The Expression of Selected Cytokine Genes in the Livers of Young Castrated Bucks after Supplementation with a Mixture of Dry Curcuma longa and Rosmarinus officinalis Extracts
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
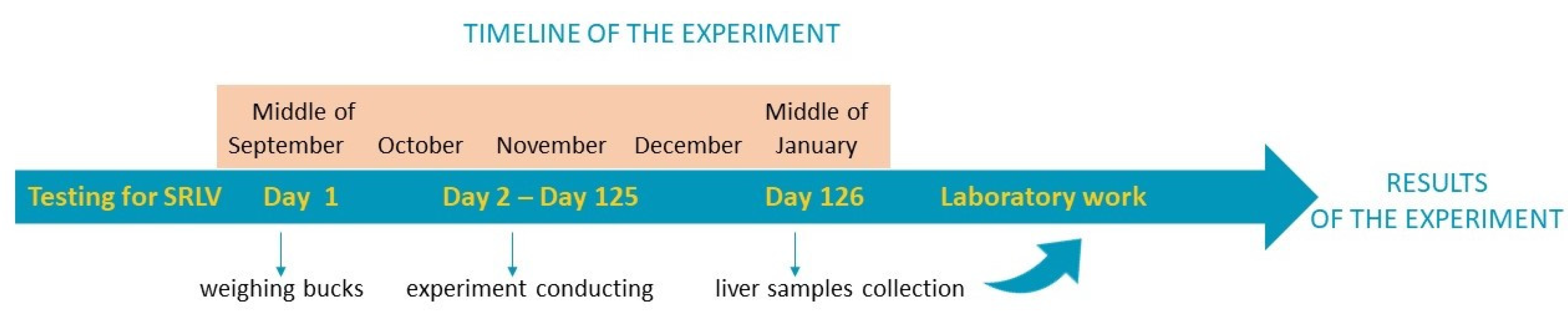
2.1. Animal Material
2.2. Expression of the Genes
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zuliani, A.; Esbjerg, L.; Grunert, K.G.; Bovolenta, S. Animal Welfare and Mountain Products from Traditional Dairy Farms: How Do Consumers Perceive Complexity? Animals 2018, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.; Costa, J.M.; Lima, M.J. Goat system productions: Advantages and disadvantages to the animal, environment and farmer. In Goat Science; InTech Open: London, UK, 2017; pp. 351–366. [Google Scholar]
- Singh, A. Immunology as applied to farm animal welfare: General principles and interventions. Res. Rev. J. Immunol. 2018, 8, 1–13. [Google Scholar]
- Waszkiewicz-Robak, B.; Obiedziński, M.W.; Biller, E.; Obiedzińska, A. Nutraceuticals in animal nutrition and their effect on selected quality characteristics of beef—A review article. Pol. J. Appl. Sci. 2017, 3, 73–77. [Google Scholar]
- Torres-Fajardo, R.A.; González-Pech, P.G.; de Jesús Torres-Acosta, J.F.; Sandoval-Castro, C.A. Nutraceutical Potential of the Low Deciduous Forest to Improve Small Ruminant Nutrition and Health: A Systematic Review. Agronomy 2021, 11, 1403. [Google Scholar] [CrossRef]
- Glombowsky, P.; Volpato, A.; Campigotto, G.; Soldá, N.M.; dos-Santos, D.S.; Bottari, N.B.; Schetinger, M.R.C.; Morsch, V.M.; Rigon, F.; Schogor, A.L.B.; et al. Dietary addition of curcumin favors weight gain and has antioxidant, anti-inflammatory and anticoccidial action in dairy calves. Rev. Colomb. Cienc. Pecu. 2020, 33, 16–31. [Google Scholar] [CrossRef]
- Chiofalo, B.; Riolo, E.B.; Fasciana, G.; Liotta, L.; Chiofalo, V. Organic management of dietary rosemary extract in dairy sheep: Effects on milk quality and clotting properties Article in Veterinary Research Communications. Vet. Res. Commun. 2010, 34, S197–S201. [Google Scholar] [CrossRef]
- Babaei, F.; Nassiri-Asl, M.; Hosseinzadeh, H. Curcumin (a constituent of turmeric): New treatment option against COVID-19. Food Sci. Nutr. 2020, 8, 5215–5227. [Google Scholar] [CrossRef]
- Toden, S.; Theiss, A.L.; Wang, X.; Goel, A. Essential turmeric oils enhance anti-inflammatory efficacy of curcumin in dextran sulfate sodium-induced colitis. Sci. Rep. 2017, 7, 814. [Google Scholar] [CrossRef]
- Zalewska, M.; Kapusta, A.; Kawecka-Grochocka, E.; Urbańska, D.M.; Czopowicz, M.; Kaba, J.; Brzozowska, P.; Bagnicka, E. Effect of Supplementation with Organic Selenium or Turmeric and Rosemary Mixture on Beta-Defensin Content in Goat Milk. Animals 2022, 12, 2948. [Google Scholar] [CrossRef]
- Urbańska, D.M.; Pawlik, M.; Korwin-Kossakowska, A.; Rutkowska, K.; Kawecka-Grochocka, E.; Czopowicz, M.; Mickiewicz, M.; Kaba, J.; Bagnicka, E. Effect of supplementation with a mixture of Curcuma longa and Rosmarinus officinalis extracts on growth performance, meat quality and lipid metabolism gene expressions in young castrated Polish White Improved bucks. J. Anim. Feed Sci. 2023, 33. in press. [Google Scholar] [CrossRef]
