Rhodotorula mucilaginosa ZTHY2 Attenuates Cyclophosphamide-Induced Immunosuppression in Mice
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Strain, Antibodies, and Reagents
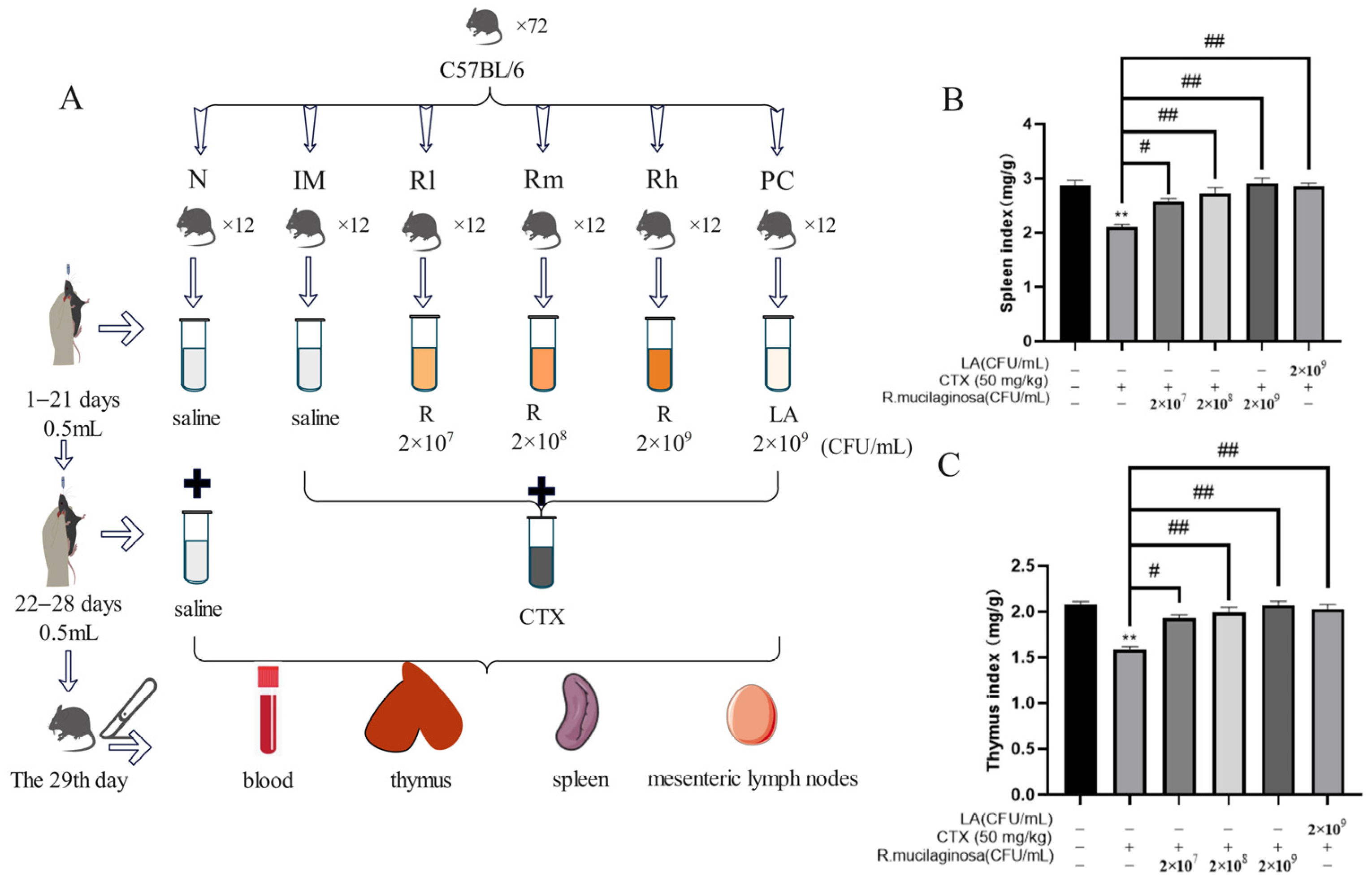
2.2. Animals, Experimental Design, and Management
2.3. Body Weight and Immunity Organ Indices
2.4. Determination of Hematological Indices
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Antioxidant Capacity Analysis
2.7. Single-Cell Suspension of Spleen, Thymus, and Mesenteric Lymph Nodes
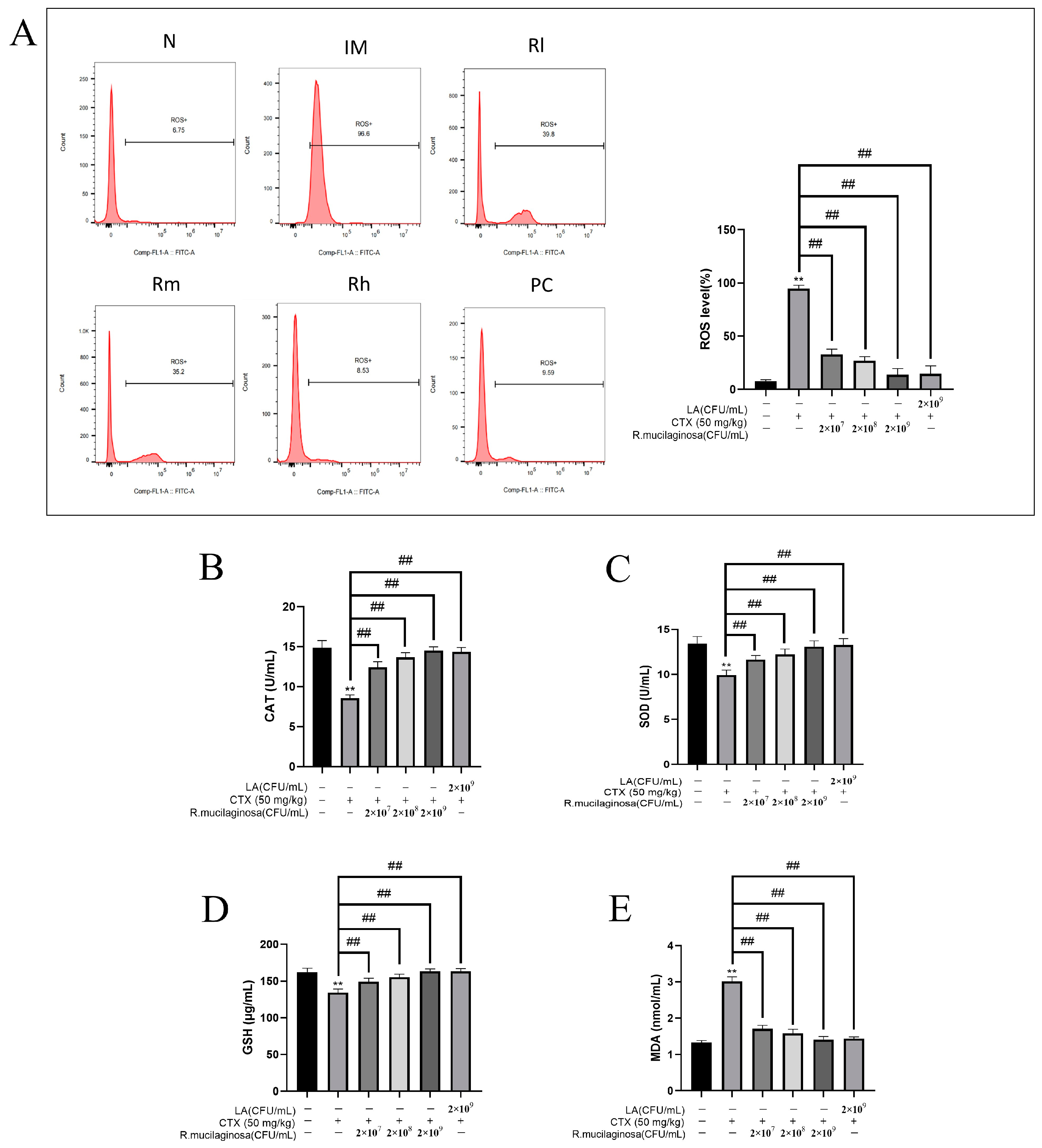
2.8. Intracellular Reactive Oxygen Species (ROS) of Spleen
2.9. Proliferation of T and B Lymphocytes in Spleen and Thymus In Vitro
2.10. Flow Cytometry Detection of T and B Cell Species of Spleen, Thymus, and Mesenteric Lymph Nodes
2.11. Statistical Analysis
3. Results
3.1. Effects of R. mucilaginosa ZTHY2 on Body Weight and Immune Organ Index in CTX-Induced Immunosuppressed Mice
3.2. Effect of R. mucilaginosa ZTHY2 on Hematological Indices in CTX-Induced Immunosuppressed Mice
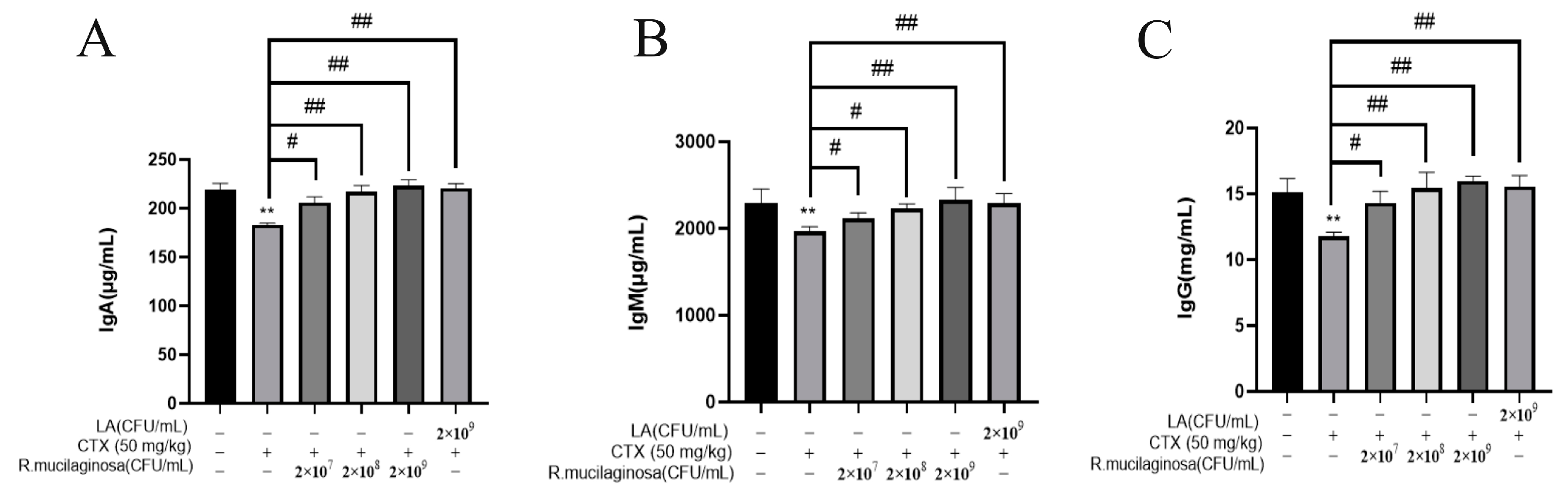
3.3. R. mucilaginosa ZTHY2 Increased the Level of Immunoglobulin in CTX-Induced Immunosuppressed Mice
3.4. R. mucilaginosa ZTHY2 Increased the Secretion of Immune Cytokines in CTX-Induced Immunosuppressed Mice
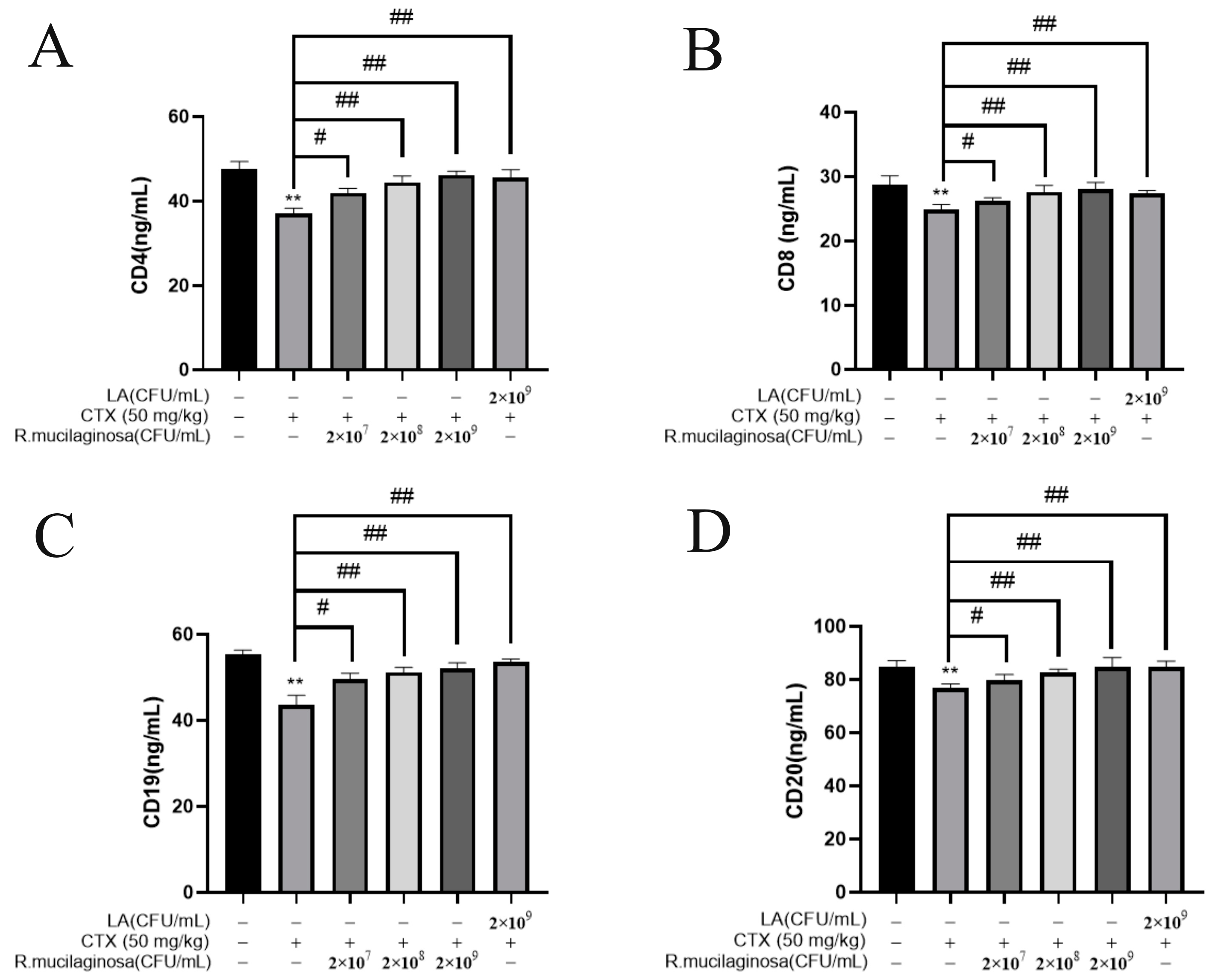
3.5. R. mucilaginosa ZTHY2 Increased the Levels of CDs in CTX-Induced Immunosuppressed Mice
3.6. R. mucilaginosa ZTHY2 Promoted the Proliferation of T and B Lymphocytes of Immunosuppressed Mice Induced by CTX In Vitro
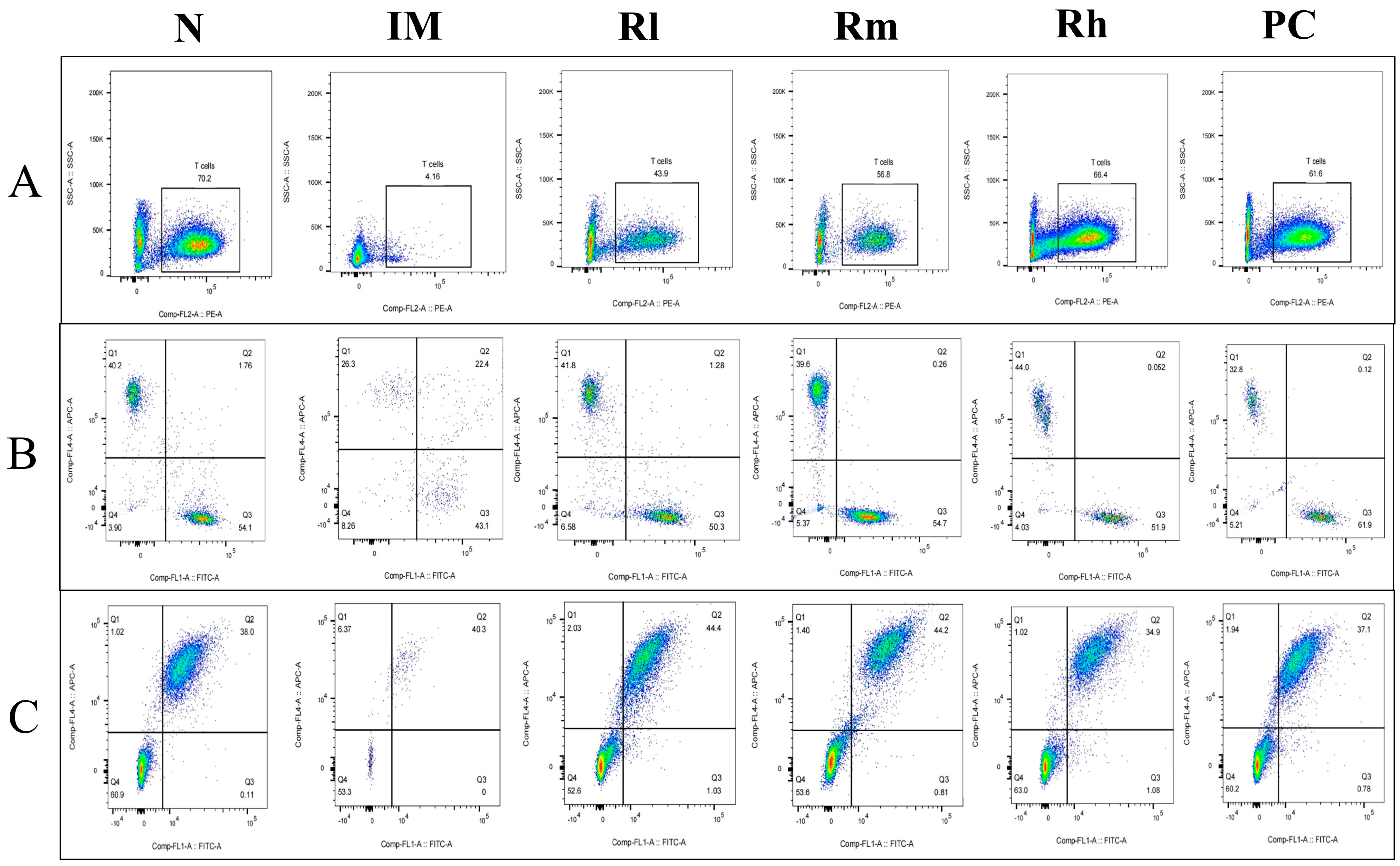
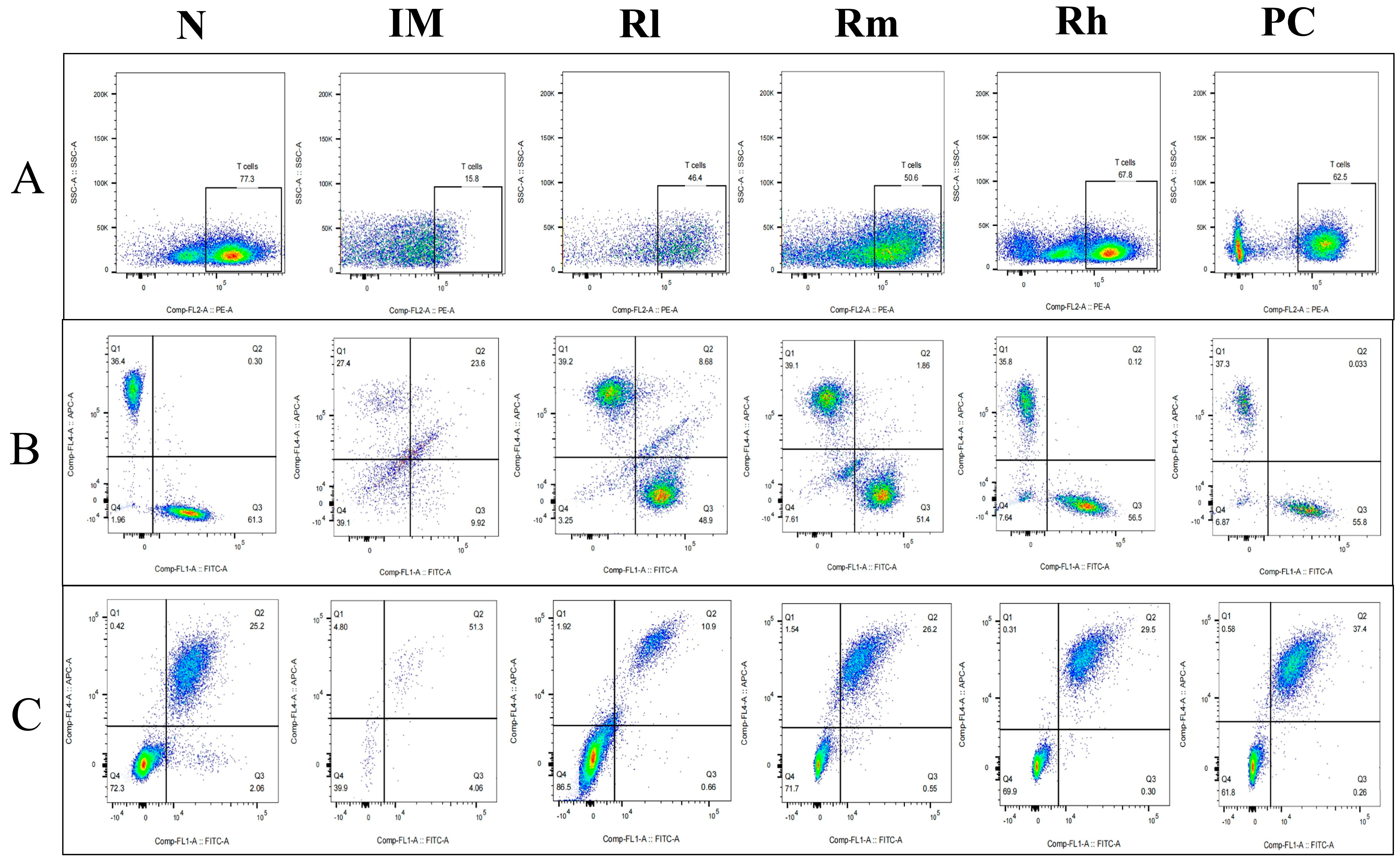
3.7. Effects of R. mucilaginosa ZTHY2 on T Cell and B Cell Subsets in CTX-Induced Immunosuppressed Mice
3.7.1. Effects of R. mucilaginosa ZTHY2 on T Cell and B Cell Subsets of Spleen in CTX-Induced Immunosuppressed Mice
3.7.2. Effects of R. mucilaginosa ZTHY2 on T Cell and B Cell Subsets of Thymus in CTX-Induced Immunosuppressed Mice
3.7.3. Effects of R. mucilaginosa ZTHY2 on T Cell and B Cell Subsets of Mesenteric Lymph Nodes in CTX-Induced Immunosuppressed Mice
3.8. Effects of R. mucilaginosa ZTHY2 on Antioxidant Capacity of Spleen in CTX-Induced Immunosuppressed Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tkacova, J.; Zara, G.; Ianiri, G.; Castoria, R.; Certik, M.; Mannazzu, I. Impairment of carotenoid biosynthesis through CAR1 gene mutation results in CoQ(10), sterols, and phytoene accumulation in Rhodotorula mucilaginosa. Appl. Microbiol. Biotechnol. 2022, 106, 317–327. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Cheng, P.; Yu, G. Rhodotorula mucilaginosa-alternative sources of natural carotenoids, lipids, and enzymes for industrial use. Heliyon 2022, 8, e11505. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, L.; Zhang, Y.; Yan, Y. Production, Characterization and Immunomodulatory Activity of an Extracellular Polysaccharide from Rhodotorula mucilaginosa YL-1 Isolated from Sea Salt Field. Mar. Drugs 2020, 18, 595. [Google Scholar] [CrossRef]
- Savini, V.; Sozio, F.; Catavitello, C.; Talia, M.; Manna, A.; Febbo, F.; Balbinot, A.; Di Bonaventura, G.; Piccolomini, R.; Parruti, G.; et al. Femoral prosthesis infection by Rhodotorula mucilaginosa. J. Clin. Microbiol. 2008, 46, 3544–3545. [Google Scholar] [CrossRef]
- Maeder, M.; Vogt, P.R.; Schaer, G.; von Graevenitz, A.; Gunthard, H.F. Aortic homograft endocarditis caused by Rhodotorula mucilaginosa. Infection 2003, 31, 181–183. [Google Scholar] [CrossRef]
