The Use of Isoflurane and Adjunctive Magnesium Chloride Provides Fast, Effective Anaesthetization of Octopus vulgaris
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Animals
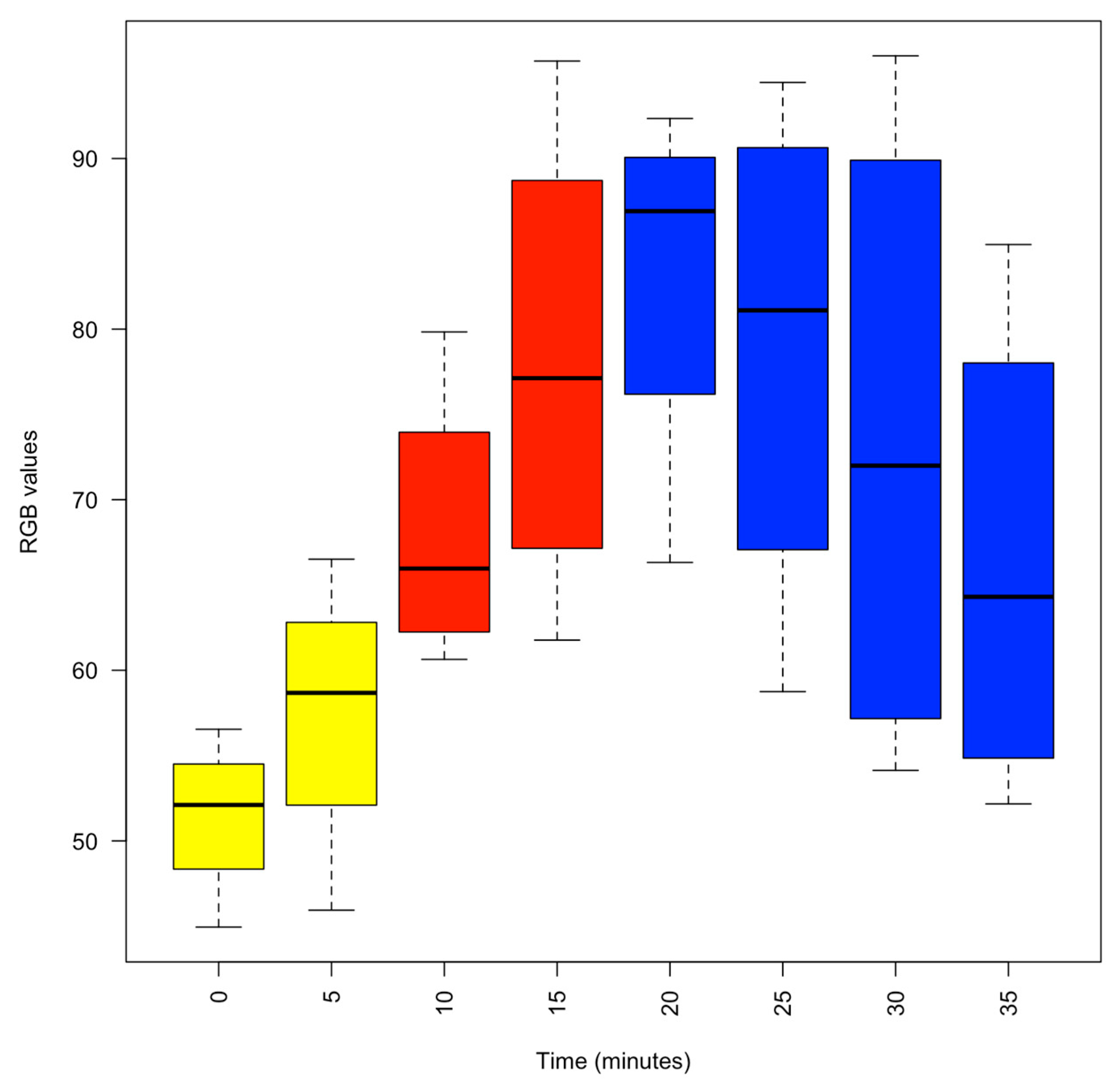
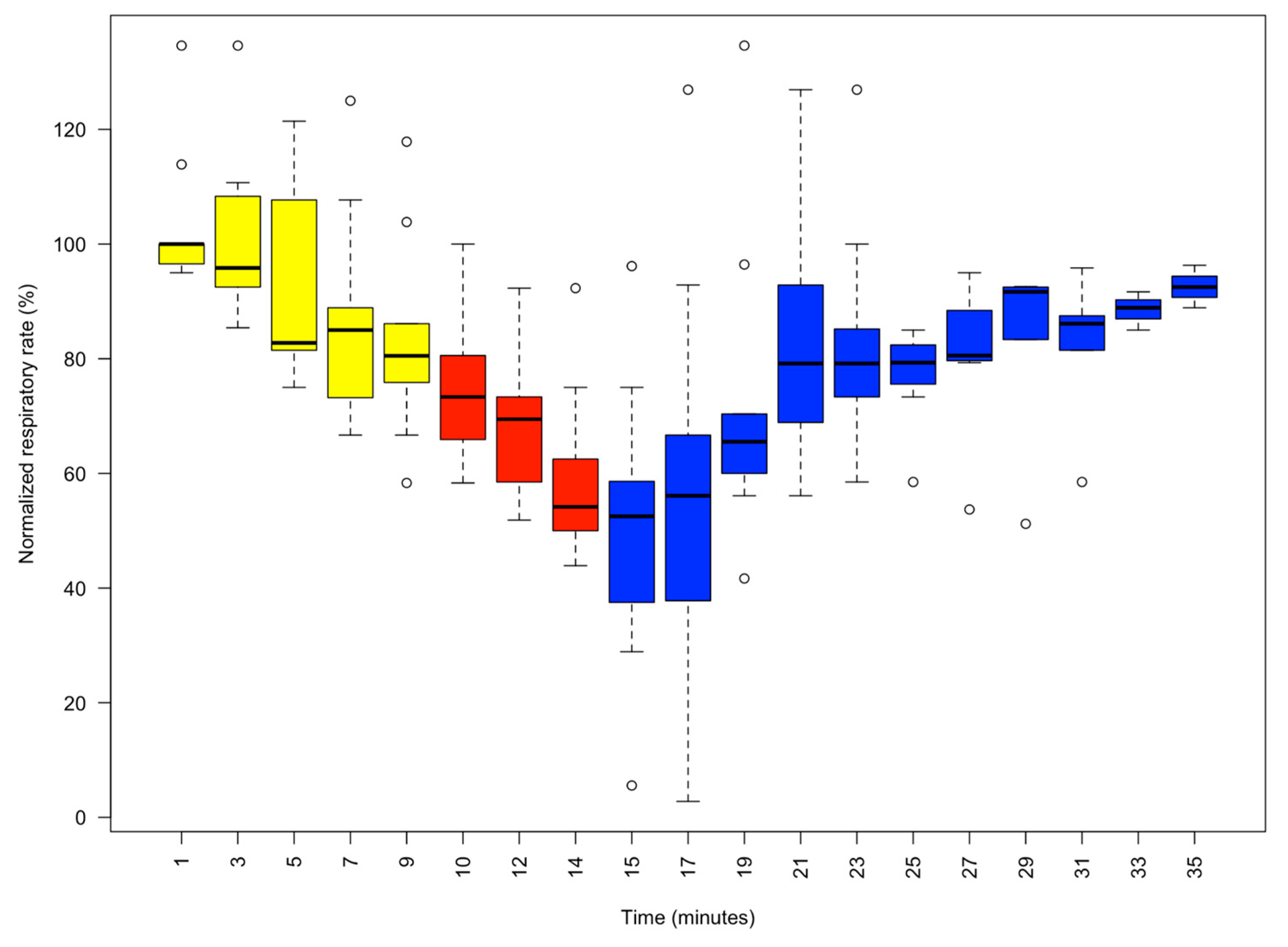
3. Results
3.1. Actions of 1% MgCl2 on Animal Behaviour
3.2. Addition of I% Isoflurane
3.3. Recovery from Anaesthesia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mather, J.A. Animal suffering: An invertebrate perspective. J. Appl. Anim. Welf. Sci. 2001, 4, 151–156. [Google Scholar] [CrossRef]
- Paolucci, M.; Di Cristo, C.; Di Cosmo, A. Immunological evidence for progesterone and estradiol receptors in the freshwater crayfish Austropotamobius pallipes. Mol. Reprod. Dev. 2002, 63, 55–62. [Google Scholar] [CrossRef]
- Passantino, A.; Elwood, R.W.; Coluccio, P. Why Protect Decapod Crustaceans Used as Models in Biomedical Research and in Ecotoxicology? Ethical and Legislative Considerations. Animals 2021, 11, 73. [Google Scholar] [CrossRef]
- Fiorito, G.; Affuso, A.; Anderson, D.B.; Basil, J.; Bonnaud, L.; Botta, G.; Cole, A.; D’Angelo, L.; De Girolamo, P.; Dennison, N.; et al. Cephalopods in neuroscience: Regulations, research and the 3Rs. Invert. Neurosci. 2014, 14, 13–36. [Google Scholar] [CrossRef]
- Winlow, W.; Polese, G.; Moghadam, H.F.; Ahmed, I.A.; Di Cosmo, A. Sense and Insensibility—An Appraisal of the Effects of Clinical Anesthetics on Gastropod and Cephalopod Molluscs as a Step to Improved Welfare of Cephalopods. Front. Physiol. 2018, 9, 1147. [Google Scholar] [CrossRef]
- Crook, R.J.; Walters, E.T. Nociceptive Behavior and Physiology of Molluscs: Animal Welfare Implications. Ilar J. 2011, 52, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Lewbart, G.A.; Mosley, C. Clinical Anesthesia and Analgesia in Invertebrates. J. Exot. Pet Med. 2012, 21, 59–70. [Google Scholar] [CrossRef]
