Phytogenics in Ginger, Origanum vulgare, and Syzygium aromaticum and Their Potential as a Feed Additive against Clostridium perfringens in Broiler Production
Abstract
Simple Summary
Abstract
1. Introduction
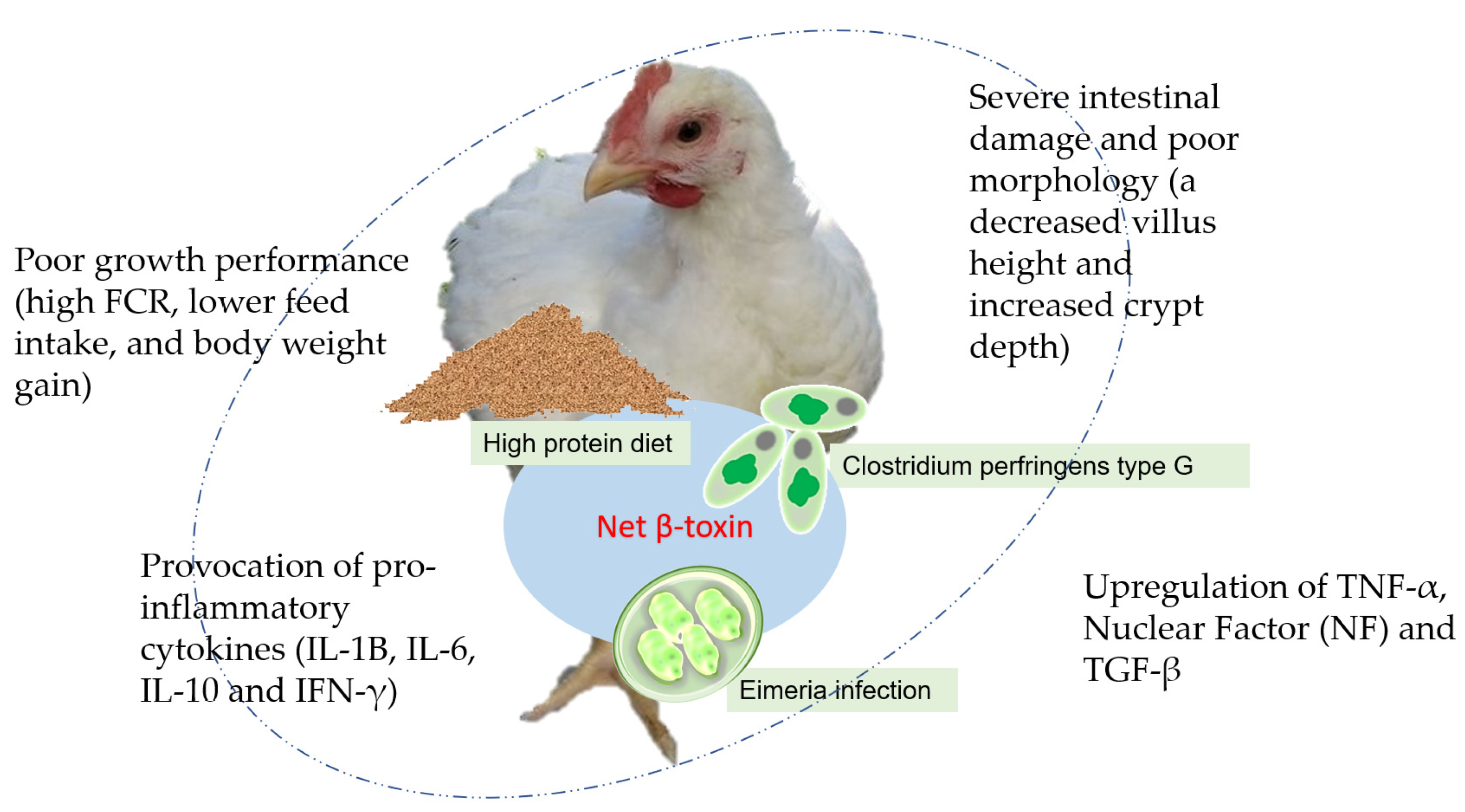
2. Effects of Plant Extracts and Their Bioactive Compounds
2.1. Ginger and Its Active Compounds
| Bioactive Components or Extracts | Parts of the Plant for Extraction | Functional Property | References |
|---|---|---|---|
| Whole phytochemical extracts | Dried rhizomes | Antioxidant and antibacterial activities | [35] |
| Hydro-alcoholic extracts | Dried rhizomes | Antibacterial effects against Staphylococcus aureus, pseudomonas aeruginosa, and Listeria monocytogenes | [36] |
| Ethanolic extracts | Fresh ginger rhizomes | Anti-microbial effects against E coli, Salmonella typhi and Bacillus subtilis | [37] |
| Gingerol (6-gingerol, 8-gingerol, and 10-gingerol) | Rhizomes | Anti-cancer activities | [38] |
| Ginger oil (6-gingerol as well as 4-, 5-, 8-, 10-, and 12-gingerols) | Ginger roots | Anti-fungal, antibacterial, anti-inflammatory, analgesic, and immunomodulatory effects | [39] |
| 6-gingerol | Rhizomes | Anti-inflammatory activities | [40] |
| 6-gingerol | Rhizomes | Anti-colitis activities | [41] |
| Gingerol | Rhizomes | Effective against E. coli, Salmonella typhi and Bacillus subtilis, with anti-fungal effects | [42] |
| 6-shogaol | Dried ginger rhizomes (gingerols converted to shogaols) | Anti-microbial effects against Gram-positive and -negative bacteria | [43] |
| 6-Shogaols | Dried rhizomes | Anti-inflammatory, antioxidant properties | [44] |
| Gingerenone-A and shogaol | Rhizomes | Anti-microbial effects against Staphylococcus aureus | [45] |
2.1.1. Effects of Ginger on the Growth Performance of Broiler Chickens
2.1.2. The Anti-Inflammatory and Immunomodulatory Properties of Ginger and Its Active Compounds
2.2. Turmeric and Its Active Compounds
2.2.1. Effects of Turmeric and Its Active Compounds on the Growth Performance of Broiler Chickens
2.2.2. The Anti-Inflammatory and Immunostimulatory Properties of Turmeric and Its Active Compounds
2.3. Alpinia spp. Extracts and Their Active Compounds
2.3.1. Effects of Alpinia spp. on the Growth Performance of Broiler Chickens
2.3.2. The Anti-Inflammatory and Immunostimulatory Properties of Alpinia spp. and Its Active Compounds
2.4. Origanum Vulgare and Its Active Compounds
2.4.1. Effects of Origanum vulgare on the Growth Performance of Broiler Chickens
2.4.2. The Anti-Inflammatory and Immunostimulatory Properties of Origanum vulgare and Its Active Compounds
2.5. Clove Extracts and Its Active Compounds
2.5.1. Effects of Cloves Extracts on the Growth Performance of Broilers
2.5.2. The Anti-Inflammatory Effects of Clove and Its Active Compounds
 —inhibitory effects;
—inhibitory effects;  —stimulatory effects;
—stimulatory effects;  —upregulation;
—upregulation;  —downregulation.
—downregulation.
 —inhibitory effects;
—inhibitory effects;  —stimulatory effects;
—stimulatory effects;  —upregulation;
—upregulation;  —downregulation.
