A Comparison of the Oral Microbiota in Healthy Dogs and Dogs with Oral Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. DeepseqTM Shotgun Metagenomic Sequencing
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Oral Microbiota of Healthy Dogs
3.3. Core Microbiota of Healthy Dogs
3.4. Oral Microbiota of Dogs with Oral Tumors
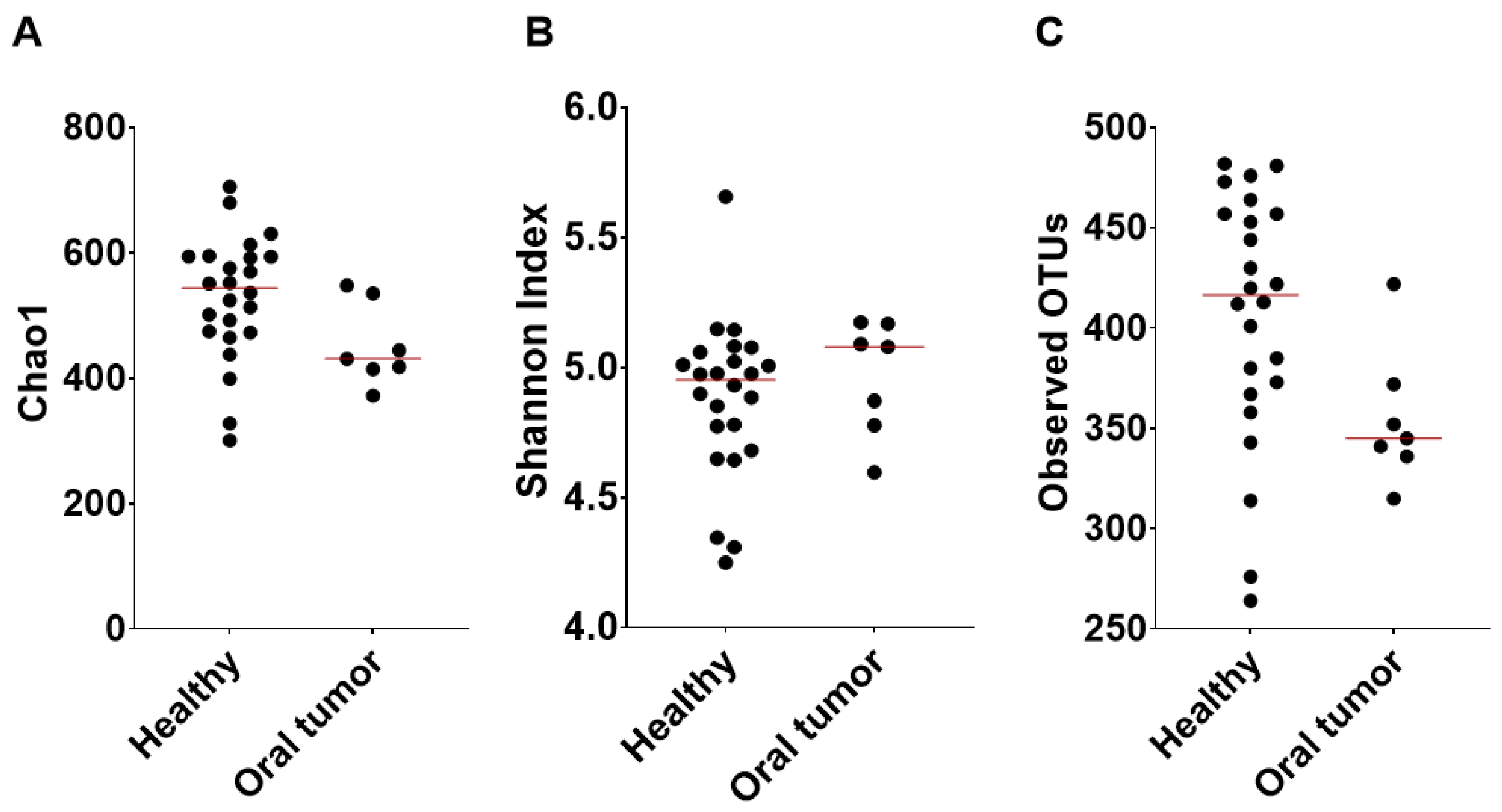
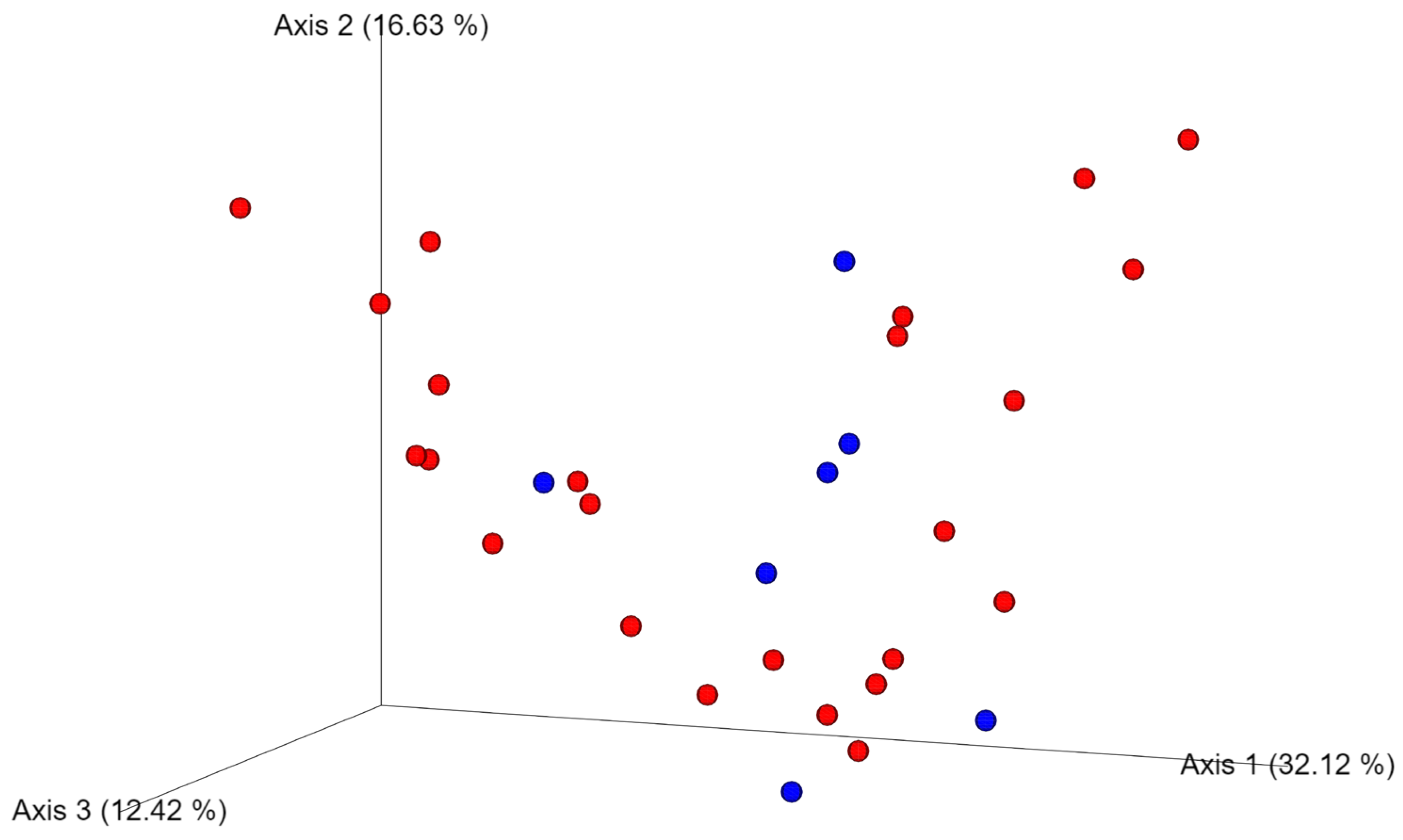
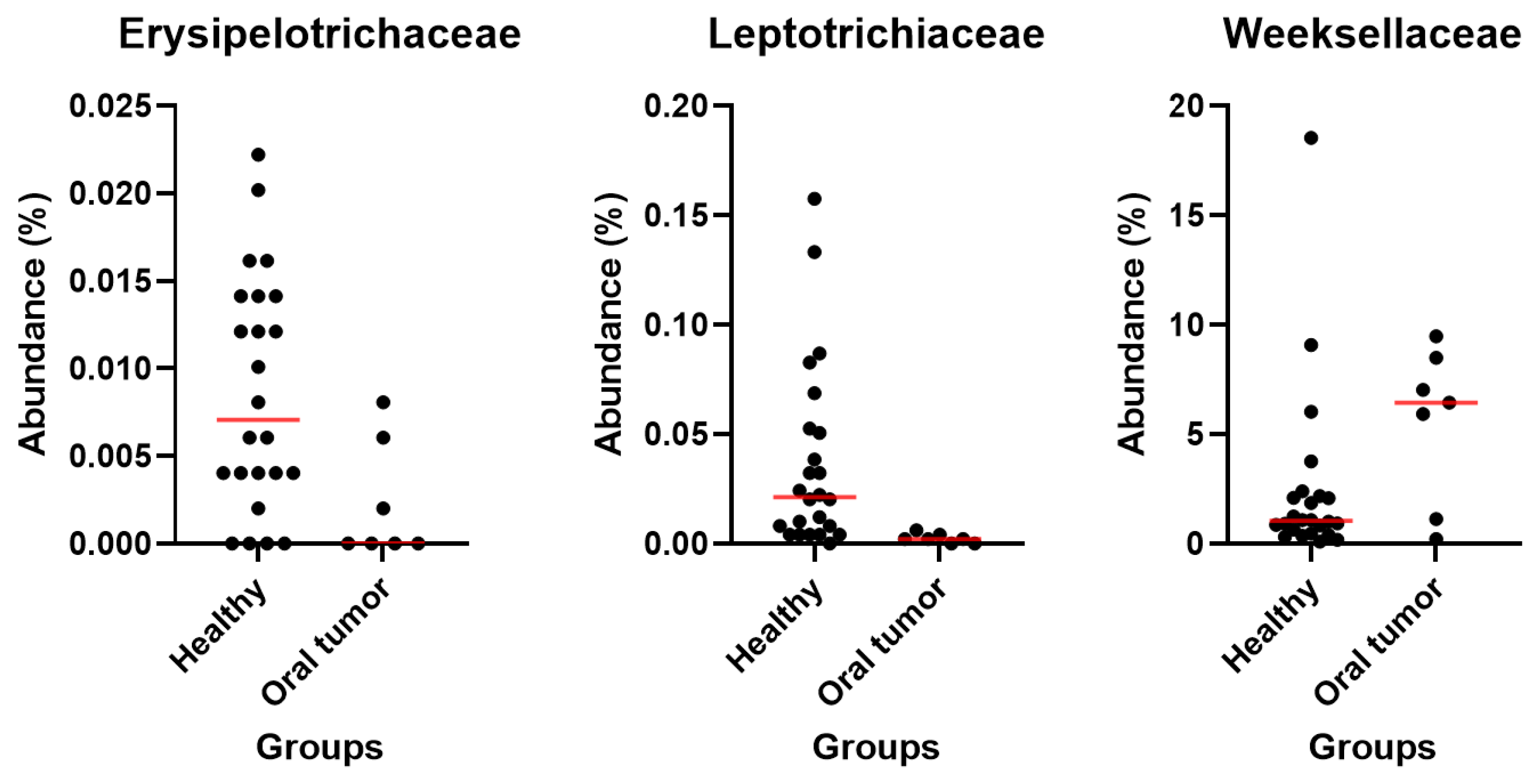
3.5. Differences in the Oral Microbiota between Healthy Dogs and Dogs with Oral Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krishnan, K.; Chen, T.; Paster, B.J. A Practical Guide to the Oral Microbiome and Its Relation to Health and Disease. Oral Dis. 2017, 23, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, F.E.; Klein, E.A.; Thompson, E.C.; Blanton, J.M.; Chen, T. The Canine Oral Microbiome. PLoS ONE 2012, 7, 36067. [Google Scholar] [CrossRef]
- Farrell, J.J.; Zhang, L.; Zhou, H.; Chia, D.; Elashoff, D.; Akin, D.; Paster, B.J.; Joshipura, K.; Wong, D.T.W. Variations of Oral Microbiota Are Associated with Pancreatic Diseases Including Pancreatic Cancer. Gut 2012, 61, 582–588. [Google Scholar] [CrossRef]
- Ruiz, J.C.A.; Jonker, A.; Bosman, A.M.; Steenkamp, G. Bacteria Profile and Antibiogram of the Bacteria Isolated from the Exposed Pulp of Dog Canine Teeth. Vet. Rec. 2018, 183, 97. [Google Scholar] [CrossRef]
- de Andrade Ferreira, F.B.; Campos Rabang, H.R.; Pinheiro, E.T.; Gadê-Neto, C.R.; Zaia, A.A.; Randi Ferraz, C.C.; de Souza-Filho, F.J.; de Almeida Gomes, B.P.F. Root Canal Microbiota of Dogs’ Teeth with Periapical Lesions Induced by Two Different Methods. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 102, 564–570. [Google Scholar] [CrossRef]
- Srečnik, Š.; Zdovc, I.; Javoršek, U.; Pirš, T.; Pavlica, Z.; Nemec, A. Microbiological Aspects of Naturally Occurring Primary Endodontic Infections in Dogs. J. Vet. Dent. 2019, 36, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Staszyk, C.; Jean Lommer, M.; Fouad, A.F.; Bicalho, R.C.; Peralta, S.; Xavier Rodrigues, M.; Nemec, A.; Fiani, N. Endodontic Microbiome of Fractured Non-Vital Teeth in Dogs Determined by 16S RRNA Gene Sequencing. Front. Vet. Sci. 2019, 6, 348. [Google Scholar] [CrossRef]
- Grice, E.A.; Kong, H.H.; Renaud, G.; Young, A.C.; Bouffard, G.G.; Blakesley, R.W.; Wolfsberg, T.G.; Turner, M.L.; Segre, J.A. A Diversity Profile of the Human Skin Microbiota. Genome Res. 2008, 18, 1043–1050. [Google Scholar] [CrossRef]
- Proctor, L.M.; Creasy, H.H.; Fettweis, J.M.; Lloyd-Price, J.; Mahurkar, A.; Zhou, W.; Buck, G.A.; Snyder, M.P.; Strauss, J.F.; Weinstock, G.M.; et al. The Integrative Human Microbiome Project the Integrative HMP (IHMP) Research Network Consortium. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef]