- Fung, H.B.; Martyn, C.A.; Shahidi, A.; Brown, S.T. Rhodotorula mucilaginosa lymphadenitis in an HIV-infected patient. Int. J. Infect. Dis. 2009, 13, e27–e29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martini, K.; Muller, H.; Huemer, H.P.; Hopfl, R. Nail psoriasis masqueraded by secondary infection with Rhodotorula mucilaginosa. Mycoses 2013, 56, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Tligui, H.; Oudaina, W.; El, F.S.; Madda, F.; Hesseissen, L. [A skin ulcer infection due to Rhodotorula mucilaginosa in an immunocompromised child]. J. Mycol. Med. 2018, 28, 215–217. [Google Scholar] [CrossRef]
- Hu, P.; Mao, J.; Zeng, Y.; Sun, Z.; Deng, H.; Chen, C.; Sun, W.; Tang, Z. Isolation, Identification, and Function of Rhodotorula mucilaginosa TZR(2014) and Its Effects on the Growth and Health of Weaned Piglets. Front. Microbiol. 2022, 13, 922136. [Google Scholar]
- Chen, X.Q.; Zhao, W.; Xie, S.W.; Xie, J.J.; Zhang, Z.H.; Tian, L.X.; Liu, Y.J.; Niu, J. Effects of dietary hydrolyzed yeast (Rhodotorula mucilaginosa) on growth performance, immune response, antioxidant capacity and histomorphology of juvenile Nile tilapia (Oreochromis niloticus). Fish. Shellfish. Immunol. 2019, 90, 30–39. [Google Scholar] [CrossRef]
- Sun, J.; Li, M.; Tang, Z.; Zhang, X.; Chen, J.; Sun, Z. Effects of Rhodotorula mucilaginosa fermentation product on the laying performance, egg quality, jejunal mucosal morphology and intestinal microbiota of hens. J. Appl. Microbiol. 2020, 128, 54–64. [Google Scholar] [PubMed]
- Kot, A.M.; Blazejak, S.; Kurcz, A.; Gientka, I.; Kieliszek, M. Rhodotorula glutinis-potential source of lipids, carotenoids, and enzymes for use in industries. Appl. Microbiol. Biotechnol. 2016, 100, 6103–6117. [Google Scholar] [PubMed]
- Kewcharoen, W.; Srisapoome, P. Probiotic effects of Bacillus spp. from Pacific white shrimp (Litopenaeus vannamei) on water quality and shrimp growth, immune responses, and resistance to Vibrio parahaemolyticus (AHPND strains). Fish. Shellfish. Immunol. 2019, 94, 175–189. [Google Scholar] [PubMed]
- Sattler, S. The Role of the Immune System Beyond the Fight Against Infection. Adv. Exp. Med. Biol. 2017, 1003, 3–14. [Google Scholar]
- McNeela, E.A.; Mills, K.H. Manipulating the immune system: Humoral versus cell-mediated immunity. Adv. Drug Deliv. Rev. 2001, 51, 43–54. [Google Scholar]
- Hogan, B.V.; Peter, M.B.; Shenoy, H.G.; Horgan, K.; Hughes, T.A. Surgery induced immunosuppression. Surgeon 2011, 9, 38–43. [Google Scholar]
- Ward, P.A. Immunosuppression after trauma. Crit. Care Med. 2005, 33, 1453–1454. [Google Scholar] [CrossRef]
- Chen, W.; Chen, S.; Nie, Y.; Li, W.; Li, H.; Zhang, X.; Chen, F.; Xie, Q. Synergistic Immunosuppression of Avian Leukosis Virus Subgroup J and Infectious Bursal Disease Virus Is Responsible for Enhanced Pathogenicity. Viruses 2022, 14, 2312. [Google Scholar] [CrossRef]
- You, L.; Nepovimova, E.; Valko, M.; Wu, Q.; Kuca, K. Mycotoxins and cellular senescence: The impact of oxidative stress, hypoxia, and immunosuppression. Arch. Toxicol. 2023, 97, 393–404. [Google Scholar]
- Depmeier, C.; Gunthard, H.F.; Steiner, U.C. Infektionen unter Immunsuppression [Infections during Immunosuppression]. Praxis 2018, 107, 689–698. [Google Scholar] [CrossRef]
- Amadori, M.; Zanotti, C. Immunoprophylaxis in intensive farming systems: The way forward. Vet. Immunol. Immunopathol. 2016, 181, 2–9. [Google Scholar] [PubMed]
- Li, S.; Han, M.; Zhang, Y.; Ishfaq, M.; Liu, R.; Wei, G.; Zhang, X.; Zhang, X. Effect of Curcumin as Feed Supplement on Immune Response and Pathological Changes of Broilers Exposed to Aflatoxin B1. Biomolecules 2022, 12, 1188. [Google Scholar]
- El-Shall, N.A.; Jiang, S.; Farag, M.R.; Azzam, M.; Al-Abdullatif, A.A.; Alhotan, R.; Dhama, K.; Hassan, F.U.; Alagawany, M. Potential of Spirulina platensis as a feed supplement for poultry to enhance growth performance and immune modulation. Front. Immunol. 2023, 14, 1072787. [Google Scholar] [PubMed]
