Simple Summary
Rhodotorula mucilaginosa is widely detected in the environment, and it produces broad metabolites such as carotenoid pigments, amino acids, polyunsaturated fatty acids, polysaccharides, and enzymatic substances. We previously isolated a strain of Rhodotorula mucilaginosa ZTHY2 from the coastal waters of the South China Sea, which showed probiotic properties. Rhodotorula mucilaginosa ZTHY2 promoted the growth of experimental animals, increased the abundance of beneficial bacteria in gut, and enhanced immune function. Given that immunosuppression is common in livestock, environmental stresses, such as overpopulation, food contamination, and chronic diseases, contribute to immunosuppression of livestock associated with declining animal production, failed immunoprophylaxis, and economic losses. Therefore, in this study, cyclophosphamide was used to generate immunosuppressed models to further evaluate the immunomodulatory effects of Rhodotorula mucilaginosa ZTHY2.
Abstract
Rhodotorula mucilaginosa (R. mucilaginosa) can enhance the immune and antioxidant function of the body. However, whether R. mucilaginosa has an immunoregulatory effect on cyclophosphamide (CTX)-induced immunosuppressed animals remains to be clarified. In this study, the R. mucilaginosa ZTHY2 that we isolated from the coastal waters of the South China Sea previously was prepared in order to investigate its immunoprotective effect on CTX-induced immunosuppression in mice, and the effects were compared to those of Lactobacillus acidophilus (LA) (a well-known probiotic). Seventy-two male SPF mice were divided into six groups: The C group (control); IM group (immunosuppressive model group) (+CTX); Rl, Rm, and Rh groups (+CTX+low, medium, and high concentration of R. mucilaginosa, respectively); and PC (positive control) group (+CTX+LA). After a 28-day feeding trial, blood samples were taken for biochemical and serum immunological analysis, and the thymus and spleen were collected to analyze the organ index, lymphocyte proliferation and differentiation, and antioxidant capacity. The findings showed that R. mucilaginosa ZTHY2 improved the spleen and thymus indices, effectively attenuated immune organ atrophy caused by CTX, and enhanced the proliferation of T and B lymphocytes induced by ConA and LPS. R. mucilaginosa ZTHY2 promoted the secretion of cytokines and immunoglobulins and significantly increased the contents of IL-2, IL-4, IL-6, TNF-α, IFN-γ, IgA, IgG, IgM, CD4, CD8, CD19, and CD20 in serum. The proportion of CD4+, CD8+, CD19+, and CD20+ lymphocytes in spleen, thymus, and mesenteric lymph nodes were increased. In addition, R. mucilaginosa ZTHY2 reduced the reactive oxygen species (ROS) and malondialdehyde (MDA) levels and increased glutathione (GSH), total superoxide dismutase (SOD), and catalase (CAT) levels. Our results indicated that R. mucilaginosa ZTHY2 can significantly enhance the immune function of immunosuppressed mice, and improving antioxidant capacity thus attenuates CTX-induced immunosuppression and immune organ atrophy.
1. Introduction
Rhodotorula mucilaginosa (R. mucilaginosa), known as “red yeast”, is widely detected in the sea, fresh water, soil, animals, and plants, etc., and it produces carotenoid pigments, resulting in orange-, pinkish-, or red-colored colonies [1]. R. mucilaginosa has been isolated and cultivated for various purposes, such as its bioproducts. R. mucilaginosa’s metabolites include amino acids, polyunsaturated fatty acids, polysaccharides, and enzymatic substances; R. mucilaginosa is thus an economic and valuable resource for biomedical and agricultural products [2,3]. R. mucilaginosa possesses valuable biotechnological features, although there have been a few case reports of fungemia, inflammation, or skin infection in humans, particularly in patients with immunodeficiency, tumors, or chronic disease [4,5,6,7,8]. However, environmentally derived R. mucilaginosa has been shown to promote growth in animals. R. mucilaginosa has been used as an additive to fodder for increasing the growth and antioxidant capacity of weaned piglets [9] and specific growth rate (SGR) and protein content of juvenile Nile tilapia [10]. An R. mucilaginosa solid-state fermentation product promoted the production of laying hens [11]. R. mucilaginosa has also been utilized by the food industry to produce enzymes, such as phenylalanine ammonia lyase (PAL) [12]. R. mucilaginosa has been shown to enhance immune responses in aquatic arthropods and vertebrates [10,13]; however, whether R. mucilaginosa helps enhance immunity of terrestrial animals remains to be determined.
The immune response of terrestrial animals depends on the innate and adaptive immune systems. The innate immune system provides the first line of defense against “non-self” factors, and the adaptive immune system consists of humoral and cell-mediated immunity to control infections [14,15]. Lymphocytes and macrophages produce and secrete inflammatory factors, such as tumor necrosis factor-α (TNF-α), interferon-(IFN)-γ, interleukin (IL)-2, IL-4, and IL-6. Both lymphocytes and macrophages play important roles in the function and homeostasis of animal immune systems. Altered homeostasis of the immune system contributes to immunosuppression associated with pathophysiological and environmental stresses [16,17]. Environmental stresses, such as overpopulation, food contamination, and chronic diseases, contribute to the immunosuppression of livestock associated with declining animal production, failed immunoprophylaxis, and economic losses [18,19,20,21]. Although natural products have been considered to help maintain the immunity of livestock [22,23,24], natural products effective in modulating immunosuppression remain to be determined.
Cyclophosphamide (CTX) is an antineoplastic drug for lymphoma and an immunosuppressant to treat autoimmune diseases, such as severe systemic lupus erythematosus [25,26]. CTX is also commonly used as a cancer treatment in companion animals [27,28,29,30], and the administration of CTX often leads to adverse effects in treated companion animals such as lethargy, moderate alopecia, vomiting, anorexia, anemia, and hematuria [31,32,33]. In addition, CTX is routinely used to induce immunosuppression in animal models associated with bone-marrow suppression, atrophy of the spleen and thymus, an imbalance of blood cells, and suppression of cytokines, such as IL-2, IL-4, TNF-α, IFN-γ, IgA, IgG, and IgM [34,35,36,37]. CTX has also been shown to suppress antioxidant superoxide dismutase (SOD) and catalase (CAT). SOD is a scavenger of oxygen free radicals, and CAT decomposes hydrogen peroxide into O2 and H2O as well as increases the lipid peroxidation product malondialdehyde (MDA), an index for oxidative damage [38,39]. Accordingly, CTX acts an optimal agent for studying immunosuppression and oxidation.
In our previous studies, we reported a novel strain of R. mucilaginosa ZTHY2 isolated from the sea of Southwest China [40], and we have successfully extracted astaxanthin from it [41]. Feeding animals with R. mucilaginosa ZTHY2 increased the thymus and spleen indices, delayed hypersensitivity, and increased IgG, IgA, IL-2, TNF-α, and INF-γ in serum [42]. Accordingly, R. mucilaginosa ZTHY2 appeared to help enhance the immune system. In this study, in order to investigate the effect of R. Mucilaginosa ZTHY2 on immunoprotective function and antioxidant capacity in CTX-induced immunosuppressed mice, the mechanisms were addressed by analyzing the immune organ index, blood biochemical factors, immunoglobulins, inflammatory factors, and lymphocyte proliferation and differentiation. Our research provides a theoretical basis for the use of R. mucilaginosa ZTHY2 as an immunopotentiator and a means of attenuating CTX-induced immunosuppression, and it gives an application perspective for exploration in other fields.
2. Materials and Methods
2.1. Microbial Strain, Antibodies, and Reagents
R. mucilaginosa ZTHY2 was isolated and identified by our team and was stored in the China Center for Type Culture Collection, CCTCC (Wuhan, China). The preservation number is M2015296. Lactobacillus acidophilus (BNCC185342) was purchased from BeNa Culture Collection (Beijing, China). Antibodies of FITC anti-mouse CD4 (100509), PE anti-rat CD3 (100205), APC anti-mouse CD8a (100711), PerCP anti-mouse CD19 (115531), APC anti-mouse CD20 (152107), and Zombie NIR™ Fixable Viability Kit were all purchased from Biolegend (San Diego, CA, USA). CTX was purchased from Yuanye biotechnology Co., Ltd. (Shanghai, China). ConA and LPS were purchased from Solarbio Life Sciences (Beijing, China).
2.2. Animals, Experimental Design, and Management
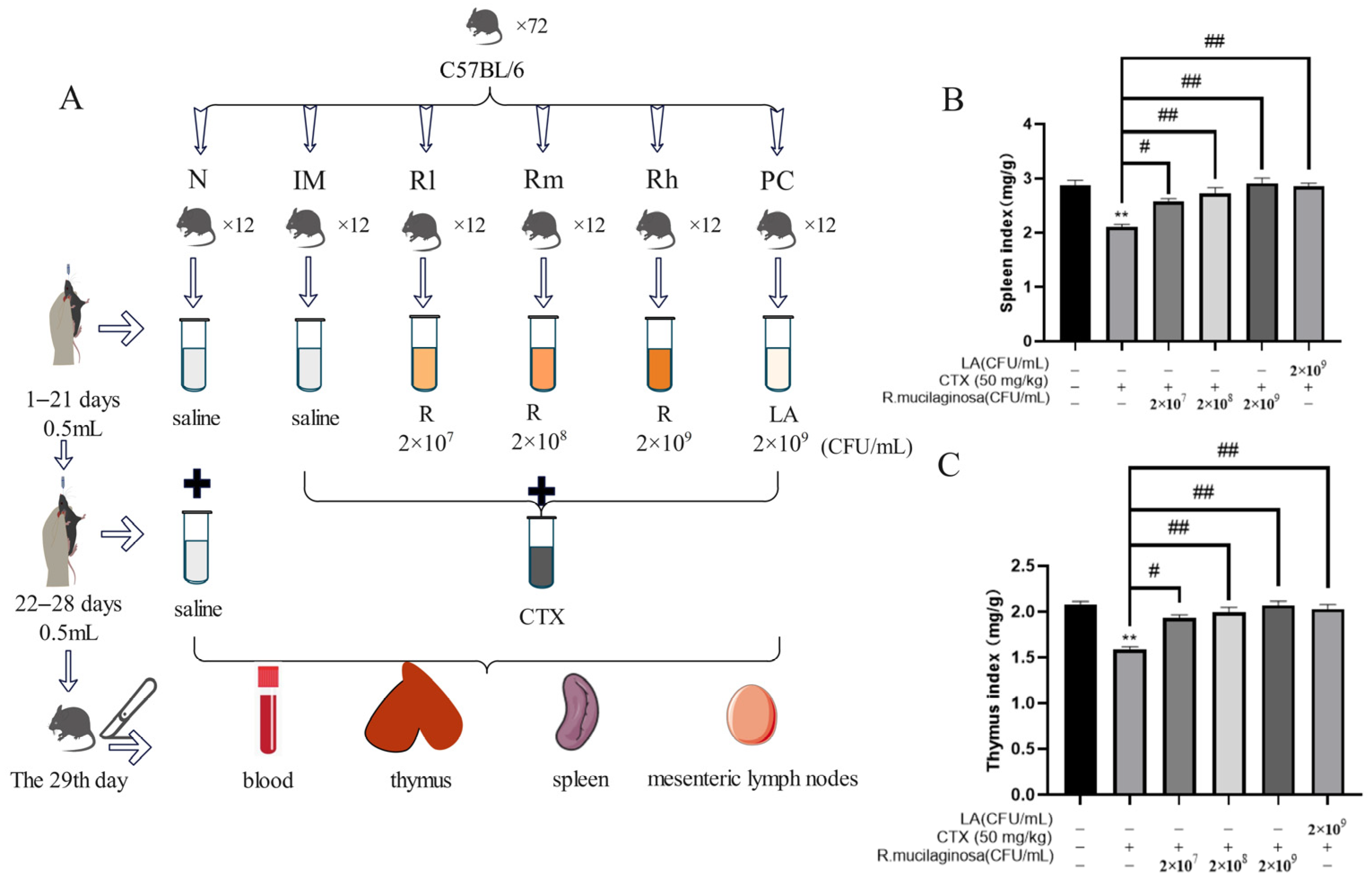
A total of 72 SPF male mice (C57BL/6), five weeks old, were used in a 28-day feeding trial (six to nine weeks old during the study). The mice, with an initial average body weight (BW) of 18.25 ± 2 g, were sourced from Guangzhou Dean Gene biological Co., Ltd. (Guangzhou, China). Mice were randomly and equally divided into six groups, namely the normal control group (N), immunosuppressive model group (IM), and positive control group (PC), and the R. mucilaginosa ZTHY2 treatment groups with low, medium, and high concentration, respectively (Rl, Rm, Rh). Each group had two replicates with six mice in each replicate. From 1 to 21 days, the N and IM groups were treated with 0.5 mL saline via gavage at each day. The PC group was treated with LA at 2 × 109 CFU/mL, 0.5 mL. The Rl, Rm, and Rh groups were treated with 0.5 mL of R. mucilaginosa ZTHY2 with concentrations of 2 × 107 CFU/mL, 2 × 108 CFU/mL, and 2 × 109 CFU/mL, respectively. From the 22nd–28th day, except for the N group, CTX was treated via gavage at 50 mg/kg for the other groups (Figure 1A). All the mice were kept in an environmentally controlled room, with the temperature maintained at (25 ± 1) °C, and the relative humidity was 65–85%. The mice had free access to water and feed. The formal experiment was carried out after one week of adaptation. All experimental protocols were approved by the Animal Ethics Committee of Guangdong Ocean University, (IACUC No. GDOU-LAE-2020-007), and the experiment was performed according to the ethical guidelines of the European Community guidelines.