- Lofthouse, S.A.; Andrews, A.E.; Elhay, M.J.; Bowles, V.M.; Meeusen, E.N.; Nash, A.D. Cytokines as adjuvants for ruminant vaccines. Int. J. Parasitol. 1996, 26, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Van Miert, A.S. Pro-inflammatory cytokines in a ruminant model: Pathophysiological, pharmacological, and therapeutic aspects. Vet. Q. 1995, 17, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Bai, J.; Zhao, Z.; Li, N.; Wang, Y.; Zhang, L. Differential expression of T helper cytokines in the liver during early pregnancy in sheep. Anim. Reprod. 2019, 16, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Bahna, S.L. Spice allergy. Ann. Allergy Asthma Immunol. 2011, 107, 191–199. [Google Scholar] [CrossRef]
- Cani, M.M.; Abed, S.A. The Protective Effect of different Feeding Methods of Ethanolic Extract of Rosmarinus officinalis on Overdose Toxicity of Hepatorenal induced by Paracetomol. J. Pharm. Sci. Res. 2018, 10, 293–297. [Google Scholar]
- Qiu, P.; Man, S.; Li, J.; Liu, J.; Zhang, L.; Yu, P.; Gao, W. Overdose intake of curcumin initiates the unbalanced state of bodies. Agric. Food Chem. 2016, 64, 2765–2771. [Google Scholar] [CrossRef] [PubMed]
- Czopowicz, M.; Szaluś-Jordanow, O.; Moroz, A.; Mickiewicz, M.; Witkowski, L.; Markowska-Daniel, I.; Bagnicka, E.; Kaba, J. Use of two commercial caprine arthritis–encephalitis immunoenzymatic assays for screening of arthritic goats. J. Vet. Diagn. Investig. 2018, 30, 36–41. [Google Scholar] [CrossRef]
- Brzóska, F.; Kowalski, Z.M.; Osięgłowski, S.; Strzetelski, J. IZ PIB-INRA Nutritional Recommendations for Ruminants and Tables of Nutritional Value of Feeds; [Zalecenia Żywieniowe dla Przeżuwaczy i Tabele Wartości Pokarmowej Pasz]; Strzetelski, J., Ed.; Foundation of the Institute of Animal Production of the National Research Institute Patronus Animalium: Warsaw, Poland, 2014; ISBN 978-83-938377-0-0. [Google Scholar]
- Urbańska, D.M.; Pawlik, M.; Korwin-Kossakowska, A.; Czopowicz, M.; Rutkowska, K.; Kawecka-Grochocka, E.; Mickiewicz, M.; Kaba, J.; Bagnicka, E. Effect of Supplementation with Curcuma longa and Rosmarinus officinalis Extract Mixture on Acute Phase Protein, Cathelicidin, Defensin and Cytolytic Protein Gene Expression in the Livers of Young Castrated Polish White Improved Bucks. Genes 2023, 14, 1932. [Google Scholar] [CrossRef]
- Brenaut, P.; Lefèvre, L.; Rau, A.; Laloë, D.; Pisoni, G.; Moroni, P.; Bevilacqua, C.; Martin, P. Contribution of mammary epithelial cells to the immune response during early stages of a bacterial infection to Staphylococcus aureus. BMC Vet. Res. 2014, 45, 16. [Google Scholar] [CrossRef]
- Jarczak, J.; Kaba, J.; Bagnicka, E. The validation of hausekeeping genes as a reference in quantitative Real Time PCR analysis. Gene 2014, 549, 280–285. [Google Scholar] [CrossRef]