- Hoseinifar, S.H.; Roosta, Z.; Hajimoradloo, A.; Vakili, F. The effects of Lactobacillus acidophilus as feed supplement on skin mucosal immune parameters, intestinal microbiota, stress resistance and growth performance of black swordtail (Xiphophorus helleri). Fish. Shellfish. Immunol. 2015, 42, 533–538. [Google Scholar]
- Guarnera, L.; Meconi, F.; Secchi, R.; Pascale, M.R.; Esposito, F.; Zizzari, A.; Rapisarda, V.M.; Rizzo, M.; Pupo, L.; Cantonetti, M. Efficacy and Safety of Cyclophosphamide Low-Dose Pre-Phase Chemotherapy in Diffuse Large B Cell Lymphoma with Gastrointestinal Involvement. Mediterr. J. Hematol. Infect. Dis. 2022, 14, e2022017. [Google Scholar] [CrossRef]
- Zhong, S.; Huang, M.; Yang, X.; Liang, L.; Wang, Y.; Romkes, M.; Duan, W.; Chan, E.; Zhou, S.F. Relationship of glutathione S-transferase genotypes with side-effects of pulsed cyclophosphamide therapy in patients with systemic lupus erythematosus. Br. J. Clin. Pharmacol. 2006, 62, 457–472. [Google Scholar]
- Childress, M.O.; Christian, J.A.; Ramos-Vara, J.A.; Rosen, N.K.; Ruple, A. Greater baseline serum C-reactive protein concentrations are associated with reduced survival in dogs receiving cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy for primary nodal diffuse large B-cell lymphoma. Vet. J. 2022, 289, 105911. [Google Scholar] [CrossRef]
- Machado, M.C.; Yamamoto, P.A.; Pippa, L.F.; de Moraes, N.V.; Neves, F.; Portela, R.D.; Barrouin-Melo, S.M.; Hielm-Bjorkman, A.; Godoy, A.; Estrela-Lima, A. Pharmacokinetics of Carboplatin in Combination with Low-Dose Cyclophosphamide in Female Dogs with Mammary Carcinoma. Animals 2022, 12, 3109. [Google Scholar]
- O’Connell, K.; Thomson, M.; Morgan, E.; Henning, J. Procarbazine, prednisolone and cyclophosphamide oral combination chemotherapy protocol for canine lymphoma. Vet. Comp. Oncol. 2022, 20, 613–622. [Google Scholar] [CrossRef]
- Todd, J.; Thomas, P.; Nguyen, S. Cyclophosphamide and prednisolone for chemotherapy naive B cell multicentric lymphoma in dogs: 32 cases (2017–2021). J. Small Anim. Pract. 2022, 63, 52–55. [Google Scholar] [CrossRef]
- Sunpongsri, S.; Kovitvadhi, A.; Rattanasrisomporn, J.; Trisaksri, V.; Jensirisak, N.; Jaroensong, T. Effectiveness and Adverse Events of Cyclophosphamide, Vincristine, and Prednisolone Chemotherapy in Feline Mediastinal Lymphoma Naturally Infected with Feline Leukemia Virus. Animals 2022, 12, 900. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, R.V. Assessment of Efficacy and Toxicity of Cyclophosphamide Chemotherapy in Canines with Malignant Mammary Tumor: A Retrospective Study. Vet. Med. Int. 2021, 2021, 5520603. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Wouda, R.M.; Borrego, J.; Chon, E. Cyclophosphamide rescue therapy for relapsed low-grade alimentary lymphoma after chlorambucil treatment in cats. J. Feline Med. Surg. 2021, 23, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Khordad, E.; Alipour, F.; Pourabbas, M.; Mansouri, S.; Salimnejad, R. Hepatoprotective Impact of Ghrelin against Cyclophosphamide-Induced Toxicity in the Male Mice. Drug Res. 2021, 71, 407–412. [Google Scholar] [CrossRef]
- Tang, F.; Zhong, Q.; Yang, Z.; Li, H.; Pan, C.; Huang, L.; Ni, T.; Deng, R.; Wang, Z.; Tan, S.; et al. Low-dose cyclophosphamide combined with IL-2 inhibits tumor growth by decreasing regulatory T cells and increasing CD8+ T cells and natural killer cells in mice. Immunobiology 2022, 227, 152212. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.I.; Rehman, A.U.; Farooqui, N.A.; Siddiqui, N.Z.; Ayub, Q.; Ramzan, M.N.; Zexu, W.; Zhang, X.; Yu, Y.; Xin, Y.; et al. Shrimp peptide hydrolysate modulates the immune response in cyclophosphamide immunosuppressed mice model. J. Food Biochem. 2022, 46, e14251. [Google Scholar] [CrossRef]
