Captivating Colors, Crucial Roles: Astaxanthin’s Antioxidant Impact on Fish Oxidative Stress and Reproductive Performance
Abstract
:Simple Summary
Abstract
1. Introduction
- Oxidative stress and fish health
- Mechanisms of astaxanthin as an antioxidant
- Astaxanthin and oxidative stress regulation in fish
- Reproductive performance and oxidative stress: interplay and implications
- Astaxanthin’s influence on fish reproductive performance
- Practical applications of astaxanthin in aquaculture
- Future directions and research gaps
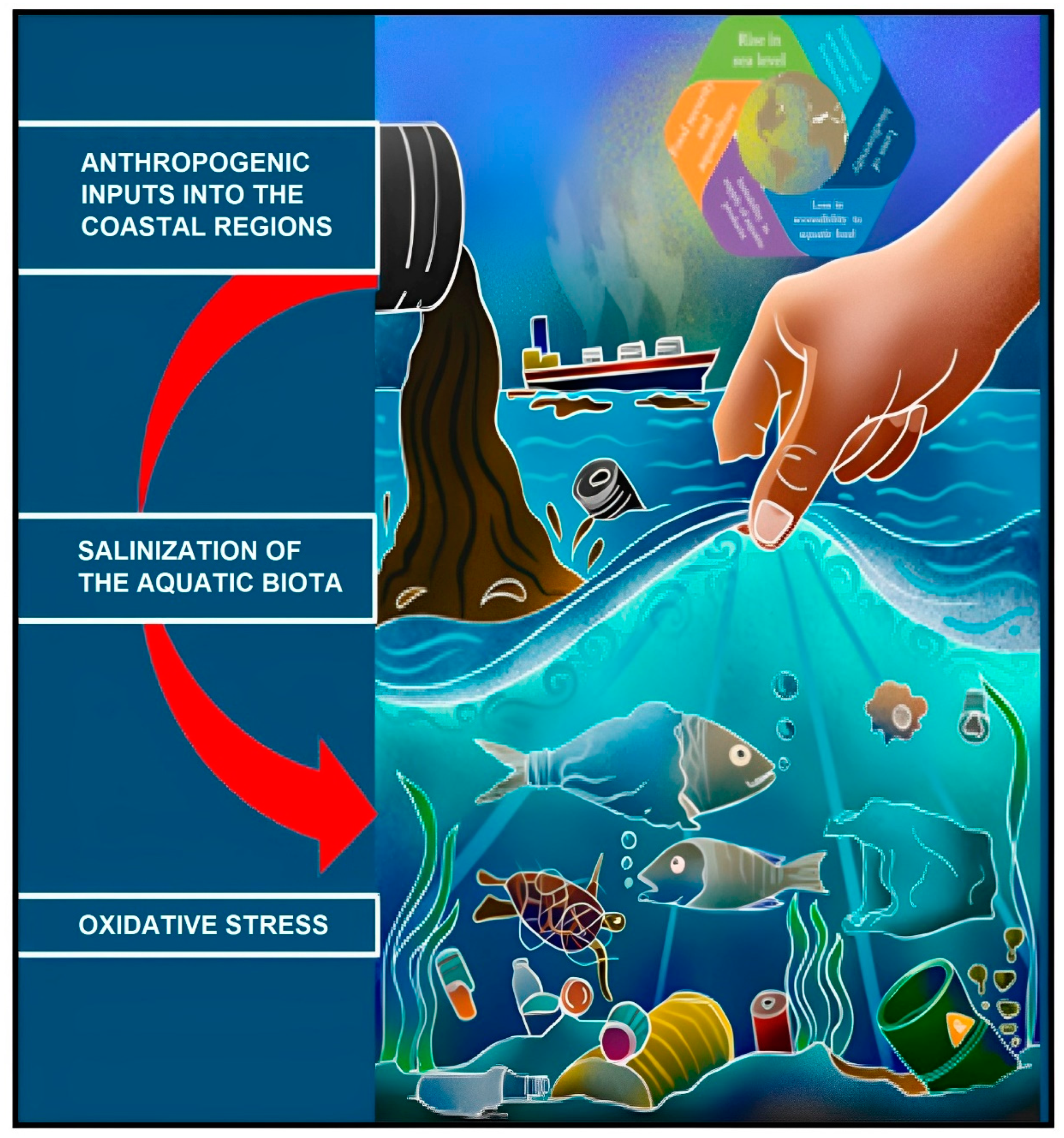
2. Oxidative Stress and Fish Health
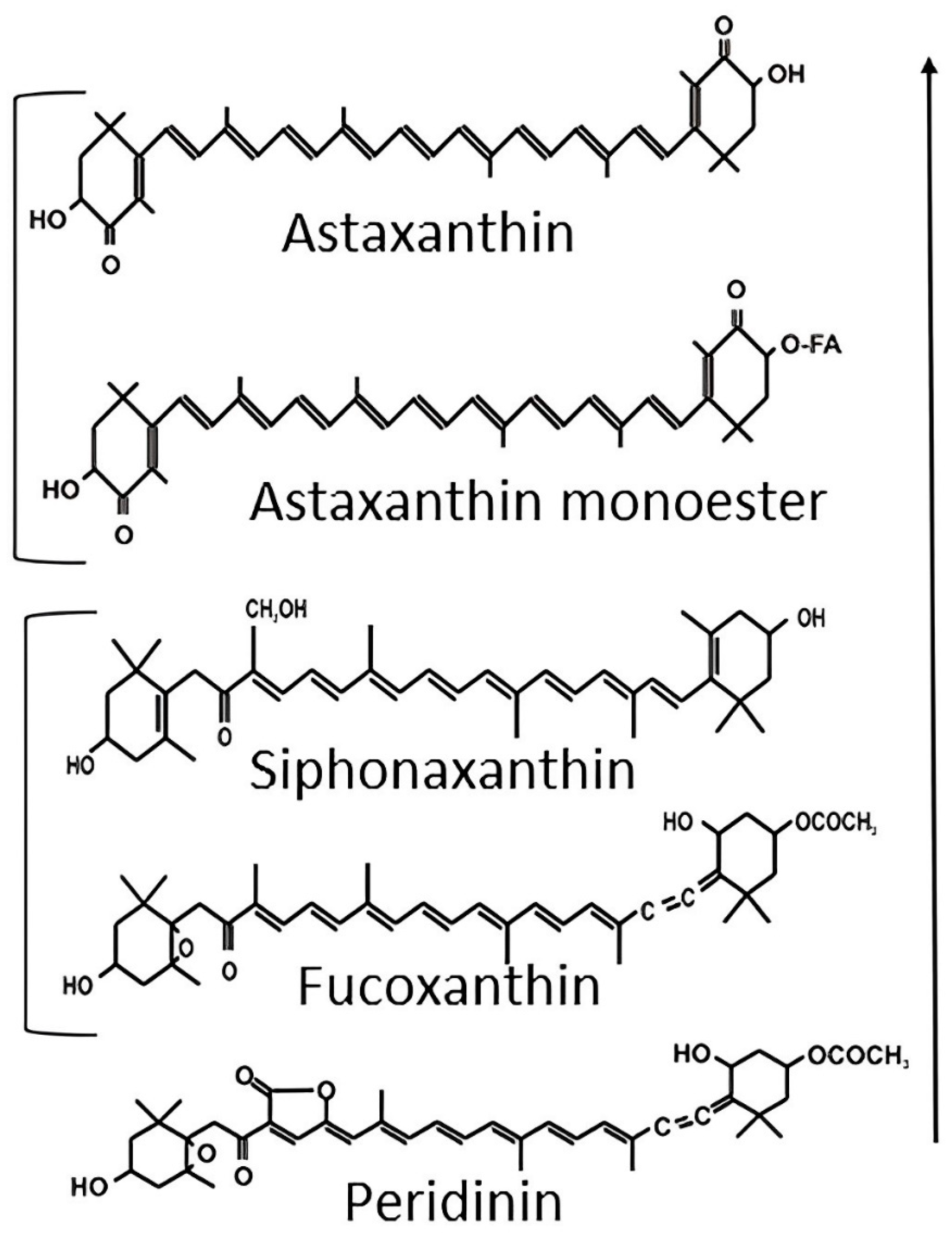
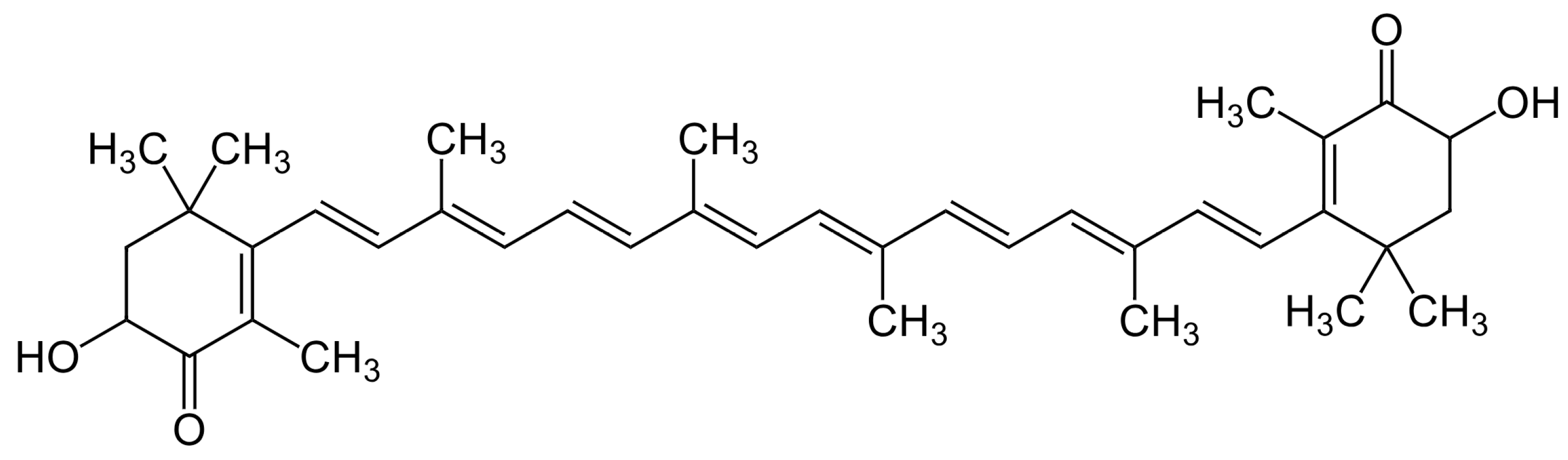
3. Mechanisms of Astaxanthin as an Antioxidant
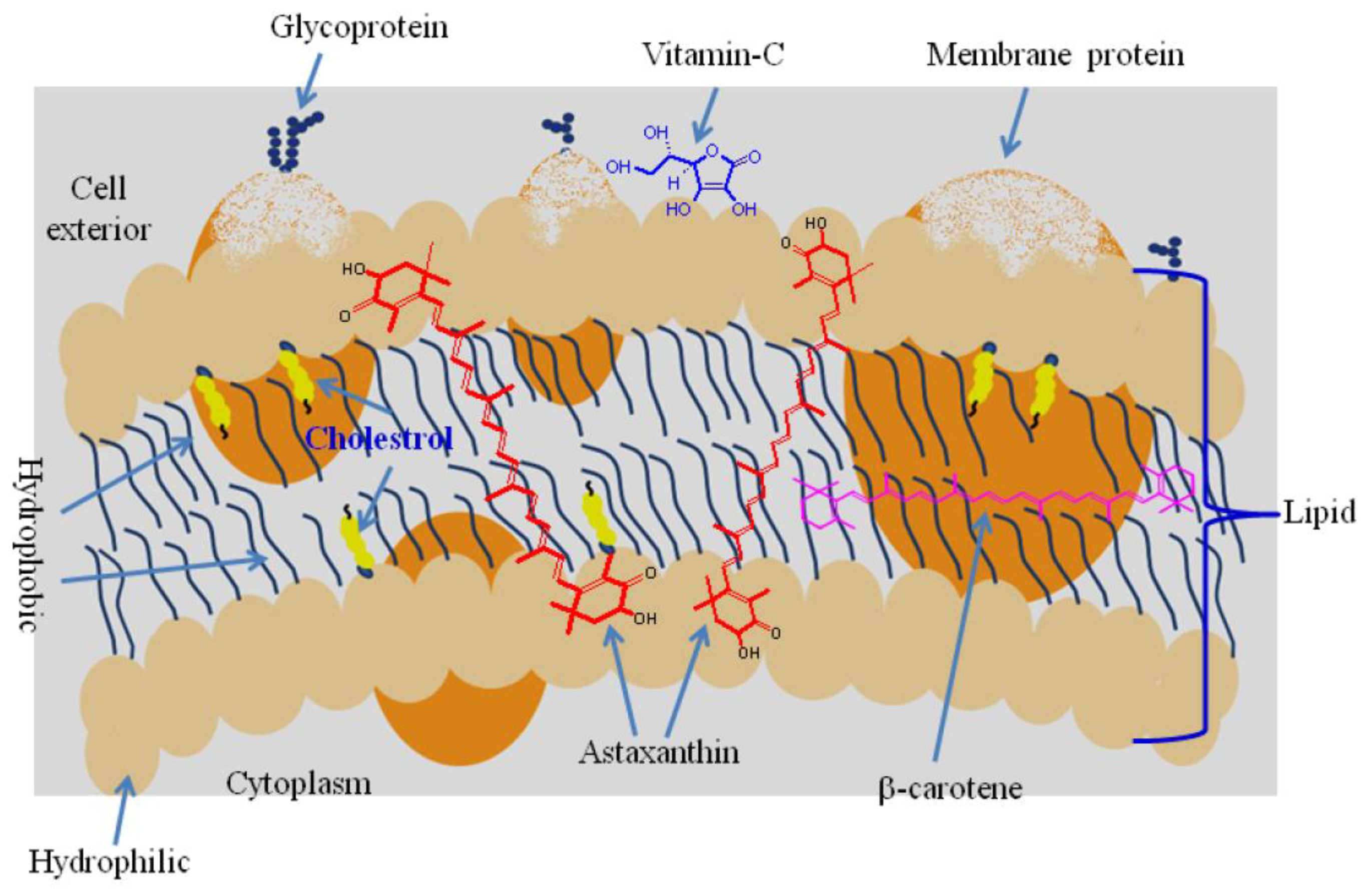
3.1. ROS Scavenging Mechanism
3.2. Lipid Peroxidation Inhibition
4. Astaxanthin and Oxidative Stress Regulation in Fish
5. Reproductive Performance and Oxidative Stress: Interplay and Implications
6. Astaxanthin’s Influence on Fish Reproductive Performance
7. Practical Applications in Aquaculture
8. Future Directions and Research Gaps
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DNA | Deoxyribonucleic acid |
| E2 | Estradiol |
| FCR | Feed conversion ratio |
| FSH | Follicle-stimulating hormone |
| H2O2 | Hydrogen peroxide |
| IU | International Unit |
| LH | Luteinizing hormone |
| MAPKs | Mitogen-activated protein kinases |
| Nrf2 | Nuclear factor erythroid-2 related factor 2 |
| NF-κB | Nuclear factor-kappa B |
| GSSG | Oxidized glutathione |
| ROS | Reactive oxygen species |
| GSH | Reduced glutathione |
| NADPH | Reduced nicotinamide adenine dinucleotide phosphate |
| 1O2 | Singlet oxygen |
| SDP | Soft-dry pellets |
| SOD | Superoxide dismutase |
| TBARS | Thiobarbituric acid-reactive substances |
| T-AOC | Total antioxidant capacity |
References
- Song, C.; Sun, C.; Liu, B.; Xu, P. Oxidative Stress in Aquatic Organisms. Antioxidants 2023, 12, 1223. [Google Scholar] [CrossRef] [PubMed]
- Shastak, Y.; Gordillo, A.; Pelletier, W. The relationship between vitamin A status and oxidative stress in animal production. J. Appl. Anim. Res. 2023, 51, 546–553. [Google Scholar] [CrossRef]
- Slaninova, A.; Smutna, M.; Modra, H.; Svobodova, Z. A review: Oxidative stress in fish induced by pesticides. Neuroendocrinol. Lett. 2009, 30 (Suppl. S1), 2. [Google Scholar] [PubMed]
