Simple Summary
The chicken digestive system is not fully developed, and the feed type and shape have essential effects on it. The growth performance and health status would be affected in various ways during this stage. Immune function and antioxidant capacity can explain this mechanism at the endocrine level. Fermented feed is a new feed type that saves food crops, has the potential to promote growth and development, improve animal welfare and help them stay healthy, and is beneficial to human food safety. We studied the effects of fermented feed on the growth performance, antioxidant activity, immune function, intestinal digestive enzyme activity, morphology, and microflora of yellow-feather chickens. The results showed that adding fermented feed increased the digestive enzyme activity, ameliorated intestinal morphology, cecal microflora, beneficial bacteria richness, immune function, and antioxidational ability of chickens without effects on growth performance. In conclusion, the fermented feed added to the chickens’ diets improved the growth performance, antioxidant activity, immune function, intestinal digestive enzyme activity, morphology, and microflora of yellow-feather chickens. This study offers a more theoretical basis for exploiting and utilizing new fermented feed resources and the sustainable and healthy development of poultry farming.
Abstract
This experiment was conducted to investigate the effects of fermented feed on growth performance, antioxidant activity, immune function, intestinal digestive enzyme activity, morphology, and microflora of yellow-feather chickens. A total of 240 one-day-old female yellow-feathered (Hexi dwarf) chickens were randomly divided into two treatment groups, with six replicates per group and 20 chickens per replicate. The control group (CK) received a basal diet, whereas the experimental group was fed a basal diet of +2.00% fermented feed (FJ). The trial lasted for 22 days. Compared with the CK, (1) the growth performance was not affected (p > 0.05); (2) immunoglobin a, immunoglobin g, immunoglobin m, interleukin-1β, and interleukin-6 were affected (p < 0.05); (3) liver superoxide dismutase, glutathione peroxidase, and catalase were higher (p < 0.05); (4) trypsin activity in the duodenum and cecal Shannon index were increased (p < 0.05); (5) the relative abundance of Actinobacteriota in cecum was increased (p < 0.05); (6) the abundance of dominant microflora of Bacteroides as well as Clostridia UCG-014_norank were increased (p < 0.05). In summary, the fermented feed improved the growth performance, antioxidant activity, immune function, intestinal digestive enzyme activity, morphology, and microflora of yellow-feather chickens.
1. Introduction
As chickens’ digestive system and immune systems are poorly developed, the feed utilization rate and disease resistance are low [1,2,3]. The feed contains a large number of anti-nutritional factors, like cellulose, hemicellulose, and pectin, that affect the chicken digestibility [4] and have a specific impact on the survival rate of chickens. Currently, there is a shortage of feed resources and low utilization of agricultural by-products for other products. Therefore, it is imperative to improve the feed conversion rate, solve the shortage of resources, and utilize the by-products to reduce industrial waste [5]. Fermented feed refers to the high-quality feed produced by the mixed probiotic fermentation using different feed raw materials, auxiliary materials, and probiotics under specific temperatures, humidity, oxygen content, and pH, which can not only reduce anti-nutritional factors in the feed but also improve the content of beneficial bacteria, amino acids, short-chain fatty acids, peptides, and other nutrients [6]. Fermented feed has a promising future in promoting animal growth performance and immunity, replacing antibiotics, realizing the reuse of industrial waste, and alleviating the shortage of feed resources as a new type of feed with safe, green, non-toxic, and replaceable antibiotics [7,8,9]. There have been 44 bacteria strains announced by the Food and Drug Administration and the Association of American Feed Control Officials that can be used in fermented feed production. In recent years, the rapid development of fermented products has had a significantly good effect on animal husbandry production [10,11,12].
Growth performance is an essential and direct index for measuring the economic benefits and competitiveness of farming. The lactic acid bacteria increase the palatability of poultry feed [13] and are used as natural preservatives in animal feed to prevent fungal growth followed by mycotoxin production [14]. Li et al. [15] and Sun et al. [16] found that fermented feed could improve the average daily feed intake and average daily gain of broilers. Antioxidant capacity and immune function are closely related to animal health, indirectly affecting the economic benefits of breeding. It was shown that chickens supplemented with fermented feed would perform better in muscle antioxidant capacity [17]. More polypeptides, fatty acids, nitrogen-free extract, and other nutrients are produced during the fermentation process by certain feed resources, which provide material and energy for immunity [18,19,20], and nutrition is the foundation of immunity [21]. However, few studies have been conducted to comprehensively evaluate the growth, immunity, antioxidant, digestion, intestine morphology, and microflora of chickens. This study aims to investigate the effects of fermented feed on the growth performance, antioxidant activity, immune function, intestinal digestive enzyme activity, morphology, and microflora of yellow-feather chickens and offer a more theoretical basis for the exploitation and utilization of new fermented feed resources and the sustainable and healthy development of poultry farming.
2. Materials and Methods
2.1. Experiment Material
Fermented feed is made by mixing beancurd residue and beer grains with bran and rice hull powder, followed by adding lactic acid bacteria and yeast (both the number of viable bacteria is 2.0 × 107 CFU/g) and then fermenting in 20 kg sealed plastic bags at 32 °C, humidity 67.0%, and pH 4.2–4.3 for 11 days. The basal diet and fermented feed used in the experiment were purchased from Jiangxi Qiling Agriculture and Animal Husbandry Co., Ltd., Ganzhou, China. All the experimental chickens received 24 h light every day and were raised on the floor, had free access to fresh drinking water, were fed twice a day at 8:00 and 17:00, respectively, and were vaccinated according to the normal immunization procedures. Automatic temperature control equipment was used to control the temperature of the hen house at 30 °C. The mental state, the disease, and the death of the chickens were observed and recorded. The experimental animals were offered by Qiling Agriculture and Animal Husbandry Farm, Ganzhou, China.
2.2. Experiment Design and Sample Collection
A total of 240 one-day-old female yellow-feathered (Hexi dwarf) chickens with similar body weights were randomly divided into two groups, with six replicates per group and 20 broilers per replicate. The control group (CK) was fed a basal diet, and the fermented feed group (FJ) was fed a basal diet of +2.00% fermented feed, determined based on previous experiments, and the experimental basal diet was corn–soybean diet, which was formulated according to the nutrient requirements published by NRC (1998; 2012). Basal diets, fermented feed composition, and nutrient levels are shown in Table 1. The trial lasted for 22 days.

Table 1.
Composition (kg/100 kg) and nutrient level of the experimental diets 1 for chickens.
Body weights were measured for each chicken at 1 and 22 d of age, and the total feed consumption of each group was recorded. Growth performance was evaluated by average daily gain (ADG), average daily feed intake (ADFI), and feed conversion rate (FCR). At the end of the experiment, one chicken that was similar in average weight was selected from each replicate to be slaughtered by carotid artery bloodletting after feed deprivation for 12 h, and about 10 mL of blood samples was collected from the wing artery of each replicate in a tube before the chicken was slaughtered. Then, the abdominal cavity was rapidly opened, and the contents were taken, respectively—about 2 g from the duodenum, jejunum, ileum cecum, and liver, which were placed in a sterile cryogenic vial and stored at −80 °C for later use. Tubular segments (2–3 cm long) in the same part were cut from the duodenum, jejunum, and ileum; cleaned with normal saline; and stored in 4% paraformaldehyde fix solution at room temperature. The serum was separated by centrifugation for 20 min at 3500 r/min at 4 °C and stored at −20 °C for later analysis.
The immunity indexes contain immunoglobin A (IgA), immunoglobin G (IgG), immunoglobin M (IgM), interleukin-1β (IL-1β), and interleukin-6 (IL-6). The antioxidant indexes contain malonaldehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT). They were all determined by the HITACHI Automatic Analyzer 3500, Ibaraki-Ken, Japan, and the kits were provided by Beijing SINO-UK Institute of Biological Technology. The catalog numbers for IgA, IgG, IgM, IL-1β, IL-6, MDA, SOD, GSH-Px, and CAT kits are ILP2673-C1, 269A-16-RUO, M2521, EP2074, EP2076, A003-1, N82750-25g-, S10152-200UN-, and A007-1, respectively.
The duodenum, jejunum, and ileum contents were taken at 0.3 g, respectively, and normal saline was added to the mass ratio of the contents: normal saline = 1:9, treated with a high-speed grinder under the ice bath condition, centrifuged at 3000 r/min for 10 min at 4 °C with Multifuge X4 Pro ThermoFisher High-performance centrifuge (Shanghai, China), and 50 μL of supernatant was taken to determine the amylase, trypsin, and lipase activities. The kit was provided by Nanjing Jiancheng Bioengineering Co., Ltd., Nanjing, China. The catalog numbers for the amylase, trypsin, and lipase kits are Ab102523, PP0100-1KT, and Ab102524, respectively.