- Wade, W.G. The Oral Microbiome in Health and Disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef]
- Chen, T.; Yu, W.-H.; Izard, J.; Baranova, O.V.; Lakshmanan, A.; Dewhirst, F.E. The Human Oral Microbiome Database: A Web Accessible Resource for Investigating Oral Microbe Taxonomic and Genomic Information. Database 2010, 6, baq013. [Google Scholar] [CrossRef]
- Allaker, R.P.; De Rosayro, R.; Young, K.A.; Hardie, J.M. Prevalence of Porphyromonas and Prevotella Species in the Dental Plaque of Dogs. Vet. Rec. 1997, 140, 147–148. [Google Scholar] [CrossRef]
- Fournier, D.; Mouton, C.; Lapierre, P.; Kato, T.; Okuda, K.; Ménard, C. Porphyromonas gulae Sp. Nov., an Anaerobic, Gram-Negative Coccobacillus from the Gingival Sulcus of Various Animal Hosts. Int. J. Syst. Evol. Microbiol. 2001, 51, 1179–1189. [Google Scholar] [CrossRef]
- Hardham, J.; Dreier, K.; Wong, J.; Sfintescu, C.; Evans, R.T. Pigmented-Anaerobic Bacteria Associated with Canine Periodontitis. Vet. Microbiol. 2005, 106, 119–128. [Google Scholar] [CrossRef]
- Elliott, D.R.; Wilson, M.; Buckley, C.M.F.; Spratt, D.A. Cultivable Oral Microbiota of Domestic Dogs. J. Clin. Microbiol. 2005, 43, 5470–5476. [Google Scholar] [CrossRef] [PubMed]
- Ruparell, A.; Inui, T.; Staunton, R.; Wallis, C.; Deusch, O.; Holcombe, L.J. The Canine Oral Microbiome: Variation in Bacterial Populations across Different Niches. BMC Microbiol. 2020, 20, 42. [Google Scholar] [CrossRef]
- Davis, E.M.; Weese, J.S. Oral Microbiome in Dogs and Cats: Dysbiosis and the Utility of Antimicrobial Therapy in the Treatment of Periodontal Disease. Vet. Clin. N. Am. Small Anim. Pract. 2022, 52, 107–119. [Google Scholar] [CrossRef]
- McDonald, J.E.; Larsen, N.; Pennington, A.; Connolly, J.; Wallis, C.; Rooks, D.J.; Hall, N.; McCarthy, A.J.; Allison, H.E. Characterising the Canine Oral Microbiome by Direct Sequencing of Reverse-Transcribed RRNA Molecules. PLoS ONE 2016, 11, e0157046. [Google Scholar] [CrossRef]
- Santibáñez, R.; Rodríguez-Salas, C.; Flores-Yáñez, C.; Garrido, D.; Thomson, P. Assessment of Changes in the Oral Microbiome That Occur in Dogs with Periodontal Disease. Vet. Sci. 2021, 8, 291. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Li, H.; Ni, C.; Du, Z.; Yan, F. Human Oral Microbiota and Its Modulation for Oral Health. Biomed. Pharmacother. 2018, 99, 883–893. [Google Scholar] [CrossRef]
- Sun, C.; Li, B.; Wang, B.; Zhao, J.; Zhang, X.; Li, T.; Li, W.; Tang, D.; Qiu, M.; Wang, X.; et al. The Role of Fusobacterium nucleatum in Colorectal Cancer: From Carcinogenesis to Clinical Management. Chronic Dis. Transl. Med. 2019, 5, 178–187. [Google Scholar] [CrossRef]