- Akram, R.; Iqbal, R.; Hussain, R.; Jabeen, F.; Ali, M. Evaluation of Oxidative stress, antioxidant enzymes and genotoxic potential of bisphenol A in fresh water bighead carp (Aristichthys nobils) fish at low concentrations. Environ. Pollut. 2021, 268, 115896. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Hao, R.; Zhang, J.; Tian, C.; Hong, Y.; Zhu, C.; Li, G. Dietary astaxanthin improves the antioxidant capacity, immunity and disease resistance of coral trout (Plectropomus leopardus). Fish Shellfish Immunol. 2022, 122, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, C.T.; Betancor, M.B.; Torrecillas, S.; Sprague, M.; Larroquet, L.; Véron, V.; Panserat, S.; Izquierdo, M.S.; Kaushik, S.J.; Fontagné-Dicharry, S. More Than an Antioxidant: Role of Dietary Astaxanthin on Lipid and Glucose Metabolism in the Liver of Rainbow Trout (Oncorhynchus mykiss). Antioxidants 2023, 12, 136. [Google Scholar] [CrossRef]
- Yao, Q.; Ma, J.; Chen, X.; Zhao, G.; Zang, J. A natural strategy for astaxanthin stabilization and color regulation: Interaction with proteins. Food Chem. 2023, 402, 134343. [Google Scholar] [CrossRef]
- Molino, A.; Rimauro, J.; Casella, P.; Cerbone, A.; Larocca, V.; Chianese, S.; Karatza, D.; Mehariya, S.; Ferraro, A.; Hristoforou, E.; et al. Extraction of astaxanthin from microalga Haematococcus pluvialis in red phase by using generally recognized as safe solventsand accelerated extraction. J. Biotechnol. 2018, 283, 51–61. [Google Scholar] [CrossRef]
- Harith, Z.T.; de Andrade Lima, M.; Charalampopoulos, D.; Chatzifragkou, A. Optimised Production and Extraction of Astaxanthin from the Yeast Xanthophyllomyces dendrorhous. Microorganisms 2020, 8, 430. [Google Scholar] [CrossRef]
- Stachowiak, B.; Szulc, P. Astaxanthin for the Food Industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef]
- Nguyen, K.D. Astaxanthin: A Comparative Case of Synthetic vs. Natural Production. Faculty Publications and Other Works—Chemical and Biomolecular Engineering, The University of Tennessee, Knoxville, TN, USA. 2013. Available online: https://trace.tennessee.edu/cgi/viewcontent.cgi?article=1094&context=utk_chembiopubs (accessed on 23 October 2023).
- Smith, C.T.; Gomez, L.A.; Chile, C.; Cortes, R.A. Astaxanthin effect on reactive oxygen species and leukocytes counts in rainbow trout (Oncorhynchus mykiss). Glob. Virtual Conf. 2013, 8, 451–454. [Google Scholar]
- Fan, K.W.; Chen, F. Production of high-value products by marine microalgae thraustochytrids. In Bioprocessing for Value-Added Products from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2007; pp. 293–323. [Google Scholar]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Balendra, V.; Singh, S.K. Therapeutic potential of astaxanthin and superoxide dismutase in Alzheimer’s disease. Open Biol. 2021, 11, 210013. [Google Scholar] [CrossRef]
- Pawluk, R.J.; de Leaniz, C.G.; Cable, J.; Tiddeman, B.; Consuegra, S. Colour plasticity in response to social context and parasitic infection in a self-fertilizing fish. R. Soc. Open Sci. 2019, 6, 181418. [Google Scholar] [CrossRef]