—downregulation.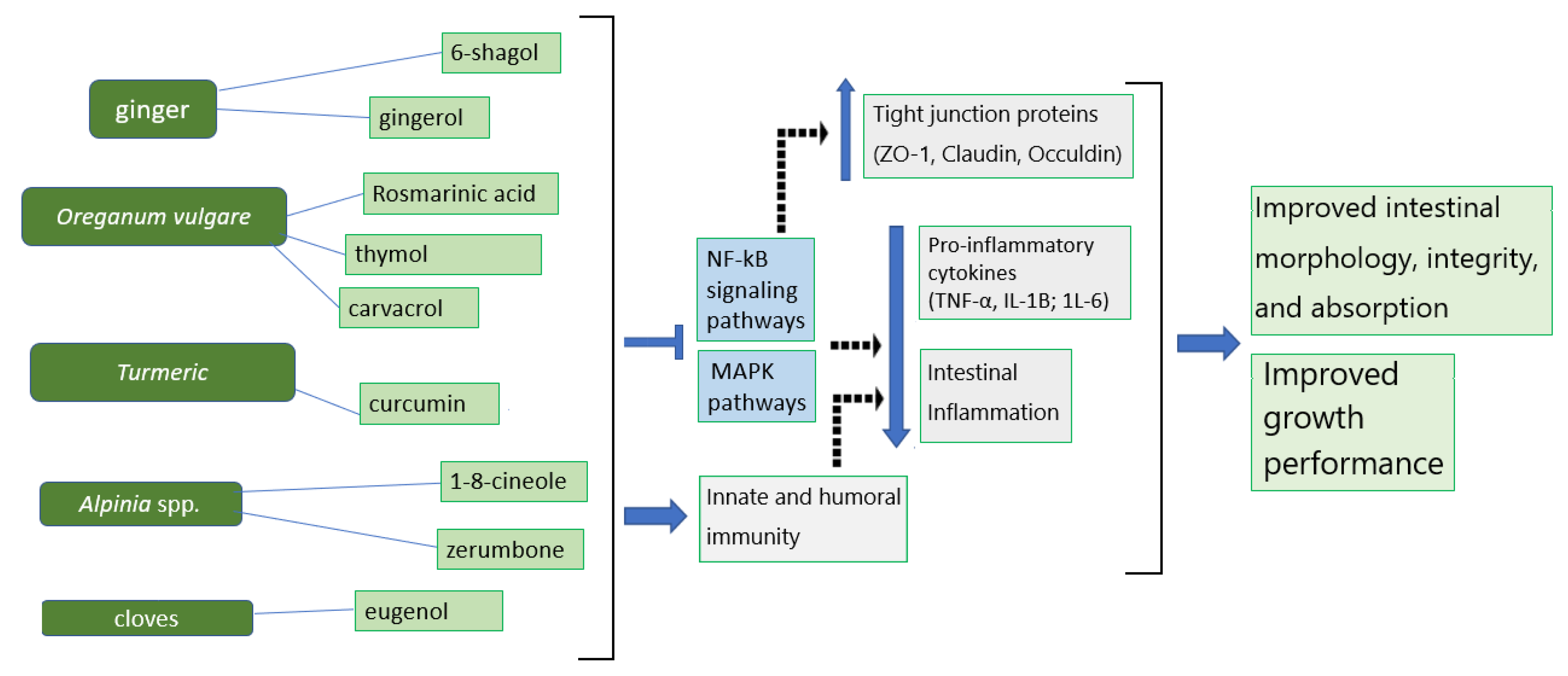
3. Proposed Mechanisms for the Phytogenics of Ginger, Origanum vulgare, and Cloves against Inflammation to Improve Intestinal Integrity
4. The Toxicity and Safety of Ginger, Origanum vulgare, and Syzygium aromaticum as Potential Feed Supplements
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Lutful, S.M. Avian colibacillosis and salmonellosis: A closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int. J. Environ. Res. Public Health 2010, 7, 89–114. [Google Scholar]
- Butaye, P.; Devriese, L.A.; Haesebrouck, F. Antimicrobial growth promoters used in animal feed: Effects of less well-known antibiotics on gram-positive bacteria. Clin. Microbiol. Rev. 2003, 16, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Paiva, D.M.; McElroy, A.P. Necrotic enteritis: Applications for the poultry industry. J. Appl. Poult. Res. 2014, 23, 557–566. [Google Scholar] [CrossRef]
- Timbermont, L.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Necrotic enteritis in broilers: An updated review on the pathogenesis. Avian Pathol. 2011, 40, 341–347. [Google Scholar] [CrossRef]
- Cooper, K.K.; Songer, J.G.; Uzal, F.A. Diagnosing clostridial enteric disease in poultry. J. Vet. Diagn. Investig. 2013, 25, 314–327. [Google Scholar] [CrossRef]
- Kaldhusdal, M.; Schneitz, C.; Hofshagen, M.; Skjerve, E. Reduced incidence of Clostridium perfringens-associated lesions and improved performance in broiler chickens treated with normal intestinal bacteria from adult fowl. Avian Dis. 2001, 45, 149–156. [Google Scholar] [CrossRef]
- Olkowski, A.A.; Wojnarowicz, C.; Chinio-Trejo, M.; Drew, M.D. Responses of broiler chickens orally challenged with Clostridium perfringens isolated from field cases of necrotic enteritis. Res. Vet. Sci. 2006, 81, 99–108. [Google Scholar] [CrossRef]
- Kaldhusdal, M.; Hofshagen, M. Barley inclusion and avoparcin supplementation in broiler diets. 2. clinical, pathological, and bacteriological findings in a mild form of necrotic enteritis. Poult. Sci. 1992, 71, 1145–1153. [Google Scholar] [CrossRef]
- Skinner, J.T.; Bauer, S.; Young, V.; Pauling, G.; Wilson, J. An economic analysis of the impact of subclinical (MiLD) necrotic enteritis in broiler chickens. Avian Dis. 2010, 54, 1237–1240. [Google Scholar] [CrossRef]
- Emami, N.K.; Dalloul, R.A. Centennial review: Recent developments in host-pathogen interactions during necrotic enteritis in poultry. Poult. Sci. 2021, 100, 101330. [Google Scholar] [CrossRef] [PubMed]
- Riva, S.; Monjo, T.P. The major predisposing factors for necrotic enteritis in broiler chickens and the use of probiotics as new strategy to prevent the disease. J. Vet. Med. Anim. Sci. 2021, 4, 1051. [Google Scholar]
- Dierick, E.; Hirvonen, O.P.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F.; Goossens, E. Rapid growth predisposes broilers to necrotic enteritis. Avian Pathol. 2019, 48, 416–422. [Google Scholar] [CrossRef]
- Justino, L.; Baptista, A.; de Souza, M.; Menck-Costa, M.F.; Pires, B.G.; Cicero, C.E.; Bracarense, A.; Kaneko, V.M.; Oba, A.; Okamoto, A.S.; et al. Evaluation of predisposing factors of necrotic enteritis in experimentally challenged broiler chickens. Animals 2022, 12, 1880. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.J. Necrotic enteritis predisposing factors in broiler chickens. Avian Pathol. 2016, 45, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, N.J.; Swick, R.A.; Geier, M.S.; Moore, R.J.; Choct, M.; Wu, S.B. A multifactorial analysis of the extent to which eimeria and fishmeal predispose broiler chickens to necrotic enteritis. Avian Dis. 2015, 59, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, T.; Zhu, H.; Liu, L.; Bi, S.; Chen, X.; Zhang, H. Effects of challenge with clostridium perfringens, eimeria and both on ileal microbiota of yellow feather broilers. Front. Microbiol. 2022, 13, 1063578–1063590. [Google Scholar] [CrossRef]
- Palliyeguru, M.; Rose, S.P. Sub-clinical necrotic enteritis: Its aetiology and predisposing factors in commercial broiler production. Worlds Poult. Sci. J. 2014, 70, 803–816. [Google Scholar] [CrossRef]
- Daneshmand, A.; Kermanshahi, H.; Mohammed, J.; Sekhavati, M.H.; Javadmanesh, A.; Ahmadian, M.; Alizadeh, M.; Razmyar, J.; Kulkarni, R.R. Intestinal changes and immune responses during Clostridium perfringens-induced necrotic enteritis in broiler chickens. Poult. Sci. 2022, 101, 101652. [Google Scholar] [CrossRef]
- Lee, K.W.; Lillehoj, H.S. Role of Clostridium perfringens necrotic enteritis b-like toxin in disease pathogenesis. Vaccines 2021, 10, 61. [Google Scholar] [CrossRef]
- Keyburn, A.L.; Boyce, J.D.; Vaz, P.; Bannam, T.L.; Ford, M.E.; Parker, D.; Di Rubbo, A.; Rood, J.I.; Moore, R.J. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008, 4, e26. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, M.; Yuan, W.; Song, Z.; Liao, S.; Qi, N.; Li, J.; Lv, M.; Wu, C.; Lin, X.; Hu, J.; et al. Experimental induction of necrotic enteritis with or without predisposing factors using net-B positive Clostridium perfringens strains. Gut Pathog. 2021, 13, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Lillehoj, H.S.; Park, M.S.; Jang, S.I.; Ritter, G.D.; Hong, Y.H.; Jeong, W.; Jeoung, H.Y.; An, D.J.; Lillehoj, E.P. Clostridium perfringens α-toxin and netB toxin antibodies and their possible role in protection against necrotic enteritis and gangrenous dermatitis in broiler chickens. Avian Dis. 2012, 56, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Akerele, G.; Al Hakeem, W.; Lourenco, J.; Selvaraj, R. The effect of necrotic enteritis challenge on production performance, cecal microbiome, and cecal tonsil transcriptome in broilers. Pathogens 2022, 11, 839. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, X.; Wang, Y.; Guo, Y.; Zhu, P.; Li, G.; Zhang, J.; Ma, Q.; Zhao, L. Dietary ellagic acid ameliorated clostridium perfringens-induced subclinical necrotic enteritis in broilers via regulating inflammation and cecal microbiota. J. Anim. Sci. Biotechnol. 2022, 13, 47–64. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, W.H.; Lee, S.; Lillehoj, H.S. Detection of chicken interleukin-10 production in intestinal epithelial cells and necrotic enteritis induced by Clostridium perfringens using capture ELISA. Vet. Immunol. Immunopathol. 2018, 204, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Lillehoj, H.S.; Allen, P.C.; Park, D.W.; Fitzcoy, S.; Bautista, D.A.; Lillehoj, E.P. Immunopathology and cytokine responses in broiler chickens coinfected with Eimeria maxima and Clostridium perfringens with the use of an animal model of necrotic enteritis. Avian Dis. 2008, 52, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Huang, X.; Yan, Z.; Gao, X.; Wang, P.; Yang, Q.; Wang, W.; Xie, K.; Gun, S. Identification and characterization of MAPK signaling pathway genes and associated lncRNAs in the ileum of piglets infected by Clostridium perfringens type C. Biomed. Res. Int. 2020, 2020, 8496872. [Google Scholar] [CrossRef]
- Abd El-Ghany, W.A.; Yazar Soyadı, Y.A. Phytobiotics in poultry industry as growth promoters, antimicrobials and immunomodulators—A Review. J. World’s Poult. Res. 2020, 10, 571–579. [Google Scholar] [CrossRef]
- Sayed, M.; Shahta, M.A.; Ali, N.; Kotob, M.; Mahmoud, U.; Mahmoud, M.; Amen, O. Evaluate the effect of some phytobiotics on the control of necrotic enteritis in broilers chicken. Assiut Vet. Med. J. 2023, 69, 89–104. [Google Scholar] [CrossRef]
- Xiao, J.; Bai, W. Bioactive phytochemicals. Crit. Rev. Food Sci. Nutr. 2019, 59, 827–829. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Elbestawy, A.R.; Gado, A.R.; Nader, M.M.; Saad, A.M.; El-Tahan, A.M.; Taha, A.E.; Salem, H.M.; El-Tarabily, K.A. Hot red pepper powder as a safe alternative to antibiotics in organic poultry feed: An updated review. Poult. Sci. 2022, 101, 101684. [Google Scholar] [CrossRef] [PubMed]
- Kikusato, M. Phytobiotics to improve health and production of broiler chickens: Functions beyond the antioxidant activity. Anim. Biosci. 2021, 34, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Kizhakkayil, J.; Sasikumar, B. Diversity, characterization and utilization of ginger: A review. Plant Genet. Resour. 2011, 9, 464–477. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.; Rahmat, A. Changes in antioxidant and antibacterial activities as well as phytochemical constituents associated with ginger storage and polyphenol oxidase activity. BMC Complement. Altern. Med. 2016, 16, 382. [Google Scholar] [CrossRef] [PubMed]
- Azadpour, M.; Azadpour, N.; Bahmani, M.; Hassanzadazar, H.; Rafieian-Kopaei, M.; Naghdi, N. Antimicrobial effect of ginger (Zingiber officinale) and mallow (Malva sylvestris) hydroalcholic extracts on four pathogen bacteria. Der Pharm. Lett. 2016, 8, 181–187. [Google Scholar]