- Ali, M.S.; Lee, E.B.; Quah, Y.; Birhanu, B.T.; Suk, K.; Lim, S.K.; Park, S.C. Heat-killed Limosilactobacillus reuteri PSC102 Ameliorates Impaired Immunity in Cyclophosphamide-induced Immunosuppressed Mice. Front. Microbiol. 2022, 13, 820838. [Google Scholar] [CrossRef]
- Baharmi, S.; Kalantari, H.; Kalantar, M.; Goudarzi, M.; Mansouri, E.; Kalantar, H. Pretreatment with Gallic Acid Mitigates Cyclophosphamide Induced Inflammation and Oxidative Stress in Mice. Curr. Mol. Pharmacol. 2022, 15, 204–212. [Google Scholar]
- Shan, C.; Miao, F. Immunomodulatory and antioxidant effects of hydroxytyrosol in cyclophosphamide-induced immunosuppressed broilers. Poult. Sci. 2022, 101, 101516. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Y.; Xie, W.; Xu, C. Isolation and identification of marine Rhodotorula in a coastal area near Leizhou Peninsula. J. Trop. Oceanogr. 2017, 36, 87–92. [Google Scholar]
- Huang, K.S.; Liao, Z.Y.; Xu, C.H.; Xie, W.T. Extraction, Separation and Purification Methods of Astaxanthin Pure Product from Rhodotorula mucilaginosa. Nat. Prod. Res. Dev. 2018, 30, 1858–1862, 1877. [Google Scholar]
- Ge, Y.; Huang, K.; Xie, W.; Xu, C.; Yao, Q.; Liu, Y. Effects of Rhodotorula mucilaginosa on the Immune Function and Gut Microbiota of Mice. Front. Fungal Biol. 2021, 2, 705696. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Tu, H.; Yin, X.; Peng, C.; Dou, C.; Yang, W.; Wu, W.; Guan, X.; Li, J.; Yan, H.; et al. Targeting Glutamine Metabolism Ameliorates Autoimmune Hepatitis via Inhibiting T Cell Activation and Differentiation. Front. Immunol. 2022, 13, 880262. [Google Scholar] [CrossRef]
- Adori, M.; Khoenkhoen, S.; Zhang, J.; Dopico, X.C.; Karlsson, H.G. Enhanced B Cell Receptor Signaling Partially Compensates for Impaired Toll-like Receptor 4 Responses in LPS-Stimulated IkappaBNS-Deficient B Cells. Cells 2023, 12, 1229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Jiang, S.; Tang, Y.; Ding, G. Effects of Low Molecular Weight Peptides from Red Shrimp (Solenocera crassicornis) Head on Immune Response in Immunosuppressed Mice. Int. J. Mol. Sci. 2023, 24, 10297. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, B.; Sun, X.; Zhang, M.; Xiao, D.; Lin, S.; Liu, Z.; Cui, W.; Lin, Y. Tetrahedral-Framework Nucleic Acid Loaded with MicroRNA-155 Enhances Immunocompetence in Cyclophosphamide-Induced Immunosuppressed Mice by Modulating Dendritic Cells and Macrophages. ACS Appl. Mater. Interfaces 2023, 15, 7793–7803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, S.; Li, H.; Cao, M.; Li, W.; Liu, X. Immunomodulatory effects of selenium-enriched peptides from soybean in cyclophosphamide-induced immunosuppressed mice. Food Sci. Nutr. 2021, 9, 6322–6334. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Li, C.; Shao, Y.; Chen, A. Polysaccharides from Spores of Cordyceps cicadae Protect against Cyclophosphamide-Induced Immunosuppression and Oxidative Stress in Mice. Foods 2022, 11, 515. [Google Scholar] [CrossRef]
- Gao, X.; Zeng, R.; Qi, J.; Ho, C.T.; Li, B.; Chen, Z.; Chen, S.; Xiao, C.; Hu, H.; Cai, M.; et al. Immunoregulatory activity of a low-molecular-weight heteropolysaccharide from Ganoderma leucocontextum fruiting bodies in vitro and in vivo. Food Chem. X 2022, 14, 100321. [Google Scholar] [CrossRef]
- Perdigon, G.; de Macias, M.; Alvarez, S.; Medici, M.; Oliver, G.; de Ruiz, H.A. Effect of a Mixture of Lactobacillus casei and Lactobacillus acidophilus Administered Orally on the Immune System in Mice. J. Food Prot. 1986, 49, 986–989. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Yan, H.; Ning, Z.; Wang, Z. Lactobacillus salivarius SNK-6 Activates Intestinal Mucosal Immune System by Regulating Cecal Microbial Community Structure in Laying Hens. Microorganisms 2022, 10, 1469. [Google Scholar] [CrossRef] [PubMed]