- Vilgrain, L.; Maps, F.; Basedow, S.; Trudnowska, E.; Madoui, M.A.; Niehoff, B.; Ayata, S.D. Copepods’ True Colors: Astaxanthin Pigmentation as an Indicator of Fitness. Ecosphere 2023, 14, e4489. [Google Scholar] [CrossRef]
- Nie, X.-P.; Zie, J.; Häubner, N.; Tallmark, B.; Snoeijs, P. Why Baltic herring and sprat are weak conduits for astaxanthin from zooplankton to piscivorous fish. Limnol. Oceanogr. 2011, 56, 1155–1167. [Google Scholar] [CrossRef]
- Alam, F.; Syed, H.; Amjad, S.; Baig, M.; Khan, T.A.; Rehman, R. Interplay between oxidative stress, SIRT1, reproductive and metabolic functions. Curr. Res. Physiol. 2021, 4, 119–124. [Google Scholar] [CrossRef]
- Samarin, A.M.; Samarin, A.M.; Policar, T. Cellular and molecular changes associated with fish oocyte ageing. Rev. Aquac. 2019, 11, 619–630. [Google Scholar] [CrossRef]
- Pintus, E.; Ros-Santaella, J.L. Impact of Oxidative Stress on Male Reproduction in Domestic and Wild Animals. Antioxidants 2021, 10, 1154. [Google Scholar] [CrossRef]
- Bhat, R.A.; Bakhshalizadeh, S.; Guerrera, M.C.; Kesbiç, O.S.; Fazio, F. Toxic effect of heavy metals on ovarian deformities, apoptotic changes, oxidative stress, and steroid hormones in rainbow trout. J. Trace Elem. Med. Biol. 2023, 75, 127106. [Google Scholar] [CrossRef]
- Shabanzadeh, S.; Vatandoust, S.; Hosseinifard, S.M.; Sheikhzadeh, N.; Shahbazfar, A.A. Dietary astaxanthin (Lucantin® Pink) mitigated oxidative stress induced by diazinon in rainbow trout (Oncorhynchus mykiss). Vet. Res. Forum 2023, 14, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.F.; Li, Y.W.; Liu, Z.H.; Chen, Q.L. Reproductive toxicity of inorganic mercury exposure in adult zebrafish: Histological damage, oxidative stress, and alterations of sex hormone and gene expression in the hypothalamic-pituitary-gonadal axis. Aquat. Toxicol. 2016, 177, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, C.B. The stress—Reproductive axis in fish: The involvement of functional neuroanatomical systems in the brain. J. Chem. Neuroanat. 2020, 112, 101904. [Google Scholar] [CrossRef]
- Brix, K.V.; De Boeck, G.; Baken, S.; Fort, D.J. Adverse Outcome Pathways for Chronic Copper Toxicity to Fish and Amphibians. Environ. Toxicol. Chem. 2022, 41, 2911–2927. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Hara, S.; Sakima, K.; Nozu, R.; Yazawa, T.; Kitano, T. Oxidative Stress Causes Masculinization of Genetically Female Medaka without Elevating Cortisol. Front. Endocrinol. 2022, 13, 878286. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Kim, J.-H.; Lee, D.-C.; Lim, H.-J.; Kang, J.-C. Toxic Effects on Oxidative Stress, Neurotoxicity, Stress, and Immune Responses in Juvenile Olive Flounder, Paralichthys olivaceus, Exposed to Waterborne Hexavalent Chromium. Biology 2022, 11, 766. [Google Scholar] [CrossRef]