- Azu, N.; Onyeagba, R.A. Antimicrobial properties of extracts of Allium cepa (Onions) and Zingiber officinale (Ginger) on Escherichia coli, Salmonella typhi and Bacillus subtilis. J. Trop. Med. 2007, 3, 1–10. [Google Scholar]
- Prasad, S.; Tyagi, A.K. Ginger and its constituents: Role in prevention and treatment of gastrointestinal cancer. Gastroenterol. Res. Pract. 2015, 2015, 142979. [Google Scholar] [CrossRef]
- Yücel, Ç.; Karatoprak, G.Ş.; Açıkara, Ö.B.; Akkol, E.K.; Barak, T.H.; Sobarzo-Sánchez, E.; Aschner, M.; Shirooie, S. Immunomodulatory and anti-inflammatory therapeutic potential of gingerols and their nanoformulations. Front. Pharmacol. 2022, 13, 902551. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Kim, T.; Kim, R.; Ha, H. The Natural product 6-gingerol inhibits inflammation-associated osteoclast differentiation via reduction of prostaglandin E2 levels. Int. J. Mol. Sci. 2018, 19, 2068. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, R.A.; Shabrmi, F.M.; Aly, S.M. Active ingredients of ginger as potential candidates in the prevention and treatment of diseases via modulation of biological activities. Int. J. Physiol. Pathophysiol. Pharmacol. 2014, 6, 125–136. [Google Scholar] [PubMed]
- Ghasemzadeh, A.; Jaafar, H.; Baghdadi, A.; Tayebi-Meigooni, A. Formation of 6-, 8- and 10-shogaol in ginger through application of different drying methods: Altered antioxidant and antimicrobial activity. Molecules 2018, 23, 1646. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Kont, I.; Primke, T.; Niebergall, L.S.; Zech, T.; Fürst, R. Ginger constituent 6-shogaol inhibits inflammation- and angiogenesis-related cell functions in primary human endothelial cells. Front. Pharmacol. 2022, 13, 844767. [Google Scholar] [CrossRef] [PubMed]
- Rampogu, S.; Baek, A.; Gajula, R.G.; Zeb, A.; Bavi, R.S.; Kumar, R.; Kim, Y.; Kwon, Y.J.; Lee, K.W. Ginger (Zingiber officinale) phytochemicals—Gingerenone-a and shogaol inhibit sahppk: Molecular docking, molecular dynamics simulations and in vitro approaches. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 16. [Google Scholar] [CrossRef]
- Friedlein, U.; Dorn-In, S.; Schwaiger, K. Antimicrobial effects of plant extracts against Clostridium perfringens with respect to food-relevant influencing factors. J. Food Prot. 2021, 84, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Karangiya, V.K.; Savsani, H.H.; Patil, S.S.; Garg, D.D.; Murthy, K.S.; Ribadiya, N.K.; Vekariya, S.J. Effect of dietary supplementation of garlic, ginger and their combination on feed intake, growth performance and economics in commercial broilers. Vet. World 2016, 9, 245–250. [Google Scholar] [CrossRef]
- Asghar, M.U.; Rahman, A.; Hayat, Z.; Rafique, M.K.; Badar, I.H.; Yar, M.K.; Ijaz, M. Exploration of Zingiber officinale effects on growth performance, immunity and gut morphology in broilers. Braz. J. Biol. 2023, 83, e250296. [Google Scholar] [CrossRef]
- Egenuka, F.C.; Okeudo, N.J.; Otti, M.I.; Obikaonu, H.O.; Aladi, N.O. Comparative effects of fresh and dry ginger as nutritional supplements on live-weight gain, carcass characteristics and meat quality of broiler chicken. Res. Sq. 2022, 1, 1–15. [Google Scholar]
- Al-Khalaifah, H.; Al-Nasser, A.; Al-Surrayai, T.; Sultan, H.; Al-Attal, D.; Al-Kandari, R.; Al-Saleem, H.; Al-Holi, A.; Dashti, F. Effect of ginger powder on production performance, antioxidant status, hematological parameters, digestibility, and plasma cholesterol content in broiler chickens. Animals 2022, 12, 901. [Google Scholar] [CrossRef]
- Safiullah; Chand, N.; Khan, R.U.; Naz, S.; Ahmad, M.; Gul, S. Effect of ginger (Zingiber officinale Roscoe) and organic selenium on growth dynamics, blood melanodialdehyde and paraoxonase in broilers exposed to heat stress. J. Appl. Anim. Res. 2019, 47, 212–216. [Google Scholar] [CrossRef]
- Wen, C.; Liu, Y.; Ye, Y.; Tao, Z.; Cheng, Z.; Wang, T.; Zhou, Y. Effects of gingerols-rich extract of ginger on growth performance, serum metabolites, meat quality and antioxidant activity of heat-stressed broilers. J. Therm. Biol. 2020, 89, 102544. [Google Scholar] [CrossRef] [PubMed]
- Shewita, R.S.; Taha, A.E. Influence of dietary supplementation of ginger powder at different levels on growth performance, haematological profiles, slaughter traits and gut morphometry of broiler chickens. S. Afr. J. Anim. Sci. 2019, 48, 997–1008. [Google Scholar] [CrossRef]
- Shukri, N.K.; Mustafa, M.A.G.; Mustafa, N.A.G. Impact of ginger powder supplementation in broiler diet on the immune statue development and small intestine morphology. Bas. J. Vet. Res. 2018, 17, 99–112. [Google Scholar]
- Duwa, H.; Amaza, I.B.; Dikko, M.; Raymond, J.; Paullyne, U. Effect of ginger (Zingiber officinale) on the growth performance and nutrient digestibility of finisher broiler chickens in semi-arid zone of Nigeria. Niger. J. Anim. Sci. 2020, 22, 279–286. [Google Scholar]
- Habibi, R.; Sadeghi, G.H.; Karimi, A. Effect of different concentrations of ginger root powder and its essential oil on growth performance, serum metabolites and antioxidant status in broiler chicks under heat stress. Br. Poult. Sci. 2014, 55, 228–237. [Google Scholar] [CrossRef]
- Ehebha, E.T.E.; Okosun, S.E.; Adomeh, E.E.; Eguaoje, A.S. Growth performance, carcass characteristics and organoleptic properties of broiler finisher fed graded levels of ginger root meal (GRM) basal diets. Sustain. Agric. Food Environ. Res. 2018, 6, 86–98. [Google Scholar] [CrossRef]
- Ali, M.; Chand, N.; Khan, R.U.; Naz, S.; Gul, S. Anticoccidial effect of garlic (Allium sativum) and ginger (Zingiber officinale) against experimentally induced coccidiosis in broiler chickens. J. Appl. Anim. Res. 2019, 47, 79–84. [Google Scholar] [CrossRef]
- Luettig, J.; Rosenthal, R.; Lee, I.F.M.; Krug, S.M.; Schulzke, J.D. The ginger component 6-shogaol prevents TNF-α-induced barrier loss via inhibition of PI3K/Akt and NF-κB signaling. Mol. Nutr. Food Res. 2016, 60, 2576–2586. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.S.; Rhee, M.H.; Sung, G.H.; Yoo, B.C.; Cho, J.Y. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediat. Inflamm. 2014, 2014, 352371. [Google Scholar] [CrossRef]
- Li, Y.; Xu, B.; Xu, M.; Chen, D.; Xiong, Y.; Lian, M.; Sun, Y.; Tang, Z.; Wang, L.; Jiang, C.; et al. 6-gingerol protects intestinal barrier from ischemia/reperfusion-induced damage via inhibition of p38 MAPK to NF-κB signalling. Pharmacol. Res. 2017, 119, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.S.; Allam, T.S.; El-Latif, A.S.A.; Ghazy, E.W. The effects of dietary supplementation of different levels of thyme (Thymus vulgaris) and Ginger (Zingiber officinale) essential oils on performance, hematological, biochemical and immunological parameters of broiler chickens. Glob. Vet. 2014, 12, 736–744. [Google Scholar]
- Herawati, H.; Anisa, A.K.; Widiatmoko, K.D.; Alam, S.S.P.; Diari, I.A.; Naprila, Z.H.; Kisya, R.L.A.; Puspabela, A.; Permata, F.S. Effect of red ginger powder (Zingiber officinale var. Rubrum) as a feed additive for starter and finisher broiler chicken to increase immunoglobulin a and immunoglobulin y expression and to prevent intestinal injury due to Salmonella enteritidis infection. Vet. World 2022, 15, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Mahassni, S.H.; Bukhari, O.A. Beneficial effects of an aqueous ginger extract on the immune system cells and antibodies, hematology, and thyroid hormones in male smokers and non-smokers. J. Nutr. Intermed. Metab. 2019, 15, 10–17. [Google Scholar] [CrossRef]
- Qorbanpour, M.; Fahim, T.; Javandel, F.; Nosrati, M.; Paz, E.; Seidavi, A.; Ragni, M.; Laudadio, V.; Tufarelli, V. Effect of dietary ginger (Zingiber officinale roscoe) and multi-strain probiotic on growth and carcass traits, blood biochemistry, immune responses and intestinal microflora in broiler chickens. Animals 2018, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Maksoud, E.M.; Daha, A.A.E.F.; Taha, N.M.; Lebda, M.A.; Sadek, K.M.; Alshahrani, M.Y.; Ahmed, A.E.; Shukry, M.; Fadl, S.E.; Elfeky, M. Effects of ginger extract and/or propolis extract on immune system parameters of vaccinated broilers. Poult. Sci. 2023, 102, 102903–102914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.A.; Kitts, D.D. Turmeric and its bioactive constituents trigger cell signaling mechanisms that protect against diabetes and cardiovascular diseases. Mol. Cell. Biochem. 2021, 476, 3785–3814. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and its major compound curcumin on health: Bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front. Pharmacol. 2020, 11, 1021–1043. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Swelum, A.A.; Arif, M.; Abo Ghanima, M.M.; Shukry, M.; Noreldin, A.; Taha, A.E.; El-Tarabily, K.A. Curcumin, the active substance of turmeric: Its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021, 101, 5747–5762. [Google Scholar] [CrossRef]