The duodenum, jejunum, and ileum tissues were fixed with 4% paraformaldehyde fix solution for 72 h, and then paraffin sections were prepared, and hematoxylin–eosin (HE) staining was performed. A 40-fold vision of view of scan images was captured by K-Viewer software (1.7.0.29) for observation. Six tissue regions with complete intestinal morphology and a clear field of view were selected for scanning. Using a micron as the standard unit, Image-pro plus 6.0 was used to measure the villus height and crypt depth of a single intestine, and the villus height/crypt depth was calculated.
The cecum contents were taken from the −80 °C refrigerator, and its total DNA was extracted using E.Z.N.A.® Stool DNA Kit (catalog number: YFXM0027), which was provided by Shanghai Lingen Biological Technology Co., Ltd., Shanghai, China. The purity and concentration of DNA extraction were detected by 1% agarose gel electrophoresis. According to the total DNA of cecum contents as a template, the rRNA gene V3–V4 region was amplified by PCR using high-fidelity DNA polymerase. The upstream primer was 515F (5′-barcode-GTGCCAGCMGCCGCGG)-3′), and the downstream primer was 907R (5-CCGTCAATTCMTTTRAGTTT-3′) for PCR amplification. The library was established, followed by the 16S rDNA sequencing on an Illumina HiSeq 2500 PE 250 platform in Shanghai Biozeron Biotechnology Co., Ltd., Shanghai, China. All the sequence splicing and filtering were performed using the fastqc software. Qiime software (Version 1.7.0) was used for OUT (Operational Taxonomic Units) clustering and statistical analysis in bioinformatics at the 97% similarity level. Based on the clustering results, α diversity index analysis, β diversity index analysis, principal component analysis, microbial classification statistics, and linear discriminant analysis effect size (lEfSe) were conducted. Statistical analysis of community structure was performed at taxonomic levels (phylum, class, order, family, genus).
2.3. Statistical Analysis of Data
Statistical analyses were conducted using SPSS 20.0 statistics software. Different treatments were analyzed using two-factor and multiple comparisons with Tukey’s multiple-range tests. Data were expressed as mean ± standard error of the mean and p < 0.05 as the significance differences.
3. Results
3.1. Effects of Fermented Feed on Growth Performance in Chicks
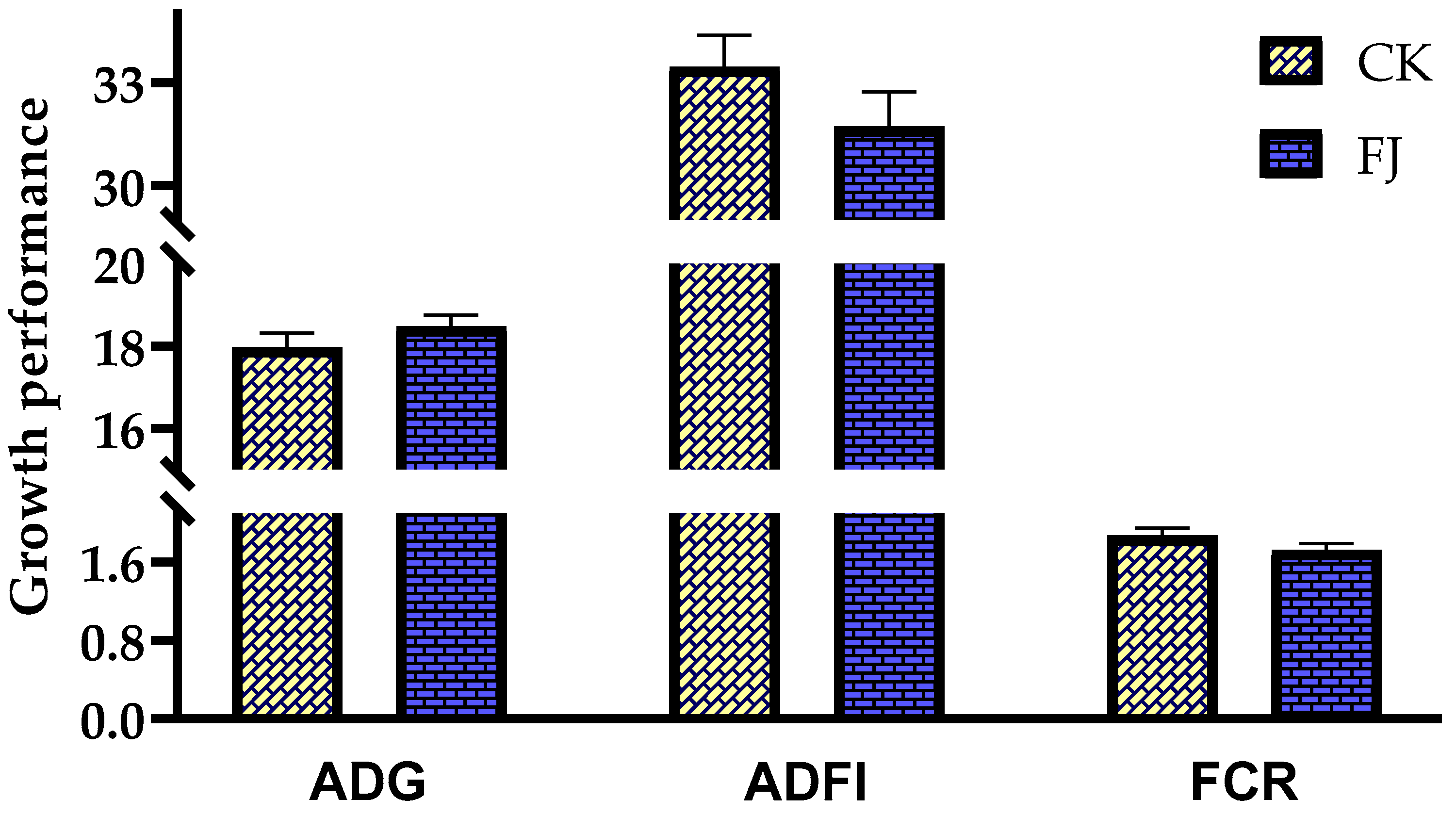
The differences in ADG, ADFI, and FCR of chicks between CK and FJ were not statistically significant (p > 0.05) (Figure 1).
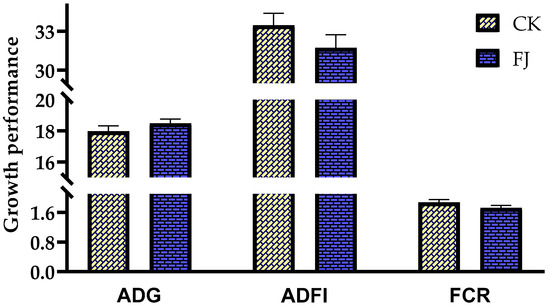
Figure 1.
Effects of fermented feed on growth performance of chicks. ADG (Average daily gain) in the CK and FJ groups was 17.96 ± 0.36 vs. 18.47± 0.28, respectively, p = 0.288; ADFI (Average daily feed intake) in the CK and FJ groups was 33.46 ± 0.93 vs. 31.73 ± 1.00, respectively, p = 0.234; FCR (Feed conversion rate) in the CK and FJ groups was 1.87 ± 0.08 vs. 1.72 ± 0.07, respectively, p = 0.192.
3.2. Effects of Fermented Feed on Serum Immune Function of Chickens
The IgA, IgG, and IgM were significantly affected (p < 0.01) by the fermented feed. The IL-1β and IL-6 were lower (p < 0.01) in FJ than those in CK (Table 2).

Table 2.
Effects of fermented feed on serum immune indexes of chickens at 22 d of age.