- Lin, W.; Luo, H.; Wu, J.; Liu, X.; Cao, B.; Hung, T.-C.; Liu, Y.; Chen, Z.; Yang, P. Distinct vulnerability to oxidative stress determines the ammonia sensitivity of crayfish (Procambarus clarkii) at different developmental stages. Ecotoxicol. Environ. Saf. 2022, 242, 113895. [Google Scholar] [CrossRef]
- Li, X.; Naseem, S.; Hussain, R.; Ghaffar, A.; Li, K.; Khan, A. Evaluation of DNA Damage, Biomarkers of Oxidative Stress, and Status of Antioxidant Enzymes in Freshwater Fish (Labeo rohita) Exposed to Pyriproxyfen. Oxidative Med. Cell. Longev. 2022, 2022, 5859266. [Google Scholar] [CrossRef]
- Wiens, L.; Banh, S.; Sotiri, E.; Jastroch, M.; Block, B.A.; Brand, M.D.; Treberg, J.R. Comparison of Mitochondrial Reactive Oxygen Species Production of Ectothermic and Endothermic Fish Muscle. Front. Physiol. 2017, 8, 704. [Google Scholar] [CrossRef]
- Onukwufor, J.O.; Berry, B.J.; Wojtovich, A.P. Physiologic Implications of Reactive Oxygen Species Production by Mitochondrial Complex I Reverse Electron Transport. Antioxidants 2019, 8, 285. [Google Scholar] [CrossRef]
- Devaux, J.B.L.; Hedges, C.P.; Birch, N.; Herbert, N.; Renshaw, G.M.C.; Hickey, A.J.R. Electron transfer and ROS production in brain mitochondria of intertidal and subtidal triplefin fish (Tripterygiidae). J. Comp. Physiol. B 2023, 193, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Biller, J.D.; Takahashi, L.S. Oxidative stress and fish immune system: Phagocytosis and leukocyte respiratory burst activity. An. Acad. Bras. Cienc. 2018, 90, 3403–3414. [Google Scholar] [CrossRef] [PubMed]
- Di Cara, F.; Andreoletti, P.; Trompier, D.; Vejux, A.; Bülow, M.H.; Sellin, J.; Lizard, G.; Cherkaoui-Malki, M.; Savary, S. Peroxisomes in Immune Response and Inflammation. Int. J. Mol. Sci. 2019, 20, 3877. [Google Scholar] [CrossRef] [PubMed]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef]
- Lismont, C.; Revenco, I.; Fransen, M. Peroxisomal Hydrogen Peroxide Metabolism and Signaling in Health and Disease. Int. J. Mol. Sci. 2019, 20, 3673. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Miralda, I.; Armstrong, C.L.; Uriarte, S.M.; Bagaitkar, J. The roles of NADPH oxidase in modulating neutrophil effector responses. Mol. Oral Microbiol. 2019, 34, 27–38. [Google Scholar] [CrossRef]
- Farombi, E.O.; Adelowo, O.A.; Ajimoko, Y.R. Biomarkers of Oxidative Stress and Heavy Metal Levels as Indicators of Environmental Pollution in African Cat Fish (Clarias gariepinus) from Nigeria Ogun River. Int. J. Environ. Res. Public Health 2007, 4, 158–165. [Google Scholar] [CrossRef]
- Hurem, S.; Fraser, T.W.; Gomes, T.; Mayer, I.; Christensen, T. Sub-lethal UV radiation during early life stages alters the behaviour, heart rate and oxidative stress parameters in zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2018, 166, 359–365. [Google Scholar] [CrossRef]