- Dai, C.; Lin, J.; Li, H.; Shen, Z.; Wang, Y.; Velkov, T.; Shen, J. The natural product curcumin as an antibacterial agent: Current achievements and problems. Antioxidants 2022, 11, 459. [Google Scholar] [CrossRef]
- Gul, P.; Bakht, J. Antimicrobial activity of turmeric extract and its potential use in food industry. J. Food Sci. Technol. 2013, 52, 2272–2279. [Google Scholar] [CrossRef] [PubMed]
- Bright, J.J. Curcumin and autoimmune disease. Adv. Exp. Med. Biol. 2007, 595, 425–451. [Google Scholar] [PubMed]
- Wang, D.; Huang, H.; Zhou, L.; Li, W.; Zhou, H.; Hou, G.; Liu, J.; Hu, L. Effects of dietary supplementation with turmeric rhizome extract on growth performance, carcass characteristics, antioxidant capability, and meat quality of Wenchang broiler chickens. Ital. J. Anim. Sci. 2015, 14, 3870–3875. [Google Scholar] [CrossRef]
- Choudhury, D.; Mahanta, J.; Sapcota, D.; Saikia, B.; Islam, R. Effect of Dietary supplementation of turmeric (Curcuma longa) powder on the performance of commercial broiler chicken. Int. J. Livest. Res. 2018, 8, 182–191. [Google Scholar] [CrossRef]
- Anyanwu, N.J.; Anyanwu, G.A.; Omumuabunike, C.S.; Okonkwo, Z.C.; Nwasike, H.U. Growth performance of broiler chickens fed raw and cooked turmeric rhizome (Curcuma longa) supplemented diets. Niger. J. Agric. Food Environ. 2021, 17, 69–77. [Google Scholar]
- Gowda, N.K.S.; Ledoux, D.R.; Rottinghaus, G.E.; Bermudez, A.J.; Chen, Y.C. Antioxidant efficacy of curcuminoids from turmeric (Curcuma longa L.) powder in broiler chickens fed diets containing aflatoxin B1. Br. J. Nutr. 2009, 102, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Hafez, M.H.; El-Kazaz, S.E.; Alharthi, B.; Ghamry, H.I.; Alshehri, M.A.; Sayed, S.; Shukry, M.; El-Sayed, Y.S. The impact of curcumin on growth performance, growth-related gene expression, oxidative stress, and immunological biomarkers in broiler chickens at different stocking densities. Animals 2022, 12, 958. [Google Scholar] [CrossRef]
- Jin, S.; Yang, H.; Liu, F.; Pang, Q.; Shan, A.; Feng, X. Effect of dietary curcumin supplementation on duck growth performance, antioxidant capacity and breast meat quality. Foods 2021, 10, 2981. [Google Scholar] [CrossRef]
- Guo, S.; Hu, J.; Ai, S.; Li, L.; Ding, B.; Zhao, D.; Wang, L.; Hou, Y. Effects of pueraria extract and curcumin on growth performance, antioxidant status and intestinal integrity of broiler chickens. Animals 2023, 13, 1276. [Google Scholar] [CrossRef]
- Badran, A. Effect of Dietary Curcumin and curcumin nanoparticles supplementation on growth performance, immune response and antioxidant of broilers chickens. Egypt. Poult. Sci. J. 2020, 40, 325–343. [Google Scholar] [CrossRef]
- Lee, S.H.; Lillehoj, H.S.; Jang, S.I.; Lillehoj, E.P.; Min, W.; Bravo, D.M. Dietary supplementation of young broiler chickens with capsicum and turmeric oleoresins increases resistance to necrotic enteritis. Br. J. Nutr. 2013, 110, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Islam, M.; Zaman, S. Effects of turmeric powder on Clostridium perfringens load in broiler chickens. SAARC J. Agric. 2020, 18, 209–218. [Google Scholar] [CrossRef]
- Yadav, S.; Teng, P.Y.; Souza dos Santos, T.; Gould, R.L.; Craig, S.W.; Lorraine Fuller, A.; Pazdro, R.; Kim, W.K. The effects of different doses of curcumin compound on growth performance, antioxidant status, and gut health of broiler chickens challenged with Eimeria Species. Poult. Sci. 2020, 99, 5936–5945. [Google Scholar] [CrossRef] [PubMed]
- Arafa, W.; Hashem, K. Anticoccidial properties of micronized curcumin against Eimeria tenella in experimentally infected broiler chickens. Egypt. Vet. Soc. Parasitol. J. 2016, 12, 1–10. [Google Scholar] [CrossRef]
- Larmonier, C.B.; Uno, J.K.; Lee, K.-M.; Karrasch, T.; Laubitz, D.; Thurston, R.; Midura-Kiela, M.T.; Ghishan, F.K.; Sartor, R.B.; Jobin, C.; et al. Limited effects of dietary curcumin on Th-1 Driven colitis in IL-10 deficient mice suggest an IL-10-dependent mechanism of protection. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G1079–G1091. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Han, Z.; Tian, L.; Chen, K.; Fan, Y.; Ye, B.; Huang, W.; Wang, C.; Huang, Z. Curcumin inhibits EMMPRIN and MMP-9 expression through AMPK-MAPK and PKC signaling in PMA induced macrophages. J. Transl. Med. 2014, 12, 266–275. [Google Scholar] [CrossRef]
- Wang, P.; Hao, X.; Li, X.; Yan, Y.; Tian, W.; Xiao, L.; Wang, Z.; Dong, J. Curcumin inhibits adverse psychological stress-induced proliferation and invasion of glioma cells via down-regulating the ERK/MAPK pathway. J. Cell. Mol. Med. 2021, 25, 7190–7203. [Google Scholar] [CrossRef]
- Epstein, J.; Docena, G.; MacDonald, T.T.; Sanderson, I.R. Curcumin suppresses p38 mitogen-activated protein kinase activation, reduces iL-1β and matrix metalloproteinase-3 and enhances IL-10 in the mucosa of children and adults with inflammatory bowel disease. Br. J. Nutr. 2009, 103, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Barquero, L.; Villegas, I.; Sánchez-Calvo, J.M.; Talero, E.; Sánchez-Fidalgo, S.; Motilva, V.; Alarcón de la Lastra, C. Curcumin, a Curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis. Int. Immunopharmacol. 2007, 7, 333–342. [Google Scholar] [CrossRef]
- Nawab, A.; Tang, S.; Li, G.; An, L.; Wu, J.; Liu, W.; Xiao, M. Dietary curcumin supplementation effects on blood immunological profile and liver enzymatic activity of laying hens after exposure to high temperature conditions. J. Therm. Biol. 2020, 90, 102573–102580. [Google Scholar] [CrossRef]
- Wang, J.; Ghosh, S.S.; Ghosh, S. Curcumin improves intestinal barrier function: Modulation of intracellular signaling, and organization of tight junctions. Am. J. Physiol. Cell Physiol. 2017, 312, C438–C445. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, H. Curcumin improved intestinal epithelial barrier integrity by up-regulating ZO-1/Occludin/Claudin-1 in septic rats. Evid. Based Complement. Altern. Med. 2022, 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- de Sá, F.D.L.; Butkevych, E.; Nattramilarasu, P.K.; Fromm, A.; Mousavi, S.; Moos, V.; Golz, J.C.; Stingl, K.; Kittler, S.; Seinige, D. Curcumin mitigates immune-induced epithelial barrier dysfunction by Campylobacter jejuni. Int. J. Mol. Sci. 2019, 20, 4830. [Google Scholar] [CrossRef] [PubMed]
- Asghar, I.; Rizvi, F.; Usmani, M.W.; Shakir, M.Z.; Mahmood, N.; Numan, M.; Ikram, M.S.; Waqar, N. Immunomodulatory effect of turmeric (Curcuma longa) in Escherichia coli induced infected broiler chicks. J. Microb. Pathog. 2022, 6, 129–134. [Google Scholar]
- Sahoo, N.; Mishra, S.K.; Swain, R.K.; Acharya, A.P.; Pattnaik, S.; Sethy, K.; Sahoo, L. Effect of turmeric and ginger supplementation on immunity, antioxidant, liver enzyme activity, gut bacterial load and histopathology of broilers. Indian J. Anim. Sci. 2019, 89, 774–779. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J.; Li, X.; Ning, X.; Sun, C.; Zhang, N.; Zhang, S. Curcumin alleviates spleen immunotoxicity induced by decabrominated diphenyl ethers (bde-209) by improving immune function and inhibiting inflammation and apoptosis in broilers. Ecotoxicol. Environ. Saf. 2023, 259, 115048–115059. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Alaidaroos, B.A.; Farsi, R.M.; Abou-Kassem, D.E.; El-Saadony, M.T.; Saad, A.M.; Shafi, M.E.; Albaqami, N.M.; Taha, A.E.; Ashour, E.A. Impacts of supplementing broiler diets with biological curcumin, zinc nanoparticles and bacillus licheniformis on growth, carcass traits, blood indices, meat quality and cecal microbial load. Animals 2021, 11, 1878. [Google Scholar] [CrossRef]
- Shwetha, H.S.; Narayana Swamy, M.; Srinivas, R.B.; Naik, J.; Kalmath, G.P.; Malathi, V.; Veena, M.P.; Rajendran, D. Nano-selenium and nano-curcumin preparation, characterization and its effect on broiler chickens to produce lean meat. Res. Sq. 2022, 1, 1–28. [Google Scholar]
- Ghosh, S.; Rangan, L. Alpinia: The gold mine of future therapeutics. 3 Biotech. 2012, 3, 173–185. [Google Scholar] [CrossRef]
- Chompoo, J.; Upadhyay, A.; Fukuta, M.; Tawata, S. Effect of Alpinia zerumbet components on antioxidant and skin diseases-related enzymes. BMC Complement. Altern. Med. 2012, 12, 106. [Google Scholar] [CrossRef]