- Vakili, F.; Roosta, Z.; Safari, R.; Raeisi, M.; Hossain, M.S.; Guerreiro, I.; Akbarzadeh, A.; Hoseinifar, S.H. Effects of dietary nutmeg (Myristica fragrans) seed meals on growth, non-specific immune indices, antioxidant status, gene expression analysis, and cold stress tolerance in zebrafish (Danio rerio). Front. Nutr. 2022, 9, 1038748. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Astill, J.; Alqazlan, N.; Shojadoost, B.; Taha-Abdelaziz, K.; Bavananthasivam, J.; Doost, J.S.; Sedeghiisfahani, N.; Sharif, S. In ovo co-administration of vitamins (A and D) and probiotic lactobacilli modulates immune responses in broiler chickens. Poult. Sci. 2022, 101, 101717. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Nie, C.; Wu, Y.; Luo, R.; Chen, C.; Niu, J.; Zhang, W. Effects of two strains of Lactobacillus isolated from the feces of calves after fecal microbiota transplantation on growth performance, immune capacity, and intestinal barrier function of weaned calves. Front. Microbiol. 2023, 14, 1249628. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.E.; Joh, E.H.; Lee, H.Y.; Ahn, Y.T.; Lee, J.H.; Huh, C.S.; Han, M.J.; Kim, D.H. Lactobacillus plantarum HY7712 ameliorates cyclophosphamide-induced immunosuppression in mice. J. Microbiol. Biotechnol. 2013, 23, 414–421. [Google Scholar] [CrossRef]
- Jang, S.E.; Joh, E.H.; Ahn, Y.T.; Huh, C.S.; Han, M.J.; Kim, D.H. Lactobacillus casei HY7213 ameliorates cyclophosphamide-induced immunosuppression in mice by activating NK, cytotoxic T cells and macrophages. Immunopharmacol. Immunotoxicol. 2013, 35, 396–402. [Google Scholar] [CrossRef]
- Xie, J.H.; Fan, S.T.; Nie, S.P.; Yu, Q.; Xiong, T.; Gong, D.; Xie, M.Y. Lactobacillus plantarum NCU116 attenuates cyclophosphamide-induced intestinal mucosal injury, metabolism and intestinal microbiota disorders in mice. Food Funct. 2016, 7, 1584–1592. [Google Scholar] [CrossRef]
- Xie, J.; Nie, S.; Yu, Q.; Yin, J.; Xiong, T.; Gong, D.; Xie, M. Lactobacillus plantarum NCU116 Attenuates Cyclophosphamide-Induced Immunosuppression and Regulates Th17/Treg Cell Immune Responses in Mice. J. Agric. Food Chem. 2016, 64, 1291–1297. [Google Scholar] [CrossRef]
- Aguilera, N.S.; Auerbach, A. T-cell lymphoproliferative processes in the spleen. Semin. Diagn. Pathol. 2020, 37, 47–56. [Google Scholar] [CrossRef]
- Kim, Y.O.; Han, S.B.; Lee, H.W.; Ahn, H.J.; Yoon, Y.D.; Jung, J.K.; Kim, H.M.; Shin, C.S. Immuno-stimulating effect of the endo-polysaccharide produced by submerged culture of Inonotus obliquus. Life Sci. 2005, 77, 2438–2456. [Google Scholar] [CrossRef]
- Zhou, R.; He, D.; Xie, J.; Zhou, Q.; Zeng, H.; Li, H.; Huang, L. The Synergistic Effects of Polysaccharides and Ginsenosides From American Ginseng (Panax quinquefolius L.) Ameliorating Cyclophosphamide-Induced Intestinal Immune Disorders and Gut Barrier Dysfunctions Based on Microbiome-Metabolomics Analysis. Front. Immunol. 2021, 12, 665901. [Google Scholar] [CrossRef]
- Zheng, S.; Zheng, H.; Zhang, R.; Piao, X.; Hu, J.; Zhu, Y.; Wang, Y. Immunomodulatory Effect of Ginsenoside Rb2 Against Cyclophosphamide-Induced Immunosuppression in Mice. Front. Pharmacol. 2022, 13, 927087. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah-Hassan, A.; Shalaby, S.I.; Khater, S.I.; El-Shetry, E.S.; Abd, E.F.H.; Elsayed, S.A. Panax ginseng is superior to vitamin E as a hepatoprotector against cyclophosphamide-induced liver damage. Complement. Ther. Med. 2019, 46, 95–102. [Google Scholar] [CrossRef]