- la Rosa, G.R.M.; Gattuso, G.; Pedullà, E.; Rapisarda, E.; Nicolosi, D.; Salmeri, M. Association of Oral Dysbiosis with Oral Cancer Development. Oncol. Lett. 2020, 19, 3045–3058. [Google Scholar] [CrossRef]
- Karpínski, T.M. Role of Oral Microbiota in Cancer Development. Microorganisms 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.L.; Kuczynski, J.; Bhattacharya, A.; Huey, B.; Corby, P.M.; Queiroz, E.L.S.; Nightingale, K.; Kerr, A.R.; DeLacure, M.D.; Veeramachaneni, R.; et al. Changes in Abundance of Oral Microbiota Associated with Oral Cancer. PLoS ONE 2014, 9, e98741. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y.; Yeh, Y.-M.; Yu, H.-Y.; Chin, C.-Y.; Hsu, C.-W.; Liu, H.; Huang, P.-J.; Hu, S.-N.; Liao, C.-T.; Chang, Y.-L.; et al. Oral Microbiota Community Dynamics Associated with Oral Squamous Cell Carcinoma Staging. Front. Microbiol. 2018, 9, 862. [Google Scholar] [CrossRef] [PubMed]
- Nagy, K.N.; Sonkodi, I.; Szgke, I.; Nagy, E.; Newman, H.N. The Microflora Associated with Human Oral Carcinomas’. Oral Oncol. 1998, 34, 304–308. [Google Scholar] [CrossRef]
- Chocolatewala, N.; Chaturvedi, P.; Desale, R. The Role of Bacteria in Oral Cancer. Indian J. Med. Paediatr. Oncol. 2010, 31, 126–131. [Google Scholar] [CrossRef]
- Lee, W.-H.; Chen, H.-M.; Yang, S.-F.; Liang, C.; Peng, C.-Y.; Lin, F.-M.; Tsai, L.-L.; Wu, B.-C.; Hsin, C.-H.; Chuang, C.-Y.; et al. Bacterial Alterations in Salivary Microbiota and Their Association in Oral Cancer. Sci. Rep. 2017, 7, 16540. [Google Scholar] [CrossRef]
- Pushalkar, S.; Mane, S.P.; Ji, X.; Li, Y.; Evans, C.; Crasta, O.R.; Morse, D.; Meagher, R.; Singh, A.; Saxena, D. Microbial Diversity in Saliva of Oral Squamous Cell Carcinoma. FEMS Immunol. Med. Microbiol. 2011, 61, 269–277. [Google Scholar] [CrossRef]
- Perera, M.; Al-Hebshi, N.; Speicher, D.J.; Perera, I.; Johnson, N.W.; Al-Hebshi, N.N. Emerging Role of Bacteria in Oral Carcinogenesis: A Review with Special Reference to Perio-Pathogenic Bacteria. J. Microbiol. 2016, 8, 32762. [Google Scholar] [CrossRef]
- Whitmore, S.E.; Lamont, R.J. Pearls Oral Bacteria and Cancer. PLoS Pathog. 2014, 10, e1003933. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of Oral Microbiome Signatures in Diagnosis and Prognosis of Oral Cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354. [Google Scholar] [CrossRef]
- Zamarian, V.; Catozzi, C.; Cuscó, A.; Stefanello, D.; Ferrari, R.; Ceciliani, F.; Francino, O.; Sánchez, A.; Grieco, V.; Zani, D.; et al. Characterization of Skin Surface and Dermal Microbiota in Dogs with Mast Cell Tumor. Sci. Rep. 2020, 10, 12634. [Google Scholar] [CrossRef]
- Gavazza, A.; Rossi, G.; Lubas, G.; Cerquetella, M.; Minamoto, Y.; Suchodolski, J.S. Faecal Microbiota in Dogs with Multicentric Lymphoma. Vet. Comp. Oncol. 2018, 16, 169–175. [Google Scholar] [CrossRef]