3.3. Effects of Fermented Feed on Antioxidational Ability in the Liver of Chickens
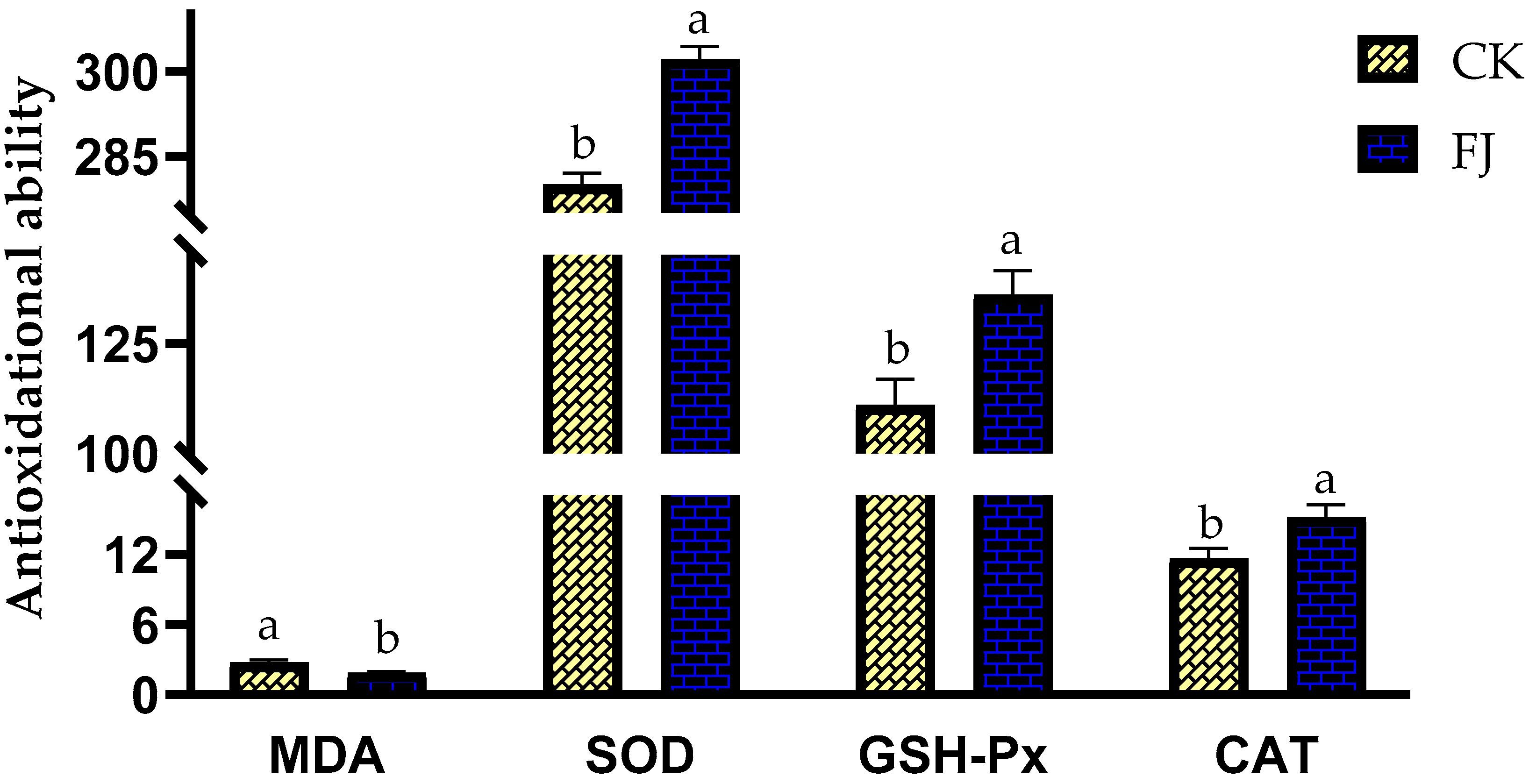
The differences in MDA, SOD, GSH-Px, and CAT in the liver of chickens between CK and FJ were statistically significant (p < 0.05). The liver MDA in FJ was lower than that in CK; however, the liver SOD, GSH-Px, and CAT of CK were higher than that of FJ (Figure 2).
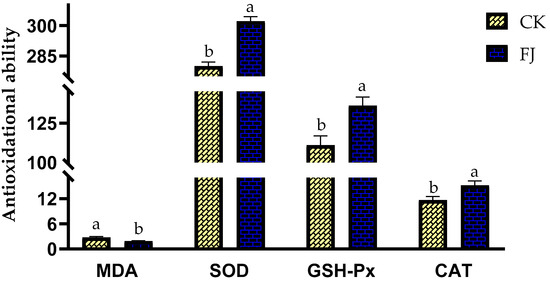
Figure 2.
Effects of fermented feed on immune index in the liver of chickens at 22 d of age. Note: The different superscripts within the same index indicate a significant difference (p < 0.05). The MDA (Malonaldehyde) levels in the CK and FJ groups were 2.74 ± 0.24 a vs. 1.87 ± 0.12 b, nmol/mg·protein, respectively, p = 0.009; The SOD (Superoxide dismutase) levels in the CK and FJ groups were 280 ± 2.06 b vs. 302 ± 2.33 a, U/mg·protein, respectively, p = <0.001; the GSH-Px (Glutathione peroxidase) levels in the CK and FJ groups were 111 ± 5.92 b vs. 136 ± 5.47 a U/mg·protein, respectively, p = 0.010; The CAT (Catalase) levels in the CK and FJ groups were 11.67 ± 0.84 b vs. 15.19 ± 1.04 a, U/mg·protein, respectively, p = 0.025.
3.4. Effects of Fermented Feed on Digestive Enzyme Activity in Chickens
Compared with CK, the duodenum trypsin activity in FJ was significantly enhanced (p < 0.05), and there were no statistically significant differences (p > 0.05) with the activities of neither trypsin in the jejunum and ileum nor amylase together with lipase in the duodenum, jejunum, and ileum in FJ (Table 3).

Table 3.
Effects of fermented feed on digestive enzyme activities of chickens at 22 d of age.
3.5. Effects of Fermented Feed on Intestinal Tissue Morphology of Chickens
The villus height, crypt depth, and height/crypt depth among the two groups did not differ significantly (p > 0.05), except the villus height of ileum in FJ was significantly lower (p < 0.01) than CK (Table 4).

Table 4.
Effects of fermented feed on intestinal tissue morphology of chicks at 22 d of age.
3.6. Effects of Fermented Feed on Microbial Diversity in the Cecum of Chickens
3.6.1. Alpha Diversity Analysis
Compared with CK, the Shannon indexes in FJ were significantly increased (p < 0.05). However, the differences between all the Chao indexes, Richness indexes, ACE indexes, Evenness indexes, and Simpson indexes were not significant (p > 0.05) (Table 5).

Table 5.
Analysis of cecal microflora α diversity index of chickens at 22 d of age.
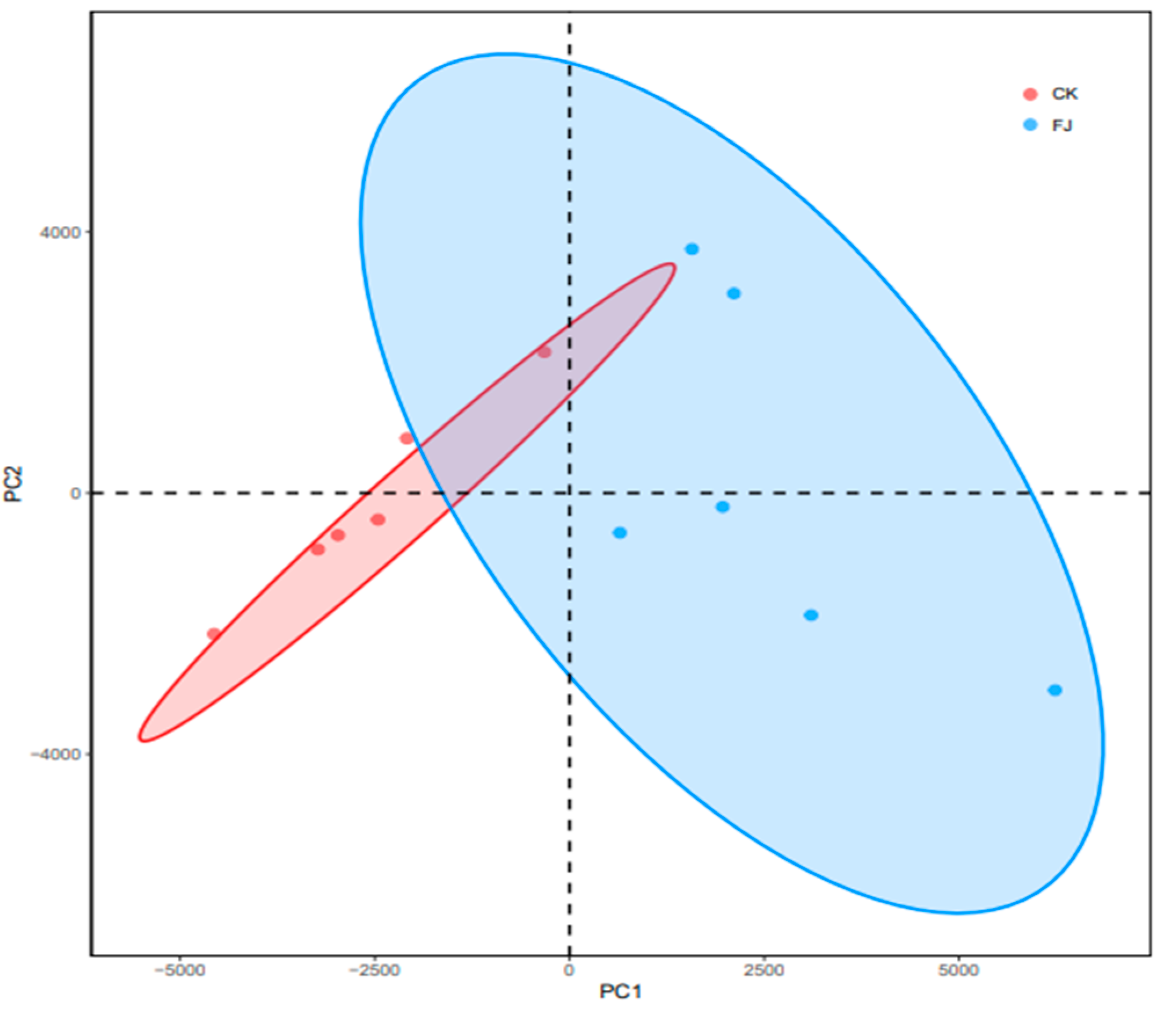
3.6.2. Beta Diversity Comparative Analysis–PCA Analysis of the Chicken Samples between the Groups
As shown in Figure 3, the microbial colony structure difference between CK and FJ was significant, indicating that the addition of fermented feed had a significant influence (p < 0.05) on the intestinal flora structure of chickens’ cecum.