- Sreejai, R.; Jaya, D. Studies on the changes in lipid peroxidation and antioxidants in fishes exposed to Hydrogen Sulfide. Toxicol. Int. 2010, 17, 71–77. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Contaminant-induced oxidative stress in fish: A mechanistic approach. Fish Physiol. Biochem. 2016, 42, 711–747. [Google Scholar] [CrossRef] [PubMed]
- Bassoy, E.Y.; Walch, M.; Martinvalet, D. Reactive Oxygen Species: Do They Play a Role in Adaptive Immunity? Front. Immunol. 2021, 12, 755856. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xu, H. Multi-faced roles of reactive oxygen species in anti-tumor T cell immune responses and combination immunotherapy. Explor. Med. 2022, 3, 77–98. [Google Scholar] [CrossRef]
- Félix, F.; Oliveira, C.C.V.; Cabrita, E. Antioxidants in Fish Sperm and the Potential Role of Melatonin. Antioxidants 2020, 10, 36. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, H.-J.; Kim, K.-W.; Hwang, I.-K.; Kim, D.-H.; Oh, C.W.; Lee, J.S.; Kang, J.-C. Growth performance, oxidative stress, and non-specific immune responses in juvenile sablefish, Anoplopoma fimbria, by changes of water temperature and salinity. Fish Physiol. Biochem. 2017, 43, 1421–1431. [Google Scholar] [CrossRef]
- Zengin, H. The effects of feeding and starvation on antioxidant defence, fatty acid composition and lipid peroxidation in reared Oncorhynchus mykiss fry. Sci. Rep. 2021, 11, 16716. [Google Scholar] [CrossRef]
- Janssens, L.; Stoks, R. Chronic predation risk reduces escape speed by increasing oxidative damage: A deadly cost of an adaptive antipredator response. PLoS ONE 2014, 9, e101273. [Google Scholar] [CrossRef]
- Strungaru, S.-A.; Robea, M.A.; Plavan, G.; Todirascu-Ciornea, E.; Ciobica, A.; Nicoara, M. Acute exposure to methylmercury chloride induces fast changes in swimming performance, cognitive processes and oxidative stress of zebrafish (Danio rerio) as reference model for fish community. J. Trace Elem. Med. Biol. 2018, 47, 115–123. [Google Scholar] [CrossRef]
- Loughland, I.; Lau, G.Y.; Jolly, J.; Seebacher, F. Rates of warming impact oxidative stress in zebrafish (Danio rerio). J. Exp. Biol. 2022, 225, jeb243740. [Google Scholar] [CrossRef]
- Bou, M.; Torgersen, J.S.; Østbye, T.-K.K.; Ruyter, B.; Wang, X.; Škugor, S.; Kristiansen, I.Ø.; Todorčević, M. DHA Modulates Immune Response and Mito-chondrial Function of Atlantic Salmon Adipocytes after LPS Treatment. Int. J. Mol. Sci. 2020, 21, 4101. [Google Scholar] [CrossRef]
- Liu, R.; Liu, R.; Song, G.; Li, Q.; Cui, Z.; Long, Y. Mitochondria Dysfunction and Cell Apoptosis Limit Resistance of Nile Tilapia (Oreochromis niloticus) to Lethal Cold Stress. Animals 2022, 12, 2382. [Google Scholar] [CrossRef]