- Stashenko, P.; Yost, S.; Choi, Y.; Danciu, T.; Chen, T.; Yoganathan, S.; Kressirer, C.; Ruiz-Tourrella, M.; Das, B.; Kokaras, A.; et al. The Oral Mouse Microbiome Promotes Tumorigenesis in Oral Squamous Cell Carcinoma. mSystems 2019, 4, 323–342. [Google Scholar] [CrossRef]
- Zheng, H.-H.; Du, C.-T.; Yu, C.; Tang, X.-Y.; Huang, R.-L.; Zhang, Y.-Z.; Gao, W.; Xie, G.-H. The Relationship of Tumor Microbiome and Oral Bacteria and Intestinal Dysbiosis in Canine Mammary Tumor. J. Mol. Sci. 2022, 23, 10928. [Google Scholar] [CrossRef] [PubMed]
- Pires De Carvalho, J.; Carrilho, M.C.; Santos Dos Anjos, D.; Dagli Hernandez, C.; Sichero, L.; Lúcia, M.; Dagli, Z. Unraveling the Risk Factors and Etiology of the Canine Oral Mucosal Melanoma: Results of an Epidemiological Questionnaire, Oral Microbiome Analysis and Investigation of Papillomavirus Infection. Cancers 2022, 14, 3397. [Google Scholar] [CrossRef]
- Zhou, P.; Hu, Y.; Wang, X.; Shen, L.; Liao, X.; Zhu, Y.; Yu, J.; Zhao, F.; Zhou, Y.; Shen, H.; et al. Microbiome in Cancer: An Exploration of Carcinogenesis, Immune Responses and Immunotherapy. Front. Immunol. 2022, 13, 877939. [Google Scholar] [CrossRef] [PubMed]
- Setthawongsin, C.; Khunbutsri, D.; Pisamai, S.; Raksajit, W.; Ngamkala, S.; Jarudecha, T.; Meekhanon, N.; Rungsipipat, A. Isolation of Oral Bacteria, Measurement of the C-Reactive Protein, and Blood Clinical Parameters in Dogs with Oral Tumor. Vet. Med. Int. 2023, 22, 2582774. [Google Scholar] [CrossRef]
- O’leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; Mcveigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference Sequence (RefSeq) Database at NCBI: Current Status, Taxonomic Expansion, and Functional Annotation. Nucleic Acids Res. 2015, 44, 733–745. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Wensel, C.R.; Pluznick, J.L.; Salzberg, S.L.; Sears, C.L. Next-Generation Sequencing: Insights to Advance Clinical Investigations of the Microbiome. J. Clin. Investig. 2022, 132, e154944. [Google Scholar] [CrossRef] [PubMed]
- Whittemore, J.C.; Price, J.M.; Moyers, T.; Suchodolski, J.S. Effects of Synbiotics on the Fecal Microbiome and Metabolomic Profiles of Healthy Research Dogs Administered Antibiotics: A Randomized, Controlled Trial. Front. Vet. Sci. 2021, 8, 665713. [Google Scholar] [CrossRef]
- Yuan, X.; Zhou, F.; Wang, H.; Xu, X.; Xu, S.; Zhang, C.; Zhang, Y.; Lu, M.; Zhang, Y.; Zhou, M.; et al. ARTICLE Systemic Antibiotics Increase Microbiota Pathogenicity and Oral Bone Loss. Int. J. Oral Sci. 2023, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; He, F.; Si, M.; Sun, P.; Chen, Q. Effects of Antibiotic Use on Saliva Antibody Content and Oral Microbiota in Sprague Dawley Rats. Front. Cell. Infect. Microbiol. 2022, 12, 721691. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.M.; Suchodolski, J.S.; Price, J.M.; Tolbert, M.K. Omeprazole Minimally Alters the Fecal Microbial Community in Six Cats: A Pilot Study. Front. Vet. Sci. 2018, 5, 320536. [Google Scholar] [CrossRef]