- Cancro, M.P.; Tomayko, M.M. Memory B cells and plasma cells: The differentiative continuum of humoral immunity. Immunol. Rev. 2021, 303, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Steinert, E.M.; Vasan, K.; Chandel, N.S. Mitochondrial Metabolism Regulation of T Cell-Mediated Immunity. Annu. Rev. Immunol. 2021, 39, 395–416. [Google Scholar] [CrossRef] [PubMed]
- Rocamora-Reverte, L.; Melzer, F.L.; Wurzner, R.; Weinberger, B. The Complex Role of Regulatory T Cells in Immunity and Aging. Front. Immunol. 2020, 11, 616949. [Google Scholar] [CrossRef]
- Frank, A.M.; Braun, A.H.; Scheib, L.; Agarwal, S.; Schneider, I.C.; Fusil, F.; Perian, S.; Sahin, U.; Thalheimer, F.B.; Verhoeyen, E.; et al. Combining T-cell-specific activation and in vivo gene delivery through CD3-targeted lentiviral vectors. Blood Adv. 2020, 4, 5702–5715. [Google Scholar]
- Younes, A.K.; Hammad, R.; Othman, M.; Sobhy, A. CD4, CD8 and natural killer cells are depressed in patients with alopecia areata: Their association with disease activity. BMC Immunol. 2022, 23, 13. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; Huang, M. Metabolic Regulation of CD8(+) T Cells: From Mechanism to Therapy. Antioxid. Redox Signal 2022, 37, 1234–1253. [Google Scholar] [CrossRef]
- Malagnino, V.; Cerva, C.; Teti, E.; Campogiani, L.; Compagno, M.; Foroghi, B.L.; Saderi, L.; Armenia, D.; Salpini, R.; Svicher, V.; et al. Poor CD4/CD8 ratio recovery in HBcAb-positive HIV patients with worse immune status is associated with significantly higher CD8 cell numbers. Sci. Rep. 2021, 11, 3965. [Google Scholar] [CrossRef]
- Perez-Mazliah, D.; Langhorne, J. CD4 T-cell subsets in malaria: TH1/TH2 revisited. Front. Immunol. 2014, 5, 671. [Google Scholar] [CrossRef]
- Tian, H.; Xing, J.; Tang, X.; Sheng, X.; Chi, H.; Zhan, W. Interactions of interleukin 2 (IL-2) and IL-2 receptors mediate the activities of B lymphocytes in flounder (Paralichthys olivaceus). Int. J. Biol. Macromol. 2023, 227, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, N.; Ravanshad, M.; Asadi, B.; Kianfar, R.; Maleki, A. Investigation of IL-2 and IFN-gamma to EBV Peptides in Stimulated Whole Blood among Multiple Sclerosis Patients and Healthy Individuals. Intervirology 2021, 64, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Salomon, B.L.; Leclerc, M.; Tosello, J.; Ronin, E.; Piaggio, E.; Cohen, J.L. Tumor Necrosis Factor alpha and Regulatory T Cells in Oncoimmunology. Front. Immunol. 2018, 9, 444. [Google Scholar] [CrossRef] [PubMed]
- Czimmerer, Z.; Halasz, L.; Daniel, B.; Varga, Z.; Bene, K.; Domokos, A.; Hoeksema, M.; Shen, Z.; Berger, W.K.; Cseh, T.; et al. The epigenetic state of IL-4-polarized macrophages enables inflammatory cistromic expansion and extended synergistic response to TLR ligands. Immunity 2022, 55, 2006–2026. [Google Scholar] [CrossRef]
- Qing, H.; Desrouleaux, R.; Israni-Winger, K.; Mineur, Y.S.; Fogelman, N.; Zhang, C.; Rashed, S.; Palm, N.W.; Sinha, R.; Picciotto, M.R.; et al. Origin and Function of Stress-Induced IL-6 in Murine Models. Cell 2020, 182, 372–387. [Google Scholar] [CrossRef]
- Yan, H.; Lu, J.; Wang, J.; Chen, L.; Wang, Y.; Li, L.; Miao, L.; Zhang, H. Prevention of Cyclophosphamide-Induced Immunosuppression in Mice With Traditional Chinese Medicine Xuanfei Baidu Decoction. Front. Pharmacol. 2021, 12, 730567. [Google Scholar] [CrossRef]
- Wang, L.; Bhardwaj, R.; Mostowski, H.; Patrone, P.N.; Kearsley, A.J.; Watson, J.; Lim, L.; Pichaandi, J.; Ornatsky, O.; Majonis, D.; et al. Establishing CD19 B-cell reference control materials for comparable and quantitative cytometric expression analysis. PLoS ONE 2021, 16, e248118. [Google Scholar] [CrossRef]