- Da Cruz, J.D.; Mpalantinos, M.A.; Ramos, A.S.; Ferreira, J.L.P.; de Oliveira, A.A.; Júnior, N.L.N.; de Silva, J.R.A.; Amaral, A.C.F. Chemical standardization, antioxidant activity and phenolic contents of cultivated Alpinia zerumbet preparations. Ind. Crops Prod. 2020, 151, 112495. [Google Scholar] [CrossRef]
- Zahra, M.H.; Salem, T.A.R.; El-Aarag, B.; Yosri, N.; EL-Ghlban, S.; Zaki, K.; Marei, A.H.; Abd El-Wahed, A.; Saeed, A.; Khatib, A.; et al. Alpinia zerumbet (Pers.): Food and medicinal plant with potential in vitro and in vivo anti-cancer activities. Molecules 2019, 24, 2495. [Google Scholar] [CrossRef] [PubMed]
- Chumroenphat, T.; Somboonwatthanakul, I.; Saensouk, S.; Siriamornpun, S. The diversity of biologically active compounds in the rhizomes of recently discovered Zingiberaceae plants native to north eastern Thailand. Pharmacogn. J. 2019, 11, 1014–1022. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, C.; Ou, Z.; Liang, X.; Shi, Y.; Chi, L.; Zhang, Z.; Zheng, X.; Li, C.; Xiang, H. Chemical profiling and bioactivity of essential oils from Alpinia officinarum Hance from ten localities in China. Ind. Crops Prod. 2020, 153, 112583. [Google Scholar] [CrossRef]
- Victório, C.P.; Alviano, D.S.; Alviano, C.S.; Lage, C.L.S. Chemical composition of the fractions of leaf oil of Alpinia zerumbet (Pers.) B.L. Burtt & R.M. Sm. and antimicrobial activity. Rev. Bras. Farmacogn. 2009, 19, 697–701. [Google Scholar]
- Victório, C.P.; Leitão, S.G.; Lage, C.L.S. Chemical composition of the leaf oils of Alpinia zerumbet (Pers.) Burtt et Smith and A. Purpurata (Vieill) K. Schum. from Rio de Janeiro, Brazil. J. Essent. Oil Res. 2010, 22, 52–54. [Google Scholar] [CrossRef]
- Costa, G.M.; Suga, R.; Oliveira, P.; Silva, F.C.; Magalhães, P.; Duarte, L.; Farias, L.M. Bioactivity of extracts from Alpinia zerumbet against sinusitis-causing bacterial pathogens. Rev. Fitos. 2015, 9, 185–194. [Google Scholar] [CrossRef][Green Version]
- Weerapreeyakul, N.; Tavichakorntrakool, R.; Lulitanond, A.; Sangka, A.; Sungkeeree, S. Antibacterial activity and bioactive compounds of 50% hydroethanolic extract of Alpinia zerumbet (Pers.) B.L. Burtt & R.M. Sm. Asian Pac. J. Trop. Biomed. 2019, 9, 204–208. [Google Scholar]
- Pogačar, M.Š.; Klančnik, A.; Bucar, F.; Langerholc, T.; Možina, S.S. Alpinia katsumadai extracts inhibit adhesion and invasion of Campylobacter jejuni in animal and human foetal small intestine cell lines. Phytother. Res. 2015, 29, 1585–1589. [Google Scholar] [CrossRef]
- Wong, L.F.; Lim, Y.Y.; Omar, M. Antioxidant and antimicrobial activities of some alpina species. J. Food Biochem. 2009, 33, 835–851. [Google Scholar] [CrossRef]
- Victorio, C.P.; Silva, D.O.e.; Alviano, D.; Alviano, C.; Kuster, R.M.; Lage, C.L.S. In vitro antimicrobial activity of Alpinia zerumbet and A. purpurata Nonpolar Fraction of Leaf Extract. Rev. Fitos 2021, 15, 136–143. [Google Scholar] [CrossRef]
- Bhuiyan, M.N.I.; Chowdhury, J.U.; Begum, J. Chemical investigation of the leaf and rhizome essential oils of Zingiber zerumbet (L.) smith from Bangladesh. Bangladesh J. Pharmacol. 2008, 4, 9–12. [Google Scholar] [CrossRef]
- AbuZahra, H.M.; Rajendran, P.; Ismail, M.B. Zerumbone exhibit protective effect against zearalenone induced toxicity via ameliorating inflammation and oxidative stress induced apoptosis. Antioxidants 2021, 10, 1593. [Google Scholar] [CrossRef] [PubMed]
- Mirghaed, A.T.; Fayaz, S.; Hoseini, S.M. Effects of dietary 1,8-cineole supplementation on serum stress and antioxidant markers of common carp (Cyprinus carpio) acutely exposed to ambient ammonia. Aquaculture 2019, 509, 8–15. [Google Scholar] [CrossRef]
- Di, Y.; Cao, A.; Zhang, Y.; Li, J.; Sun, Y.; Geng, S.; Li, Y.; Zhang, L. Effects of Dietary 1,8-Cineole supplementation on growth performance, antioxidant capacity, immunity, and intestine health of broilers. Animals 2022, 12, 2415–2425. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Luo, M.; Ma, W.; Ma, S.; Wang, Y.; Zhang, K. Protective effects of 1,8-cineole microcapsules against inflammation and gut microbiota imbalance associated weight loss induced by heat stress in broiler chicken. Front. Pharmacol. 2021, 11, 585945–585956. [Google Scholar] [CrossRef] [PubMed]
- Platel, K.; Srinivasan, K. Digestive stimulant action of spices: A myth or reality? Indian J. Med. Res. 2004, 119, 167–179. [Google Scholar]
- Cimrin, T. Effect of Cinnamaldehyde and 1, 8-Cineole on performance, egg quality and some blood parameters of laying hens. Indian J. Anim. Sci. 2019, 89, 435–441. [Google Scholar] [CrossRef]
- Samadi, S.; Wajizah, S.; Tarman, A. Administration of Zingiber zerumbet extract on performances and haematological parameters of broiler chickens. IOP Conf. Ser. Earth Environ. Sci. 2020, 497, 012048–012054. [Google Scholar] [CrossRef]
- Rafiqi, R.; Ryanda, M.A.; Fuadi, M.A.; Riwa, N.; Wahyudi, I.; Ilham, I.; Allaily, A.; Wajizah, S.; Samadi, S. Influence of Zingiber Zerumbet Extracts as Feed Additive on Performance, Carcass Characteristics and Inhibition of Escherichia coli Bacteria of Commercial Broiler Chickens. J. Kedokt. Hewan 2022, 16, 39–44. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, J.; Fang, C.; Tang, F. 1,8-Cineol attenuates LPS-induced acute pulmonary inflammation in mice. Inflammation 2013, 37, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Juergens, U.R.; Engelen, T.; Racké, K.; Stöber, M.; Gillissen, A.; Vetter, H. Inhibitory activity of 1,8-cineol (eucalyptol) on cytokine production in cultured human lymphocytes and monocytes. Pulm. Pharmacol. Ther. 2004, 17, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, T.; de Oliveira, M.P.; Lima, A.; Bezerra-Santos, C.; Piuvezam, M. Gamma-terpinene modulates acute inflammatory response in mice. Planta Med. 2015, 81, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Jo, M.; Hong, J.E.; Park, C.O.; Lee, C.G.; Yun, M.; Rhee, K.J. Zerumbone suppresses enterotoxigenic bacteroides fragilis infection-induced colonic inflammation through inhibition of NF-κB. Int. J. Mol. Sci. 2019, 20, 4560. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Yun, J.M. Molecular mechanism of the protective effect of zerumbone on lipopolysaccharide-induced inflammation of THP-1 cell-derived macrophages. J. Med. Food 2019, 22, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.d.A.; Jantan, I.; Harikrishnan, H.; Ghazalee, S. Standardized extract of Zingiber zerumbet suppresses LPS-induced pro-inflammatory responses through NF-κB, MAPK and PI3K-Akt signaling pathways in U937 macrophages. Phytomedicine 2019, 54, 195–205. [Google Scholar] [CrossRef]
- Lee, C.Y. Zerumbone from Zingiber zerumbet ameliorates lipopolysaccharide-induced ICAM-1 and cytokines expression via P38 MAPK/JNK-IκB/NF-κB Pathway in mouse model of acute lung injury. Chin. J. Physiol. 2018, 61, 171–180. [Google Scholar] [CrossRef]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A recent insight regarding the phytochemistry and bioactivity of Origanum vulgare L. essential oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef]
- Khorsand, G.J.; Morshedloo, M.R.; Mumivand, H.; Bistgani, Z.E.; Maggi, F.; Khademi, A. Natural diversity in phenolic components and antioxidant properties of oregano (Origanum vulgare L.) accessions, grown under the same conditions. Sci. Rep. 2022, 12, 5813. [Google Scholar] [CrossRef]
- Vazirian, M.; Mohammadi, M.; Farzaei, M.H.; Amin, G.; Amanzadeh, Y. Chemical composition and antioxidant activity of Origanum vulgare subsp. vulgare essential oil from Iran. Dir. Open Access J. 2015, 2, 41–46. [Google Scholar]
- Russo, M.; Galletti, G.C.; Bocchini, P.; Carnacini, A. Essential oil chemical composition of wild populations of Italian oregano spice (Origanum vulgare Ssp. Hirtum (Link) Ietswaart): A preliminary evaluation of their use in chemotaxonomy by cluster analysis. 1. inflorescences. J. Agric. Food Chem. 1998, 46, 3741–3746. [Google Scholar] [CrossRef]
- Ilić, Z.; Stanojević, L.; Milenković, L.; Šunić, L.; Milenković, A.; Stanojević, J.; Cvetković, D. The yield, chemical composition, and antioxidant activities of essential oils from different plant parts of the wild and cultivated oregano (Origanum vulgare L.). Horticulture 2022, 8, 1042. [Google Scholar] [CrossRef]
- Oniga, I.; Pușcaș, C.; Silaghi-Dumitrescu, R.; Olah, N.K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.; Benedec, D.; Pop, C. Origanum vulgare ssp. vulgare: Chemical composition and biological studies. Molecules 2018, 23, 2077. [Google Scholar] [CrossRef] [PubMed]