- Elwood, R.W. Pain and suffering in invertebrates? Ilar J. 2011, 52, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Crook, R.J.; Hanlon, R.T.; Walters, E.T. Squid have nociceptors that display widespread long-term sensitization and spontaneous activity after bodily injury. J. Neurosci. 2013, 33, 10021–10026. [Google Scholar] [CrossRef] [PubMed]
- Sneddon, L.U. Pain in aquatic animals. J. Exp. Biol. 2015, 218, 967–976. [Google Scholar] [CrossRef]
- Crook, R.J. Behavioral and neurophysiological evidence suggests affective pain experience in octopus. iScience 2021, 24, 102229. [Google Scholar] [CrossRef]
- Elwood, R.W. Behavioural Indicators of Pain and Suffering in Arthropods and Might Pain Bite Back? Animals 2023, 13, 2602. [Google Scholar] [CrossRef]
- García-Franco, M. Anaesthetics for the squid Sepioteuthis sepioidea (mollusca: Cephalopoda). Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1992, 103, 121–123. [Google Scholar] [CrossRef]
- Pugliese, C.; Mazza, R.; Andrews, P.L.; Cerra, M.C.; Fiorito, G.; Gattuso, A. Effect of Different Formulations of Magnesium Chloride Used as Anesthetic Agents on the Performance of the Isolated Heart of Octopus vulgaris. Front. Physiol. 2016, 7, 610. [Google Scholar] [CrossRef]
- Butler-Struben, H.M.; Brophy, S.M.; Johnson, N.A.; Crook, R.J. In Vivo Recording of Neural and Behavioral Correlates of Anesthesia Induction, Reversal, and Euthanasia in Cephalopod Molluscs. Front. Physiol. 2018, 9, 109. [Google Scholar] [CrossRef]
- Abbo, L.A.; Himebaugh, N.E.; DeMelo, L.M.; Hanlon, R.T.; Crook, R.J. Anesthetic Efficacy of Magnesium Chloride and Ethyl Alcohol in Temperate Octopus and Cuttlefish Species. J. Am. Assoc. Lab. Anim. Sci. 2021, 60, 556–567. [Google Scholar] [CrossRef] [PubMed]
- James, M.F.M. Clinical Use of Magnesium Infusions in Anesthesia. Anesth. Analg. 1992, 74, 129–136. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, G.S., Jr.; Castro-Alves, L.J.; Khan, J.H.; McCarthy, R.J. Perioperative systemic magnesium to minimize postoperative pain: A meta-analysis of randomized controlled trials. Anesthesiology 2013, 119, 178–190. [Google Scholar] [CrossRef]