- Jarczak, J.; Słoniewska, D.; Kaba, J.; Bagnicka, E. The expression of cytokines in the milk somatic cells, blood leukocytes and serum of goats infected with small ruminant lentivirus. BMC Vet. Res. 2019, 15, 424. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Fagergren, P.; Smith, H.R.; Daunais, J.B.; Nader, M.A.; Porrino, L.J.; Hurd, Y.L. Temporal upregulation of prodynorphin mRNA in the primate striatum after cocaine self-administration. Eur. J. Neurosci. 2003, 17, 2212–2218. [Google Scholar] [CrossRef]
- Bruce, K.D.; Cagampang, F.R.; Argenton, M.; Zhang, J.; Ethirajan, P.L.; Burdge, G.C.; Bateman, A.C.; Clough, G.F.; Poston, L.; Hanson, M.A.; et al. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology 2009, 50, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Bagnicka, E.; Sikora, J.; Kaba, J.; Gruszecki, T.M. Goat breeding in Poland. In Proceedings of the European Regional Conference on Goats “Sustainable Goat Breeding and Goat Farming in Central and Eastern European Countries”, Debrecen, Hungary; Oradea, Romania, 7–13 April 2014; pp. 91–98, ISBN 978-92-5-109123-4. [Google Scholar]
- Teixeira, A.; Silva, S.; Guedes, C.; Rodrigues, S. Sheep and goat meat processed products quality: A review. Foods 2020, 9, 960. [Google Scholar] [CrossRef] [PubMed]
- Harel, M.; Fauteux-Daniel, S.; Girard-Guyonvarc’h, C.; Gabay, C. Balance between Interleukin-18 and Interleukin-18 binding protein in auto-inflammatory diseases. Cytokine 2022, 150, 155781. [Google Scholar] [CrossRef]
- Beheshtipour, J.; Raeeszadeh, M. Evaluation of Interleukin-10 and Pro-inflammatory Cytokine Profile in Calves Naturally Infected with Neonatal Calf Diarrhea Syndrome. Arch. Razi. Inst. 2020, 75, 213–218. [Google Scholar] [CrossRef]
- Glassman, C.R.; Su, L.; Majri-Morrison, S.S.; Winkelmann, H.; Mo, F.; Li, P.; Pérez-Cruz, M.; Ho, P.P.; Koliesnik, I.; Nagy, N.; et al. Calibration of cell-intrinsic interleukin-2 response thresholds guides design of a regulatory T cell biased agonist. eLife 2021, 10, e65777. [Google Scholar] [CrossRef]
- Kang, B.Y.; Song, Y.J.; Kim, K.M.; Choe, Y.K.; Hwang, S.Y.; Kim, T.S. Curcumin inhibits Th1 cytokine profile in CD4 + T cells by suppressing interleukin-12 production in macrophages. Br. J. Pharmacol. 1999, 128, 380–384. [Google Scholar] [CrossRef]
- Bicalho, M.L.S.; Zinicola, M.; Machado, V.S.; Lima, F.S.; Teixeira, A.G.V.; Narbus, C.; Xavier, M.R.; Higgins, H.; Bicalho, R.C. Effects of recombinant bovine interleukin-8 (rbIL-8) treatment on health, metabolism, and lactation performance in Holstein cattle I: Production and functional characterization of rbIL-8 in vitro and in vivo. J. Dairy Sci. 2019, 102, 10304–10315. [Google Scholar] [CrossRef]
- Fiorenza, F.; dos Santos Amaral, C.; de Almeida da Anunciação, A.R.; Portela, V.V.M.; Marey, M.A.; Miyamoto, A.; Antoniazzi, A.Q. Possible impact of neutrophils on immune responses during early pregnancy in ruminants Mariani. Anim. Reprod. 2021, 18, e20210048. [Google Scholar] [CrossRef] [PubMed]