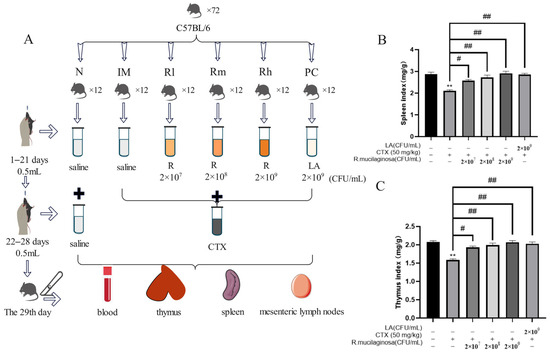
Figure 1.
Effects of R. mucilaginosa ZTHY2 on the spleen and thymus indices in CTX-induced immunosuppressed mice. (A) The protocol of animal experiments. (B) The spleen index. (C) The thymus index. N: normal control group. IM: immunosuppressive model. R: R. mucilaginosa ZTHY2. Rl: R. mucilaginosa ZTHY2 with low concentration. Rm: R. mucilaginosa ZTHY2 with medium concentration. Rh: R. mucilaginosa ZTHY2 with high concentration; PC: positive control. CTX: cyclophosphamide. LA: Lactobacillus acidophilus. Comparison with N group, differences are indicated by “*”, ** (p < 0.01). Comparison with IM group, differences are indicated by “#”, # (p < 0.05), ## (p < 0.01).
2.3. Body Weight and Immunity Organ Indices
The mice were weighed on day 0 (before the experiment), day 22 (before treatment with CTX) and day 29 (before dissecting the mice). At the 29th day, all the mouse blood was collected, followed by neck amputation, and studied with the gross anatomy to collect immune organs (the spleen, thymus, and mesenteric lymph nodes). The spleen and thymus were weighed to calculate the immune organ index as follows.
2.4. Determination of Hematological Indices
Mouse blood was collected from the eyeballs, of which 50 μL anticoagulant blood from each mouse was analyzed using an automatic blood cell analyzer (URIT-5180, Medical Electronic Co., Ltd., Guilin, China) to analyze the changes in leukocytes (WBCs), monocytes (MONs), lymphocytes (LYMs), neutrophils (NEUs), basophils (BASOs), and eosinophils (EOSs).
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
Blood was collected without anticoagulant, and the serum samples were obtained by centrifugation at 3000 rpm (10 min, 4 °C) after coagulation. The immunoglobulins IgA, IgG, and IgM; the interleukins IL-2, IL-4, and IL-6; tumor necrosis factor-α (TNF-α); interferon-γ (IFN-γ); and clusters of differentiation CD4, CD8, CD19, and CD20 in the serum were analyzed using commercial ELISA kits for mice (catalog numbers: IgA, QS42791; IgG, QS49338; IgM, QS42794; IL-2, QS42903; IL4, QS42901; IL-6, QS42899; TNF-α, QS42868; IFN-γ, QS42918; CD4, QS40227; CD8, QS440266; CD19, QS445871; CD20, QS40582; Beijing Gersion Bio-Technology Co., Ltd., Beijing, China).
2.6. Antioxidant Capacity Analysis
To analyze the antioxidant capacity of mice, the serum samples were obtained by centrifugation at 3000 rpm (10 min, 4 °C) after blood coagulation. Afterward, MDA contents were analyzed using commercial kits (catalog number: S0131S; Beyotime, Shanghai, China). Antioxidant enzyme activity, including SOD and CAT, was analyzed using commercial kits (catalog numbers: SOD, BC0170; CAT, BC0200; Solarbio Life Sciences, Beijing, China). Glutathione peroxidase (GSH-Px) was analyzed using commercial kits (catalog number: A005-1-1. Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.7. Single-Cell Suspension of Spleen, Thymus, and Mesenteric Lymph Nodes
The spleen, thymus, and mesenteric lymph nodes were collected and digested with pancreatic enzyme until the tissue became mushy. Then, digestion was terminated with twice the volume of cell culture medium. Next, the single-cell suspension was obtained with a 70 μm cell filter.
2.8. Intracellular Reactive Oxygen Species (ROS) of Spleen
The spleen single-cell suspension was centrifuged at 1200 rpm for 5 min and re-suspended with 1:500 diluted 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) for three hours, then washed by PBS and detected using a FACSVantage cytometer (SE, Becton Dickinson, Franklin Lakes, NJ, USA), with the excitation setting at 488 nm, and signals were acquired at the FL-2 channel. At least 10,000 cells per sample were acquired in histograms, and the data were analyzed with CytExpert (2.3.0.84) software. The DCFH-DA was purchased from Sigma (St. Louis, MO, USA) and dissolved in ethanol to produce a 1 mmol/L stock solution.
2.9. Proliferation of T and B Lymphocytes in Spleen and Thymus In Vitro
The spleen and thymus single-cell suspension were centrifuged at 500× g for 5 min, then suspended with red blood cell lysate for 3 min, and then centrifuged, and 2 mL of RPMI-1640 culture medium was used for suspension. The cell was inoculated in 96-well cell culture plates, with 1.0 × 104 cell/well. ConA was added to the spleen single-cell suspension to stimulate T cell proliferation, and LPS was added to the thymus single-cell suspension to stimulate B cell proliferation. The cell proliferation was detected using CCK-8 (Abmole, Houston, TX, USA) following the manufacturer’s instructions.
2.10. Flow Cytometry Detection of T and B Cell Species of Spleen, Thymus, and Mesenteric Lymph Nodes
A single-cell suspension of 100 μL was taken and adjusted to a total amount of 1 × 106 cells, centrifuged at 500× g for 5 min, re-suspended with Zombie NIR dye, and incubated at room temperature for 15–30 min away from light. PBS-BSA was used to clean the cells, and a 100 μL suspension was retained after centrifugation. Next, 1 μL FITC-CD4 antibody, 1.25 μL ACP-CD8 antibody, and 1.25 μL PE-CD3 antibody were added to the cells and incubated at room temperature for 15–30 min to detect T cell types, while 1 μL FITC-CD19 antibody and 1.25 μL CD20 antibody were added to the cells and incubated at room temperature for 15–30 min to detect B cell types. Moreover, 300 μL red cell lysate was added to each tube incubated away from light for 10 min, centrifuged at 300× g for 5 min, and then 1 mL PBS-BSA was added to the mixture, which was centrifuged again to discard the supernatant, followed by machine detection. The cells were detected using a flow cytometer.
2.11. Statistical Analysis
The statistical analysis of the data was carried out using SPSS 22.0. Statistical significance among multiple comparisons was determined via one-way ANOVA, followed by Ducan’s test using GraphPad Prism 5. The experimental results are expressed as mean ± standard deviation; a p-value less than 0.05 was considered to indicate a significant difference, and a p-value less than 0.01 was considered to indicate an extremely significant difference.
3. Results
3.1. Effects of R. mucilaginosa ZTHY2 on Body Weight and Immune Organ Index in CTX-Induced Immunosuppressed Mice
To address whether R. mucilaginosa ZTHY2 was able to enhance or protect immunity from suppression, we treated CTX-induced immunosuppressed mice and determined animal body weight, followed by isolation of the spleen and thymus for studies, as depicted in Figure 1A. As shown in Table 1, there was no significant difference in body weight among all groups at the initial weight (Day 0) and after gavage with R. mucilaginosa ZTHY2 or LA for 21 days (Day 22). After treatment with CTX for one week, the body weight of the IM group was significantly decreased (p < 0.01). Compared with the IM group, the body weight of the R groups and PC group was significantly increased (p < 0.05) (Day 29). As shown in Figure 1B,C, compared with the N group, the spleen and thymus indices in the IM group were significantly decreased (p < 0.01). Compared with the IM group, the spleen and thymus indices in the PC and R groups were significantly increased (p < 0.01). There was no significant difference between the Rh and N groups (p > 0.05), indicating that R. mucilaginosa ZTHY2 could effectively attenuate the atrophy of the spleen and thymus induced by CTX.

Table 1.
Changes in body weight of mice.
3.2. Effect of R. mucilaginosa ZTHY2 on Hematological Indices in CTX-Induced Immunosuppressed Mice
The results of biochemical analysis of the mice’s blood showed that, as shown in Table 2, compared with the N group, the numbers of WBCs, LYMs, MONs, NEUs, and EOSs were significantly decreased in the IM group (p < 0.01). Compared with the IM group, the numbers of WBCs, LYMs, MONs, and NEUs in the R groups and the PC group were significantly increased (p < 0.05). There was no difference in any of the detected indicators between the Rh group and the PC group. This showed that R. mucilaginosa ZTHY2 can attenuate the blood toxicity caused by CTX.

Table 2.
Hematological indices of mice.
3.3. R. mucilaginosa ZTHY2 Increased the Level of Immunoglobulin in CTX-Induced Immunosuppressed Mice
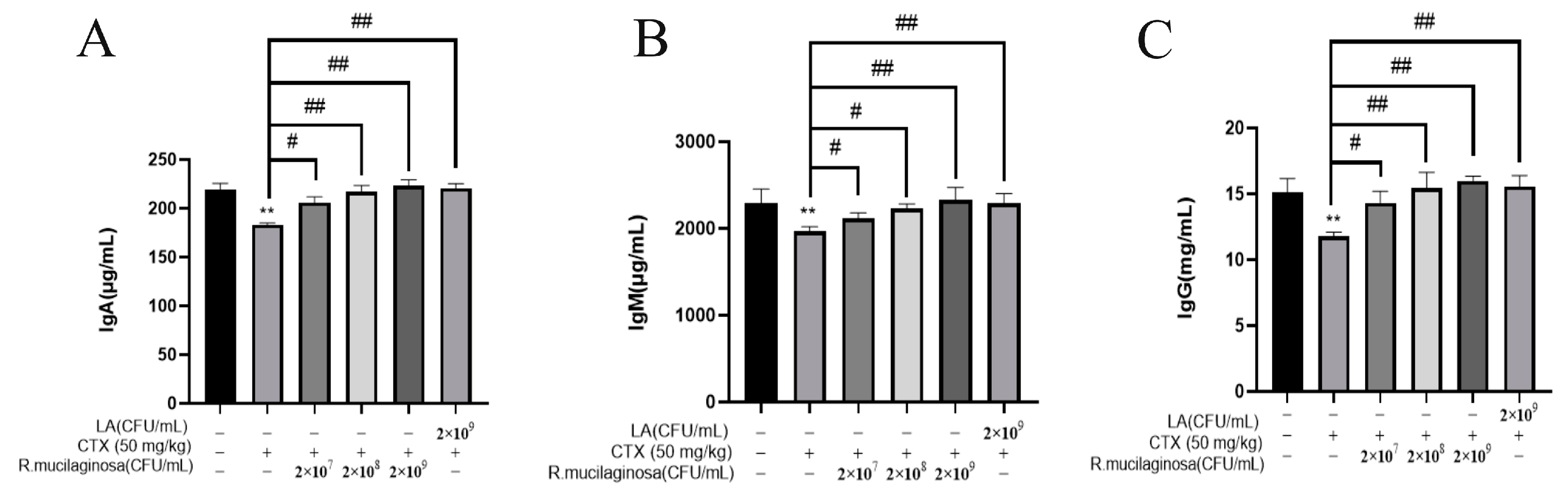
To detect the effect of R. mucilaginosa ZTHY2 on immunoglobulin levels in CTX-induced immunosuppressed mice, the ELISA method was adopted to analyze the serum immunoglobulins IgA, IgG, and IgM in mice. As shown in Figure 2, compared with the N group, the levels of IgA (Figure 2A), IgG (Figure 2B), and IgM (Figure 2C) in serum in the IM group were significantly decreased (p < 0.01). Compared with the IM group, the levels of IgG, IgA, and IgM in the Rl group were significantly increased (p < 0.05) and extremely significantly increased (p < 0.01) in the Rm, Rh, and PC groups. The promotion effect of immunoglobulin by R. mucilaginosa ZTHY2 was dose-dependent. This showed that R. mucilaginosa ZTHY2 can attenuate the adverse effect of CTX on the immunoglobulins.
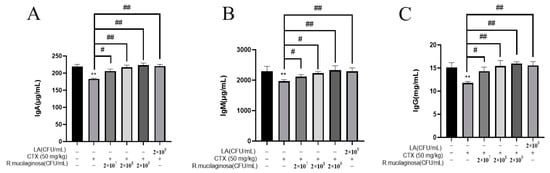
Figure 2.
R. mucilaginosa ZTHY2 increased the level of immunoglobulin in CTX-induced immunosuppressed mice. The IgG, IgA, and IgM in the serum were detected by the ELISA. (A) The level of IgA. (B) The level of IgM. (C) The level of IgG. Comparison with N group, differences are indicated by “*”, ** (p < 0.01); comparison with IM group, differences are indicated by “#”, # (p < 0.05), ## (p < 0.01).