- Fathi Moghadam, H.; Yar, T.; Qazzaz, M.M.; Ahmed, I.A.; Winlow, W. A Comparative Study of Cell Specific Effects of Systemic and Volatile Anesthetics on Identified Motor Neurons and Interneurons of Lymnaea stagnalis (L.), Both in the Isolated Brain and in Single Cell Culture. Front. Physiol. 2019, 10, 583. [Google Scholar] [CrossRef] [PubMed]
- Polese, G.; Winlow, W.; Di Cosmo, A. Dose-dependent effects of the clinical anesthetic isoflurane on Octopus vulgaris: A contribution to cephalopod welfare. J. Aquat. Anim. Health 2014, 26, 285–294. [Google Scholar] [CrossRef]
- Gutnick, T.; Neef, A.; Cherninskyi, A.; Ziadi-Künzli, F.; Di Cosmo, A.; Lipp, H.-P.; Kuba, M.J. Recording electrical activity from the brain of behaving octopus. Curr. Biol. 2023, 33, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, R.; Lieb, W.R.; Franks, N.P. The effects of temperature on the interactions between volatile general anaesthetics and a neuronal nicotinic acetylcholine receptor. Br. J. Pharmacol. 1995, 116, 2949–2956. [Google Scholar] [CrossRef][Green Version]
- Messenger, J.B. Cephalopod chromatophores: Neurobiology and natural history. Biol. Rev. Camb. Philos. Soc. 2001, 76, 473–528. [Google Scholar] [CrossRef] [PubMed]
- Berge, K.H.; Warner, D.O. Drugs that affect the respiratory system. In Foundations of Anesthesia, Basic and Clinical Sciences; Hemmings, H., Hopkins, P., Eds.; Mosby: London, UK, 2000; pp. 491–498. [Google Scholar]
- Di Cosmo, A.; Polese, G.; Bertapelle, C.; Palumbo, A.L.; Zullo, L.C. Cefalopodi. Benessere ed Animal Care Dell’animale da Laboratorio; Le Point Veterinaire Italie: Milano, Italy, 2015. [Google Scholar]