- Jones, S.M.; Gaier, A.; Enomoto, H.; Ishii, P.; Pilla, R.; Price, J.; Suchodolski, J.; Steiner, J.M.; Papich, M.G.; Messenger, K.; et al. The Effect of Combined Carprofen and Omeprazole Administration on Gastrointestinal Permeability and Inflammation in Dogs. J. Vet. Intern. Med. 2020, 34, 1886–1893. [Google Scholar] [CrossRef]
- Mishiro, T.; Oka, K.; Kuroki, Y.; Takahashi, M.; Tatsumi, K.; Saitoh, T.; Tobita, H.; Ishimura, N.; Sato, S.; Ishihara, S.; et al. Oral Microbiome Alterations of Healthy Volunteers with Proton Pump Inhibitor. J. Gastroenterol. Hepatol. 2018, 33, 1059–1066. [Google Scholar] [CrossRef]
- Atherly, T.; Rossi, G.; White, R.; Seo, Y.J.; Wang, C.; Ackermann, M.; Breuer, M.; Allenspach, K.; Mochel, J.P.; Jergens, A.E. Glucocorticoid and Dietary Effects on Mucosal Microbiota in Canine Inflammatory Bowel Disease. PLoS ONE 2019, 14, e0226780. [Google Scholar] [CrossRef]
- Galeano Niño, J.L.; Wu, H.; LaCourse, K.D.; Kempchinsky, A.G.; Baryiames, A.; Barber, B.; Futran, N.; Houlton, J.; Sather, C.; Sicinska, E.; et al. Effect of the Intratumoral Microbiota on Spatial and Cellular Heterogeneity in Cancer. Nature 2022, 611, 810–817. [Google Scholar] [CrossRef]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The Human Tumor Microbiome Is Composed of Tumor Type-Specific Intracellular Bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Poore, G.D.; Kopylova, E.; Zhu, Q.; Carpenter, C.; Fraraccio, S.; Wandro, S.; Kosciolek, T.; Janssen, S.; Metcalf, J.; Song, S.J.; et al. Microbiome Analyses of Blood and Tissues Suggest Cancer Diagnostic Approach. Nature 2020, 579, 567. [Google Scholar] [CrossRef]
- Zeng, B.; Tan, J.; Guo, G.; Li, Z.; Yang, L.; Lao, X.; Wang, D.; Ma, J.; Zhang, S.; Liao, G.; et al. The Oral Cancer Microbiome Contains Tumor Space–Specific and Clinicopathology-Specific Bacteria. Front. Cell. Infect. Microbiol. 2022, 12, 942328. [Google Scholar] [CrossRef]
- Anderson, J.G.; Paster, B.J.; Kokaras, A.; Chent, T. Characterization of the Oral Microbiome in Canine Chronic Ulcerative Stomatitis. J. Immun. Res. 2021, 7, 1037. [Google Scholar] [CrossRef]
- Oba, P.M.; Sieja, K.M.; Keating, S.C.J.; Hristova, T.; Somrak, A.J.; Swanson, K.S. Oral Microbiota Populations of Adult Dogs Consuming Wet or Dry Foods. J. Anim. Sci. 2022, 100, skac200. [Google Scholar] [CrossRef]
- Bauer, A.E.; Stella, J.; Lemmons, M.; Croney, C.C. Evaluating the Validity and Reliability of a Visual Dental Scale for Detection of Periodontal Disease (PD) in Non-Anesthetized Dogs (Canis Familiaris). PLoS ONE 2018, 13, e0203930. [Google Scholar] [CrossRef] [PubMed]