- Otsuka, T.; Matsui, H. Fish Models for Exploring Mitochondrial Dysfunction Affecting Neurodegenerative Disorders. Int. J. Mol. Sci. 2023, 24, 7079. [Google Scholar] [CrossRef]
- Bal, A.; Paital, B. Anthropization, Salinity and Oxidative Stress in Animals in the Coastal Zone. Environ. Sci. Proc. 2023, 25, 7. [Google Scholar]
- Janssens, L.; Stoks, R. Oxidative stress mediates rapid compensatory growth and its costs. Funct. Ecol. 2020, 34, 2087–2097. [Google Scholar] [CrossRef]
- Lackner, R. Oxidative Stress in Fish by Environmental Pollutants. In Fish Ecotoxicology; Braunbeck, T., Hinton, D.E., Streit, B., Eds.; Birkhäuser: Basel, Switzerland, 1998; pp. 203–224. [Google Scholar]
- Serradell, A.; Montero, D.; Terova, G.; Rimoldi, S.; Makol, A.; Acosta, F.; Bajek, A.; Haffray, P.; Allal, F.; Torrecillas, S. Functional Additives in a Selected European Sea Bass (Dicentrarchus labrax) Genotype: Effects on the Stress Response and Gill Antioxidant Response to Hydrogen Peroxide (H2O2) Treatment. Animals 2023, 13, 2265. [Google Scholar] [CrossRef] [PubMed]
- Venditti, P.; Gravato, C.; Napolitano, G. Editorial: Environmental pollutant and oxidative stress in terrestrial and aquatic organisms. Front. Physiol. 2022, 13, 1073582. [Google Scholar] [CrossRef]
- Castro, J.S.; Braz-Mota, S.; Campos, D.F.; Souza, S.S.; Val, A.L. High Temperature, pH, and Hypoxia Cause Oxidative Stress and Impair the Spermatic Performance of the Amazon Fish Colossoma macropomum. Front. Physiol. 2020, 11, 772. [Google Scholar] [CrossRef] [PubMed]
- Osório, J.; Stiller, K.T.; Reiten, B.-K.; Kolarevic, J.; Johansen, L.-H.; Afonso, F.; Lazado, C.C. Intermittent administration of peracetic acid is a mild environmental stressor that elicits mucosal and systemic adaptive responses from Atlantic salmon post-smolts. BMC Zool. 2022, 7, 1. [Google Scholar] [CrossRef]
- Francis, G.; Makkar, H.P.S.; Becker, K. Antinutritional Factors Present in Plant-Derived Alternate Fish Feed Ingredients and Their Effects in Fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Gao, Y.; He, Z.; Vector, H.; Zhao, B.; Li, Z.; He, J.; Lee, J.-Y.; Chu, Z. Effect of Stocking Density on Growth, Oxidative Stress and HSP 70 of Pacific White Shrimp Litopenaeus vannamei. Turk. J. Fish. Aquat. Sci. 2017, 17, 877–884. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saikia, S.K. Oxidative stress in fish: A review. J. Sci. Res. 2020, 12, 145–160. [Google Scholar] [CrossRef]
- Francis-Floyd, R. Dissolved Oxygen for Fish Production. University of Florida Fact Sheet FA-27. 2019. Available online: https://freshwater-aquaculture.extension.org/wp-content/uploads/2019/08/Dissolved_Oxygen_for_Fish_Production.pdf (accessed on 17 September 2023).