- Kolypetri, S.; Kostoglou, D.; Nikolaou, A.; Kourkoutas, Y.; Giaouris, E. Chemical composition, antibacterial and antibiofilm actions of oregano (Origanum vulgare subsp. Hirtum) essential oil against Salmonella typhimurium and Listeria monocytogenes. Foods 2023, 12, 2893. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Khan, S.T.; Khan, N.A.; Mahmood, A.; Al-Kedhairy, A.A.; Alkhathlan, H.Z. The composition of the essential oil and aqueous distillate of Origanum vulgare L. growing in saudi arabia and evaluation of their antibacterial activity. Arab. J. Chem. 2018, 11, 1189–1200. [Google Scholar] [CrossRef]
- Hao, Y.; Li, J.; Shi, L. A carvacrol-rich essential oil extracted from oregano (Origanum vulgare “hot & spicy”) exerts potent antibacterial effects against Staphylococcus aureus. Front. Microbiol. 2021, 12, 741861. [Google Scholar]
- Baj, T.; Biernasiuk, A.; Wróbel, R.; Malm, A. Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of candida albicans and candida glabrata. Open Chem. 2020, 18, 108–118. [Google Scholar] [CrossRef]
- Sik, B.; Kapcsándi, V.; Székelyhidi, R.; Hanczné, E.L.; Ajtony, Z. Recent advances in the analysis of rosmarinic acid from herbs in the lamiaceae family. Nat. Prod. Commun. 2019, 14, 1934578X19864216. [Google Scholar] [CrossRef]
- Forte, C.; Branciari, R.; Pacetti, D.; Miraglia, D.; Ranucci, D.; Acuti, G.; Balzano, M.; Frega, N.G.; Trabalza-Marinucci, M. Dietary oregano (Origanum vulgare L.) aqueous extract improves oxidative stability and consumer acceptance of meat enriched with CLA and n-3 PUFA in broilers. Poult. Sci. 2018, 97, 1774–1785. [Google Scholar] [CrossRef]
- Ri, C.S.; Jiang, X.R.; Kim, M.H.; Wang, J.; Zhang, H.J.; Wu, S.G.; Bontempo, V.; Qi, G.H. Effects of Dietary Oregano Powder Supplementation on the Growth Performance, Antioxidant Status and Meat Quality of Broiler Chicks. Ital. J. Anim. Sci. 2017, 16, 246–252. [Google Scholar] [CrossRef]
- Shang, R.; Chen, L.; Xin, Y.; Wang, G.; Li, R.; Li, S.; Li, L. Evaluation of rosmarinic acid on broiler growth performance, serum biochemistry, liver antioxidant activity, and muscle tissue composition. Animals 2022, 12, 3313. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, D.; Beghelli, D.; Trabalza-Marinucci, M.; Branciari, R.; Forte, C.; Olivieri, O.; Badillo Pazmay, G.V.; Cavallucci, C.; Acuti, G. Dietary effects of a mix derived from oregano (Origanum vulgare L.) essential oil and sweet chestnut (Castanea sativa Mill.) wood extract on pig performance, oxidative status and pork quality traits. Meat Sci. 2015, 100, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Gandova, V.; Lazarov, A.; Fidan, H.; Dimov, M.; Stankov, S.; Denev, P.; Ercisli, S.; Stoyanova, A.; Gulen, H.; Assouguem, A. Physicochemical and biological properties of carvacrol. Open Chem. 2023, 21, 20220319. [Google Scholar] [CrossRef]
- Juneja, V.K.; Friedman, M. Carvacrol, cinnamaldehyde, oregano oil, and thymol inhibit Clostridium perfringens spore germination and outgrowth in ground turkey during chilling. J. Food Prot. 2007, 70, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Sabah, K.H.; Yousseff, F.M.; Elghoneimy, A.A. Antibacterial effect of Origanum vulgare and associated haematological and serum biochemical changes in chickens. Vet. Med. J. 2009, 7, 576–605. [Google Scholar] [CrossRef]
- Umar, A.A.; Kware, A.A.; Abdurrahman, B.; Alhaji, B.A. Evaluation of oregano (Origanum vulgare) leaf powder on performance indices of finisher broiler chickens in Sokoto, Nigeria. Biomed. J. Sci. Technol. Res. 2023, 52, 43263–43274. [Google Scholar]
- Ampode, K.M.; Mendoza, F.C. Oregano (Origanum vulgare Linn.) powder as phytobiotic feed additives improves the growth performance, lymphoid organs, and economic traits in broiler chickens. Adv. Anim. Vet. Sci. 2021, 10, 434–441. [Google Scholar]
- Beghelli, D.; Alunn, O.A.; Cardinali, R.; Bistoni, O.; Caterbi, S.; de Cosmo, A.; Dal Bosco, A.; Castellini, C.; Gerli, R. Effects of oregano (Origanum vulgare L.) and rosemary (Rosmarinus officinalis L.) aqueous extracts on in vitro rabbit immune responses. MOJ Immunol. 2016, 4, 474–479. [Google Scholar]
- Ruan, D.; Fan, Q.; Fouad, A.M.; Sun, Y.; Huang, S.; Wu, A.; Lin, C.; Kuang, Z.; Zhang, C.; Jiang, S. Effects of dietary oregano essential oil supplementation on growth performance, intestinal antioxidative capacity, immunity, and intestinal microbiota in yellow-feathered chickens. J. Anim. Sci. 2021, 99, 1–11. [Google Scholar] [CrossRef]
- Zaazaa, A.; Mudalal, S.; Alzuheir, I.; Samara, M.; Jalboush, N.; Fayyad, A.; Petracci, M. The impact of thyme and oregano essential oils dietary supplementation on broiler health, growth performance, and prevalence of growth-related breast muscle abnormalities. Animals 2022, 12, 3065. [Google Scholar] [CrossRef]
- Salama, A. Influence of dietary oregano plant extract supplementation on growth performance and economic efficiency of broiler chicks. Benha Med. J. 2023, 44, 15–19. [Google Scholar] [CrossRef]
- Vlaicu, P.A.; Panaite, T.D.; Olteanu, M.; Turcu, R.P.; Saracila, M.; Criste, R.D. Effect of the dietary oregano (Origanum vulgare L.) powder and oil on the performance, carcass and organs development of broilers reared under heat stress (32 °C). Sci. Papers Ser. Anim. Sci. 2018, 69, 207–2013. [Google Scholar]
- Gül, M.; Yilmaz, E.; Sezmiş, G.; Yildirim, B.A.; Kaya, A.; Önel, S.E. Effect of oregano (Oreganum syriacum L.) essential oil and cage density on performance parameters, egg quality criteria, some blood biochemical parameters, blood antioxidant capacity, and intestinal histopathology in laying hens. GSC Biol. Pharm. Sci. 2020, 13, 136–145. [Google Scholar]
- Gao, F.; Zhang, L.; Li, H.; Xia, F.; Bai, H.; Piao, X.; Sun, Z.; Cui, H.; Shi, L. Dietary oregano essential oil supplementation influences production performance and gut microbiota in late-phase laying hens fed wheat-based diets. Animals 2022, 12, 3007. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.M.; Anderson, G.; Arguelles-Ramos, M.; Ali, A.A.B. Effect of dietary essential oil of oregano on performance parameters, gastrointestinal traits, blood lipid profile, and antioxidant capacity of laying hens during the pullet phase. Front. Vet. Sci. 2022, 3, 1072712. [Google Scholar] [CrossRef]
- Hashemipour, H.; Kermanshahi, H.; Golian, A.; Veldkamp, T. Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult. Sci. 2013, 92, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Wang, W.; Gan, L.; Li, Z.; Guo, S.; Guo, Y. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2016, 7, 19–28. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Zhang, K.; Tian, G.; Ding, X.; Bai, S.; Zeng, Q. Effects of thymol and carvacrol eutectic on growth performance, serum biochemical parameters, and intestinal health in broiler chickens. Animals 2023, 13, 2242. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jin, X.; Wu, Q.; Long, L.; Li, Y.; Zhang, Q.; Liu, A.; Chen, X.; Geng, Z.; Zhang, C. Effects of encapsulated thymol and carvacrol mixture on growth performance, antioxidant capacity, immune function and intestinal health of broilers. Ital. J. Anim. Sci. 2022, 21, 1651–1659. [Google Scholar] [CrossRef]
- Bravo, D.; Pirgozliev, V.; Rose, S.P. A mixture of carvacrol, cinnamaldehyde, and capsicum oleoresin improves energy utilization and growth performance of broiler chickens fed maize-based diet. J. Anim. Sci. 2014, 92, 1531–1536. [Google Scholar] [CrossRef]
- Awaad, M.H.H.; Elmenawey, M.; Ahmed, K.A. Effect of a specific combination of carvacrol, cinnamaldehyde, and capsicum oleoresin on the growth performance, carcass quality and gut integrity of broiler chickens. Vet. World 2014, 7, 284–290. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Wang, Y.; Su, C.; Wang, L.; Lv, X.; Cui, G.; Ji, L.; Huang, Y.; Zhang, H.; Chen, W. Dietary cinnamaldehyde with carvacrol or thymol improves the egg quality and intestinal health independent of gut microbiota in post-peak laying hens. Front. Vet. Sci. 2022, 9, 994089. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Gan, L.; Li, Z.; Wang, W.; Liu, D.; Guo, Y. In vitro antibacterial activity of thymol and carvacrol and their effects on broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2015, 6, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Huang, G.; Luo, Z.; Hu, Y.; Liu, D. Oregano (Origanum vulgare L.) essential oil feed supplement protected broilers chickens against Clostridium perfringens induced necrotic enteritis. Agriculture 2021, 12, 18. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Huang, S.; Huang, Y.; Shi, H.; Bai, X. Effects of dietary essential oil supplementation on growth performance, carcass yield, meat quality, and intestinal tight junctions of broilers with or without Eimeria challenge. Poult. Sci. 2023, 102, 102874–102884. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.D.; Song, M.H.; Yun, W.; Lee, J.H.; Kim, H.B.; Cho, J.H. Effect of carvacrol essential oils on growth performance and intestinal barrier function in broilers with lipopolysaccharide challenge. Anim. Prod. Sci. 2020, 60, 545. [Google Scholar] [CrossRef]