- Šerstņova, K.; Pilmane, M.; Vitenberga-Verza, Z.; Melderis, I.; Gontar, Ł.; Kochański, M.; Drutowska, A.; Maróti, G.; Prieto-Simón, B. Expression of anti-inflammatory markers IL-2, IL-10, TGF-β1, βDEF-2, βDEF-3 and Cathelicidin LL37 in dairy cattle milk with different health status of the udder. Pol. J. Vet. 2022, 25, 237–248. [Google Scholar]
- Rahman, A.N.A.; Balasubramaniam, V.R.M.T.; Yap, W.B. Potential of Interleukin (IL)-12 Group as Antivirals: Severe Viral Disease Prevention and Management. Int. J. Mol. Sci. 2023, 24, 7350. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.; Pyo, J.Y.; Ahn, S.S.; Song, J.J.; Park, Y.B.; Lee, S.W. Serum interleukin-16 significantly correlates with the Vasculitis Damage Index in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Res. Ther. 2020, 22, 73. [Google Scholar] [CrossRef]
- Elbana, A.M.; Elgamal, E.; Hashim, O.; Emran, T.M.; Alkhrsawy, A.A. Pro-inflammatory versus anti-inflammatory cytokines in psoriatic patients (case–control study). J. Cosmet. Dermatol. 2020, 21, 6302–6307. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Aggarwal, B. „Spicing Up” of the immune system by curcumin. J. Clin. Immunol. 2007, 27, 19–35. [Google Scholar] [CrossRef]
- Sun, L.; Hurez, V.J.; Thibodeaux, S.R.; Kious, M.J.; Liu, A.; Lin, P.; Murthy, K.; Pandeswara, S.; Shin, T.; Curiel, T.J. Aged regulatory T cells protect from autoimmune inflammation despite reduced STAT3 activation and decreased constraint of IL-17 producing T cells. Aging Cell 2012, 11, 509–519. [Google Scholar] [CrossRef]
- Guo, C.; Li, H.; Sun, D.; Liu, J.; Mao, S. Effects of abomasal supplementation of quercetin on performance, inflammatory cytokines, and matrix metalloproteinase genes expression in goats fed a high-grain diet. Livest. Sci. 2018, 209, 20–24. [Google Scholar] [CrossRef]
- Shah, T.; Malhi, M.; Kachiwal, A.B.; Bhutto, B.; Shah, Q.A.; Lei, Y.; Soomro, S.A.; Soomro, J.; Kalhoro, N.H.; Gui, H. Ameliorative effects of supranutritional selenium on TLR-4-NF-kB-TNF-α-mediated hepatic oxidative injury and inflammation in goats fed high concentrate diet. Food Sci. Nutr. 2022, 10, 3842–3854. [Google Scholar] [CrossRef]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Costa, R.A.; Crameri, R.; Duan, S.; Eiwegger, T.; Eljaszewicz, A.; Ferstl, R.; et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2016, 138, 984–1010. [Google Scholar] [CrossRef]
- Dong, H.; Wang, S.; Jia, Y.; Ni, Y.; Zhang, Y.; Zhuang, S.; Shen, X.; Zhao, R. Long-Term Effects of Subacute Ruminal Acidosis (SARA) on Milk Quality and Hepatic Gene Expression in Lactating Goats Fed aHigh-Concentrate Diet. PLoS ONE 2013, 8, e82850. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Zhang, K.; Xu, T.; Jin, D.; Seyfert, H.M.; Shen, X.; Zhuang, S. Feeding a high-grain diet reduces the percentage of LPS clearance and enhances immune gene expression in goat liver. BMC Vet. Res. 2015, 11, e82850. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. 2003, 3, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Yoshimoto, T.; Tsutsui, H.; Okamura, H. Interleukin-18 Regulates Both Th1 and Th2 Responses. Annu Rev. Immunol. 2001, 19, 423–471. [Google Scholar] [CrossRef]