- Ahn, C.H.; Lee, S.; Song, H.M.; Park, J.R.; Joo, J.C. Assessment of Water Quality and Thermal Stress for an Artificial Fish Shelter in an Urban Small Pond during Early Summer. Water 2019, 11, 139. [Google Scholar] [CrossRef]
- Mensoor, M.; Said, A. Determination of Heavy Metals in Freshwater Fishes of the Tigris River in Baghdad. Fishes 2018, 3, 23. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Robinson, B.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Farraji, H.; Vakili, M. Pesticides in aquatic environments and their removal by adsorption methods. Chemosphere 2020, 253, 126646. [Google Scholar] [CrossRef]
- EIO (Eurofish International Organisation). Guide to Recirculation Aquaculture: Chapter 2. eurofish.dk. 2017. Available online: https://eurofish.dk/guide-to-recirculation-aquaculture-chapter-2-continued-1/#:~:text=In%20general%2C%20ammonia%20is%20toxic,ammonia%20is%20to%20be%20ensured (accessed on 17 September 2023).
- Robertson-Bryan Inc. pH Requirements of Freshwater Aquatic Life. Technical Memorandum Robertson-Bryan, Inc. 2004. Available online: https://www.waterboards.ca.gov/waterrights/water_issues/programs/bay_delta/deltaflow/docs/exhibits/bigbreak/dscbb_exh5.pdf (accessed on 17 September 2023).
- Kültz, D. Physiological mechanisms used by fish to cope with salinity stress. J. Exp. Biol. 2015, 218 Pt 12, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.N.; Mahamed, A.H.; Alarcon, J.F.; Al Suwailem, A.; Agustí, S. Adverse Effects of Ultraviolet Radiation on Growth, Behavior, Skin Condition, Physiology, and Immune Function in Gilthead Seabream (Sparus aurata). Front. Mar. Sci. 2020, 7, 306. [Google Scholar] [CrossRef]
- Ziarati, M.; Zorriehzahra, M.J.; Hassantabar, F.; Mehrabi, Z.; Dhawan, M.; Sharun, K.; Bin Emran, T.; Dhama, K.; Chaicumpa, W.; Shamsi, S. Zoonotic diseases of fish and their prevention and control. Vet. Q. 2022, 42, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M. Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean Coast. Manag. 2009, 52, 342–347. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Guo, Z.-X.; Ye, C.-X.; Wang, A.-L. Effect of dietary astaxanthin on the growth performance, non-specific immunity, and antioxidant capacity of pufferfish (Takifugu obscurus) under high temperature stress. Fish Physiol. Biochem. 2017, 44, 209–218. [Google Scholar] [CrossRef]
- Rizzardi, N.; Pezzolesi, L.; Samorì, C.; Senese, F.; Zalambani, C.; Pitacco, W.; Calonghi, N.; Bergamini, C.; Prata, C.; Fato, R. Natural Astaxanthin Is a Green Antioxidant Able to Counteract Lipid Peroxidation and Ferroptotic Cell Death. Int. J. Mol. Sci. 2022, 23, 15137. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, G. Antioxidant Protection from UV- and Light-Stress Related to Carotenoid Structures. Antioxidants 2019, 8, 219. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Abdol Wahab, N.R.; Affandi, M.M.R.M.M.; Fakurazi, S.; Alias, E.; Hassan, H. Nanocarrier System: State-of-the-Art in Oral Delivery of Astaxanthin. Antioxidants 2022, 11, 1676. [Google Scholar] [CrossRef]
- Dhankhar, J.; Kadian, S.S.; Sharma, A. Astaxanthin: A Potential Carotenoid. Int. J. Pharm. Sci. Res. 2012, 3, 5. [Google Scholar]
- Dogra, V.; Kim, C. Singlet Oxygen Metabolism: From Genesis to Signaling. Front. Plant Sci. 2020, 10, 1640. [Google Scholar] [CrossRef]
- Liu, J.; Mai, K.; Xu, W.; Zhang, Y.; Zhou, H.; Ai, Q. Effects of dietary glutamine on survival, growth performance, activities of digestive enzyme, antioxidant status and hypoxia stress resistance of half-smooth tongue sole (Cynoglossus semilaevis Günther) post larvae. Aquaculture 2015, 446, 48–56. [Google Scholar] [CrossRef]
- Sharma, N.K.; Akhtar, M.; Pandey, N.; Singh, R.; Singh, A.K. Seasonal variation in thermal tolerance, oxygen consumption, antioxidative enzymes and non-specific immune indices of Indian hill trout, Barilius bendelisis (Hamilton, 1807) from central Himalaya, India. J. Therm. Biol. 2015, 52, 166–176. [Google Scholar] [CrossRef]
- Brotosudarmo, T.H.P.; Limantara, L.; Setiyono, E.; Heriyanto. Structures of Astaxanthin and Their Consequences for Therapeutic Application. Int. J. Food Sci. 2020, 2020, 2156582. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khosravi, S.; Chang, K.H.; Lee, S.M. Effects of Dietary Inclusion of Astaxanthin on Growth, Muscle Pigmentation and Antioxidant Capacity of Juvenile Rainbow Trout (Oncorhynchus mykiss). Prev. Nutr. Food Sci. 2016, 21, 281–288. [Google Scholar] [CrossRef]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed]