- Maselli, V.; Polese, G.; Al-Soudy, A.S.; Buglione, M.; Di Cosmo, A. Cognitive Stimulation Induces Differential Gene Expression in Octopus vulgaris: The Key Role of Protocadherins. Biology 2020, 9, 196. [Google Scholar] [CrossRef]
- Maselli, V.; Al-Soudy, A.S.; Buglione, M.; Aria, M.; Polese, G.; Di Cosmo, A. Sensorial Hierarchy in Octopus vulgaris’s Food Choice: Chemical vs. Visual. Animals 2020, 10, 457. [Google Scholar] [CrossRef]
- Collins, T.J. ImageJ for microscopy. Biotechniques 2007, 43, 25–30. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Pauca, A.L.; Dripps, R.D. Clinical experience with isoflurane (Forane): Preliminary communication. BJA Br. J. Anaesth. 1973, 45, 697–703. [Google Scholar] [CrossRef]
- Guedel, A.E. Inhalation Anesthesia: A Fundamental Guide. Anesth. Analg. 1937, 16, 119–120. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Cosmo, A.; Maselli, V.; Cirillo, E.; Norcia, M.; de Zoysa, H.K.S.; Polese, G.; Winlow, W. The Use of Isoflurane and Adjunctive Magnesium Chloride Provides Fast, Effective Anaesthetization of Octopus vulgaris. Animals 2023, 13, 3579. https://doi.org/10.3390/ani13223579
Di Cosmo A, Maselli V, Cirillo E, Norcia M, de Zoysa HKS, Polese G, Winlow W. The Use of Isoflurane and Adjunctive Magnesium Chloride Provides Fast, Effective Anaesthetization of Octopus vulgaris. Animals. 2023; 13(22):3579. https://doi.org/10.3390/ani13223579
Chicago/Turabian StyleDi Cosmo, Anna, Valeria Maselli, Emanuela Cirillo, Mariangela Norcia, Heethaka K. S. de Zoysa, Gianluca Polese, and William Winlow. 2023. "The Use of Isoflurane and Adjunctive Magnesium Chloride Provides Fast, Effective Anaesthetization of Octopus vulgaris" Animals 13, no. 22: 3579. https://doi.org/10.3390/ani13223579
APA StyleDi Cosmo, A., Maselli, V., Cirillo, E., Norcia, M., de Zoysa, H. K. S., Polese, G., & Winlow, W. (2023). The Use of Isoflurane and Adjunctive Magnesium Chloride Provides Fast, Effective Anaesthetization of Octopus vulgaris. Animals, 13(22), 3579. https://doi.org/10.3390/ani13223579