Figure 3.
PCA analysis of microorganisms in cecal contents of chickens at 22 d of age.
3.6.3. Relative Abundance of Cecal Microflora at Phylum and Genus Levels of Chickens
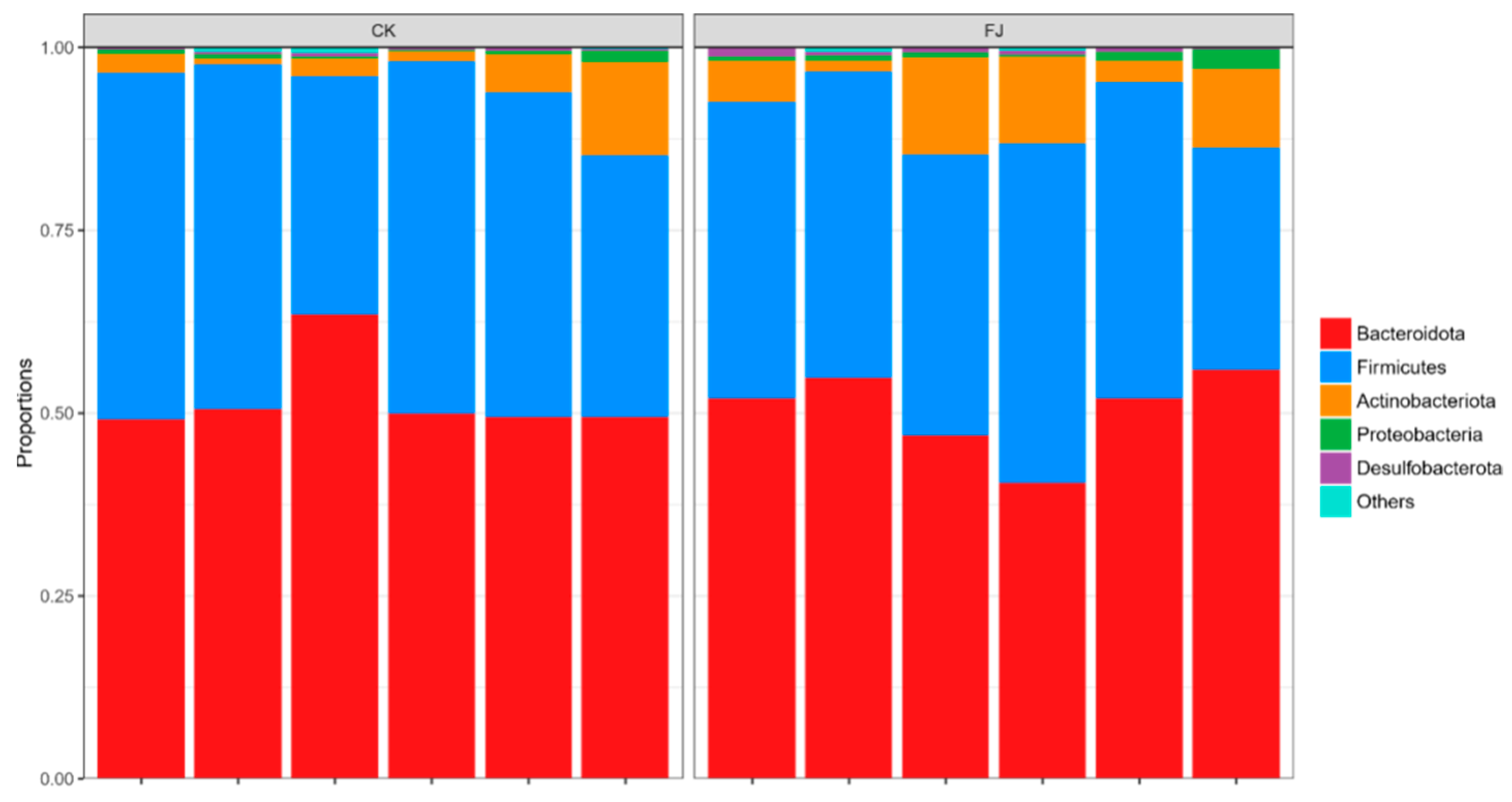
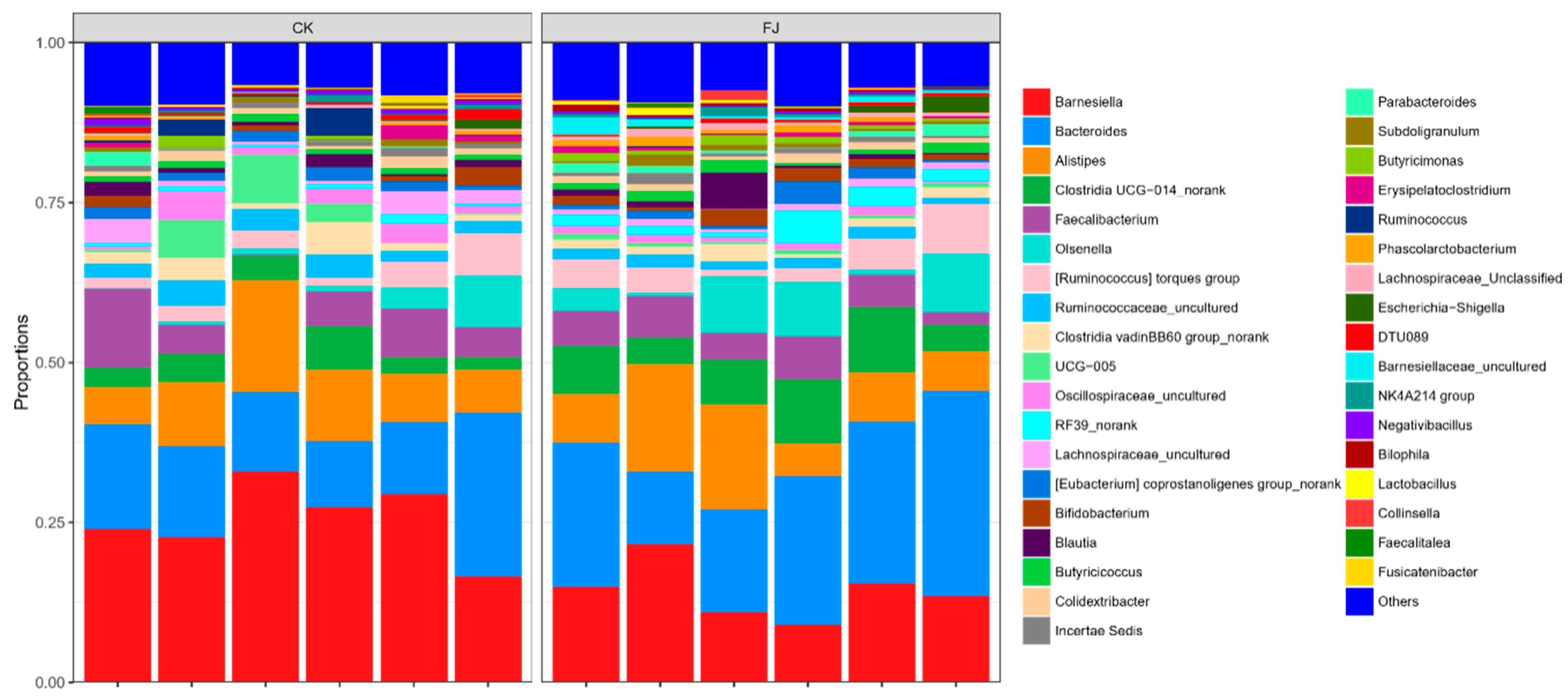
Based on the analysis at the phylum level, it can be seen that the dominant microflora in cecum contents was mainly Bacteroidota, Firmicutes, Actinobateriota, Proteobacteria, and Desulfobacterota. Firmicutes was the main bacteria in the cecum intestinal flora. Compared with CK, the Desulfobacterota was extremely significantly increased (p < 0.01), and Actinobateriota was significantly increased (p < 0.05), while the differences of Bacteroidota, Firmicutes, and Proteobacteria were not significant (p > 0.05) in FJ (Figure 4).

Figure 4.
Relative abundance of cecal microflora at phylum level of chickens at 22 d of age.
The figure that was carried out by the analysis at the genus level indicated the dominant flora of intestinal contents bacteria in cecum mainly included Barnesiella, Bacteroides, Alistipes, Clostridia UCG-014_norank, and Faecalibacterium. The highest two bacteria contents were Barnesiella and Bacteroides, the dominant fungi in the microecological environment of cecum. It can be seen after analysis that the dominant microflora abundance of Bacteroides and Clostridia UCG-014_norank were significantly increased (p < 0.05), and Barnesiella was significantly decreased (p < 0.05). At the same time, both Alistipes and Faecalibacterium were not significantly affected (p > 0.05) by FJ compared to CK (Figure 5).