- Oba, P.M.; Carroll, M.Q.; Alexander, C.; Somrak, A.J.; Keating, S.C.J.; Sage, A.M.; Swanson, K.S. Dental Chews Positively Shift the Oral Microbiota of Adult Dogs. J. Anim. Sci. 2021, 99, skab100. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Geng, F.; Shi, X.; Li, Y.; Zhang, X.; Zhao, X.; Pan, Y. The Prevalence Rate of Periodontal Pathogens and Its Association with Oral Squamous Cell Carcinoma. Appl. Microbiol. Biotechnol. 2019, 103, 1393–1404. [Google Scholar] [CrossRef]
- Brennan, C.A.; Garrett, W.S. Fusobacterium nucleatum—Symbiont, Opportunist and Oncobacterium. Nat. Rev. Microbiol. 2019, 17, 156–166. [Google Scholar] [CrossRef]
- Bernardi, S.; Continenza, M.A.; Al-Ahmad, A.; Karygianni, L.; Follo, M.; Filippi, A.; Macchiarelli, G. Streptococcus Spp. and Fusobacterium nucleatum in Tongue Dorsum Biofilm from Halitosis Patients: A Fluorescence in Situ Hybridization (FISH) and Confocal Laser Scanning Microscopy (CLSM) Study. New Microbiol. 2019, 42, 1121–7138. [Google Scholar]
- Minerbi, A.; Shen, S. Gut Microbiome in Anesthesiology and Pain Medicine. Anesthesiology 2022, 137, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, X.; Wu, H. Juvenile Rats Show Altered Gut Microbiota After Exposure to Isoflurane as Neonates. Neurochem. Res. 2019, 44, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhang, Z.; Guo, N.; Li, X.; Yang, M.; Peng, Y.; Ma, X.; Yu, K.; Wang, C. Effects of Sevoflurane Inhalation Anesthesia on the Intestinal Microbiome in Mice. Front. Cell. Infect. Microbiol. 2021, 11, 633527. [Google Scholar] [CrossRef]
- Guo, N.; Zhang, Z.; Han, C.; Chen, L.; Zheng, X.; Yu, K.; Zhang, Z.; Wang, C. Effects of Continuous Intravenous Infusion of Propofol on Intestinal Flora in Rats. Biomed. Pharmacother. 2021, 134, 111080. [Google Scholar] [CrossRef] [PubMed]


| Healthy Group | Oral Tumor Group | p-Value | |
|---|---|---|---|
| Age (years) | 0.5026 | ||
| Mean | 7.5 | 8.3 | |
| Min-Max | 2–14.3 | 5.4–11.9 | |
| Weight (kg) | 0.3469 | ||
| Mean | 18.5 | 23.6 | |
| Min-Max | 3–42 | 6–50 | |
| Sex | 0.2015 | ||
| Female (%) | 13 (54.2) | 1 (14.3) | |
| Male (%) | 11 (45.8) | 6 (85.7) | |
| Breeds (n) | Airedale Terrier, Beagle, Cavalier King Charles Spaniel, English Cocker Spaniel, French Bulldog (3), German Boxer (2), German Pointer, German Shepherd, German Spitz (3), Giant Schnauzer, Keeshond, Labrador retriever, Lagotto Romagnolo (2), Pomeranian, Russian Greyhound, Tibetan terrier (2), and Whippet | American Staffordshire Terrier, American Bulldog, Chihuahua, Cross-breed (2), Labrador Retriever, and Shih-Tzu | NA |
| Phylum | Median (%) | Range (%) |
|---|---|---|
| Bacteroidota | 66.1 | 15.6–86.7 |
| Proteobacteria | 28.2 | 4.6–69.3 |
