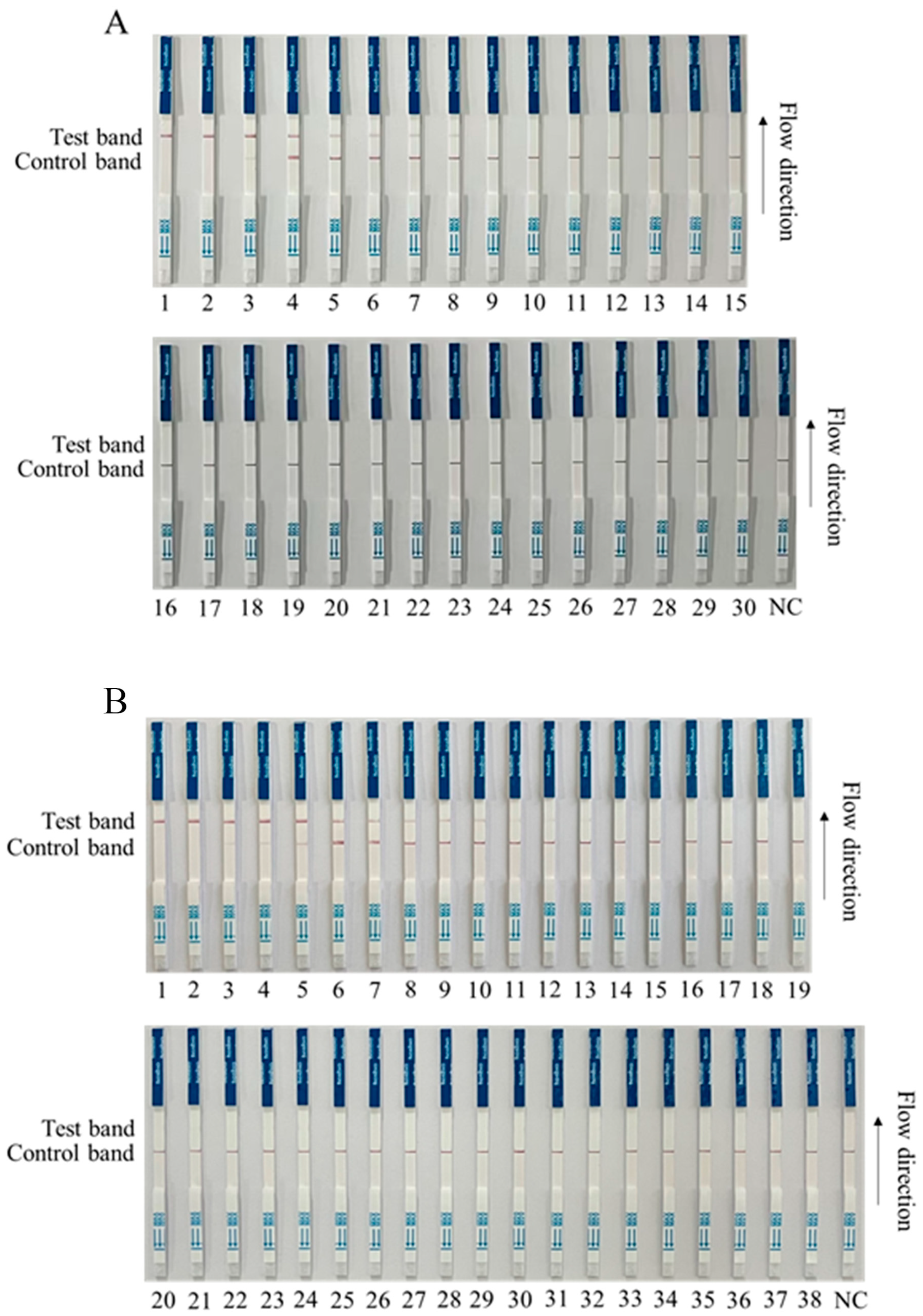
3.4. Detection of Clinical Samples by RPA-CRISPR/Cas12a-LFS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Galindo, I.; Alonso, C. African Swine Fever Virus: A Review. Viruses 2017, 9, 103. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, W.; Yang, W.; Zhang, J.; Li, D.; Zheng, H. Structure of African Swine Fever Virus and Associated Molecular Mechanisms Underlying Infection and Immunosuppression: A Review. Front. Immunol. 2021, 12, 715582. [Google Scholar] [CrossRef]
- Ata, E.B.; Li, Z.J.; Shi, C.W.; Yang, G.L.; Yang, W.T.; Wang, C.F. African swine fever virus: A raised global upsurge and a continuous threaten to pig husbandry. Microb. Pathog. 2022, 167, 105561. [Google Scholar] [CrossRef]
- Xin, G.; Kuang, Q.; Le, S.; Wu, W.; Gao, Q.; Gao, H.; Xu, Z.; Zheng, Z.; Lu, G.; Gong, L.; et al. Origin, genomic diversity and evolution of African swine fever virus in East Asia. Virus Evol. 2023, 9, vead060. [Google Scholar] [CrossRef] [PubMed]
- Urbano, A.C.; Ferreira, F. African swine fever control and prevention: An update on vaccine development. Emerg. Microbes Infect. 2022, 11, 2021–2033. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Li, Z.; Yan, Q.; Li, Y.; Xiong, W.; Wu, K.; Li, X.; Fan, S.; Zhao, M.; Ding, H.; et al. Development of Diagnostic Tests Provides Technical Support for the Control of African Swine Fever. Vaccines 2021, 9, 343. [Google Scholar] [CrossRef] [PubMed]
- Oura, C.A.; Edwards, L.; Batten, C.A. Virological diagnosis of African swine fever—Comparative study of available tests. Virus Res. 2013, 173, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Wang, X.; Zhu, Z.; Yu, Y.; Chang, D.; Zhang, X.; Li, D.; Sun, F.; Zhou, L.; Xu, J.; et al. Quality Management for Point-Of-Care Testing of Pathogen Nucleic Acids: Chinese Expert Consensus. Front. Cell. Infect. Microbiol. 2021, 11, 755508. [Google Scholar] [CrossRef] [PubMed]
- Padzil, F.; Mariatulqabtiah, A.R.; Tan, W.S.; Ho, K.L.; Isa, N.M.; Lau, H.Y.; Abu, J.; Chuang, K.P. Loop-Mediated Isothermal Amplification (LAMP) as a Promising Point-of-Care Diagnostic Strategy in Avian Virus Research. Animals 2021, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Sciuto, E.L.; Leonardi, A.A.; Calabrese, G.; Luca, G.; Coniglio, M.A.; Irrera, A.; Conoci, S. Nucleic Acids Analytical Methods for Viral Infection Diagnosis: State-of-the-Art and Future Perspectives. Biomolecules 2021, 11, 1585. [Google Scholar] [CrossRef]
- Wu, X.; Chan, C.; Springs, S.L.; Lee, Y.H.; Lu, T.K.; Yu, H. A warm-start digital CRISPR/Cas-based method for the quantitative detection of nucleic acids. Anal. Chim. Acta 2022, 1196, 339494. [Google Scholar] [CrossRef]