- Harrison, O.J.; Srinivasan, N.; Pott, J.; Schiering, C.; Krausgruber, T.; Ilott, N.E.; Maloy, K.J. Epithelial-derived IL-18 regulates Th17 cell differentiation and Foxp3+ Treg cell function in the intestine. Mucosal Immunol. 2015, 8, 1226–1236. [Google Scholar] [CrossRef]
- Sanders, N.L.; Mishra, A. Role of interleukin-18 in the pathophysiology of allergic diseases. Cytokine Growth Factor Rev. 2016, 32, 31–39. [Google Scholar] [CrossRef]
- Horras, C.J.; Lamb, C.L.; Mitchell, K.A. Regulation of hepatocyte fate by interferon-γ. Cytokine Growth Factor Rev. 2011, 22, 35–43. [Google Scholar] [CrossRef]
- Vinicius, L.F.; Borba, H.H.L.; Bonetti, A.F.; Leonart, L.P.; Pontarolo, R. Cytokines and Interferons: Types and Functions. In Autoantibodies and Cytokines; Khan, W.A., Ed.; IntechOpen: London, UK, 2019; Chapter 4; pp. 65–87. [Google Scholar] [CrossRef]
- McKenna, O.E.; Asam, C.; Araujo, G.R.; Roulias, A.; Goulart, L.R.; Ferreira, F. How relevant is panallergen sensitization in the development of allergies? Pediatr. Allergy Immunol. 2016, 27, 560–568. [Google Scholar]
- Onyimba, F.; Crowe, S.E.; Johnson, S.; Leung, J. Food Allergies and Intolerances: A Clinical Approach to the Diagnosis and Management of Adverse Reactions to Food. Clin. Gastroenterol. Hepatol. 2021, 19, 2230–2240. [Google Scholar] [CrossRef]


| Group of Genes | Gene Name | Gene Symbol | Primer Sequence | Product Size (bp *) | GenBank ^/UniProt Accession | Reference | |
|---|---|---|---|---|---|---|---|
| References | Cyclophilin A | PPIA | F | GGATTTATGTGCCAGGGTGGTGA | 120 | AY_247029.1 | [22] |
| R | CAAGATGCCAGGACCTGTATG | ||||||
| Battenin | CLN3 | F | TTCTGACTCCTTGGGACACA | 62 | NM_001075174 | [22] | |
| R | CAACCTGCCCACCTATCAGT | ||||||
| Cytokines | Interleukin-1α | IL-1α | F | TGAACGACGCCCTCAATCAA | 366 | D63350.1 | [23] |
| R | CTTGCCATGTGCACCAGTTT | ||||||
| Interleukin-1β | IL-1β | F | GCAGCTGGAGGAAGTAGACC | 231 | D63351.1 | [23] | |
| R | TGGCTTTCTTTAGGGAGAGAGG | ||||||
| Interleukin-2 | IL-2 | F | ATGTACCAGATACCACTCTTGTCTT | 467 | AY603404.1 | [23] | |
| R | TCAAGTCATTGTTGAGTAGAT | ||||||
| Interleukin-4 | IL-4 | F | TAGCTTCTCCTGATAAACTA | 534 | U34273.1 | [23] | |
| R | ATGAGTTATAAATATATAAATA | ||||||
| Interleukin-6 | IL-6 | F | TCTTCACAAGCGCCTTCAGT | 120 | D86569.1 | [23] | |
| R | CTGCTTGGGGTGGTGTCATT | ||||||
| Interleukin-8 | IL-8 | F | TGAGAG TGGGCCACACTGC | 103 | JN559767.1 | [21] | |
| R | CACAACCTTCTGCACCCACTT | ||||||
| Interleukin-10 | IL-10 | F | CGGCGCTGTCATCGTTTT | 82 | DQ837159.1 | [23] | |
| R | TCTTGGAGCATATTGAAGACTCTCTTC | ||||||
| Interleukin-12 | IL-12 | F | CACCAAAGATAAAACCAGCACAGT | 125 | AY603407.1 | [23] | |
| R | GTCTTTCCAGAAGCCAGACAATG | ||||||
| Interleukin-16 | IL-16 | F | AAAAGACCTCTGCGGGACTG | 213 | AF481158.1 | [23] | |
| R | TCAGGCAACGCCTTGATGAT | ||||||
| Interleukin-18 | IL-18 | F | TCCTAAGAAGCTATTGAGCACAGGC | 619 | AY605263.1 | [23] | |
| R | ATTTTAATATCTAGTCTGGTTTTG | ||||||
| Interferon α | IFN-α | F | CACCTTCCAGCTCTTCAGCA | 96 | FJ959074.1 | [23] | |