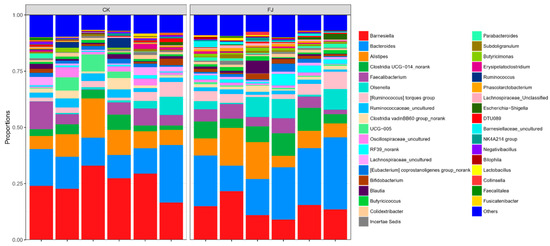
Figure 5.
Relative abundance of cecal microflora at genus level of chickens at 22 d of age.
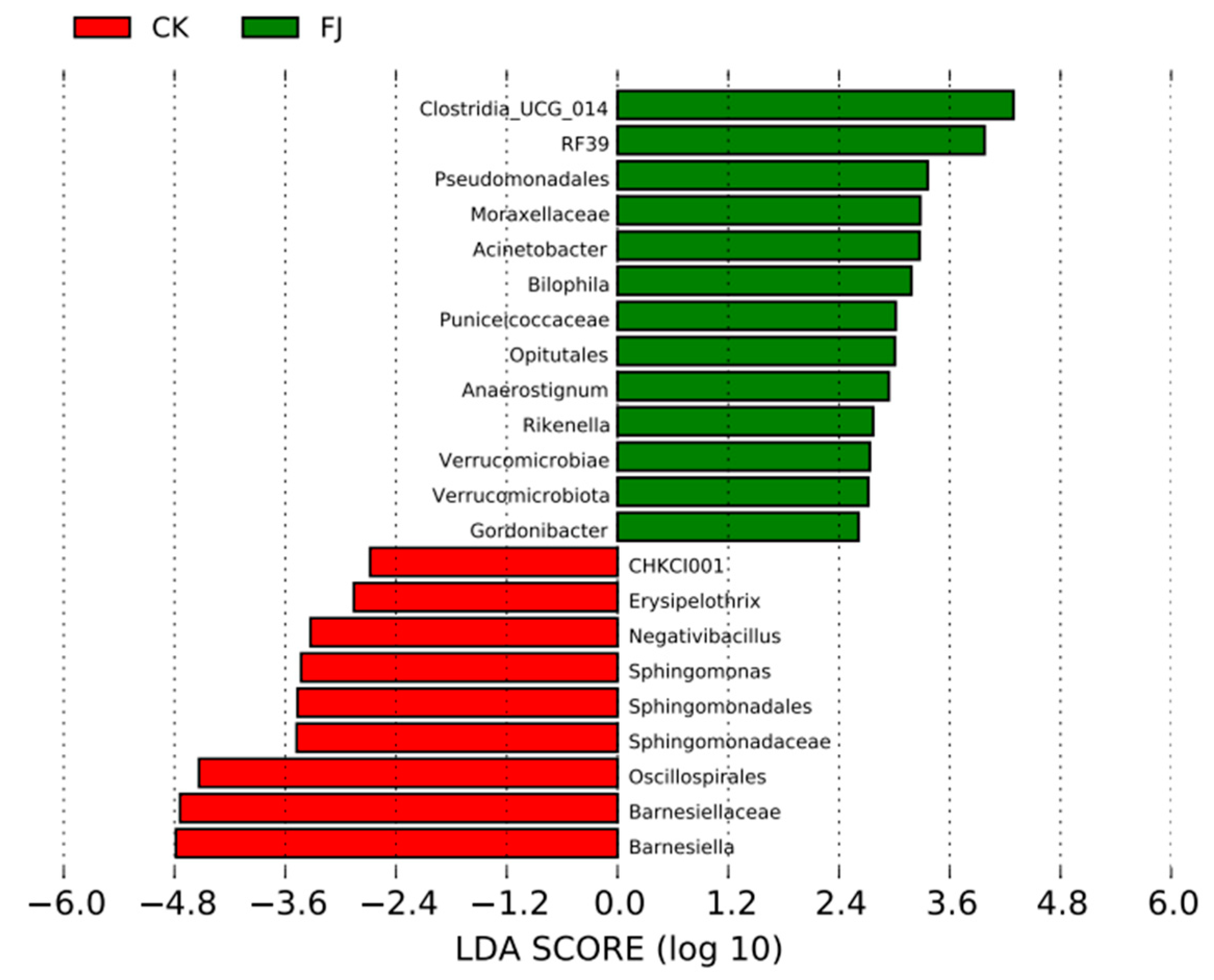
3.6.4. Lefse Difference Analysis of the Chicken Samples between the Groups
Only the species with LDA values greater than 2.4 were displayed in Figure 4, which were used to evaluate species groups with significant differences. There were 13 species groups with significant differences in FJ, which were mainly about Clostridia_UCG_014, RF39, Pseudomonadales, Moraxellaceae, Acinetobacter, Bilophila, Puniceicoccaceae, Opitutales, and so on. While CHKCI001, Erysipelothrix, Negativibacillus, Sphingomonas, Sphingomonadales, Sphingomonadaceae, and Oscillospirales were the leading nine significantly different species groups (Figure 6).
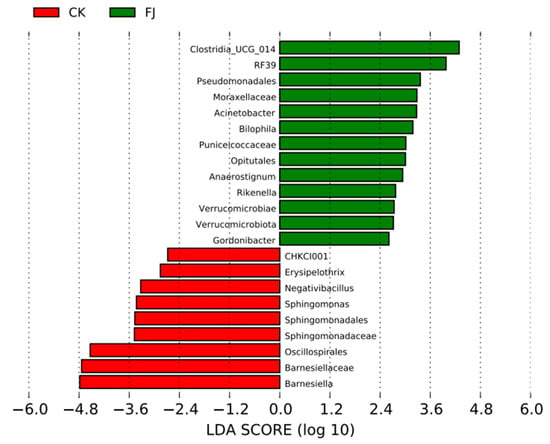
Figure 6.
Column diagram of LefSe analysis of cecal contents in chickens at 22 d of age.
4. Discussion
4.1. Effects of Fermented Feed on the Growth Performance of Chickens
Various amino acids, peptides, organic acids, vitamins, and other nutrients are generated during the feed fermentation [6]. In addition, the anti-nutritional factor contents can be reduced by fermentation [22,23,24], thereby improving the decomposition, digestion, absorption, and utilization of nutrients in animals. There are not only chemical changes occurring in fermentation but also physical changes happening. The feed pH value, hardness, shape, and taste will be changed after fermentation [25,26]. Both chemical and physical changes could affect the feed palatability and the animal growth performance. Zhu et al. [26] found that the feed intake of chickens fed 10% and 15% dried or 10% wet fermented feed during the whole period (1–42 d) was higher than those fed the control diet, while feed intake and feed conversion ratio were not affected by those fermented feed during the starter stage (1–21 d). Omar et al. [27] found that adding fermented fava bean by-products of 15%, 25%, and 35% improved the feed intake of broiler chickens. Xie et al. [28] found that the weight gain increased significantly after 10% of the olive leaf residues fermented by solid-state fermentation were added to daily chicken feed for 28 days. This experiment showed that the chickens’ ADG, ADFI, and FCR were not affected by fermented feed, which may be due to some of the following reasons: (1) The chickens were fed in hot and damp weather, harming growth performance, as the weather induces heat stress, promoting catabolism in chickens [29] and decreasing the feed intake [30]. (2) The development of the digestive system is immature, making the absorption of nutrients incomplete. (3) The stomach content of chickens is limited, and the yellow-feather broilers have a large feed intake, which makes it easy to reach the maximum stomach content, and they cannot continue to increase feed intake.
4.2. Effects of Fermented Feed on Immune Function in the Serum of Chickens
Specific immunity mainly consists of humoral immunity and cellular immunity [31]. The immune system is mediated by cells, soluble factors, interacting cells, and tissues [32]. Immunity is the biological basis of organisms resisting pathogenic microorganisms, bacteria, and viruses. The function of immunity is mainly reflected by immune indicators. Immunoglobulin (Ig), which mainly includes IgG, IgM, and IgA, refers to any class of structurally related proteins in the serum and the immune system cells [33]. The systemic inflammatory response is triggered by some cytokines, including interleukin 1 (IL-1), IL-6, and tumor necrosis factor (TNF-α), which lead to multiple inflammatory cascades [34]. The immune system enhancement is illustrated by the decrease of the proinflammatory cytokines content. Zhu et al. [26] found that the serum IgA, IgG, and IgM of broilers in groups fed with 10%, 15%, and 25% dried fermented feed and 10% wet fermented feed were higher than the control group, except for IgG content in the 25% dried fermented feed group. Wang et al. [35] found that fermented soybean meal decreased the plasma IL-1β and IL-6 concentration of weaned piglets after the enterotoxigenic Escherichia coli (ETEC) K88 challenge. And several previous studies have suggested that fermentation could boost immunity [36,37,38]. The results of this experiment showed that the serum IgA, IgG, and IgM of chicks in FJ were significantly increased, while the levels of proinflammatory factors IL-1β and IL-6 in serum decreased significantly compared with the CK. The results showed that adding fermented feed during the brooding period could significantly improve the serum immunity function of chickens.