- Chen, J.S.; Ma, E.; Harrington, L.B.; Da Costa, M.; Tian, X.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, R.; Yang, M.; Peng, S.; Cheng, Y.; Chen, C. Conformational Dynamics and Cleavage Sites of Cas12a Are Modulated by Complementarity between crRNA and DNA. iScience 2019, 19, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Ren, K.; Qiu, X.; Zheng, J.; Guo, M.; Guan, X.; Liu, H.; Li, N.; Zhang, B.; Yang, D.; et al. The crystal structure of Cpf1 in complex with CRISPR RNA. Nature 2016, 532, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Swarts, D.C.; van der Oost, J.; Jinek, M. Structural Basis for Guide RNA Processing and Seed-Dependent DNA Targeting by CRISPR-Cas12a. Mol. Cell 2017, 66, 221–233.e4. [Google Scholar] [CrossRef]
- Qian, W.; Huang, J.; Wang, X.; Wang, T.; Li, Y. CRISPR-Cas12a combined with reverse transcription recombinase polymerase amplification for sensitive and specific detection of human norovirus genotype GII.4. Virology 2021, 564, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; Zeng, H.; Liu, X.; Jiang, W.; Wang, Y.; Ouyang, W.; Tang, X. RPA-Cas12a-FS: A frontline nucleic acid rapid detection system for food safety based on CRISPR-Cas12a combined with recombinase polymerase amplification. Food Chem. 2021, 334, 127608. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Xiao, B.; Deng, H.; Gong, K.; Li, K.; Li, L.; Hao, W. Development of a RPA-CRISPR-Cas12a Assay for Rapid, Simple, and Sensitive Detection of Mycoplasma hominis. Front. Microbiol. 2022, 13, 842415. [Google Scholar] [CrossRef]
- Talwar, C.S.; Park, K.H.; Ahn, W.C.; Kim, Y.S.; Kwon, O.S.; Yong, D.; Kang, T.; Woo, E. Detection of Infectious Viruses Using CRISPR-Cas12-Based Assay. Biosensors 2021, 11, 301. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, Y.; Cai, G.; Meng, G.; Shi, S. Rapid and sensitive RPA-Cas12a-fluorescence assay for point-of-care detection of African swine fever virus. PLoS ONE 2021, 16, e0254815. [Google Scholar] [CrossRef]
- Xiong, Y.; Cao, G.; Chen, X.; Yang, J.; Shi, M.; Wang, Y.; Nie, F.; Huo, D.; Hou, C. One-pot platform for rapid detecting virus utilizing recombinase polymerase amplification and CRISPR/Cas12a. Appl. Microbiol. Biotechnol. 2022, 106, 4607–4616. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Miao, C.; Liu, W.; Zhang, G.; Shao, J.; Chang, H. Structure and function of African swine fever virus proteins: Current understanding. Front. Microbiol. 2023, 14, 1043129. [Google Scholar] [CrossRef] [PubMed]