3.4. R. mucilaginosa ZTHY2 Increased the Secretion of Immune Cytokines in CTX-Induced Immunosuppressed Mice
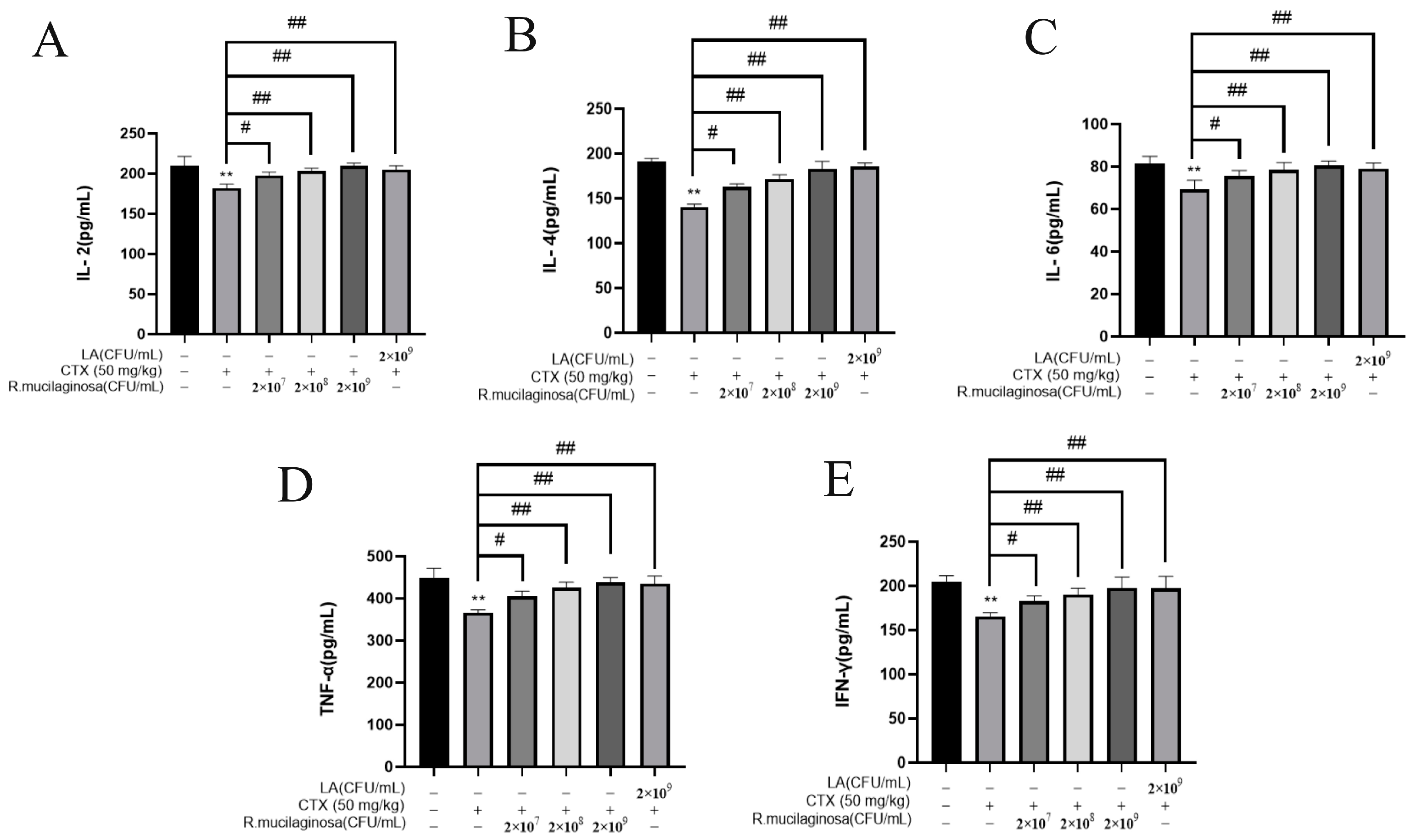
To detect the effect of R. mucilaginosa ZTHY2 on immune cytokines in immunosuppressed mice, the ELISA was used to analyze the IL-2, IL-4, IL-6, TNF-α, and IFN-γ in the serum. As shown in Figure 3, the protein levels of IL-2 (Figure 3A), IL-4 (Figure 3B), IL-6 (Figure 3C), TNF-α (Figure 3D), and IFN-γ (Figure 3E) in the IM group were significantly decreased compared with the N group (p < 0.01). Compared with the IM group, protein levels of L-2, IL-4, IL-6, TNF-α, and IFN-γ were significantly increased in the Rl group (p < 0.05) and extremely significantly increased in the Rm, Rh, and PC groups (p < 0.01), which indicated that R. mucilaginosa ZTHY2 could promote the secretion of immune cytokines in serum and attenuated the immunosuppressant response induced by CTX.
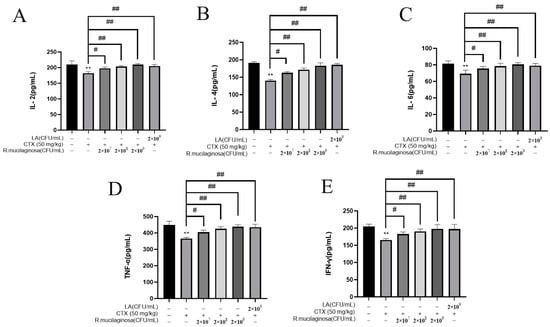
Figure 3.
R. mucilaginosa ZTHY2 increased the secretion of immune cytokines in CTX-induced immunosuppressed mice. The IL-2, IL-4, IL-6, TNF-α, and IFN-γ in the serum were detected by the ELISA. (A) The level of IL-2. (B) The level of IL-4. (C) The level of IL-6. (D) The level of TNF-α. (E) The level of IFN-γ. Comparison with N group, differences are indicated by “*”, ** (p < 0.01); comparison with IM group, differences are indicated by “#”, # (p < 0.05), ## (p < 0.01).
3.5. R. mucilaginosa ZTHY2 Increased the Levels of CDs in CTX-Induced Immunosuppressed Mice
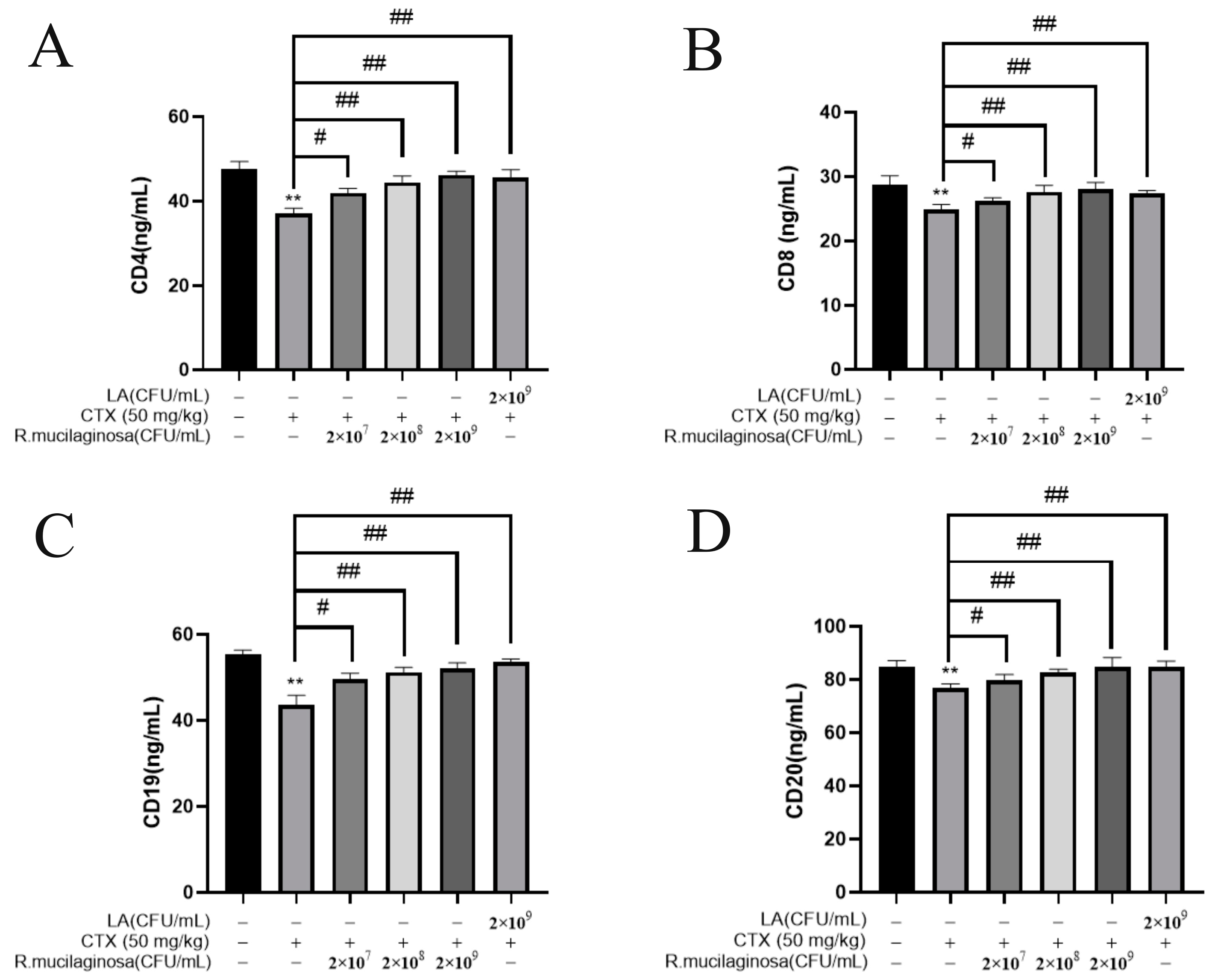
In order to investigate the effect of R. mucilaginosa ZTHY2 on leukocyte differentiation and maturation, the ELISA was used to analyze CD4, CD8, CD19, and CD20 in the serum. As shown in Figure 4, the protein levels of CD4 (Figure 4A), CD8 (Figure 4B), CD19 (Figure 4C), and CD20 (Figure 4D) in the IM group were significantly decreased compared with the N group (p < 0.01). The protein levels of CD4, CD8, CD19, and CD20 in the Rl group were significantly increased compared with the IM group (p < 0.05). The protein levels in the Rm, Rh, and PC groups were significantly increased (p < 0.01). The protein levels of CD4 and CD20 in the Rh group were not significantly different from those in the N group (p > 0.05). These results indicated that R. mucilaginosa ZTHY2 could increase the proportion of CD4, CD8, CD19, and CD20 in immunosuppressed mice, thereby regulating the immune function of the body.
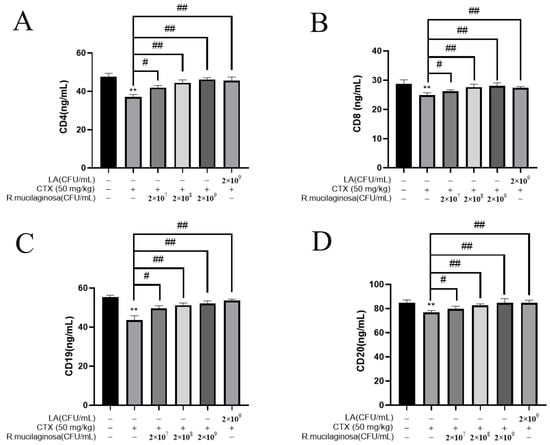
Figure 4.
R. mucilaginosa ZTHY2 increased the levels of CDs in CTX-induced immunosuppressed mice. The CD4, CD8, CD19, and CD20 in the serum were detected by the ELISA. (A) The level of CD4. (B) The level of CD8. (C) The level of CD19. (D) The level of CD20. Comparison with N group, differences are indicated by “*”, ** (p < 0.01); comparison with IM group, differences are indicated by “#”, # (p < 0.05), ## (p < 0.01).
3.6. R. mucilaginosa ZTHY2 Promoted the Proliferation of T and B Lymphocytes of Immunosuppressed Mice Induced by CTX In Vitro
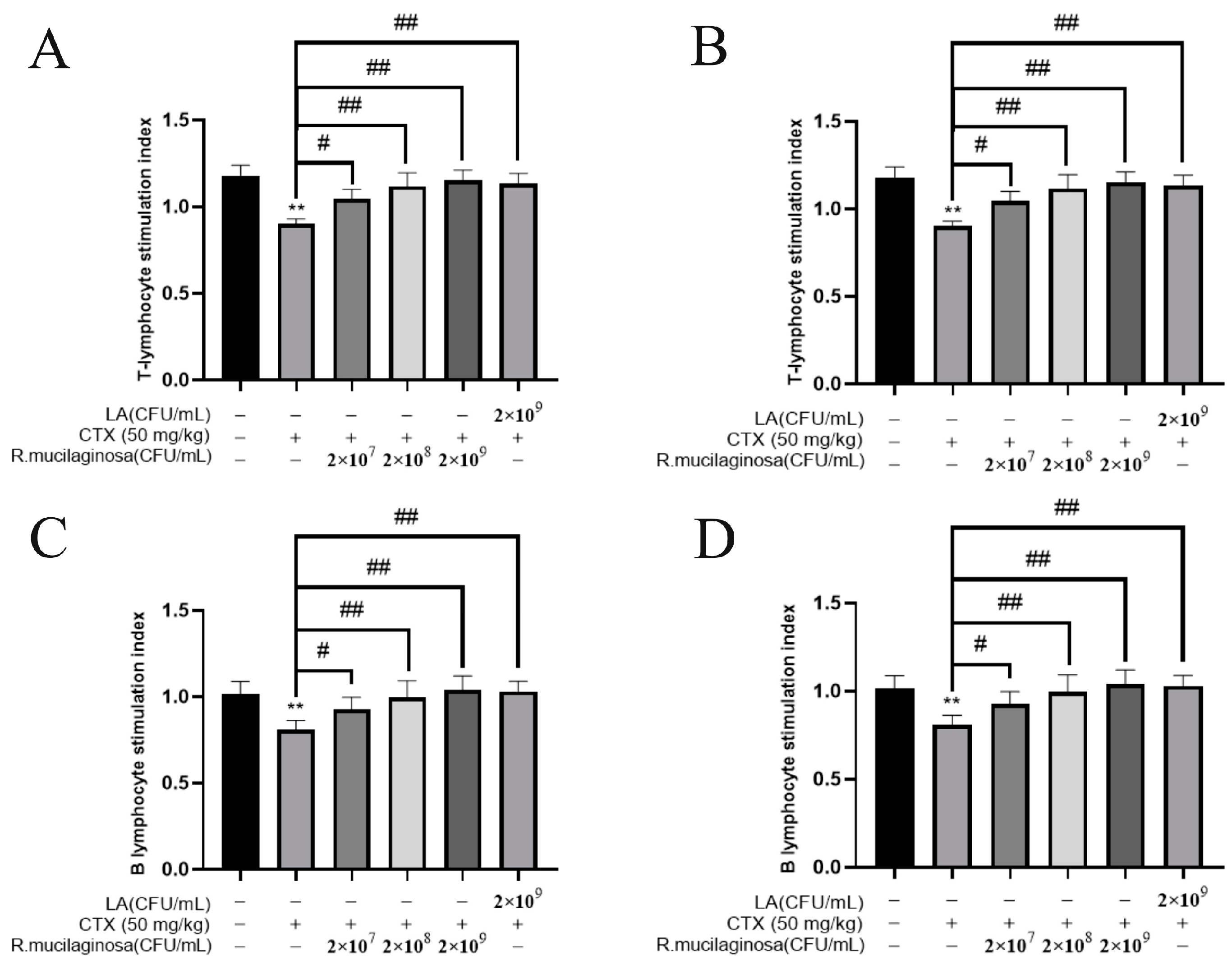
To determine whether R. mucilaginosa ZTHY2 could promote lymphocyte proliferation, ConA, known to activate the T cells [43], and LPS, widely used to activate the B cells [44], were used to induce the proliferation of lymphocytes in vitro. As shown in Figure 5, the proliferation of spleen T (Figure 5A) and B (Figure 5B) lymphocytes and thymus T (Figure 5C) and B (Figure 5D) lymphocytes in the MI groups was significantly decreased compared with the N group (p < 0.01). Compared with the IM group, the proliferation was significantly increased in the Rl (p < 0.05), Rm and Rh (p < 0.01), and PC groups (p < 0.01), and the trend was dependent on the concentration. This showed that the proliferation of T and B lymphocytes in the spleen and thymus of immunosuppressed mice was enhanced by R. mucilaginosa ZTHY2.