| Actinobacteriota | 2.1 | 0.3–12.5 |
| Desulfobacterota | 0.8 | 0.1–4.4 |
| Firmicutes | 0.8 | 0.2–11.1 |
| Spirochaetota | 0.5 | <0.1–2.4 |
| Firmicutes_A | 0.4 | 0.1–1.1 |
| Fusobacteriota | 0.3 | <0.1–0.6 |
| Unclassified Phylum from Bacteria | 0.2 | 0.1–5.6 |
| Campylobacterota | 0.2 | <0.1–2.6 |
| Patescibacteria | <0.1 | 0–0.2 |
| Firmicutes_C | <0.1 | 0–0.5 |
| Species | Median (%) | Range (%) |
|---|---|---|
| Porphyromonas A cangingivalis | 15.9 | 4.9–31.3 |
| Porphyromonas gulae | 10.9 | 0.4–50.7 |
| Conchiformibius steedae | 6.2 | 0.7–40.4 |
| Porphyromonas A canoris | 4.1 | 0.5–23.9 |
| Porphyromonas gingivicanis | 4.0 | 0.1–13.2 |
| Neisseria Weaveri | 1.9 | 0.1–9.5 |
| Frederiksenia canicola | 1.6 | 0.2–17.2 |
| Capnocytophaga cynodegmi | 1.3 | 0.2–7.6 |
| Capnocytophaga canimorsus | 1.3 | 0.2–9.2 |
| Capnocytophaga canis | 1.1 | 0.4–5.5 |
| Bergeyella zoohelcum | 1.0 | 0.1–18.5 |
| Histophilus haemoglobinophilus | 0.9 | 0.3–18.6 |
| Pasteurella dagmatis | 0.9 | 0.0–17.3 |
| Neisseria zoodegmatis | 0.8 | 0.1–7.0 |
| Desulfomicrobium orale | 0.8 | 0.1–4.3 |
| Porphyromonas gingivalis | 0.8 | 0.1–3.1 |
| Species | Median (%) | Range (%) |
|---|---|---|
| Porphyromonas A cangingivalis | 15.87 | 4.88–31.34 |
| Porphyromonas gulae | 10.86 | 0.35–50.73 |
| Conchiformibius steedae | 6.18 | 0.65–40.44 |
| Porphyromonas A canoris | 4.05 | 0.51–23.85 |
| Porphyromonas gingivicanis | 3.98 | 0.12–13.22 |
| Neisseria weaveri | 1.89 | 0.08–9.48 |
| Frederiksenia canicola | 1.63 | 0.15–17.19 |
| Capnocytophaga cynodegmi | 1.30 | 0.16–7.64 |
| Capnocytophaga canimo rsus | 1.26 | 0.16–9.23 |
| Capnocytophaga canis | 1.08 | 0.39–5.48 |
| Bergeyella zoohelcum | 1.01 | 0.09–18.49 |
| Histophilus haemoglobinophilus | 0.91 | 0.32–18.57 |
| Pasteurella dagmatis | 0.91 | 0.02–17.30 |
| Neisseria zoodegmatis | 0.81 | 0.06–7.05 |
| Desulfomicrobium orale | 0.80 | 0.13–4.30 |
| Porphyromonas gingivalis | 0.80 | 0.13–3.13 |
| Porphyromonas Other | 0.71 | 0.10–2.82 |
| Pasteurella canis | 0.59 | 0.13–2.21 |
| Porphyromonas crevioricanis | 0.57 | 0.01–4.04 |
| Pasteurella multocida A | 0.55 | 0.01–3.16 |
| Unidentified species from Order Bacteroidales | 0.54 | 0.10–1.54 |
| Neisseria animaloris | 0.50 | 0.08–13.69 |
| Actinomyces GCF 016598775.1 | 0.49 | <0.01–2.40 |
| Capnocytophaga Other | 0.48 | 0.08–2.03 |
| Unidentified species from Family Porphyromonadaceae | 0.47 | 0.04–0.83 |
| Neisseria Other | 0.45 | 0.02–2.77 |
| Eikenella shayeganii | 0.37 | 0.07–3.20 |
| Tannerella forsythia | 0.31 | 0.04–0.93 |
| Unidentified species from Family Pasteurellaceae | 0.30 | 0.05–1.16 |
| Mycoplasmopsis A canis | 0.30 | 0.02–3.83 |
| Neisseria canis | 0.23 | 0.01–1.95 |
| Other | 0.20 | 0.1–5.57 |
| Treponema B denticola | 0.17 | 0.01–0.79 |