- Zhao, B.C.; Wang, T.H.; Chen, J.; Qiu, B.H.; Xu, Y.R.; Zhang, Q.; Li, J.J.; Wang, C.J.; Nie, Q.F.; Li, J.L. Effects of dietary supplementation with a carvacrol–cinnamaldehyde–thymol blend on growth performance and intestinal health of nursery pigs. Porc. Health Manag. 2023, 9, 24–33. [Google Scholar] [CrossRef]
- Khalaf, M.M.; Hassan, S.M.; Sayed, A.M.; Abo-Youssef, A.M. Carvacrol mitigates vancomycin-induced nephrotoxicity via regulation of IkBα/p38MAPK and Keap1/Nrf2 signaling pathways: An experimental study with in silico evidence. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8738–8755. [Google Scholar]
- Moens, U.; Kostenko, S.; Sveinbjørnsson, B. The role of mitogen-activated protein kinase-activated protein kinases (MAPKAPKs) in inflammation. Genes 2013, 4, 101–133. [Google Scholar] [CrossRef]
- Liu, S.D.; Song, M.H.; Yun, W.; Lee, J.H.; Kim, H.B.; Cho, J.H. Effect of carvacrol essential oils on immune response and inflammation-related genes expression in broilers challenged by lipopolysaccharide. Poult. Sci. 2019, 98, 2026–2033. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Kuete, V. Syzygium aromaticum. In Medicinal Spices and Vegetables from Africa; Academic Press: Cambridge, MA, USA, 2017; pp. 611–625. [Google Scholar]
- Esmaeili, F.; Zahmatkeshan, M.; Yousefpoor, Y.; Alipanah, H.; Safari, E.; Osanloo, M. Anti-inflammatory and anti-nociceptive effects of cinnamon and clove essential oils nanogels: An in vivo study. BMC Complement. Med. Ther. 2022, 22, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Kovács, J.K.; Felső, P.; Makszin, L.; Pápai, Z.; Horváth, G.; Ábrahám, H.; Palkovics, T.; Böszörményi, A.; Emődy, L.; Schneider, G. Antimicrobial and virulence-modulating effects of clove essential oil on the foodborne pathogen Campylobacter jejuni. Appl. Environ. Microbiol. 2016, 82, 6158–6166. [Google Scholar] [CrossRef] [PubMed]
- Chaieb, K.; Zmantar, T.; Ksouri, R.; Hajlaoui, H.; Mahdouani, K.; Abdelly, C.; Bakhrouf, A. Antioxidant properties of the essential oil of Eugenia caryophyllata and its antifungal activity against a large number of clinical candida species. Mycoses 2007, 50, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Bachiega, T.F.; de Sousa, J.P.B.; Bastos, J.K.; Sforcin, J.M. Clove and eugenol in noncytotoxic concentrations exert immunomodulatory/anti-inflammatory action on cytokine production by murine macrophages. J. Pharm. Pharmacol. 2012, 64, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Nikousaleh, A.; Prakash, J. Antioxidant components and properties of dry heat-treated clove in different extraction solvents. J. Food Sci. Technol. 2015, 53, 1993–2000. [Google Scholar] [CrossRef]
- Eid, N.M.; Dahshan, A.H.M.; El-Nahass, E.S.; Shalaby, B.; Ali, A. Anti-clostridial activity of the thyme and clove essential oils against experimentally induced necrotic enteritis in commercial broiler chickens. Vet. Sci. Res. Rev. 2018, 4, 25–34. [Google Scholar]
- Agostini, P.S.; Solà-Oriol, D.; Nofrarías, M.; Barroeta, A.C.; Gasa, J.; Manzanilla, E.G. Role of in-feed clove supplementation on growth performance, intestinal microbiology, and morphology in broiler chicken. Livest. Sci. 2012, 147, 113–118. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Ghazanfari, S.; Moradi, M.A. Effect of supplementing clove essential oil to the diet on microflora population, intestinal morphology, blood parameters and performance of broilers. Eur. Poult. Sci. 2014, 78, 1–11. [Google Scholar] [CrossRef]
- Islam, R.; Sultana, N.; Bhakta, S.; Haque, Z.; Hasan, A.; Siddique, M.P.; Islam, M.R. Modulation of growth performance, gut morphometry, and cecal microbiota in broilers by clove (Syzygium aromaticum) and tulsi (Ocimum sanctum) supplementation. Poult. Sci. 2023, 102, 102266–102276. [Google Scholar] [CrossRef]
- Al-Mufarrej, S.I.; Al-Baadani, H.H.; Fazea, E.H. Effect of level of inclusion of clove (Syzygium aromaticum) powder in the diet on growth and histological changes in the intestines and livers of broiler chickens. S. Afr. J. Anim. Sci. 2019, 49, 166–175. [Google Scholar] [CrossRef]
- Suliman, G.M.; Alowaimer, A.N.; Al-Mufarrej, S.I.; Hussein, E.O.S.; Fazea, E.H.; Naiel, M.A.E.; Alhotan, R.A.; Swelum, A.A. The effects of clove seed (Syzygium aromaticum) dietary administration on carcass characteristics, meat quality, and sensory attributes of broiler chickens. Poult. Sci. 2021, 100, 100904–100915. [Google Scholar] [CrossRef]
- Mahrous, H.; ElFar, A.; Sadek, K.; Abdel-Latif, M. Effects of different levels of clove bud (Syzygium aromaticum) dietary supplementation on immunity, antioxidant status, and performance in broiler chickens. Alex. J. Vet. Sci. 2017, 54, 29–39. [Google Scholar] [CrossRef]
- Daniel, A.N.; Sartoretto, S.M.; Schmidt, G.; Caparroz-Assef, S.M.; Bersani-Amado, C.A.; Cuman, R.K.N. Anti-inflammatory and antinociceptive activities a of eugenol essential oil in experimental animal models. Rev. Bras. Farmacogn. 2009, 19, 212–217. [Google Scholar] [CrossRef]
- Al-Mufarrej, S.I.; Fazea, E.H.; Al-Baadani, H.H.; Qaid, M.M. Effects of clove powder supplementation on performance, blood biochemistry, and immune responses in broiler chickens. S. Afr. J. Anim. Sci. 2019, 49, 835–844. [Google Scholar] [CrossRef]
- Sang, R.; Ge, B.; Li, H.; Zhou, H.; Yan, K.; Wang, W.; Cui, Q.; Zhang, X. Taraxasterol alleviates aflatoxin b1-induced liver damage in broiler chickens via regulation of oxidative stress, apoptosis and autophagy. Ecotoxicol. Environ. Saf. 2023, 251, 114546–114555. [Google Scholar] [CrossRef]
- Shibata, M.; Nakajima, K. High serum aspartate aminotransferase, underweight, and weight loss in older people: Results of the KITCHEN-4. Healthcare 2020, 8, 69. [Google Scholar] [CrossRef]
- Hussein, M.M.A.; Abd El-Hack, M.E.; Mahgoub, S.A.; Saadeldin, I.M.; Swelum, A.A. Effects of clove (Syzygium aromaticum) oil on quail growth, carcass traits, blood components, meat quality, and intestinal microbiota. Poult. Sci. 2019, 98, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Iji, P.A.; Choct, M. Dietary modulation of gut microflora in broiler chickens: A review of the role of six kinds of alternatives to in-feed antibiotics. Worlds Poult. Sci. J. 2009, 65, 97–114. [Google Scholar] [CrossRef]
- Prakatur, I.; Miskulin, M.; Pavic, M.; Marjanovic, K.; Blazicevic, V.; Miskulin, I.; Domacinovic, M. Intestinal morphology in broiler chickens supplemented with propolis and bee pollen. Animals 2019, 9, 301. [Google Scholar] [CrossRef]
- Wang, K.; Chen, D.; Yu, B.; He, J.; Mao, X.; Huang, Z.; Yan, H.; Wu, A.; Luo, Y.; Zheng, P. Eugenol alleviates transmissible gastroenteritis virus-induced intestinal epithelial injury by regulating NF-κB signaling pathway. Front. Immunol. 2022, 13, 921613–921627. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020, 91, 13357–13368. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zheng, S.; Wei, S.; Tian, Q.; Tao, Y.; Bo, R.; Liu, M.; Li, J. The protective effect and potential mechanisms of eugenol against Salmonella in vivo and in vitro. Poult. Sci. 2022, 101, 101801–101812. [Google Scholar] [CrossRef] [PubMed]
- Meligy, A.M.A.; El-Hamid, M.I.A.; Yonis, A.E.; Elhaddad, G.Y.; Abdel-Raheem, S.M.; El-Ghareeb, W.R.; Mohamed, M.H.A.; Ismail, H.; Ibrahim, D. Liposomal encapsulated oregano, cinnamon, and clove oils enhanced the performance, bacterial metabolites antioxidant potential, and intestinal microbiota of broiler chickens. Poult. Sci. 2023, 102, 102683–102696. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Eldemery, F.; Metwally, A.S.; Abd-Allah, E.M.; Mohamed, D.T.; Ismail, T.A.; Hamed, T.A.; Al Sadik, G.M.; Neamat-Allah, A.N.F.; Abd El-Hamid, M.I. Dietary eugenol nanoemulsion potentiated performance of broiler chickens: Orchestration of digestive enzymes, intestinal barrier functions and cytokines related gene expression with a consequence of attenuating the severity of E. coli O78 infection. Front. Vet. Sci. 2022, 9, 847580–847596. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt-Mernak, M.I.; Pinheiro, N.M.; da Silva, R.C.; Ponci, V.; Banzato, R.; Pinheiro, A.J.M.C.R.; Olivo, C.R.; Tibério, I.F.L.C.; Lima Neto, L.G.; Santana, F.P.R.; et al. Effects of eugenol and dehydrodieugenol b from nectandra leucantha against lipopolysaccharide (LPS)-induced experimental acute lung inflammation. J. Nat. Prod. 2021, 84, 2282–2294. [Google Scholar] [CrossRef]
- Rodrigues, T.G.; Fernandes, A.; Sousa, J.P.B.; Bastos, J.K.; Sforcin, J.M. In vitro and in vivo effects of clove on pro-inflammatory cytokines production by macrophages. Nat. Prod. Res. 2009, 23, 319–326. [Google Scholar] [CrossRef]