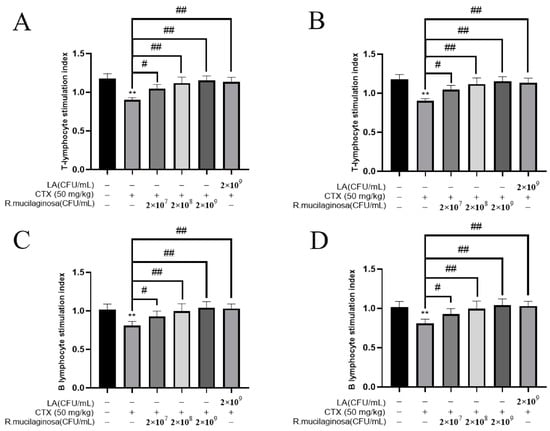
Figure 5.
R. mucilaginosa ZTHY2 promoted the proliferation of T and B lymphocytes of immunosuppressed mice induced by CTX in vitro. Single-cell suspensions of the spleen and thymus were prepared, and lymphocyte proliferation was detected using CCK-8 after stimulation with ConA and LPS. (A) Proliferation of T lymphocytes of the spleen stimulated by ConA. (B) Proliferations of B lymphocytes of the spleen stimulated by ConA. (C) Proliferations of T lymphocytes of the thymus stimulated by LPS. (D) Proliferations of B lymphocytes of the thymus stimulated by LPS. Comparison with N group, differences are indicated by “*”, ** (p < 0.01); comparison with IM group, differences are indicated by “#”, # (p < 0.05), ## (p < 0.01).
3.7. Effects of R. mucilaginosa ZTHY2 on T Cell and B Cell Subsets in CTX-Induced Immunosuppressed Mice
To further analyze the effects of R. mucilaginosa ZTHY2 on T cell and B cell subsets in CTX-induced immunosuppressed mice, the levels of CD3, CD4, and CD8 were detected via flow cytometry to verify the T cell differentiation, and CD19 and CD20 were detected to verify the B cell differentiation.
3.7.1. Effects of R. mucilaginosa ZTHY2 on T Cell and B Cell Subsets of Spleen in CTX-Induced Immunosuppressed Mice
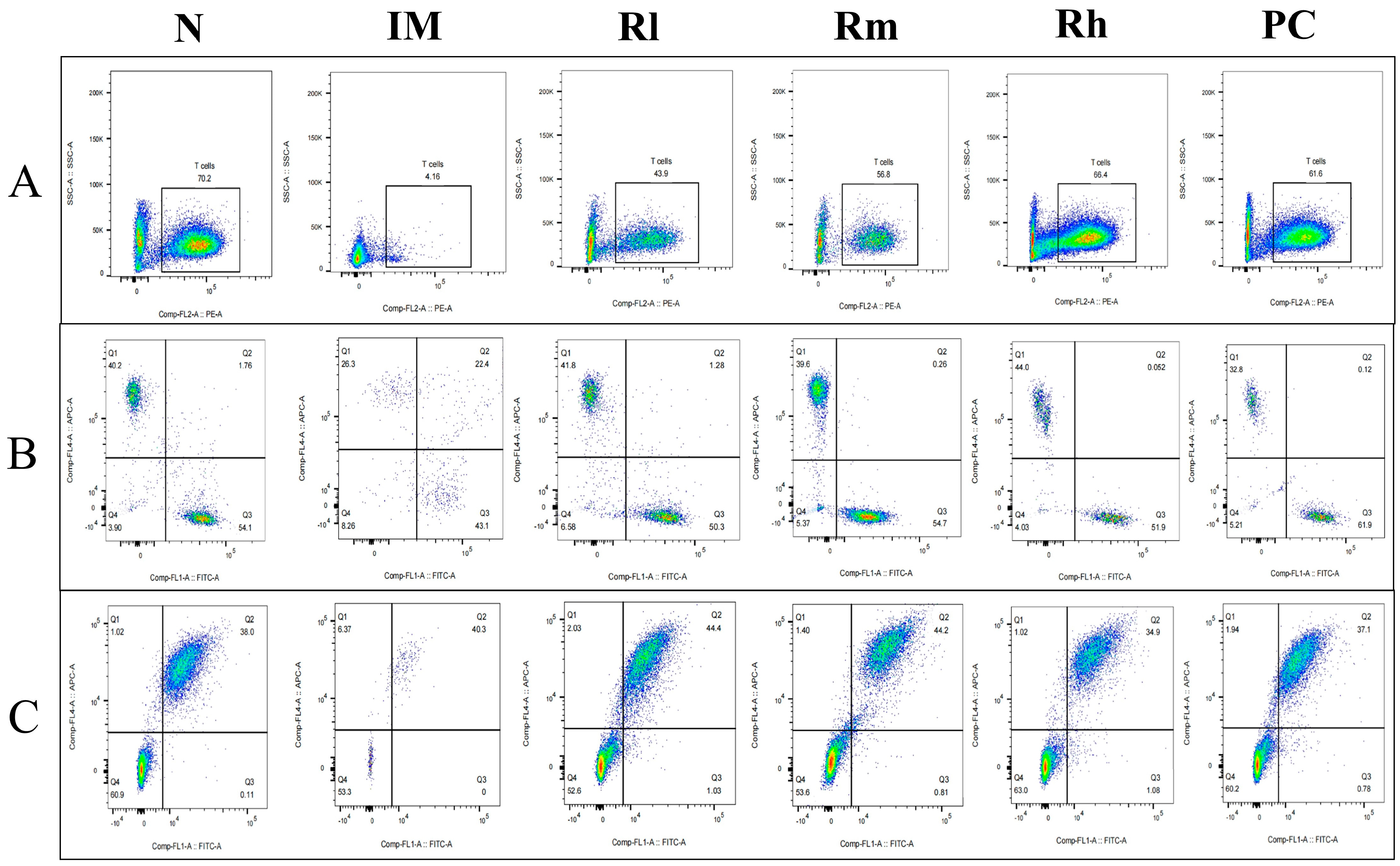
The analysis of lymphocyte subsets in the spleen showed that compared with the N group, the percentages of CD3+, CD4+, CD8+, CD19+, and CD20+ lymphocytes and the ratio of CD4+/CD8+ in the IM group were significantly decreased (p < 0.01). Compared with the IM group, the percentages of CD3+, CD4+, CD8+, CD19+, and CD20+ lymphocytes and the ratio of CD4+/CD8+ were significantly increased in the R groups and the PC group (p < 0.05). There was no significant difference in the percentages of CD4+, CD8+, and CD19+ between the R groups and PC group (p > 0.05) and CD3+ and CD20+ cells between the Rm and Rh groups compared to the PC group (p > 0.05). There was no significant difference in CD3+, CD4+, and CD20+ cells between the Rh and N groups (p > 0.05) (Figure 6 and Table 3). The results indicated that R. mucilaginosa ZTHY2 could significantly stimulate both T and B lymphocyte differentiation of spleen in CTX-induced immunosuppressed mice, and gavage with R. mucilaginosa ZTHY2 at the concentration of 2 × 108 CFU/mL had the same effect as LA at 2 × 109 CFU/mL.
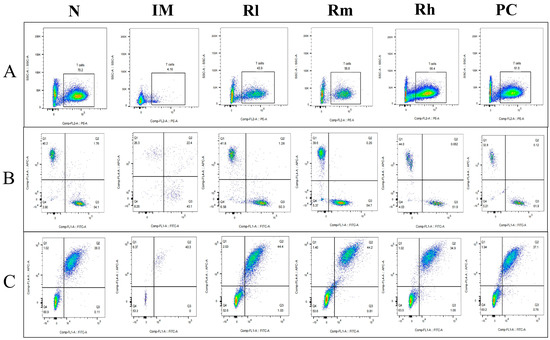
Figure 6.
Expression of marker molecules of lymphocyte subpopulations in mouse spleen. Single-cell suspensions of spleen were prepared, and CD3+, CD4+, CD8+, CD19+, and CD20+ lymphocytes were detected using a flow cytometer. (A) Detection of CD3+ lymphocytes. (B) Detection of CD4+ and CD8+ lymphocytes. (C) Detection of CD19+ and CD20+ lymphocytes. N: normal control group. IM: immunosuppressive model group. Rl: R. mucilaginosa ZTHY2 with low concentration. Rm: R. mucilaginosa ZTHY2 with medium concentration. Rh: R. mucilaginosa ZTHY2 with high concentration. PC: positive control.

Table 3.
Detection of T and B lymph subpopulations in mouse spleen.
3.7.2. Effects of R. mucilaginosa ZTHY2 on T Cell and B Cell Subsets of Thymus in CTX-Induced Immunosuppressed Mice
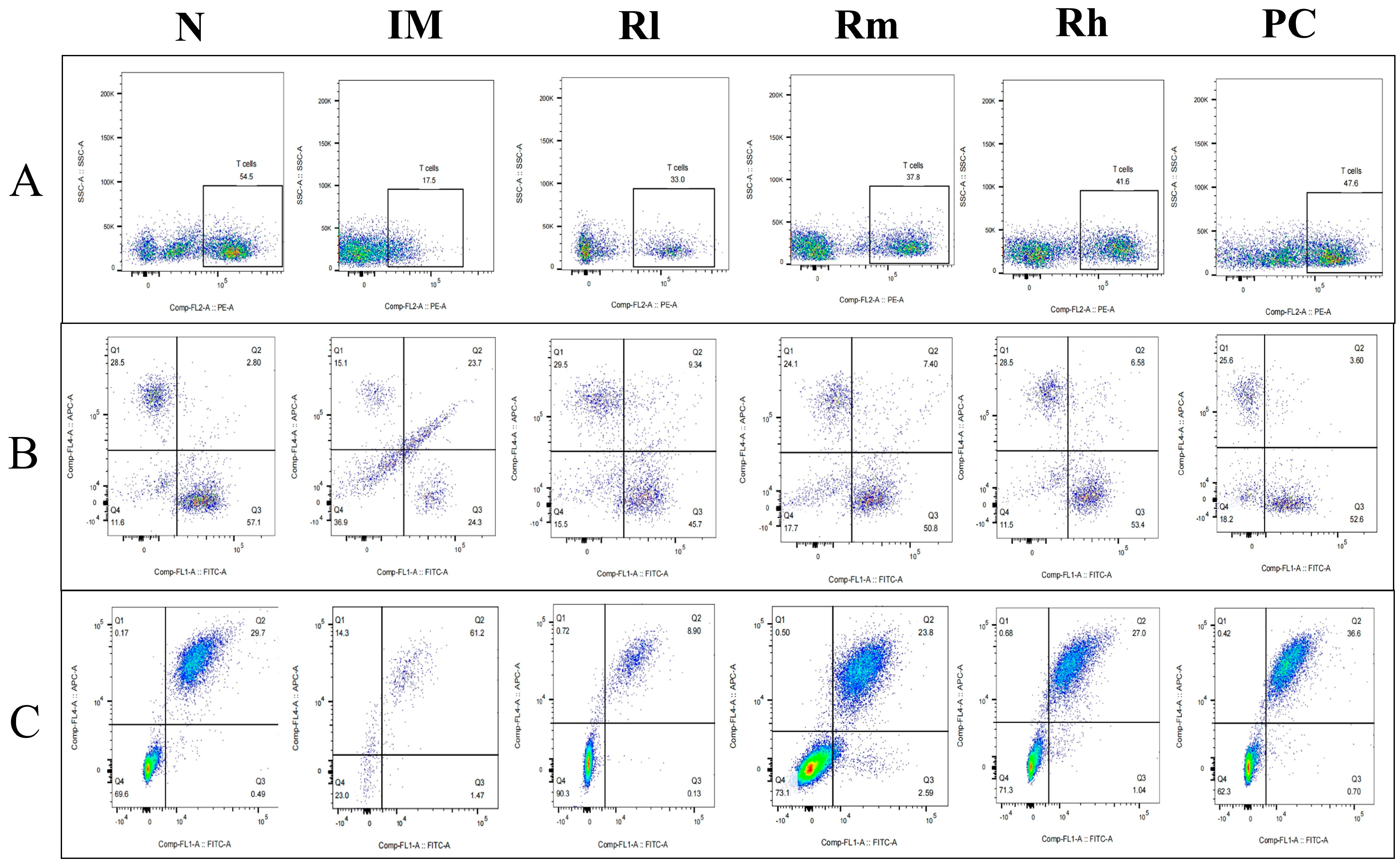
The analysis of lymphocyte subsets in the thymus showed that compared with those in the N group, the percentage of CD3+, CD4+, CD8+, CD19+, and CD20+ cells and the ratio of CD4+/CD8+ in the IM group were significantly decreased (p < 0.01). Compared with those in the IM group, the percentages of CD3+, CD4+, CD8+, CD19+, and CD20+ lymphocytes in the R groups and PC group were significantly increased (p < 0.05). There was no significant difference in CD3+, CD4+, CD8+, CD19+, CD20+ percentage and the ratio of CD4+/CD8+ among the Rm, Rh, and PC groups (p > 0.05) and no significant difference in CD3+, CD4+, and CD19+ percentage between Rh and N (p > 0.05) (Figure 7 and Table 4). The results indicated that R. mucilaginosa ZTHY2 could significantly stimulate lymphocyte differentiation of the thymus in CTX-induced immunosuppressed mice, and gavage with R. mucilaginosa ZTHY2 at the concentration of 2 × 108 CFU/mL had the same effect as LA at 2 × 109 CFU/mL.