| R | GTCAGTGAGCTGCTGATCCA | ||||||
| Interferon β | IFN-β | F | ACAGCAGTTCCGGAAGGAAG | 212 | JX458085.1 | [23] | |
| R | TCGGTCGTGTCTCCCATAGT | ||||||
| Interferon γ | IFN-γ | F | TAGCTAAGGGTGGGCCTCTTTTCTCA | 384 | AY603405.1 | [23] | |
| R | TGCAGGCAGGAGAACCATTACATTGA | ||||||
| Tumor necrosis factor α | TNF-α | F | AGAAGGGAGATCGCCTCAGT | 171 | X14828.1 | [23] | |
| R | AGAAGGGGATGAGGAGGGTC | ||||||
| Chemokine 4 | CCL4 | F | CAGCCGTGGTATTCCAGACC | 109 | XM_005693171.3 | [21] | |
| R | CTCGGAGCAGCTCAGTTCAGT | ||||||
| Gene Name | Gene Symbol | Immune Activity | References |
|---|---|---|---|
| Interleukin-1α | IL-1α | Proinflammatory | [29] |
| Interleukin-1β | IL-1β | Proinflammatory | [30] |
| Interleukin-2 | IL-2 | Pro- and anti-inflammatory | [31] |
| Interleukin-4 | IL-4 | Pro- and anti-inflammatory | [32] |
| Interleukin-6 | IL-6 | Proinflammatory | [30] |
| Interleukin-8 | IL-8 | Proinflammatory | [33] |
| Interleukin-10 | IL-10 | Anti-inflammatory | [30,34,35] |
| Interleukin-12 | IL-12 | Proinflammatory | [36] |
| Interleukin-16 | IL-16 | Pro- and anti-inflammatory | [37] |
| Interleukin-18 | IL-18 | Proinflammatory | [30] |
| Interferon α | IFN-α | Proinflammatory | [34] |
| Interferon β | IFN-β | Proinflammatory | [34] |
| Interferon γ | IFN-γ | Proinflammatory | [38] |
| Tumor necrosis factor α | TNF-α | Proinflammatory | [30] |
| Chemokine 4 | CCL4 | Proinflammatory | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbańska, D.M.; Pawlik, M.; Korwin-Kossakowska, A.; Rutkowska, K.; Kawecka-Grochocka, E.; Czopowicz, M.; Mickiewicz, M.; Kaba, J.; Bagnicka, E. The Expression of Selected Cytokine Genes in the Livers of Young Castrated Bucks after Supplementation with a Mixture of Dry Curcuma longa and Rosmarinus officinalis Extracts. Animals 2023, 13, 3489. https://doi.org/10.3390/ani13223489
Urbańska DM, Pawlik M, Korwin-Kossakowska A, Rutkowska K, Kawecka-Grochocka E, Czopowicz M, Mickiewicz M, Kaba J, Bagnicka E. The Expression of Selected Cytokine Genes in the Livers of Young Castrated Bucks after Supplementation with a Mixture of Dry Curcuma longa and Rosmarinus officinalis Extracts. Animals. 2023; 13(22):3489. https://doi.org/10.3390/ani13223489
Chicago/Turabian StyleUrbańska, Daria Maria, Marek Pawlik, Agnieszka Korwin-Kossakowska, Karolina Rutkowska, Ewelina Kawecka-Grochocka, Michał Czopowicz, Marcin Mickiewicz, Jarosław Kaba, and Emilia Bagnicka. 2023. "The Expression of Selected Cytokine Genes in the Livers of Young Castrated Bucks after Supplementation with a Mixture of Dry Curcuma longa and Rosmarinus officinalis Extracts" Animals 13, no. 22: 3489. https://doi.org/10.3390/ani13223489
APA StyleUrbańska, D. M., Pawlik, M., Korwin-Kossakowska, A., Rutkowska, K., Kawecka-Grochocka, E., Czopowicz, M., Mickiewicz, M., Kaba, J., & Bagnicka, E. (2023). The Expression of Selected Cytokine Genes in the Livers of Young Castrated Bucks after Supplementation with a Mixture of Dry Curcuma longa and Rosmarinus officinalis Extracts. Animals, 13(22), 3489. https://doi.org/10.3390/ani13223489