- Payandeh, Z.; Bahrami, A.A.; Hoseinpoor, R.; Mortazavi, Y.; Rajabibazl, M.; Rahimpour, A.; Taromchi, A.H.; Khalil, S. The applications of anti-CD20 antibodies to treat various B cells disorders. Biomed. Pharmacother. 2019, 109, 2415–2426. [Google Scholar] [CrossRef]
- Werner, A.; Nimmerjahn, F. HINGEneering IgG for enhanced immune activation. Sci. Immunol. 2022, 7, q4797. [Google Scholar] [CrossRef]
- Wang, J.; Wu, C.S.; Hu, Y.Z.; Yang, L.; Zhang, X.J.; Zhang, Y.A. Plasmablasts induced by chitosan oligosaccharide secrete natural IgM to enhance the humoral immunity in grass carp. Carbohydr. Polym. 2022, 281, 119073. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Z.; Wang, C.; Li, E.; Qin, J.G.; Chen, L. Dietary supplementation of selenium yeast enhances the antioxidant capacity and immune response of juvenile Eriocheir sinensis under nitrite stress. Fish. Shellfish. Immunol. 2019, 87, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, Y.; Luo, K.; Zhang, S.; Wei, C.; Wang, L.; Qiu, Y.; Tian, X. ‘Biotic’ potential of the red yeast Rhodotorula mucilaginosa strain JM-01 on the growth, shell pigmentation, and immune defense attributes of the shrimp, Penaeus vannamei. Aquaculture 2023, 572, 739543. [Google Scholar] [CrossRef]
- Marefati, N.; Ghorani, V.; Shakeri, F.; Boskabady, M.; Kianian, F.; Rezaee, R.; Boskabady, M.H. A review of anti-inflammatory, antioxidant, and immunomodulatory effects of Allium cepa and its main constituents. Pharm. Biol. 2021, 59, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, Z.Z.; Cheng, Y.L.; Lin, W.; Qu, C. Resveratrol protects against oxidative damage of retinal pigment epithelium cells by modulating SOD/MDA activity and activating Bcl-2 expression. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 378–388. [Google Scholar]
- Li, Z.; Chen, M.; Wang, W.; Liu, Q.; Li, N.; He, B.; Jiang, Y.; Ma, J. Mn-SOD alleviates methotrexate-related hepatocellular injury via GSK-3beta affecting anti-oxidative stress of HO-1 and Drp1. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2022, 47, 1191–1199. [Google Scholar] [PubMed]
- Kwon, K.; Jung, J.; Sahu, A.; Tae, G. Nanoreactor for cascade reaction between SOD and CAT and its tissue regeneration effect. J. Control. Release 2022, 344, 160–172. [Google Scholar] [CrossRef]
- Jiang, F.; Xu, X.R.; Li, W.M.; Xia, K.; Wang, L.F.; Yang, X.C. Monotropein alleviates H2O2-induced inflammation, oxidative stress and apoptosis via NF-kappaB/AP-1 signaling. Mol. Med. Rep. 2020, 22, 4828–4836. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Xue, Y.; Fang, H.; Wu, Z. Serum levels of reduced glutathione, oxidized glutathione, and glutathione reductase activity in minor recurrent aphthous stomatitis patients. J. Dent. Sci. 2023, 18, 1103–1108. [Google Scholar] [CrossRef]



| Group | Day 0 (g) | Day 22 (g) | Day 29 (g) |
|---|---|---|---|
| N | 21.68 ± 0.74 a | 22.91 ± 0.48 a | 23.48 ± 0.47 Aa |
| IM | 21.00 ± 0.65 a | 22.83 ± 0.37 a | 21.66 ± 0.48 Bc |
| Rl | 20.58 ± 0.45 a | 22.73 ± 0.65 a | 22.70 ± 0.59 ABb |
| Rm | 21.03 ± 0.92 a | 22.75 ± 0.58 a | 22.90 ± 0.54 ABb |
| Rh | 21.33 ± 0.45 a | 22.90 ± 0.58 a | 23.68 ± 0.58 Aa |
| PC | 20.78 ± 0.59 a | 22.61 ± 0.81 a | 22.98 ± 0.81 ABb |
| Group | WBCs (109/L) | LYMs (%) | MONs (%) | NEUs (%) | EOSs (%) |
|---|---|---|---|---|---|
| N | 2.72 ± 0.93 Aa | 79.12 ± 1.81 Aa | 21.80 ± 9.10 Aa | 23.67 ± 1.78 ABa | 0.81 ± 0.14 |
| IM | 0.66 ± 0.03 Cc | 61.76 ± 0.84 Cc | 10.47 ± 4.85 Cc | 16.59 ± 10.48 Bb | 0.68 ± 0.10 |
| Rl | 1.21 ± 0.55 BCb | 68.91 ± 3.89 BCb | 16.64 ± 12.87 BCb | 20.25 ± 1.94 Aab | 0.72 ± 0.10 |
| Rm | 1.34 ± 0.40 BCb | 76.96 ± 1.72 ABb | 18.33 ± 6.87 Bb | 21.96 ± 7.52 Aab | 0.75 ± 0.04 |