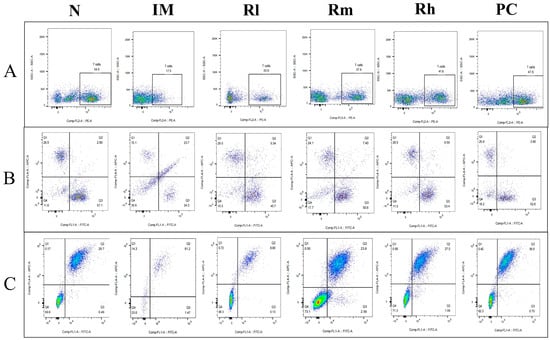
Figure 7.
Expression of marker molecules of lymphocyte subpopulations in mice thymus. Single-cell suspensions of thymus were prepared, and CD3+, CD4+, CD8+, CD19+, and CD20+ lymphocytes were detected using a flow cytometer. (A) Detection of CD3+ lymphocytes. (B) Detection of CD4+ and CD8+ lymphocytes. (C) Detection of CD19+ and CD20+ lymphocytes. N: normal control group. IM: immunosuppressive model group. Rl: R. mucilaginosa ZTHY2 with low concentration. Rm: R. mucilaginosa ZTHY2 with medium concentration. Rh: R. mucilaginosa ZTHY2 with high concentration. PC: positive control.

Table 4.
Detection of T and B lymph subpopulations in mouse thymus.
3.7.3. Effects of R. mucilaginosa ZTHY2 on T Cell and B Cell Subsets of Mesenteric Lymph Nodes in CTX-Induced Immunosuppressed Mice
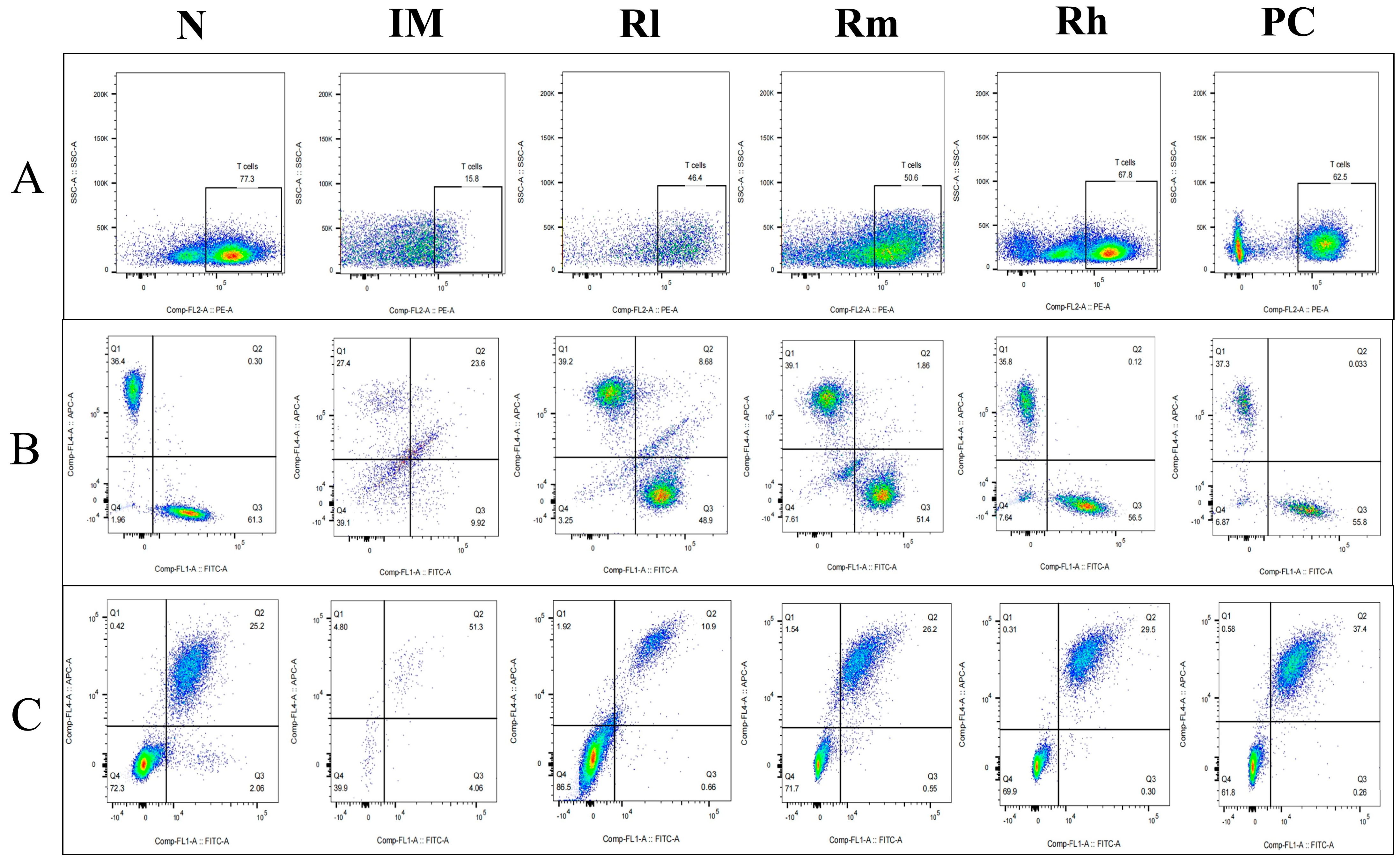
The analysis of lymphocyte subsets in mesenteric lymph nodes showed that compared with those in the N group, the percentage of CD3+, CD4+, CD8+, CD19+, and CD20+ lymphocytes and the ratio of CD4+/CD8+ in the IM group were significantly decreased (p < 0.01). Compared with those in the IM group, the percentages of CD3+, CD4+, CD8+, and CD20+ lymphocytes in the R groups and PC group were significantly increased (p < 0.01). There was no significant difference in CD3+, CD4+, CD8+, and CD20+ lymphocytes percentage among the R groups and PC group (p > 0.05). There was no significant difference in CD3+, CD4+, and the ratio of CD4+/CD8+ among the Rm, Rh, and N groups (p > 0.05) (Figure 8 and Table 5). The results indicated that R. mucilaginosa ZTHY2 could significantly stimulate lymphocyte differentiation of mesenteric lymph nodes in CTX-induced immunosuppressed mice, and gavage with R. mucilaginosa ZTHY2 at the concentration of 2 × 108 CFU/mL had the same effect as LA at 2 × 109 CFU/mL.
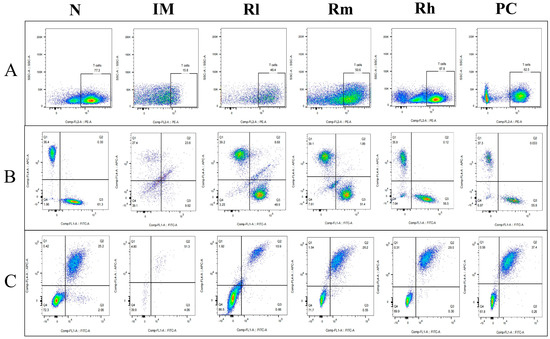
Figure 8.
Expression of marker molecules of lymphocyte subpopulations in mice mesenteric lymph nodes. Single-cell suspensions of mesenteric lymph nodes were prepared, and CD3+, CD4+, CD8+, CD19+, and CD20+ lymphocytes were detected using a flow cytometer. (A) Detection of CD3+ lymphocytes. (B) Detection of CD4+ and CD8+ lymphocytes. (C) Detection of CD19+ and CD20+ lymphocytes. N: normal control group. IM: immunosuppressive model group. Rl: R. mucilaginosa ZTHY2 with low concentration. Rm: R. mucilaginosa ZTHY2 with medium concentration. Rh: R. mucilaginosa ZTHY2 with high concentration. PC: positive control.

Table 5.
Detection of T lymph subpopulations in mouse mesenteric lymph nodes.
3.8. Effects of R. mucilaginosa ZTHY2 on Antioxidant Capacity of Spleen in CTX-Induced Immunosuppressed Mice
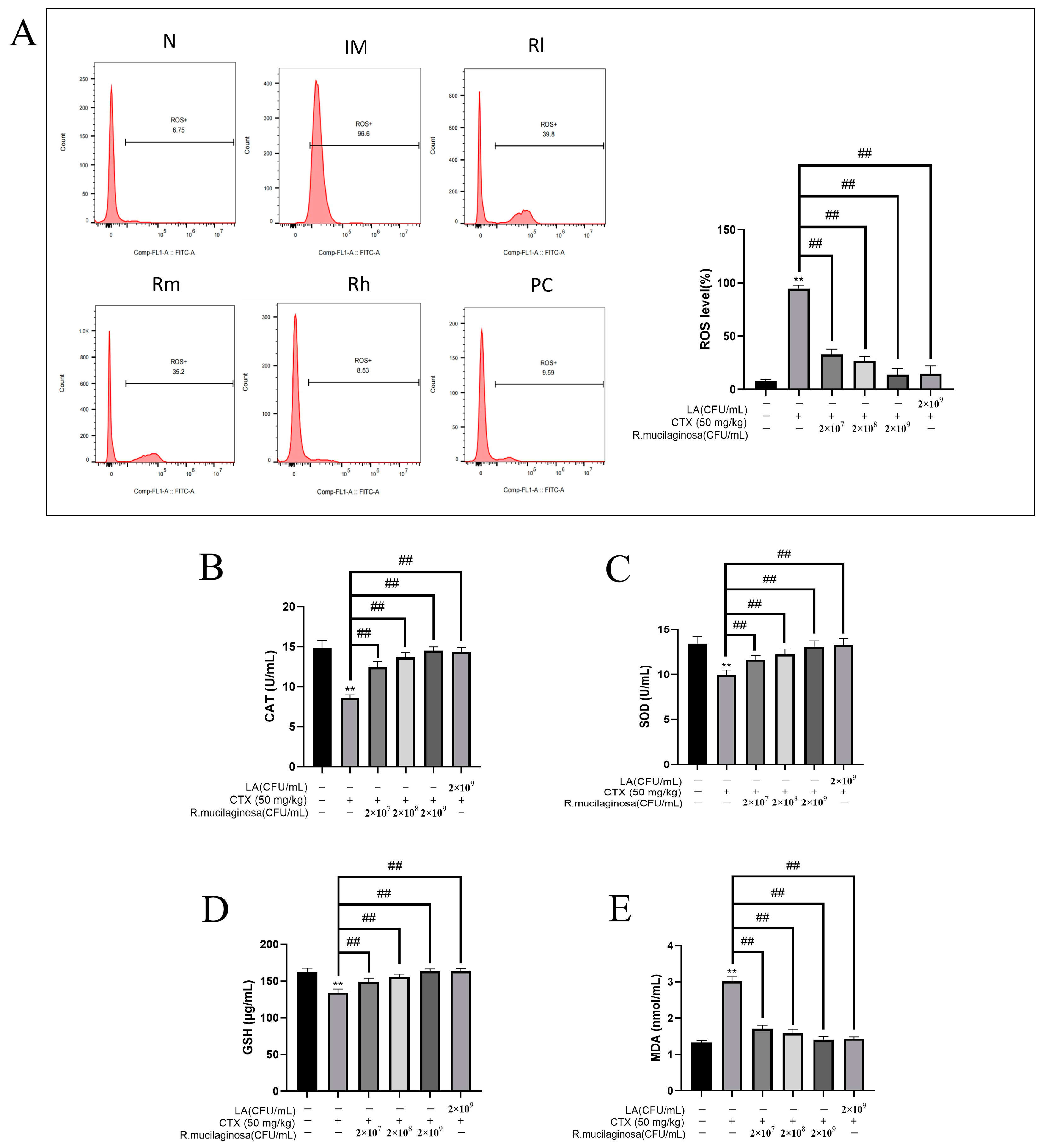
The antioxidant capacity of the body is also closely related to immune function and health, so in order to investigate the effect of R. mucilaginosa ZTHY2 on the oxidation capacity, the MDA, ROS, CAT, GSH, and SOD of the spleen were detected. As shown in Figure 9, compared with those in the N group, the levels of ROS (Figure 9A) and MDA (Figure 9E) in the IM group were significantly increased (p < 0.01), while the levels of CAT (Figure 9B), SOD (Figure 9C), and GSH (Figure 9D) were significantly decreased (p < 0.01). The results were significantly reversed in both the R groups and the PC group and dependent on the concentration of R. mucilaginosa ZTHY2 (p < 0.01). There were no significant difference in SOD, CAT, and GSH contents between the Rh group and the N group (p > 0.05). The results indicated that R. mucilaginosa ZTHY2 could effectively increase the content of GSH, SOD, and CAT in immunosuppressed mice, remove excess ROS in the spleen (p < 0.01), and then reduce MDA produced by peroxidation.