4.3. Effects of Fermented Feed on Antioxidational Ability of Chickens
The oxygen toxicity in living organisms mainly comes from the oxygen free radicals produced by its partial reduction, also known as reactive oxygen species (ROS), which are by-products of normal cellular metabolism [39]. It may lead to oxidative stress and damage to the cells and biomolecules when animal ROS is excessive [40]. Lipid and protein oxidations are generally initiated by ROS [41]. The ability of ROS to facilitate protein oxidation was enhanced with malondialdehyde (MDA) [42]. MDA is commonly used as an index of oxidative stress, which is highly reactive and disseminates and magnifies oxidative damage during lipid peroxidation [43]. Superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) belong to the first level of the antioxidant defense network [44]. Zhu et al. [45] reported that the T-AOC, SOD, and GSH-Px levels in the serum of chickens were markedly elevated by fermented feed, while the serum MDA level was significantly reduced by that. Zhang et al. [46] found that liver MDA in the control group was less than that in the Aspergillus niger fermentation group and Candida utilis combined with the Aspergillus niger fermentation group. Niu et al. [47] reported that fermented Ginkgo biloba leaves supplementing could improve the broiler gut’s antioxidant activity. In addition, the antioxidational ability was improved by fermented feed in different tissues and organs of animals. In this study, therefore, it is inferred that the liver antioxidational ability of chickens was promoted by adding fermented feed to the basal feed.
4.4. Effects of Fermented Feed on Intestinal Digestive Enzyme Activity of Chickens
Animal growth performance is determined by feed utilization, which is closely related to the digestion enzyme activity [27]. The digestive enzymes decompose the significant molecular substances into small ones, which the intestine absorbs more efficiently [48]. This chemical digestion can raise the feed utilization rate to improve animals’ growth performance [27,48]. Amylase is a critical enzyme in hydrolyzing starch and polysaccharides into disaccharides and oligosaccharides [49]. Proteins and peptide chains can be hydrolyzed by trypsin into easily absorbable polypeptides and amino acids [50]. Monoglycerides and free-fatty acids can be efficiently absorbed by organisms and decomposed from lipids by lipase [51]. Feng et al. [52] found that the addition of fermented soybean meal replacing soybean meal in 1–21-day-old broilers’ diet significantly increased trypsin, chymotrypsin, and lipase in the small intestine. It was found that replacing soybean meal with fermented cottonseed meal in the diet improved the amylase and protease activities in intestinal content after 21 days and also improved the protease activity in intestinal digesta of 42-day-old broilers [53]. It was shown that the AMY2A gene expression was significantly upregulated after feeding chickens with fermented fava bean by-products. In contrast, the PNLIP gene expression was significantly upregulated with increasing fermented fava bean levels [27]. The results demonstrated that the duodenal amylase activity of chickens could be significantly improved by adding fermented feed. In contrast, the duodenum amylase, jejunum, ileum amylase, trypase, and lipase were not significantly improved. Although the overall trend was increased, there were a few results with significant differences. The differences of multiple data sets may be caused by the short feeding period.
4.5. Effects of Fermented Feed on Intestinal Tissue Morphology of Chickens
The intestinal epithelium is covered by a superficial columnar epithelium layer and divided into crypt and villus. The crypt is invaginated into the hypoblast layer mesenchyme, and the villus is oriented towards the intestinal lumen [54]. It was found that nutrient absorption is mainly performed in the small intestine, and the villus height (VH), crypt depth (CD), and VH/CD determined the absorption efficiency of nutrients in diets [55]. Sun et al. [53] found that the broilers fed fermented cottonseed meal have higher VH and VH/CD in the jejuna and duodena. The feed fermentation creates organic acids, which stimulate gastro-intestinal cell proliferation and potentially increase the intestinal surface area by improving the VH [56,57]. The higher VH/CD indicated a more vital integrated functional state of the small intestine [58]. It was shown that the chicken fed high doses of fermented feed improved the villus height in the duodenum and a higher VH/CD in the duodenum and ileum [59]. The results of this experiment showed that the supplementation of fermented feed could reduce the crypt depth of the duodenum, jejunum, and ileum and increase the villus height as well as having higher VH/CD in the duodenum and jejunum. This suggests that fermented feed can improve intestinal structure, echoing the above results of improving the growth performance of broilers.
4.6. Effects of Supplementing Fermented Feed on Microbial Diversity in the Cecum of Chickens
The stability, resistance, and resilience of intestinal microflora are the most basic ecological characteristics that play an essential role in animal health, and it was estimated that 100 trillion bacteria from 500 to 1000 species exist in the intestine [60]. Trillions of commensal microbial flora constitute a microecology [61]. Intestinal microecology balance is inextricably linked with animals’ health level, immune ability, and growth performance [62]. Lv et al. found that fermented feed changed the diversity and richness of animal intestinal flora, which provided a better environment for the colonization and growth of beneficial bacteria [63], so we collected caecum contents of chickens for further exploration. It was reported that Firmicutes and Bacteroidota are the top two dominant bacterial phyla in poultry intestines [64,65]. The results of this study showed that Bacteroidota and Firmicutes, accounting for more than 90% of the total cecum microbiotas, were the dominant phylum microbiotas, which were similar to the previous study. Bacteroidota and Firmicutes participate in glycolysis and promote the absorption and metabolism of nutrients [66]. The dietary addition of fermented feed significantly increased the relative abundance of Desulfobacterota in the cecum of chickens, indicating that fermented feed promoted the colonization of Desulfobacterota in the cecum of chicks.
Nevertheless, Desulfobacterota, a sulfate-reducing phylum, is harmful to the intestine, which reduces sulfate to hydrogen sulfide [67]. The analysis results at the genus level showed that feeding fermented feed significantly increased the number of Bacteroides. This Gram-negative obligately anaerobic non-spore-former bacterium regulates intestinal flora’s microecological balance to facilitate intestinal flora’s colonization and participate in the degradation of polysaccharides generating acetate and propionate [68]. Bacteroides play a role in maintaining intestinal homeostasis [69] and transforming bile acids, thus improving the deposition rate of nutrients [70]. We found that adding fermented feed to diets significantly improved the relative abundance of Bacteroides and Clostridia UCG-014_norank in the cecum.
In contrast, the relative abundance of Alistipes and Faecalibacterium tended to increase with an insignificant difference, indicating that fermented feed improved the abundance of bacteria, promoted the growth of beneficial bacteria, and improved the fermentation function of cecum bacteria. Faecalibacterium are widely found in animal intestines, mainly fermented to produce short-chain fatty acids, which can inhibit pathogens producing pathogenic factors, provide energy for intestinal epithelial cells, and are widely used to develop probiotics and biological drugs. In summary, the fermented feed affected the composition of the recipient animals’ intestinal flora, which increased the proportion of beneficial bacteria such as Actinobateriota, Bacteroides, and Clostridia UCG-014_norank.
5. Conclusions
In this study, the 2.00% fermented feed improved the growth performance, antioxidant activity, immune function, intestinal digestive enzyme activity, morphology, and microflora of yellow-feather chickens.
Author Contributions
F.X. and H.W. interpreted the data for the study and drafted the manuscript; J.X., T.Z. and L.H. analyzed the data for the study; W.X. and L.L. were responsible for the design and direction of the experiment. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Key Research and Development Program of Zhejiang Province (No. 2021C02034) and Zhejiang Provincial Special Commissioner Team Projects of Science & Technology (No. Xianju Chicken Industry, 2020–2024).
Institutional Review Board Statement
The Chinese guidelines for animal welfare conducted in this study and with the animal welfare standards of the College of Animal Science and Technology, Northeast Agricultural University (NEAU-2022-0913).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data presented are original and not inappropriately selected, manipulated, enhanced, or fabricated.
Conflicts of Interest
We declare no financial or personal relationships with other people or organizations that might inappropriately influence our work, and we have no professional or personal interest in any product.