| Rh | 1.81 ± 0.02 Bb | 78.74 ± 2.09 Aa | 20.25 ± 6.13 Aab | 23.24 ± 0.38 ABa | 0.78 ± 0.04 |
| PC | 1.87 ± 0.29 Bb | 77.24 ± 6.68 ABa | 21.21 ± 8.96 Aa | 22.41 ± 1.05 ABab | 0.81 ± 0.10 |
| Group | T Lymph Subpopulations | B Lymph Subpopulations | ||||
|---|---|---|---|---|---|---|
| CD3+ (%) | CD4+ (%) | CD8+ (%) | CD4+/CD8+ | CD19+ (%) | CD20+ (%) | |
| N | 56.63 ± 7.75 Aa | 36.33 ± 2.03 Aa | 35.91 ± 6.37 Aa | 3.26 ± 2.73 Aa | 43.54 ± 4.01 Aa | 36.11 ± 3.97 Aa |
| IM | 8.24 ± 8.66 Cc | 8.17 ± 4.71 Bb | 6.71 ± 2.90 Cc | 0.98 ± 3.45 Cc | 26.82 ± 5.42 Cc | 21.55 ± 4.17 Bc |
| Rl | 49.35 ± 5.77 Bb | 27.5 ± 2.32 Aa | 25.98 ± 3.72 Bb | 1.07 ± 4.21 Cc | 33.02 ± 4.53 BCb | 25.80 ± 3.68 Bb |
| Rm | 52.02 ± 4.63 ABab | 34.45 ± 2.53 Aa | 27.42 ± 3.26 Bb | 1.64 ± 3.69 ABab | 35.03 ± 4.68 Bb | 27.90 ± 5.98 Ab |
| Rh | 55.28 ± 2.84 ABa | 35.35 ± 1.93 Aa | 31.33 ± 5.16 Bb | 2.30 ± 5.40 ABab | 37.56 ± 5.62 Bb | 31.57 ± 4.34 Aab |
| PC | 53.52 ± 5.35 ABab | 34.17 ± 3.04 Aa | 30.96 ± 4.72 Bb | 2.49 ± 4.87 ABab | 38.9 ± 5.61 Bb | 31.88 ± 3.94 Aab |
| Group | T Lymph Subpopulations | B Lymph Subpopulations | ||||
|---|---|---|---|---|---|---|
| CD3+ (%) | CD4+ (%) | CD8+ (%) | CD4+/CD8+ | CD19+ (%) | CD20+ (%) | |
| N | 52.63 ± 2.75 Aa | 38.84 ± 12.03 Aa | 27.59 ± 2.41 Aa | 2.25 ± 4.813 Aa | 29.13 ± 1.64 Aa | 26.96 ± 4.42 Aa |
| IM | 10.24 ± 4.26 Cc | 11.81 ± 4.71 Cc | 9.01 ± 5.32 Cc | 0.88 ± 2.41 Cc | 12.82 ± 3.42 Cc | 10.99 ± 1.73 Cc |
| Rl | 41.35 ± 5.24 Bb | 30.28 ± 3.02 Ba | 17.98 ± 6.42 Bbc | 0.91 ± 5.31 Cc | 20.16 ± 3.28 Bb | 22.49 ± 1.92 Bb |
| Rm | 43.02 ± 6.73 Bab | 33.456 ± 6.57 Aab | 20.42 ± 5.12 Bb | 1.89 ± 3.83 ABab | 22.39 ± 3.31 Bb | 23.72 ± 2.14 Bb |
| Rh | 49.88 ± 4.04 ABa | 36.35 ± 4.33 Aa | 25.52 ± 4.53 Bb | 2.01 ± 2.57 ABab | 26.71 ± 2.27 ABa | 25.59 ± 3.71 Bb |
| PC | 49.52 ± 2.11 ABab | 36.17 ± 3.54 Aa | 25.95 ± 3.72 Bb | 2.04 ± 2.91 ABab | 26.09 ± 3.016 ABa | 25.88 ± 2.14 Bb |
| Group | T Lymph Subpopulations | B Lymph Subpopulations | ||||
|---|---|---|---|---|---|---|
| CD3+ (%) | CD4+ (%) | CD8+ (%) | CD4+/CD8+ | CD19+ (%) | CD20+ (%) | |
| N | 48.31 ± 2.51 Aa | 27.02 ± 0.21 Aa | 25.91 ± 6.37 Aa | 4.03 ± 0.63 Aa | 30.71 ± 1.41 Aa | 33.04 ± 1.77 Aa |
| IM | 9.61 ± 2.83 Cc | 8.91 ± 1.21 Cc | 9.21 ± 1.04 Cc | 1.08 ± 0.94 Cc | 14.92 ± 1.21 Cc | 15.05 ± 0.97 Cc |
| Rl | 41.21 ± 1.41 Bb | 22.69 ± 1.22 ABa | 16.15 ± 1.72 Bb | 2.16 ± 1.17 bc | 21.97 ± 0.95 BCb | 26.71 ± 1.38 Bb |
| Rm | 43.26 ± 2.15 Abab | 24.65 ± 0.93 ABa | 17.03 ± 2.22 Bb | 2.94 ± 1.32 ABab | 23.18 ± 1.65 BCb | 27.82 ± 1.32 Bb |
| Rh | 46.84 ± 0.98 Aba | 26.65 ± 1.41 Aa | 18.91 ± 0.19 Bb | 3.01 ± 0.92 ABab | 27.18 ± 1.24 Bab | 29.53 ± 1.98 Bab |
| PC | 45.61 ± 1.61 Abab | 26.03 ± 0.94 Aa | 18.56 ± 1.42 Bb | 3.19 ± 1.52 ABab | 27.09 ± 1.32 Bab | 29.88 ± 0.99 Bab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, K.; Deng, X.; Xie, W.; Chen, J.; Lin, H.; Chen, Z. Rhodotorula mucilaginosa ZTHY2 Attenuates Cyclophosphamide-Induced Immunosuppression in Mice. Animals 2023, 13, 3376. https://doi.org/10.3390/ani13213376
Kang K, Deng X, Xie W, Chen J, Lin H, Chen Z. Rhodotorula mucilaginosa ZTHY2 Attenuates Cyclophosphamide-Induced Immunosuppression in Mice. Animals. 2023; 13(21):3376. https://doi.org/10.3390/ani13213376
Chicago/Turabian StyleKang, Kai, Xinyi Deng, Weitian Xie, Jinjun Chen, Hongying Lin, and Zhibao Chen. 2023. "Rhodotorula mucilaginosa ZTHY2 Attenuates Cyclophosphamide-Induced Immunosuppression in Mice" Animals 13, no. 21: 3376. https://doi.org/10.3390/ani13213376
APA StyleKang, K., Deng, X., Xie, W., Chen, J., Lin, H., & Chen, Z. (2023). Rhodotorula mucilaginosa ZTHY2 Attenuates Cyclophosphamide-Induced Immunosuppression in Mice. Animals, 13(21), 3376. https://doi.org/10.3390/ani13213376