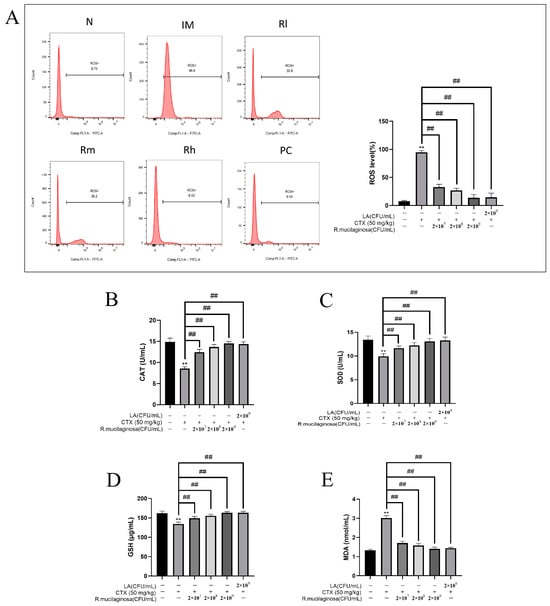
Figure 9.
Effects of R. mucilaginosa ZTHY2 on antioxidant capacity in CTX-induced immunosuppressed mice. (A) ROS level of spleen in mice. (B) CAT level in the serum. (C) SOD level in the serum. (D) GSH level in the serum. (E) MDA level in the serum. N: normal control group. IM: immunosuppressive model. R: R. mucilaginosa ZTHY2. Rl: R. mucilaginosa ZTHY2 with low concentration. Rm: R. mucilaginosa ZTHY2 with medium concentration. Rh: R. mucilaginosa ZTHY2 with high concentration. PC: positive control. Comparison with the N group, differences are indicated by “*”, ** (p < 0.01); comparison with IM group, differences are indicated by “#”, ## (p < 0.01).
4. Discussion
Immunosuppression is a condition characterized by temporary or permanent immune dysfunction. In intensive livestock and poultry farms, immunosuppression is easy to see, and feed additives that can enhance immunity and attenuate immunosuppression are needed. R. mucilaginosa ZTHY2 exhibits immunostimulatory properties and regulates the gastrointestinal microbiome [42]. Furthermore, our study confirmed its ability to enhance immune response and antioxidant capacity in mice under immunosuppressive conditions. R. mucilaginosa ZTHY2 reduced weight loss and the atrophy of immune organs, decreased proliferation and differentiation of immune cells, and reduced production of immune factors induced by CTX. Our results show that R. mucilaginosa ZTHY2 has good potential for use as an immunomodulator. At present, there are many studies exploring immunomodulators, such as peptides from soybean, nucleic acid combined with microRNA, extracts of plants and medicinal herbs, and peptides from aquatic organisms [45,46,47,48,49]. However, R. mucilaginosa ZTHY2 has a great advantage in that it is widely available in the ocean, it is easy to isolate and culture, and the culture cost is low; hence, it has good application prospects and is worth further research.
The activity of LA in stimulating the immune system has been verified in a variety of species. The immunomodulatory effects of LA were demonstrated in mice as early as 1986 [50]. Subsequently, they have been confirmed in a variety of animals such as laying hens, broiler chickens, zebrafish, and weaned calves [51,52,53,54]. Different strains of LA ameliorated the CTX-induced immunosuppression in mice, and the protective function of LA from CTX was reflected in LA reversing the body and spleen weights, blood RBC and WBC levels, and splenocyte and bone-marrow cells; increased T cell proliferation; induced expression of IL-2 and IFN-gamma in T cells; the restored phagocytosis of macrophages in mice; and cytotoxicity of NK and cytotoxic T cells [55,56,57,58]. Accordingly, LA was used as a positive control in our experiment.
The spleen and thymus are the important immune organs, and the spleen and thymus indices can reflect the immune function of the body. Splenic lymphocyte proliferation is an important biomarker of activation of cellular and humoral immune responses. T and B lymphocyte proliferation is usually induced by mitogen ConA and LPS [59,60]. Studies have shown that CTX decreased the immune organ indices and inhibited the T and B lymphocyte proliferation [61,62]. In this study, the spleen and thymus indices decreased significantly, and the proliferation ability of T and B lymphocytes decreased; these indicated that the immunosuppressive model was successfully established. With the protection of R. mucilaginosa ZTHY2, the spleen and thymus indices of mice were improved in a concentration-dependent manner, suggesting that R. mucilaginosa ZTHY2 could protect against the damage of CTX to the spleen and thymus.
The blood actively engages in metabolic reactions and plays a crucial role in maintaining homeostasis within the body. White blood cells are composed of lymphocytes, neutrophils, monocytes, eosinophils, and basophils. Studies have shown that CTX induces a significant decrease in the number of immune cells, such as leukocytes, monocytes, and lymphocytes, resulting in low immunity [63]. In this experiment, the blood indices of mice in the IM group were significantly decreased compared to those in the N group, indicating that CTX inhibited the hemocytogenesis in mice. On the contrary, the blood indices of mice in the R groups were significantly improved, especially the leukocytes, which are an important part of the body’s defense system, indicating that R. mucilaginosa ZTHY2 can enhance the body’s immune function and remove the toxic effect of CTX on blood cells.
The innate and adaptive immune systems are further divided into cellular immunity and humoral immunity. T and B lymphocytes are responsible for cellular immunity and humoral immunity, respectively [64,65]. Classically, based on the surface markers of T cells, T lymphocyte subsets can be divided into CD3+, CD4+, and CD8+ T cells. CD3+ T lymphocytes exist on the surface of all T lymphocytes and closely bind to T cell receptors to form a TCR–CD3 complex containing eight peptide chains, which jointly participate in the recognition and signaling of antigens by T cells [66,67]. CD4+ represents the cellular immune secretion function and T helper cell (Th cell) subsets. Th cells induce cytokine differentiation and mediate cellular and humoral immune responses. CD8+ T cells can kill labeled cells and inhibit antigen-presenting cells, and the percentage of CD8+ is a measure of the killing ability of cellular immune and tissue damage [68,69]. Generally, the number of T cell subsets is relatively stable. When the number of T cells or the ratio of CD4+/CD8+ is reduced, it often indicates immunosuppression, like malignant tumors or viral infections, and when the ratio is increased, autoimmune diseases may occur [70]. In addition, the immune function of the body can be evaluated by analyzing the content of CD4 and CD8 molecules. CD4 can be further divided into Th1 and Th2 subsets; Th1 cells produce cytokines such as IL-2, TNF-α, and IFN-γ, and Th2 cells produce IL-4, IL-5, IL-6, IL-10, and IL-13 [71]. IL-2 can regulate the function of B lymphocytes and natural killer cells, which reflects the function of cellular immunity [72]. IFN-γ and IL-2 have a synergistic effect on inhibiting viral replication, activating macrophages, and enhancement the cellular immune function [73]. TNF-α can selectively kill tumor cells, stimulate lymphocyte proliferation, and activate NK cells [74]. IL-4 can promote IgE-mediated humoral immune response by activating B cells to synthesize IgE and transform IgG into IgE [75]. IL-6 can induce B cell differentiation and maturation and participate in immune response and inflammation [76]. Under physiological conditions, Th1/Th2 cells in the body are in a state of dynamic balance. When the body is dysfunctional, the levels of Th1 and Th2 cells are adjusted to ensure the normal function. Studies have shown that when animals suffer from CTX, the secretion levels of CD3+, CD4+, CD8+, and CD4+/CD8+ are reduced, resulting in an imbalance of Th1/Th2 cell proportion, decreased immune function, and induced secondary infection [77]. The findings of our study are consistent with this conclusion. The results demonstrated a significant decrease in the levels of immune cytokines in the serum of mice in the IM group, as well as the secretion of CD3+, CD4+, CD8+ lymphocytes, and the ratio of CD4+/CD8+ in spleen, thymus, and mesenteric lymph nodes. Gavage with R. mucilaginosa ZTHY2 could significantly increase the levels of immune cytokines, CD4, and CD8 molecules in immunosuppressed mice, increased the percentage of CD3+, CD4+, CD8+ lymphocytes and the ratio of CD4+/CD8+, restoring the balance of Th1/Th2 cells and thus enhancing the cellular immune function of immunosuppressed mice.
CD19 is an antigen involved in B cell proliferation, differentiation, and antibody production and can promote the signal transduction of B cell receptors [78]. CD20 is a surface marker of B cells and plays an important regulatory role in the differentiation and proliferation of B cells [79]. B lymphocytes produce immunoglobulin, an important component of humoral immunity, in response to antigen stimulation. IgA has an anti-infection effect, IgG is the main immunoglobulin produced by B lymphocyte activation, and IgM is the earliest immunoglobulin produced by the immune system in response to antigens, and its proportion in the blood is only 10% [80,81]. In this study, gavage of R. mucilaginosa ZTHY2 in immunosuppressed mice increased the level of IgA, IgG, and CD19 and CD20 content in serum; promoted the secretion of CD19+ and CD20+ B lymphocytes; and regulated humoral immune function. Our results were consistent with those of other species like Penaeus vannamei and juvenile Atlantic salmon [82,83].
External factors or a decline in autoimmunity may cause immune suppression, leading to a decrease in immune and antioxidant function [84]. When the antioxidant capacity is reduced, excess ROS are generated, and their reaction with unsaturated fatty acids produces MDA, a lipid peroxidation product. The content of MDA is an important index to evaluate oxidative damage [85]. SOD is involved in regulating the redox reaction and maintaining the oxidation function by scavenging oxygen free radicals [86]. CAT is mainly present in chloroplasts of plants and in the liver and red blood cells of animals, in especially high concentrations in the liver, and it can decompose hydrogen peroxide into O2 and H2O. If hydrogen peroxide cannot be decomposed in time, it causes serious damage to the body [87,88]. There are two forms of glutathione: reduced glutathione and oxidized glutathione. Reduced glutathione, which is generally 90–95% in content, works in concert with other cellular antioxidants to remove free radicals; it can thereby relieve oxidative stress [89]. Studies on R. mucilaginosa in Nile tilapia showed that SOD activity was improved both in serum and the liver, as well as the T-AOC in the serum, and the MDA content was notably reduced in the liver [10]. Our results showed that R. mucilaginosa ZTHY2 could decrease ROS and MDA levels in the spleen and increase SOD and CAT activities in immunosuppressed mice, which is consistent with the results in tilapia. These results suggest that R. mucilaginosa ZTHY2 can promote antioxidant levels in both normal and immunosuppressed animals.
5. Conclusions
Mice were fed with R. mucilaginosa ZTHY2 for 28 days and then treated with CTX. The results showed that R. mucilaginosa ZTHY2 could enhance immune function and antioxidant capacity against CTX. Our study provides theoretical support for the use of R. mucilaginosa ZTHY2 as a feed additive in livestock and poultry as an immunomodulator to improve disease resistance and adaptability, as well as a reagent to attenuate the adverse effects of CTX.
Author Contributions
Conceptualization, Z.C.; writing the article, K.K.; validation, X.D. and W.X.; resources, J.C. and Z.C.; funding supervision, H.L.; funding acquisition, Z.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by program for scientific research start-up funds of Guangdong Ocean University, Effects and Molecular Mechanism of Astaxanthin from R. mucilaginosa on AFB1Induced Hepatotoxicity in Broilers (Grant No. R20061), and Guangdong Provincial Key Areas of Higher Education Project, Research and Development of Astaxanthin, a Feed-Resistant Product of R. mucilaginosa (Grant No. 2020ZDZX1043).
Institutional Review Board Statement
All experimental protocols were approved by the Animal Ethics Committee of Guangdong Ocean University (IACUC No. GDOU-LAE-2020-007), and were performed according to the ethical guidelines of the European Community.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that all the data used in the article supporting this study are available within the article.