- Suarez, C.; Gutierrez-Berzal, J.; Andres, G.; Salas, M.L.; Rodriguez, J.M. African swine fever virus protein p17 is essential for the progression of viral membrane precursors toward icosahedral intermediates. J. Virol. 2010, 84, 7484–7499. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Wang, H.; Liu, X.; Shao, Q.; Ao, D.; Xu, Y.; Jiang, S.; Luo, J.; Zhang, J.; Chen, N.; et al. African Swine Fever Virus Structural Protein p17 Inhibits Cell Proliferation through ER Stress-ROS Mediated Cell Cycle Arrest. Viruses 2020, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qiao, S.; Li, G.; Tong, W.; Dong, S.; Liu, J.; Guo, Z.; Zheng, H.; Zhao, R.; Tong, G.; et al. The Indirect ELISA and Monoclonal Antibody against African Swine Fever Virus p17 Revealed Efficient Detection and Application Prospects. Viruses 2022, 15, 50. [Google Scholar] [CrossRef]
- King, D.P.; Reid, S.M.; Hutchings, G.H.; Grierson, S.S.; Wilkinson, P.J.; Dixon, L.K.; Bastos, A.D.; Drew, T.W. Development of a TaqMan PCR assay with internal amplification control for the detection of African swine fever virus. J. Virol. Methods 2003, 107, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Tong, X.; Han, Y.; Zhang, K.; Zhang, Y.; Chen, Q.; Duan, J.; Lei, X.; Huang, M.; Qiu, Y.; et al. Fast and sensitive detection of SARS-CoV-2 RNA using suboptimal protospacer adjacent motifs for Cas12a. Nat. Biomed. Eng. 2022, 6, 286–297. [Google Scholar] [CrossRef]
- Liu, S.; Tao, D.; Liao, Y.; Yang, Y.; Sun, S.; Zhao, Y.; Yang, P.; Tang, Y.; Chen, B.; Liu, Y.; et al. Highly Sensitive CRISPR/Cas12a-Based Fluorescence Detection of Porcine Reproductive and Respiratory Syndrome Virus. ACS Synth. Biol. 2021, 10, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Shi, Z.; Ma, Y.; Wang, L.; Cao, L.; Luo, J.; Wan, Y.; Song, R.; Yan, Y.; Yuan, K.; et al. LAMP assay coupled with CRISPR/Cas12a system for portable detection of African swine fever virus. Transbound. Emerg. Dis. 2022, 69, e216–e223. [Google Scholar] [CrossRef]
- Wang, X.; He, S.; Zhao, N.; Liu, X.; Cao, Y.; Zhang, G.; Wang, G.; Guo, C. Development and clinical application of a novel CRISPR-Cas12a based assay for the detection of African swine fever virus. BMC Microbiol. 2020, 20, 282. [Google Scholar] [CrossRef]
- Wu, J.; Mukama, O.; Wu, W.; Li, Z.; Habimana, J.D.; Zhang, Y.; Zeng, R.; Nie, C.; Zeng, L. A CRISPR/Cas12a Based Universal Lateral Flow Biosensor for the Sensitive and Specific Detection of African Swine-Fever Viruses in Whole Blood. Biosensors 2020, 10, 203. [Google Scholar] [CrossRef]
- Wei, N.; Zheng, B.; Niu, J.; Chen, T.; Ye, J.; Si, Y.; Cao, S. Rapid Detection of Genotype II African Swine Fever Virus Using CRISPR Cas13a-Based Lateral Flow Strip. Viruses 2022, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Li, F.; Chen, Q.; Wu, J.; Duan, J.; Lei, X.; Zhang, Y.; Zhao, D.; Bu, Z.; Yin, H. Rapid detection of African swine fever virus using Cas12a-based portable paper diagnostics. Cell Discov. 