References
- Wickramasuriya, S.S.; Park, I.; Lee, K.; Lee, Y.; Kim, W.H.; Nam, H.; Lillehoj, H.S. Role of physiology, immunity, microbiota, and infectious diseases in the gut health of poultry. Vaccines 2022, 10, 172. [Google Scholar] [CrossRef]
- Biesek, J.; Banaszak, M.; Kądziołka, K.; Wlaźlak, S.; Adamski, M. Growth of broiler chickens, and physical features of the digestive system, and leg bones after aluminosilicates used. Sci. Rep. 2022, 12, 20425. [Google Scholar] [CrossRef] [PubMed]
- Broom, L.J.; Kogut, M.H. The role of the gut microbiome in shaping the immune system of chickens. Vet. Immunol. Immunopathol. 2018, 204, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Shuaib, M.; Paneru, D.; Hafeez, A.; Tahir, M.; Kim, W.K. The chemical composition of soyhulls and their effect on amino acid and nutrient digestibility in laying hens during the peak of production. Animals 2023, 13, 2808. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Bai, Z.; Ma, W.; Guo, M.; Jiang, R.; Liu, J.; Oenema, O.; Velthof, G.L.; Whitmore, A.P.; Crawford, J.; et al. Exploring future food provision scenarios for China. Environ. Sci. Technol. 2019, 53, 1385–1393. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, X.; Qiao, S. Advances in research on solid-state fermented feed and its utilization: The pioneer of private customization for intestinal microorganisms. Anim. Nutr. 2021, 7, 905–916. [Google Scholar] [CrossRef]
- Missotten, J.A.; Michiels, J.; Dierick, N.; Ovyn, A.; Akbarian, A.; De Smet, S. Effect of fermented moist feed on performance, gut bacteria and gut histo-morphology in broilers. Br. Poult. Sci. 2013, 54, 627–634. [Google Scholar] [CrossRef]
- Heres, L.; Engel, B.; van Knapen, F.; de Jong, M.C.; Wagenaar, J.A.; Urlings, H.A. Fermented liquid feed reduces susceptibility of broilers for Salmonella enteritidis. Poult. Sci. 2003, 82, 603–611. [Google Scholar] [CrossRef]
- Heres, L.; Engel, B.; Van Knapen, F.; Wagenaar, J.A.; Urlings, B.A. Effect of fermented feed on the susceptibility for Campylobacter jejuni colonisation in broiler chickens with and without concurrent inoculation of Salmonella enteritidis. Int. J. Food Microbiol. 2003, 87, 75–86. [Google Scholar] [CrossRef]
- Yan, J.; Zhou, B.; Xi, Y.; Huan, H.; Li, M.; Yu, J.; Zhu, H.; Dai, Z.; Ying, S.; Zhou, W.; et al. Fermented feed regulates growth performance and the cecal microbiota community in geese. Poult. Sci. 2019, 98, 4673–4684. [Google Scholar] [CrossRef]
- Wang, C.; Wei, S.; Xu, B.; Hao, L.; Su, W.; Jin, M.; Wang, Y. Bacillus subtilis and Enterococcus faecium co-fermented feed regulates lactating sow’s performance, immune status and gut microbiota. Microb. Biotechnol. 2021, 14, 614–627. [Google Scholar] [CrossRef]
- Zhu, Y.; Tao, Z.; Chen, X.; Xiao, J.; Zhang, Y.; Wang, Z. Effects of broussonetia papyrifera-fermented feed on production performance, egg quality, and caecal microbiota of laying hens during the late laying period. Ital. J. Anim. Sci. 2022, 21, 659–672. [Google Scholar] [CrossRef]
- Yeh, R.H.; Hsieh, C.W.; Chen, K.L. Screening lactic acid bacteria to manufacture two-stage fermented feed and pelleting to investigate the feeding effect on broilers. Poult. Sci. 2018, 97, 236–246. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Yan, B.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Lactic acid bacteria as antifungal and anti-mycotoxigenic agents: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1403–1436. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, B.; Wu, Z.; Wang, W.; Li, C.; Liu, G.; Cai, H. Effects of fermented soybean meal supplementation on the growth performance and cecal microbiota community of broiler chickens. Animals 2020, 10, 1098. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chen, D.; Cai, H.; Chang, W.; Wang, Z.; Liu, G.; Deng, X.; Chen, Z. Effects of fermenting the plant fraction of a complete feed on the growth performance, nutrient utilization, antioxidant functions, meat quality, and intestinal microbiota of broilers. Animals 2022, 12, 2870. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; He, W.; Zhang, H.; Wang, J.; Qi, G.; Guo, N.; Zhang, X.; Wu, S. Bio-fermented malic acid facilitates the production of high-quality chicken via enhancing muscle antioxidant capacity of broilers. Antioxidants 2022, 11, 2309. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, J.; Chen, J.; Pirzado, S.A.; Li, Y.; Cai, H.; Liu, G. Effects of fermentation on standardized ileal digestibility of amino acids and apparent metabolizable energy in rapeseed meal fed to broiler chickens. Animals 2020, 10, 1774. [Google Scholar] [CrossRef]
- Li, P.; Ji, X.; Deng, X.; Hu, S.; Wang, J.; Ding, K.; Liu, N. Effect of rapeseed meal degraded by enzymolysis and fermentation on the growth performance, nutrient digestibility and health status of broilers. Arch. Anim. Nutr. 2022, 76, 221–232. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, M.; Li, F.; Qin, M.; Yang, Q.; Yu, H.; Xu, J.; Liu, Y.; Tong, T. Evaluation of fermented soybean meal to replace a portion fish meal on growth performance, antioxidant capacity, immunity, and mTOR signaling pathway of coho salmon (Oncorhynchus kisutch). Aquac. Nutr. 2023, 2023, 2558173. [Google Scholar] [CrossRef]
- Collins, N.; Belkaid, Y. Control of immunity via nutritional interventions. Immunity 2022, 55, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Olukomaiya, O.O.; Adiamo, O.Q.; Fernando, W.C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Effect of solid-state fermentation on proximate composition, anti-nutritional factor, microbiological and functional properties of lupin flour. Food Chem. 2020, 315, 126238. [Google Scholar] [CrossRef] [PubMed]
- Vlassa, M.; Filip, M.; Țăranu, I.; Marin, D.; Untea, A.E.; Ropotă, M.; Dragomir, C.; Sărăcilă, M. The yeast fermentation effect on content of bioactive, nutritional and anti-nutritional factors in rapeseed meal. Foods 2022, 11, 2972. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Sahu, N.P.; Deo, A.D.; Kumar, S. Solid state fermentation of de-oiled rice bran: Effect on in vitro protein digestibility, fatty acid profile and anti-nutritional factors. Food Res. Int. 2019, 119, 1–5. [Google Scholar] [CrossRef]
- Gustaw, K.; Niedźwiedź, I.; Rachwał, K.; Polak-Berecka, M. New insight into bacterial interaction with the matrix of plant-based fermented foods. Foods 2021, 10, 1603. [Google Scholar] [CrossRef]
- Zhu, X.; Tao, L.; Liu, H.; Yang, G. Effects of fermented feed on growth performance, immune organ indices, serum biochemical parameters, cecal odorous compound production, and the microbiota community in broilers. Poult. Sci. 2023, 102, 102629. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.E.; Al-Khalaifah, H.S.; Ismail, T.A.; Abd El-Aziz, R.M.; El-Mandrawy, S.A.M.; Shalaby, S.I.; Ibrahim, D. Performance, serum biochemical and immunological parameters, and digestive enzyme and intestinal barrier-related gene expression of broiler chickens fed fermented fava bean by-products as a substitute for conventional feed. Front. Vet. Sci. 2021, 8, 696841. [Google Scholar] [CrossRef]
- Xie, P.J.; Huang, L.X.; Zhang, C.H.; Zhang, Y.L. Nutrient assessment of olive leaf residues processed by solid-state fermentation as an innovative feedstuff additive. J. Appl. Microbiol. 2016, 121, 28–40. [Google Scholar] [CrossRef]
- Wang, Z.; Shao, D.; Wu, S.; Song, Z.; Shi, S. Heat stress-induced intestinal barrier damage and dimethylglycine alleviates via improving the metabolism function of microbiota gut brain axis. Ecotoxicol. Environ. Saf. 2022, 244, 114053. [Google Scholar] [CrossRef]
- Mazzoni, M.; Zampiga, M.; Clavenzani, P.; Lattanzio, G.; Tagliavia, C.; Sirri, F. Effect of chronic heat stress on gastrointestinal histology and expression of feed intake-regulatory hormones in broiler chickens. Animal 2022, 16, 100600. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Sun, Q.; Knopf, J.; Herrmann, M.; Lin, L.; Jiang, J.; Shao, C.; Li, P.; He, X.; et al. Immune response in COVID-19: What is next? Cell Death Differ. 2022, 29, 1107–1122. [Google Scholar] [CrossRef]
- Varadé, J.; Magadán, S.; González-Fernández, Á. Human immunology and immunotherapy: Main achievements and challenges. Cell Mol. Immunol. 2021, 18, 805–828. [Google Scholar] [CrossRef] [PubMed]
- Megha, K.B.; Mohanan, P.V. Role of immunoglobulin and antibodies in disease management. Int. J. Biol. Macromol. 2021, 169, 28–38. [Google Scholar] [CrossRef]