Acknowledgments
We are grateful to Hwa-Chain Robert Wang for his scientific and editorial critical review of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Tkacova, J.; Zara, G.; Ianiri, G.; Castoria, R.; Certik, M.; Mannazzu, I. Impairment of carotenoid biosynthesis through CAR1 gene mutation results in CoQ(10), sterols, and phytoene accumulation in Rhodotorula mucilaginosa. Appl. Microbiol. Biotechnol. 2022, 106, 317–327. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Cheng, P.; Yu, G. Rhodotorula mucilaginosa-alternative sources of natural carotenoids, lipids, and enzymes for industrial use. Heliyon 2022, 8, e11505. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, L.; Zhang, Y.; Yan, Y. Production, Characterization and Immunomodulatory Activity of an Extracellular Polysaccharide from Rhodotorula mucilaginosa YL-1 Isolated from Sea Salt Field. Mar. Drugs 2020, 18, 595. [Google Scholar] [CrossRef]
- Savini, V.; Sozio, F.; Catavitello, C.; Talia, M.; Manna, A.; Febbo, F.; Balbinot, A.; Di Bonaventura, G.; Piccolomini, R.; Parruti, G.; et al. Femoral prosthesis infection by Rhodotorula mucilaginosa. J. Clin. Microbiol. 2008, 46, 3544–3545. [Google Scholar] [CrossRef]
- Maeder, M.; Vogt, P.R.; Schaer, G.; von Graevenitz, A.; Gunthard, H.F. Aortic homograft endocarditis caused by Rhodotorula mucilaginosa. Infection 2003, 31, 181–183. [Google Scholar] [CrossRef]
- Fung, H.B.; Martyn, C.A.; Shahidi, A.; Brown, S.T. Rhodotorula mucilaginosa lymphadenitis in an HIV-infected patient. Int. J. Infect. Dis. 2009, 13, e27–e29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martini, K.; Muller, H.; Huemer, H.P.; Hopfl, R. Nail psoriasis masqueraded by secondary infection with Rhodotorula mucilaginosa. Mycoses 2013, 56, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Tligui, H.; Oudaina, W.; El, F.S.; Madda, F.; Hesseissen, L. [A skin ulcer infection due to Rhodotorula mucilaginosa in an immunocompromised child]. J. Mycol. Med. 2018, 28, 215–217. [Google Scholar] [CrossRef]
- Hu, P.; Mao, J.; Zeng, Y.; Sun, Z.; Deng, H.; Chen, C.; Sun, W.; Tang, Z. Isolation, Identification, and Function of Rhodotorula mucilaginosa TZR(2014) and Its Effects on the Growth and Health of Weaned Piglets. Front. Microbiol. 2022, 13, 922136. [Google Scholar]
- Chen, X.Q.; Zhao, W.; Xie, S.W.; Xie, J.J.; Zhang, Z.H.; Tian, L.X.; Liu, Y.J.; Niu, J. Effects of dietary hydrolyzed yeast (Rhodotorula mucilaginosa) on growth performance, immune response, antioxidant capacity and histomorphology of juvenile Nile tilapia (Oreochromis niloticus). Fish. Shellfish. Immunol. 2019, 90, 30–39. [Google Scholar] [CrossRef]
- Sun, J.; Li, M.; Tang, Z.; Zhang, X.; Chen, J.; Sun, Z. Effects of Rhodotorula mucilaginosa fermentation product on the laying performance, egg quality, jejunal mucosal morphology and intestinal microbiota of hens. J. Appl. Microbiol. 2020, 128, 54–64. [Google Scholar] [PubMed]
- Kot, A.M.; Blazejak, S.; Kurcz, A.; Gientka, I.; Kieliszek, M. Rhodotorula glutinis-potential source of lipids, carotenoids, and enzymes for use in industries. Appl. Microbiol. Biotechnol. 2016, 100, 6103–6117. [Google Scholar] [PubMed]
- Kewcharoen, W.; Srisapoome, P. Probiotic effects of Bacillus spp. from Pacific white shrimp (Litopenaeus vannamei) on water quality and shrimp growth, immune responses, and resistance to Vibrio parahaemolyticus (AHPND strains). Fish. Shellfish. Immunol. 2019, 94, 175–189. [Google Scholar] [PubMed]
- Sattler, S. The Role of the Immune System Beyond the Fight Against Infection. Adv. Exp. Med. Biol. 2017, 1003, 3–14. [Google Scholar]
- McNeela, E.A.; Mills, K.H. Manipulating the immune system: Humoral versus cell-mediated immunity. Adv. Drug Deliv. Rev. 2001, 51, 43–54. [Google Scholar]
- Hogan, B.V.; Peter, M.B.; Shenoy, H.G.; Horgan, K.; Hughes, T.A. Surgery induced immunosuppression. Surgeon 2011, 9, 38–43. [Google Scholar]
- Ward, P.A. Immunosuppression after trauma. Crit. Care Med. 2005, 33, 1453–1454. [Google Scholar] [CrossRef]
- Chen, W.; Chen, S.; Nie, Y.; Li, W.; Li, H.; Zhang, X.; Chen, F.; Xie, Q. Synergistic Immunosuppression of Avian Leukosis Virus Subgroup J and Infectious Bursal Disease Virus Is Responsible for Enhanced Pathogenicity. Viruses 2022, 14, 2312. [Google Scholar] [CrossRef]
- You, L.; Nepovimova, E.; Valko, M.; Wu, Q.; Kuca, K. Mycotoxins and cellular senescence: The impact of oxidative stress, hypoxia, and immunosuppression. Arch. Toxicol. 2023, 97, 393–404. [Google Scholar]
- Depmeier, C.; Gunthard, H.F.; Steiner, U.C. Infektionen unter Immunsuppression [Infections during Immunosuppression]. Praxis 2018, 107, 689–698. [Google Scholar] [CrossRef]
- Amadori, M.; Zanotti, C. Immunoprophylaxis in intensive farming systems: The way forward. Vet. Immunol. Immunopathol. 2016, 181, 2–9. [Google Scholar] [PubMed]
- Li, S.; Han, M.; Zhang, Y.; Ishfaq, M.; Liu, R.; Wei, G.; Zhang, X.; Zhang, X. Effect of Curcumin as Feed Supplement on Immune Response and Pathological Changes of Broilers Exposed to Aflatoxin B1. Biomolecules 2022, 12, 1188. [Google Scholar]
- El-Shall, N.A.; Jiang, S.; Farag, M.R.; Azzam, M.; Al-Abdullatif, A.A.; Alhotan, R.; Dhama, K.; Hassan, F.U.; Alagawany, M. Potential of Spirulina platensis as a feed supplement for poultry to enhance growth performance and immune modulation. Front. Immunol. 2023, 14, 1072787. [Google Scholar] [PubMed]
- Hoseinifar, S.H.; Roosta, Z.; Hajimoradloo, A.; Vakili, F. The effects of Lactobacillus acidophilus as feed supplement on skin mucosal immune parameters, intestinal microbiota, stress resistance and growth performance of black swordtail (Xiphophorus helleri). Fish. Shellfish. Immunol. 2015, 42, 533–538. [Google Scholar]
- Guarnera, L.; Meconi, F.; Secchi, R.; Pascale, M.R.; Esposito, F.; Zizzari, A.; Rapisarda, V.M.; Rizzo, M.; Pupo, L.; Cantonetti, M. Efficacy and Safety of Cyclophosphamide Low-Dose Pre-Phase Chemotherapy in Diffuse Large B Cell Lymphoma with Gastrointestinal Involvement. Mediterr. J. Hematol. Infect. Dis. 2022, 14, e2022017. [Google Scholar] [CrossRef]
- Zhong, S.; Huang, M.; Yang, X.; Liang, L.; Wang, Y.; Romkes, M.; Duan, W.; Chan, E.; Zhou, S.F. Relationship of glutathione S-transferase genotypes with side-effects of pulsed cyclophosphamide therapy in patients with systemic lupus erythematosus. Br. J. Clin. Pharmacol. 2006, 62, 457–472. [Google Scholar]
- Childress, M.O.; Christian, J.A.; Ramos-Vara, J.A.; Rosen, N.K.; Ruple, A. Greater baseline serum C-reactive protein concentrations are associated with reduced survival in dogs receiving cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy for primary nodal diffuse large B-cell lymphoma. Vet. J. 2022, 289, 105911. [Google Scholar] [CrossRef]
- Machado, M.C.; Yamamoto, P.A.; Pippa, L.F.; de Moraes, N.V.; Neves, F.; Portela, R.D.; Barrouin-Melo, S.M.; Hielm-Bjorkman, A.; Godoy, A.; Estrela-Lima, A. Pharmacokinetics of Carboplatin in Combination with Low-Dose Cyclophosphamide in Female Dogs with Mammary Carcinoma. Animals 2022, 12, 3109. [Google Scholar]
- O’Connell, K.; Thomson, M.; Morgan, E.; Henning, J. Procarbazine, prednisolone and cyclophosphamide oral combination chemotherapy protocol for canine lymphoma. Vet. Comp. Oncol. 2022, 20, 613–622. [Google Scholar] [CrossRef]
- Todd, J.; Thomas, P.; Nguyen, S. Cyclophosphamide and prednisolone for chemotherapy naive B cell multicentric lymphoma in dogs: 32 cases (2017–2021). J. Small Anim. Pract. 2022, 63, 52–55. [Google Scholar] [CrossRef]
- Sunpongsri, S.; Kovitvadhi, A.; Rattanasrisomporn, J.; Trisaksri, V.; Jensirisak, N.; Jaroensong, T. Effectiveness and Adverse Events of Cyclophosphamide, Vincristine, and Prednisolone Chemotherapy in Feline Mediastinal Lymphoma Naturally Infected with Feline Leukemia Virus. Animals 2022, 12, 900. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, R.V. Assessment of Efficacy and Toxicity of Cyclophosphamide Chemotherapy in Canines with Malignant Mammary Tumor: A Retrospective Study. Vet. Med. Int. 2021, 2021, 5520603. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Wouda, R.M.; Borrego, J.; Chon, E. Cyclophosphamide rescue therapy for relapsed low-grade alimentary lymphoma after chlorambucil treatment in cats. J. Feline Med. Surg. 2021, 23, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Khordad, E.; Alipour, F.; Pourabbas, M.; Mansouri, S.; Salimnejad, R. Hepatoprotective Impact of Ghrelin against Cyclophosphamide-Induced Toxicity in the Male Mice. Drug Res. 2021, 71, 407–412. [Google Scholar] [CrossRef]
- Tang, F.; Zhong, Q.; Yang, Z.; Li, H.; Pan, C.; Huang, L.; Ni, T.; Deng, R.; Wang, Z.; Tan, S.; et al. Low-dose cyclophosphamide combined with IL-2 inhibits tumor growth by decreasing regulatory T cells and increasing CD8+ T cells and natural killer cells in mice. Immunobiology 2022, 227, 152212. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.I.; Rehman, A.U.; Farooqui, N.A.; Siddiqui, N.Z.; Ayub, Q.; Ramzan, M.N.; Zexu, W.; Zhang, X.; Yu, Y.; Xin, Y.; et al. Shrimp peptide hydrolysate modulates the immune response in cyclophosphamide immunosuppressed mice model. J. Food Biochem. 2022, 46, e14251. [Google Scholar] [CrossRef]
- Ali, M.S.; Lee, E.B.; Quah, Y.; Birhanu, B.T.; Suk, K.; Lim, S.K.; Park, S.C. Heat-killed Limosilactobacillus reuteri PSC102 Ameliorates Impaired Immunity in Cyclophosphamide-induced Immunosuppressed Mice. Front. Microbiol. 2022, 13, 820838. [Google Scholar] [CrossRef]
- Baharmi, S.; Kalantari, H.; Kalantar, M.; Goudarzi, M.; Mansouri, E.; Kalantar, H. Pretreatment with Gallic Acid Mitigates Cyclophosphamide Induced Inflammation and Oxidative Stress in Mice. Curr. Mol. Pharmacol. 2022, 15, 204–212. [Google Scholar]
- Shan, C.; Miao, F. Immunomodulatory and antioxidant effects of hydroxytyrosol in cyclophosphamide-induced immunosuppressed broilers. Poult. Sci. 2022, 101, 101516. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Y.; Xie, W.; Xu, C. Isolation and identification of marine Rhodotorula in a coastal area near Leizhou Peninsula. J. Trop. Oceanogr. 2017, 36, 87–92. [Google Scholar]
- Huang, K.S.; Liao, Z.Y.; Xu, C.H.; Xie, W.T. Extraction, Separation and Purification Methods of Astaxanthin Pure Product from Rhodotorula mucilaginosa. Nat. Prod. Res. Dev. 2018, 30, 1858–1862, 1877. [Google Scholar]
- Ge, Y.; Huang, K.; Xie, W.; Xu, C.; Yao, Q.; Liu, Y. Effects of Rhodotorula mucilaginosa on the Immune Function and Gut Microbiota of Mice. Front. Fungal Biol. 2021, 2, 705696. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Tu, H.; Yin, X.; Peng, C.; Dou, C.; Yang, W.; Wu, W.; Guan, X.; Li, J.; Yan, H.; et al. Targeting Glutamine Metabolism Ameliorates Autoimmune Hepatitis via Inhibiting T Cell Activation and Differentiation. Front. Immunol. 2022, 13, 880262. [Google Scholar] [CrossRef]
- Adori, M.; Khoenkhoen, S.; Zhang, J.; Dopico, X.C.; Karlsson, H.G. Enhanced B Cell Receptor Signaling Partially Compensates for Impaired Toll-like Receptor 4 Responses in LPS-Stimulated IkappaBNS-Deficient B Cells. Cells 2023, 12, 1229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Jiang, S.; Tang, Y.; Ding, G. Effects of Low Molecular Weight Peptides from Red Shrimp (Solenocera crassicornis) Head on Immune Response in Immunosuppressed Mice. Int. J. Mol. Sci. 2023, 24, 10297. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, B.; Sun, X.; Zhang, M.; Xiao, D.; Lin, S.; Liu, Z.; Cui, W.; Lin, Y. Tetrahedral-Framework Nucleic Acid Loaded with MicroRNA-155 Enhances Immunocompetence in Cyclophosphamide-Induced Immunosuppressed Mice by Modulating Dendritic Cells and Macrophages. ACS Appl. Mater. Interfaces 2023, 15, 7793–7803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, S.; Li, H.; Cao, M.; Li, W.; Liu, X. Immunomodulatory effects of selenium-enriched peptides from soybean in cyclophosphamide-induced immunosuppressed mice. Food Sci. Nutr. 2021, 9, 6322–6334. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Li, C.; Shao, Y.; Chen, A. Polysaccharides from Spores of Cordyceps cicadae Protect against Cyclophosphamide-Induced Immunosuppression and Oxidative Stress in Mice. Foods 2022, 11, 515. [Google Scholar] [CrossRef]