2020, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhao, D.; Wang, J.; Zhang, Y.; Wang, M.; Gao, Y.; Li, F.; Wang, J.; Bu, Z.; Rao, Z.; et al. Architecture of African swine fever virus and implications for viral assembly. Science 2019, 366, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xie, M.; Wu, W.; Chen, Z. Structures and Functional Diversities of ASFV Proteins. Viruses 2021, 13, 2124. [Google Scholar] [CrossRef]
- Huang, S.; Wang, X.; Chen, X.; Liu, X.; Xu, Q.; Zhang, L.; Huang, G.; Wu, J. Rapid and sensitive detection of Pseudomonas aeruginosa by isothermal amplification combined with Cas12a-mediated detection. Sci. Rep. 2023, 13, 19199. [Google Scholar] [CrossRef]
- Cao, G.; Xiong, Y.; Nie, F.; Chen, X.; Peng, L.; Li, Y.; Yang, M.; Huo, D.; Hou, C. Non-nucleic acid extraction and ultra-sensitive detection of African swine fever virus via CRISPR/Cas12a. Appl. Microbiol. Biotechnol. 2022, 106, 4695–4704. [Google Scholar] [CrossRef]

| Sample Types | Sample Numbers | Results (Positive/Negative) | |
|---|---|---|---|
| RPA-Cas12a-LFS | qPCR | ||
| Heart | 2 | 1/1 | 1/1 |
| Liver | 4 | 2/2 | 2/2 |
| Spleen | 4 | 2/2 | 2/2 |
| Lung | 6 | 2/4 | 2/4 |
| Kidney | 4 | 2/2 | 2/2 |
| Oral swab | 2 | 0/2 | 0/2 |
| Blood | 26 | 9/17 | 9/17 |
| Serum | 20 | 3/17 | 3/17 |
| Total | 68 | 21/47 | 21/47 |
| Positive rates | 44.7% | 44.7% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Jiang, S.; Xia, N.; Zhang, Y.; Zhang, J.; Liu, A.; Zhang, C.; Chen, N.; Meurens, F.; Zheng, W.; et al. Rapid Visual Detection of African Swine Fever Virus with a CRISPR/Cas12a Lateral Flow Strip Based on Structural Protein Gene D117L. Animals 2023, 13, 3712. https://doi.org/10.3390/ani13233712
Zhang D, Jiang S, Xia N, Zhang Y, Zhang J, Liu A, Zhang C, Chen N, Meurens F, Zheng W, et al. Rapid Visual Detection of African Swine Fever Virus with a CRISPR/Cas12a Lateral Flow Strip Based on Structural Protein Gene D117L. Animals. 2023; 13(23):3712. https://doi.org/10.3390/ani13233712
Chicago/Turabian StyleZhang, Desheng, Sen Jiang, Nengwen Xia, Youwen Zhang, Jiajia Zhang, Anjing Liu, Chenyang Zhang, Nanhua Chen, Francois Meurens, Wanglong Zheng, and et al. 2023. "Rapid Visual Detection of African Swine Fever Virus with a CRISPR/Cas12a Lateral Flow Strip Based on Structural Protein Gene D117L" Animals 13, no. 23: 3712. https://doi.org/10.3390/ani13233712
APA StyleZhang, D., Jiang, S., Xia, N., Zhang, Y., Zhang, J., Liu, A., Zhang, C., Chen, N., Meurens, F., Zheng, W., & Zhu, J. (2023). Rapid Visual Detection of African Swine Fever Virus with a CRISPR/Cas12a Lateral Flow Strip Based on Structural Protein Gene D117L. Animals, 13(23), 3712. https://doi.org/10.3390/ani13233712