| Unidentified species from Family Neisseriaceae | 0.14 | 0.01–0.53 |
| Porphyromonas circumdentaria | 0.14 | 0.02–0.24 |
| Prevotella Other | 0.11 | 0.01–0.22 |
| Unidentified species from Family Bacteroidaceae | 0.11 | 0.01–0.26 |
| Treponema B Other | 0.11 | 0.01–0.39 |
| Moraxella Other | 0.09 | 0.03–0.29 |
| Corynebacterium mustelae | 0.09 | 0.01–4.69 |
| Fusobacterium Other | 0.07 | <0.01–0.18 |
| Streptococcus minor | 0.06 | 0.01–2.00 |
| Gemella palaticanis | 0.06 | 0.01–0.67 |
| Pasteurella Other | 0.06 | 0.02–0.66 |
| Unidentified species from Class Gammaproteobacteria | 0.06 | 0.02–0.99 |
| Moraxella canis | 0.06 | 0.01–0.88 |
| Unidentified species from Family Moraxellaceae | 0.05 | 0.01–0.12 |
| Unidentified species from Order Flavobacteriales | 0.05 | 0.01–0.48 |
| Porphyromonas A Other | 0.05 | <0.01–0.20 |
| Prevotella intermedia | 0.05 | 0.01–0.33 |
| Streptococcus Other | 0.04 | <0.01–4.67 |
| Unidentified species from Class Bacteroidia | 0.04 | 0.01–0.10 |
| Neisseria wadsworthii | 0.04 | <0.01–0.35 |
| Capnocytophaga stomatis | 0.04 | 0.01–3.18 |
| Campylobacter A Other | 0.04 | <0.01–0.17 |
| Bacteroides Other | 0.03 | <0.01–1.86 |
| Unidentified species from Class Clostridia | 0.03 | 0.01–0.06 |
| Unidentified species from Family Campylobacteraceae | 0.02 | <0.01–0.10 |
| Unidentified species from Order Enterobacterales | 0.02 | <0.01–0.07 |
| Histophilus somni | 0.02 | <0.01–0.17 |
| Actinomyces Other | 0.01 | <0.01–0.27 |
| Pasteurella multocida | 0.01 | <0.01–0.48 |
| Treponema Other | 0.01 | <0.01–0.12 |
| Acinetobacter Other | 0.01 | <0.01–0.05 |
| Pauljensenia Other | 0.01 | <0.01–0.04 |
| Unidentified species from Order Burkholderiales | 0.01 | <0.01–0.04 |
| Unidentified species from Class Actinomycetia | 0.01 | <0.01–0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisjak, A.; Correa Lopes, B.; Pilla, R.; Nemec, A.; Suchodolski, J.S.; Tozon, N. A Comparison of the Oral Microbiota in Healthy Dogs and Dogs with Oral Tumors. Animals 2023, 13, 3594. https://doi.org/10.3390/ani13233594
Lisjak A, Correa Lopes B, Pilla R, Nemec A, Suchodolski JS, Tozon N. A Comparison of the Oral Microbiota in Healthy Dogs and Dogs with Oral Tumors. Animals. 2023; 13(23):3594. https://doi.org/10.3390/ani13233594
Chicago/Turabian StyleLisjak, Anja, Bruna Correa Lopes, Rachel Pilla, Ana Nemec, Jan S. Suchodolski, and Nataša Tozon. 2023. "A Comparison of the Oral Microbiota in Healthy Dogs and Dogs with Oral Tumors" Animals 13, no. 23: 3594. https://doi.org/10.3390/ani13233594
APA StyleLisjak, A., Correa Lopes, B., Pilla, R., Nemec, A., Suchodolski, J. S., & Tozon, N. (2023). A Comparison of the Oral Microbiota in Healthy Dogs and Dogs with Oral Tumors. Animals, 13(23), 3594. https://doi.org/10.3390/ani13233594