- Manik, M.; Singh, R.K. Role of toll-like receptors in modulation of cytokine storm signaling in SARS-CoV-2-induced COVID-19. J. Med. Virol. 2022, 94, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Y.; Hao, X.; Duan, Y.; Meng, Z.; An, X.; Qi, J. Dietary fermented soybean meal replacement alleviates diarrhea in weaned piglets challenged with enterotoxigenic Escherichia coli K88 by modulating inflammatory cytokine levels and cecal microbiota composition. BMC Vet. Res. 2020, 16, 245. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, D.; Długosz, E.; Zawistowska-Deniziak, A. Functional properties of food origin Lactobacillus in the gastrointestinal ecosystem—In vitro study. Probiotics Antimicrob. Proteins 2019, 11, 820–829. [Google Scholar] [CrossRef]
- Li, S.C.; Lin, H.P.; Chang, J.S.; Shih, C.K. Lactobacillus acidophilus-fermented germinated brown rice suppresses preneoplastic lesions of the colon in rats. Nutrients 2019, 11, 2718. [Google Scholar] [CrossRef]
- Shakya, S.; Danshiitsoodol, N.; Sugimoto, S.; Noda, M.; Sugiyama, M. Anti-oxidant and anti-inflammatory substance generated newly in Paeoniae Radix Alba extract fermented with plant-derived Lactobacillus brevis 174A. Antioxidants 2021, 10, 1071. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Miazek, K.; Beton, K.; Śliwińska, A.; Brożek-Płuska, B. The effect of β-carotene, tocopherols and ascorbic acid as anti-oxidant molecules on human and animal in vitro/in vivo studies: A review of research design and analytical techniques used. Biomolecules 2022, 12, 1087. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Emara, A.M.; Gan, X.; Li, H. Effects of malondialdehyde as a byproduct of lipid oxidation on protein oxidation in rabbit meat. Food Chem. 2019, 288, 405–412. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Escrivá, C.; Dromant, M.; Borrás, C.; Viña, J. Lipid peroxidation as measured by chromatographic determination of malondialdehyde. Human plasma reference values in health and disease. Arch. Biochem. Biophys. 2021, 709, 108941. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant defence systems and oxidative stress in poultry biology: An update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zhang, B.; Li, J.; Zhu, L. Effects of fermented feed on growth performance, immune response, and antioxidant capacity in laying hen chicks and the underlying molecular mechanism involving nuclear factor-κB. Poult. Sci. 2020, 99, 2573–2580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Sun, Z.Y.; Cao, F.L.; Ahmad, H.; Yang, X.H.; Zhao, L.G.; Wang, T. Effects of dietary supplementation with fermented ginkgo leaves on antioxidant capacity, intestinal morphology and microbial ecology in broiler chicks. Br. Poult. Sci. 2015, 56, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Zhang, J.F.; Wan, X.L.; Huang, Q.; He, J.T.; Zhang, X.H.; Zhao, L.G.; Zhang, L.L.; Wang, T. Effect of fermented Ginkgo biloba leaves on nutrient utilisation, intestinal digestive function and antioxidant capacity in broilers. Br. Poult. Sci. 2019, 60, 47–55. [Google Scholar] [CrossRef]
- Liu, X.; Ju, Y.; Huang, L.; Liu, M.; Bo, J.; Zhou, T.; Zhang, Y.; Liu, C.; Feng, M.; Zhang, S.; et al. Effects of a new fermented soya bean meal on growth performance, serum biochemistry profile, intestinal immune status and digestive enzyme activities in piglets. J. Anim. Physiol. Anim. Nutr. 2022, 106, 1046–1059. [Google Scholar] [CrossRef]
- Gallego-Lobillo, P.; Ferreira-Lazarte, A.; Hernández-Hernández, O.; Villamiel, M. In vitro digestion of polysaccharides: InfoGest protocol and use of small intestinal extract from rat. Food Res. Int. 2021, 140, 110054. [Google Scholar] [CrossRef]
- Patil, U.; Saetang, J.; Zhang, B.; Benjakul, S. Use of tuna visceral pepsin in combination with trypsin as digestion aid: Enhanced protein hydrolysis and bioavailability. Foods 2022, 12, 125. [Google Scholar] [CrossRef]
- Kozan, D.W.; Derrick, J.T.; Ludington, W.B.; Farber, S.A. From worms to humans: Understanding intestinal lipid metabolism via model organisms. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2023, 1868, 159290. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Liu, X.; Xu, Z.R.; Wang, Y.Z.; Liu, J.X. Effects of fermented soybean meal on digestive enzyme activities and intestinal morphology in broilers. Poult. Sci. 2007, 86, 1149–1154. [Google Scholar] [CrossRef]
- Sun, H.; Tang, J.W.; Yao, X.H.; Wu, Y.F.; Wang, X.; Feng, J. Effects of dietary inclusion of fermented cottonseed meal on growth, cecal microbial population, small intestinal morphology, and digestive enzyme activity of broilers. Trop. Anim. Health Prod. 2013, 45, 987–993. [Google Scholar] [CrossRef]
- DeSesso, J.M. Comparative anatomy, pre-and postnatal changes during the development and maturation of the small intestine: Life-stage influences on exposure. Birth Defects Res. 2022, 114, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Dalia, A.M.; Loh, T.C.; Sazili, A.Q.; Samsudin, A.A. Influence of bacterial organic selenium on blood parameters, immune response, selenium retention and intestinal morphology of broiler chickens. BMC Vet. Res. 2020, 16, 365. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, J.; Mahfuz, S.; Long, S.; Wu, D.; Gao, J.; Piao, X. Supplementation of mixed organic acids improves growth performance, meat quality, gut morphology and volatile fatty acids of broiler chicken. Animals 2021, 11, 3020. [Google Scholar] [CrossRef]
- Dai, D.; Qiu, K.; Zhang, H.J.; Wu, S.G.; Han, Y.M.; Wu, Y.Y.; Qi, G.H.; Wang, J. Organic acids as alternatives for antibiotic growth promoters alter the intestinal structure and microbiota and improve the growth performance in broilers. Front. Microbiol. 2021, 11, 618144. [Google Scholar] [CrossRef]
- Farahat, M.; Ibrahim, D.; Kishawy, A.T.Y.; Abdallah, H.M.; Hernandez-Santana, A.; Attia, G. Effect of cereal type and plant extract addition on the growth performance, intestinal morphology, caecal microflora, and gut barriers gene expression of broiler chickens. Animal 2021, 15, 100056. [Google Scholar] [CrossRef]
- Peng, W.; Talpur, M.Z.; Zeng, Y.; Xie, P.; Li, J.; Wang, S.; Wang, L.; Zhu, X.; Gao, P.; Jiang, Q.; et al. Influence of fermented feed additive on gut morphology, immune status, and microbiota in broilers. BMC Vet. Res. 2022, 18, 218. [Google Scholar] [CrossRef]
- Xiang, Y.; Wen, H.; Yu, Y.; Li, M.; Fu, X.; Huang, S. Gut-on-chip: Recreating human intestine in vitro. J. Tissue Eng. 2020, 11, 2041731420965318. [Google Scholar] [CrossRef]
- El-Sayed, A.; Aleya, L.; Kamel, M. The link among microbiota, epigenetics, and disease development. Environ. Sci. Pollut. Res. 2021, 28, 28926–28964. [Google Scholar] [CrossRef]
- Ding, S.; Yan, W.; Ma, Y.; Fang, J. The impact of probiotics on gut health via alternation of immune status of monogastric animals. Anim. Nutr. 2021, 7, 24–30. [Google Scholar] [CrossRef]
- Lv, J.; Guo, L.; Chen, B.; Hao, K.; Ma, H.; Liu, Y.; Min, Y. Effects of different probiotic fermented feeds on production performance and intestinal health of laying hens. Poult. Sci. 2022, 101, 101570. [Google Scholar] [CrossRef]
- Segura-Wang, M.; Grabner, N.; Koestelbauer, A.; Klose, V.; Ghanbari, M. Genome-resolved metagenomics of the chicken gut microbiome. Front. Microbiol. 2021, 12, 726923. [Google Scholar] [CrossRef]
- Xiao, S.S.; Mi, J.D.; Mei, L.; Liang, J.; Feng, K.X.; Wu, Y.B.; Liao, X.D.; Wang, Y. Microbial diversity and community variation in the intestines of layer chickens. Animals 2021, 11, 840. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Nie, Q.; He, H.; Tan, H.; Geng, F.; Ji, H.; Hu, J.; Nie, S. Gut firmicutes: Relationship with dietary fiber and role in host homeostasis. Crit. Rev. Food Sci. Nutr. 2022, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Chen, N.; Liu, T.; Feng, C. Effects of adding different carbon sources on the microbial behavior of sulfate-reducing bacteria in sulfate-containing wastewater. J. Clean. Prod. 2023, 392, 136332. [Google Scholar] [CrossRef]
- Zafar, H.; Saier, M.H., Jr. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1848158. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P. Glycan utilisation system in Bacteroides and Bifidobacteria and their roles in gut stability and health. Appl. Microbiol. Biotechnol. 2019, 103, 7287–7315. [Google Scholar] [CrossRef]
- Guo, X.; Okpara, E.S.; Hu, W.; Yan, C.; Wang, Y.; Liang, Q.; Chiang, J.Y.L.; Han, S. Interactive relationships between intestinal flora and bile acids. Int. J. Mol. Sci. 2022, 23, 8343. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).