- Yang, C.; Chowdhury, M.A.; Huo, Y.; Gong, J. Phytogenic compounds as alternatives to in-feed antibiotics: Potentials and challenges in application. Pathogens 2015, 4, 137–156. [Google Scholar] [CrossRef]
- Iwiński, H.; Chodkowska, K.A.; Drabik, K.; Batkowska, J.; Karwowska, M.; Kuropka, P.; Szumowski, A.; Szumny, A.; Różański, H. The impact of a phytobiotic mixture on broiler chicken health and meat safety. Animals 2023, 13, 2155. [Google Scholar] [CrossRef]
- Benny, M.; Shylaja, M.R.; Antony, B.; Gupta, N.K.; Mary, R.; Anto, A.; Jacob, S. Acute and sub-acute toxicity studies with ginger extract in rats. Int. J. Pharm. Sci. Res. 2020, 12, 2799–2809. [Google Scholar]
- Herawati, H. The effect of feeding red ginger as phytobiotic on body weight gain, feed conversion and internal organs condition of broiler. Int. J. Poult. Sci. 2010, 9, 963–967. [Google Scholar]
- Khan, R.U.; Naz, S.; Nikousefat, Z.; Tufarelli, V.; Javdani, M.; Qureshi, M.S.; Laudadio, V. Potential applications of ginger (Zingiber officinale) in poultry diets. Worlds Poult. Sci. J. 2012, 68, 245–252. [Google Scholar] [CrossRef]
- Repetto, M.G.; Boveris, A. Bioactivity of sesquiterpenes: Compounds that protect from alcohol-induced gastric mucosal lesions and oxidative damage. Mini Rev. Med. Chem. 2010, 10, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, M.L.; Chacko, K.M.; Kuruvilla, B.T. Systematic and comprehensive investigation of the toxicity of curcuminoid-essential oil complex: A bioavailable turmeric formulation. Mol. Med. Rep. 2016, 13, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Llana-Ruiz-Cabello, M.; Maisanaba, S.; Puerto, M.; Pichardo, S.; Jos, A.; Moyano, R.; Cameán, A.M. A subchronic 90-day oral toxicity study of Origanum vulgare essential oil in rats. Food Chem. Toxicol. 2017, 101, 36–47. [Google Scholar] [CrossRef]
- Liaqat, I.; Mahreen, A.; Arshad, M.; Arshad, N. Antimicrobial and toxicological evaluation of Origanum vulgare: An in vivo study. Braz. J. Biol. 2023, 83, e244551. [Google Scholar] [CrossRef]
- Mishra, R.K.; Singh, S.K. Safety assessment of Syzygium aromaticum flower bud (clove) extract with respect to testicular function in mice. Food Chem. Toxicol. 2008, 46, 3333–3338. [Google Scholar] [CrossRef]
- Hafsah, H.; Damayanti, A.P.; Syahrir, S.; Tahir, T.; Rahmasari, F.; Alsahab, M.R. Immune organs and growth performance of male laying hens with use of eugenol clove leaf oil as a substitute of antibiotic in feed. Agroland Agric. Sci. J. 2022, 9, 52–58. [Google Scholar] [CrossRef]
| Bioactive Components or Extracts | Parts of the Plant for Extraction | Functional Property | References |
|---|---|---|---|
| Curcumin | Rhizomes | Effective for cardiovascular diseases, diabetes, and cancers, with neuroprotection, anti-inflammatory, and antioxidant functions | [68,69] |
| Anti-bacterial, anti-protozoal, antiviral, immunomodulatory, and anti-fungal effects | [69] | ||
| Antibacterial effects against various Gram-negative and -positive bacteria, including A. baumannii, E. faecalis, K. pneumoniae, P. aeruginosa, Bacillus subtilis (B. subtilis), Staphylococcus epidermidis, Bacillus cereus (B. cereus), Listeria innocua, Streptococcus pyogenes, S. aureus, Helicobacter pylori (H. pylori), Escherichia coli (E. coli), Salmonella enterica serotype Typhimurium, and Streptococcus mutants | [70] | ||
| Effective against Streptococcus pyogenes, S. aureus, Enterococcus faecalis, and Pseudomonas aeruginosa | [71] |
| Bioactive Components or Extracts | Parts of Plants for Extraction | Functional Property | References |
|---|---|---|---|
| 5,6-dehydrokawain (DK), dihydro-5,6-dehydrokawain (DDK) | Rhizomes | Antioxidant properties and is an effective inhibitor of collagenase, elastase, hyaluronidase, and tyrosinase | [100] |
| Leaves | Possesses the highest antioxidant properties and anti-aging effects | [101] | |
| CH2Cl2 and MeOH extracts | Flowers | Possesses a higher potentiality of anti-tumor effects and antioxidant properties by upregulating superoxide dismutase (SOD) and catalase (CAT) in the liver | [102] |
| Phenolic compounds such as curcumin, 6-gingerol, eugenol, and vitamin C | Rhizomes | Antioxidant properties | [103] |
| Essential oils such as 1,8-cineole, α-farnesene, γ-cadinene, α-terpineol, α-bergamotene, and globulol | Rhizomes | Anti-microbial properties against Gram-positive bacteria in addition to anti-fungal activities. | [104,105] |
| 4-terpineol, 1,8-cineole, γ-terpinolene, sabinene, and monoterpenes | Leaves | Effective against Staphylococcus aureus and E. coli | [106] |
| Triterpenoids, flavonoids, alkaloids | Flowers | Effective against bacterial pathogens causing sinusitis, including Porphyromonas gingivalis, Fusobacterium nucleatum, Fusobacterium necrophorum, Streptococcus pneumoniae, and Prevotella intermedia. | [107] |
| Hydroxybenzoic acids, hydroxycinnamic acids, and flavonoid extracts | Fresh rhizomes | Effective against Eschiricia coli, Staphylococcus aureus, and Shigella fleneri. | [108] |
| Ethanol extracts | Dried seeds of A. katsumadai | Anti-adhesive effects against Campylobacter jejuni | [109] |
| Methanol extracts | Flowers and rhizomes | Effective against Micrococcus luteus, and treatment for intestinal infections and other diseases. | [110] |
| Palmitic acid (n-hexadecanoic acid) | Leaves from adult plants | Anti-fungal activities against Cryptococcus neoformans, Fonsecaea pedrosoi, Trichophytoon rubrum, Microsporium canis and M. gypseum | [111] |
| Zerumbone, a-caryophyllene, and camphene | Hydrodistillation of Alpinia zerumbet leaves and rhizomes. | Effective against food contaminants that cause hepatotoxicity; possesses anti-inflammatory properties | [112,113] |
| Bioactive Components or Extracts | Parts of the Plant for Extraction | Functional Property | References |
|---|---|---|---|
| Rosmarinic acid | Dried leaves | Antioxidant activities | [129,130] |
| Thymol, γ-terpinene, carvacrol, p-cymene, and elemol | Dried aerial parts | A stronger antioxidant activity than Vitamin E | [130] |
| Thymol and carvacrol | Flowers | An antioxidant function | [131] |
| Sesquiterpene hydrocarbon-germacrene, (e)-caryophyllene monoterpene hydrocarbon-sabinene, and oxygen-containing monoterpenes-terpinen-4-ol | Flowers | An antioxidant function | [132] |
| Rosmarinic and chlorogenic acids | Aerial part | Anti-microbial properties against Salmonella enteritidis and Aspergillus niger with hepatoprotective effects | [133] |
| Thymol and carvacrol | Flowers | Effective against Gram-negative bacteria, with anti-fungal properties | [131] |
| Carvacrol, β-fenchyl alcohol, thymol, and γ-terpinene | Dried aerial part | Antibacterial properties against Gram-positive and -negative strain bacteria, Brochothrix thermosphacta, E. coli, Listeria innocua Listeria monocytogenes, Pseudomonas putida, Salmonella typhimurium, and Shewanella putrefaciens | [134,135] |
| Carvacrol | Aerial parts | Antibacterial effects against Staphyloccus aureus | [136] |
| Carvacrol (the main compound) and thymol | Leaves | Fungicidal activities against C. albicans and C. glabrata | [137] |
| Bioactive Components of Cloves | Biological Property | References |
|---|---|---|
| Eugenol | Anti-inflammatory effects | [172] |
| Antibacterial properties against E. coli, Staphylococcus aureus, and Pseudomonas aeruginosa, Clostridium perfringens, and Campylobacter jejuni | [46,173] | |
| Antioxidant properties | [174] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdez, G.; Shyur, L.-F.; Wang, S.-Y.; Chen, S.-E. Phytogenics in Ginger, Origanum vulgare, and Syzygium aromaticum and Their Potential as a Feed Additive against Clostridium perfringens in Broiler Production. Animals 2023, 13, 3643. https://doi.org/10.3390/ani13233643
Valdez G, Shyur L-F, Wang S-Y, Chen S-E. Phytogenics in Ginger, Origanum vulgare, and Syzygium aromaticum and Their Potential as a Feed Additive against Clostridium perfringens in Broiler Production. Animals. 2023; 13(23):3643. https://doi.org/10.3390/ani13233643
Chicago/Turabian StyleValdez, Gilmour, Lie-Fen Shyur, Sheng-Yang Wang, and Shuen-Ei Chen. 2023. "Phytogenics in Ginger, Origanum vulgare, and Syzygium aromaticum and Their Potential as a Feed Additive against Clostridium perfringens in Broiler Production" Animals 13, no. 23: 3643. https://doi.org/10.3390/ani13233643
APA StyleValdez, G., Shyur, L.-F., Wang, S.-Y., & Chen, S.-E. (2023). Phytogenics in Ginger, Origanum vulgare, and Syzygium aromaticum and Their Potential as a Feed Additive against Clostridium perfringens in Broiler Production. Animals, 13(23), 3643. https://doi.org/10.3390/ani13233643