- Gao, X.; Zeng, R.; Qi, J.; Ho, C.T.; Li, B.; Chen, Z.; Chen, S.; Xiao, C.; Hu, H.; Cai, M.; et al. Immunoregulatory activity of a low-molecular-weight heteropolysaccharide from Ganoderma leucocontextum fruiting bodies in vitro and in vivo. Food Chem. X 2022, 14, 100321. [Google Scholar] [CrossRef]
- Perdigon, G.; de Macias, M.; Alvarez, S.; Medici, M.; Oliver, G.; de Ruiz, H.A. Effect of a Mixture of Lactobacillus casei and Lactobacillus acidophilus Administered Orally on the Immune System in Mice. J. Food Prot. 1986, 49, 986–989. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Yan, H.; Ning, Z.; Wang, Z. Lactobacillus salivarius SNK-6 Activates Intestinal Mucosal Immune System by Regulating Cecal Microbial Community Structure in Laying Hens. Microorganisms 2022, 10, 1469. [Google Scholar] [CrossRef] [PubMed]
- Vakili, F.; Roosta, Z.; Safari, R.; Raeisi, M.; Hossain, M.S.; Guerreiro, I.; Akbarzadeh, A.; Hoseinifar, S.H. Effects of dietary nutmeg (Myristica fragrans) seed meals on growth, non-specific immune indices, antioxidant status, gene expression analysis, and cold stress tolerance in zebrafish (Danio rerio). Front. Nutr. 2022, 9, 1038748. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Astill, J.; Alqazlan, N.; Shojadoost, B.; Taha-Abdelaziz, K.; Bavananthasivam, J.; Doost, J.S.; Sedeghiisfahani, N.; Sharif, S. In ovo co-administration of vitamins (A and D) and probiotic lactobacilli modulates immune responses in broiler chickens. Poult. Sci. 2022, 101, 101717. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Nie, C.; Wu, Y.; Luo, R.; Chen, C.; Niu, J.; Zhang, W. Effects of two strains of Lactobacillus isolated from the feces of calves after fecal microbiota transplantation on growth performance, immune capacity, and intestinal barrier function of weaned calves. Front. Microbiol. 2023, 14, 1249628. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.E.; Joh, E.H.; Lee, H.Y.; Ahn, Y.T.; Lee, J.H.; Huh, C.S.; Han, M.J.; Kim, D.H. Lactobacillus plantarum HY7712 ameliorates cyclophosphamide-induced immunosuppression in mice. J. Microbiol. Biotechnol. 2013, 23, 414–421. [Google Scholar] [CrossRef]
- Jang, S.E.; Joh, E.H.; Ahn, Y.T.; Huh, C.S.; Han, M.J.; Kim, D.H. Lactobacillus casei HY7213 ameliorates cyclophosphamide-induced immunosuppression in mice by activating NK, cytotoxic T cells and macrophages. Immunopharmacol. Immunotoxicol. 2013, 35, 396–402. [Google Scholar] [CrossRef]
- Xie, J.H.; Fan, S.T.; Nie, S.P.; Yu, Q.; Xiong, T.; Gong, D.; Xie, M.Y. Lactobacillus plantarum NCU116 attenuates cyclophosphamide-induced intestinal mucosal injury, metabolism and intestinal microbiota disorders in mice. Food Funct. 2016, 7, 1584–1592. [Google Scholar] [CrossRef]
- Xie, J.; Nie, S.; Yu, Q.; Yin, J.; Xiong, T.; Gong, D.; Xie, M. Lactobacillus plantarum NCU116 Attenuates Cyclophosphamide-Induced Immunosuppression and Regulates Th17/Treg Cell Immune Responses in Mice. J. Agric. Food Chem. 2016, 64, 1291–1297. [Google Scholar] [CrossRef]
- Aguilera, N.S.; Auerbach, A. T-cell lymphoproliferative processes in the spleen. Semin. Diagn. Pathol. 2020, 37, 47–56. [Google Scholar] [CrossRef]
- Kim, Y.O.; Han, S.B.; Lee, H.W.; Ahn, H.J.; Yoon, Y.D.; Jung, J.K.; Kim, H.M.; Shin, C.S. Immuno-stimulating effect of the endo-polysaccharide produced by submerged culture of Inonotus obliquus. Life Sci. 2005, 77, 2438–2456. [Google Scholar] [CrossRef]
- Zhou, R.; He, D.; Xie, J.; Zhou, Q.; Zeng, H.; Li, H.; Huang, L. The Synergistic Effects of Polysaccharides and Ginsenosides From American Ginseng (Panax quinquefolius L.) Ameliorating Cyclophosphamide-Induced Intestinal Immune Disorders and Gut Barrier Dysfunctions Based on Microbiome-Metabolomics Analysis. Front. Immunol. 2021, 12, 665901. [Google Scholar] [CrossRef]
- Zheng, S.; Zheng, H.; Zhang, R.; Piao, X.; Hu, J.; Zhu, Y.; Wang, Y. Immunomodulatory Effect of Ginsenoside Rb2 Against Cyclophosphamide-Induced Immunosuppression in Mice. Front. Pharmacol. 2022, 13, 927087. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah-Hassan, A.; Shalaby, S.I.; Khater, S.I.; El-Shetry, E.S.; Abd, E.F.H.; Elsayed, S.A. Panax ginseng is superior to vitamin E as a hepatoprotector against cyclophosphamide-induced liver damage. Complement. Ther. Med. 2019, 46, 95–102. [Google Scholar] [CrossRef]
- Cancro, M.P.; Tomayko, M.M. Memory B cells and plasma cells: The differentiative continuum of humoral immunity. Immunol. Rev. 2021, 303, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Steinert, E.M.; Vasan, K.; Chandel, N.S. Mitochondrial Metabolism Regulation of T Cell-Mediated Immunity. Annu. Rev. Immunol. 2021, 39, 395–416. [Google Scholar] [CrossRef] [PubMed]
- Rocamora-Reverte, L.; Melzer, F.L.; Wurzner, R.; Weinberger, B. The Complex Role of Regulatory T Cells in Immunity and Aging. Front. Immunol. 2020, 11, 616949. [Google Scholar] [CrossRef]
- Frank, A.M.; Braun, A.H.; Scheib, L.; Agarwal, S.; Schneider, I.C.; Fusil, F.; Perian, S.; Sahin, U.; Thalheimer, F.B.; Verhoeyen, E.; et al. Combining T-cell-specific activation and in vivo gene delivery through CD3-targeted lentiviral vectors. Blood Adv. 2020, 4, 5702–5715. [Google Scholar]
- Younes, A.K.; Hammad, R.; Othman, M.; Sobhy, A. CD4, CD8 and natural killer cells are depressed in patients with alopecia areata: Their association with disease activity. BMC Immunol. 2022, 23, 13. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; Huang, M. Metabolic Regulation of CD8(+) T Cells: From Mechanism to Therapy. Antioxid. Redox Signal 2022, 37, 1234–1253. [Google Scholar] [CrossRef]
- Malagnino, V.; Cerva, C.; Teti, E.; Campogiani, L.; Compagno, M.; Foroghi, B.L.; Saderi, L.; Armenia, D.; Salpini, R.; Svicher, V.; et al. Poor CD4/CD8 ratio recovery in HBcAb-positive HIV patients with worse immune status is associated with significantly higher CD8 cell numbers. Sci. Rep. 2021, 11, 3965. [Google Scholar] [CrossRef]
- Perez-Mazliah, D.; Langhorne, J. CD4 T-cell subsets in malaria: TH1/TH2 revisited. Front. Immunol. 2014, 5, 671. [Google Scholar] [CrossRef]
- Tian, H.; Xing, J.; Tang, X.; Sheng, X.; Chi, H.; Zhan, W. Interactions of interleukin 2 (IL-2) and IL-2 receptors mediate the activities of B lymphocytes in flounder (Paralichthys olivaceus). Int. J. Biol. Macromol. 2023, 227, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, N.; Ravanshad, M.; Asadi, B.; Kianfar, R.; Maleki, A. Investigation of IL-2 and IFN-gamma to EBV Peptides in Stimulated Whole Blood among Multiple Sclerosis Patients and Healthy Individuals. Intervirology 2021, 64, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Salomon, B.L.; Leclerc, M.; Tosello, J.; Ronin, E.; Piaggio, E.; Cohen, J.L. Tumor Necrosis Factor alpha and Regulatory T Cells in Oncoimmunology. Front. Immunol. 2018, 9, 444. [Google Scholar] [CrossRef] [PubMed]
- Czimmerer, Z.; Halasz, L.; Daniel, B.; Varga, Z.; Bene, K.; Domokos, A.; Hoeksema, M.; Shen, Z.; Berger, W.K.; Cseh, T.; et al. The epigenetic state of IL-4-polarized macrophages enables inflammatory cistromic expansion and extended synergistic response to TLR ligands. Immunity 2022, 55, 2006–2026. [Google Scholar] [CrossRef]
- Qing, H.; Desrouleaux, R.; Israni-Winger, K.; Mineur, Y.S.; Fogelman, N.; Zhang, C.; Rashed, S.; Palm, N.W.; Sinha, R.; Picciotto, M.R.; et al. Origin and Function of Stress-Induced IL-6 in Murine Models. Cell 2020, 182, 372–387. [Google Scholar] [CrossRef]
- Yan, H.; Lu, J.; Wang, J.; Chen, L.; Wang, Y.; Li, L.; Miao, L.; Zhang, H. Prevention of Cyclophosphamide-Induced Immunosuppression in Mice With Traditional Chinese Medicine Xuanfei Baidu Decoction. Front. Pharmacol. 2021, 12, 730567. [Google Scholar] [CrossRef]
- Wang, L.; Bhardwaj, R.; Mostowski, H.; Patrone, P.N.; Kearsley, A.J.; Watson, J.; Lim, L.; Pichaandi, J.; Ornatsky, O.; Majonis, D.; et al. Establishing CD19 B-cell reference control materials for comparable and quantitative cytometric expression analysis. PLoS ONE 2021, 16, e248118. [Google Scholar] [CrossRef]
- Payandeh, Z.; Bahrami, A.A.; Hoseinpoor, R.; Mortazavi, Y.; Rajabibazl, M.; Rahimpour, A.; Taromchi, A.H.; Khalil, S. The applications of anti-CD20 antibodies to treat various B cells disorders. Biomed. Pharmacother. 2019, 109, 2415–2426. [Google Scholar] [CrossRef]
- Werner, A.; Nimmerjahn, F. HINGEneering IgG for enhanced immune activation. Sci. Immunol. 2022, 7, q4797. [Google Scholar] [CrossRef]
- Wang, J.; Wu, C.S.; Hu, Y.Z.; Yang, L.; Zhang, X.J.; Zhang, Y.A. Plasmablasts induced by chitosan oligosaccharide secrete natural IgM to enhance the humoral immunity in grass carp. Carbohydr. Polym. 2022, 281, 119073. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Z.; Wang, C.; Li, E.; Qin, J.G.; Chen, L. Dietary supplementation of selenium yeast enhances the antioxidant capacity and immune response of juvenile Eriocheir sinensis under nitrite stress. Fish. Shellfish. Immunol. 2019, 87, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, Y.; Luo, K.; Zhang, S.; Wei, C.; Wang, L.; Qiu, Y.; Tian, X. ‘Biotic’ potential of the red yeast Rhodotorula mucilaginosa strain JM-01 on the growth, shell pigmentation, and immune defense attributes of the shrimp, Penaeus vannamei. Aquaculture 2023, 572, 739543. [Google Scholar] [CrossRef]
- Marefati, N.; Ghorani, V.; Shakeri, F.; Boskabady, M.; Kianian, F.; Rezaee, R.; Boskabady, M.H. A review of anti-inflammatory, antioxidant, and immunomodulatory effects of Allium cepa and its main constituents. Pharm. Biol. 2021, 59, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, Z.Z.; Cheng, Y.L.; Lin, W.; Qu, C. Resveratrol protects against oxidative damage of retinal pigment epithelium cells by modulating SOD/MDA activity and activating Bcl-2 expression. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 378–388. [Google Scholar]
- Li, Z.; Chen, M.; Wang, W.; Liu, Q.; Li, N.; He, B.; Jiang, Y.; Ma, J. Mn-SOD alleviates methotrexate-related hepatocellular injury via GSK-3beta affecting anti-oxidative stress of HO-1 and Drp1. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2022, 47, 1191–1199. [Google Scholar] [PubMed]
- Kwon, K.; Jung, J.; Sahu, A.; Tae, G. Nanoreactor for cascade reaction between SOD and CAT and its tissue regeneration effect. J. Control. Release 2022, 344, 160–172. [Google Scholar] [CrossRef]
- Jiang, F.; Xu, X.R.; Li, W.M.; Xia, K.; Wang, L.F.; Yang, X.C. Monotropein alleviates H2O2-induced inflammation, oxidative stress and apoptosis via NF-kappaB/AP-1 signaling. Mol. Med. Rep. 2020, 22, 4828–4836. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Xue, Y.; Fang, H.; Wu, Z. Serum levels of reduced glutathione, oxidized glutathione, and glutathione reductase activity in minor recurrent aphthous stomatitis patients. J. Dent. Sci. 2023, 18, 1103–1108. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).