- Woodall, A.A.; Britton, G.; Jackson, M.J. Carotenoids and protection of phospholipids in solution or in liposomes against oxidation by peroxyl radicals: Relationship between carotenoid structure and protective ability. Biochim. Biophys. Acta-Gen. Subj. 1997, 1336, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, A. Natural Foods and Indian Herbs of Cardiovascular Interest. Pharm. Pharmacol. Int. J. 2019, 7, 60–84. [Google Scholar] [CrossRef]
- McNulty, H.P.; Byun, J.; Lockwood, S.F.; Jacob, R.F.; Mason, R.P. Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Biochim. Biophys. Acta-Biomembr. 2007, 1768, 167–174. [Google Scholar] [CrossRef]
- Morilla, M.J.; Ghosal, K.; Romero, E.L. More Than Pigments: The Potential of Astaxanthin and Bacterioruberin-Based Nanomedicines. Pharmaceutics 2023, 15, 1828. [Google Scholar] [CrossRef]
- Kidd, P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern. Med. Rev. 2011, 16, 355–364. [Google Scholar]
- Kamezaki, C.; Nakashima, A.; Yamada, A.; Uenishi, S.; Ishibashi, H.; Shibuya, N.; Hama, S.; Hosoi, S.; Yamashita, E.; Kogure, K. Synergistic antioxidative effect of astaxanthin and tocotrienol by co-encapsulated in liposomes. J. Clin. Biochem. Nutr. 2016, 59, 100–106. [Google Scholar] [CrossRef]
- Fernando, F.; Candebat, C.L.; Strugnell, J.M.; Andreakis, N.; Nankervis, L. Dietary supplementation of astaxanthin modulates skin color and liver antioxidant status of giant grouper (Epinephelus lanceolatus). Aquac. Rep. 2022, 26, 101266. [Google Scholar] [CrossRef]
- Böhm, F.; Edge, R.; Land, E.J.; McGarvey, D.J.; Truscott, T.G. Carotenoids enhance vitamin e antioxidant efficiency. J. Am. Chem. Soc. 1997, 119, 621–622. [Google Scholar] [CrossRef]
- Kalinowski, C.T.; Larroquet, L.; Véron, V.; Robaina, L.; Izquierdo, M.S.; Panserat, S.; Kaushik, S.; Fontagné-Dicharry, S. Influence of Dietary Astaxanthin on the Hepatic Oxidative Stress Response Caused by Episodic Hyperoxia in Rainbow Trout. Antioxidants 2019, 8, 626. [Google Scholar] [CrossRef]
- Hassanzadeh, P.; Ahmadvand, M.; Aslani, S.; Sheikhzadeh, N.; Mousavi, S.; Khatibi, S.A.; Ahmadifar, E. Dietary astaxanthin mitigated paraquat-induced oxidative stress in rainbow trout (Oncorhynchus mykiss) fillet. Aquac. Res. 2022, 53, 5300–5309. [Google Scholar] [CrossRef]
- Xie, J.-J.; Chen, X.; Niu, J.; Wang, J.; Wang, Y.; Liu, Q.-Q. Effects of astaxanthin on antioxidant capacity of golden pompano (Trachinotus ovatus) in vivo and in vitro. Fish. Aquat. Sci. 2017, 20, 6. [Google Scholar] [CrossRef]
- Davinelli, S.; Saso, L.; D’angeli, F.; Calabrese, V.; Intrieri, M.; Scapagnini, G. Astaxanthin as a Modulator of Nrf2, NF-κB, and Their Crosstalk: Molecular Mechanisms and Possible Clinical Applications. Molecules 2022, 27, 502. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, H. Astaxanthin Modulation of Signaling Pathways That Regulate Autophagy. Mar. Drugs 2019, 17, 546. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Badenetti, L.; Manzoli, R.; Rubin, M.; Cozza, G.; Moro, E. Monitoring Nrf2/ARE Pathway Activity with a New Zebrafish Reporter System. Int. J. Mol. Sci. 2023, 24, 6804. [Google Scholar] [CrossRef]
- Xie, W.J.; Hou, G.; Wang, L.; Wang, S.S.; Xiong, X.X. Astaxanthin suppresses lipopolysaccharide-induced myocardial injury by regulating MAPK and PI3K/AKT/mTOR/GSK3β signaling. Mol. Med. Rep. 2020, 22, 3338–3346. [Google Scholar] [CrossRef]
- Deng, W.; Yang, T.; Dong, R.; Yan, Y.; Jiang, Q. Astaxanthin protects tilapia head kidney cells against polystyrene microplastics-induced inflammation through MAPK and NF-κB signaling pathways. Aquaculture 2023, 574, 739686. [Google Scholar] [CrossRef]
- Bjerkeng, B.; Storebakken, T.; Liaaen-Jensen, S. Response to carotenoids by rainbow trout in the sea: Resorption and metabolism of dietary astaxanthin and canthaxanthin. Aquaculture 1990, 91, 153–162. [Google Scholar] [CrossRef]
- Saleh, N.; Wassef, E.A.; Shalaby, S.M. The role of dietary astaxanthin in European sea bass (Dicentrarchus labrax) growth, immunity, antioxidant competence and stress tolerance. Egypt. J. Aquat. Biol. Fish. 2018, 22, 189–200. [Google Scholar] [CrossRef]
- Liu, F.; Shi, H.Z.; Guo, Q.S.; Yu, Y.B.; Wang, A.M.; Lv, F.; Shen, W.B. Effects of astaxanthin and emodin on the growth, stress resistance and disease resistance of yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2016, 51, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.H.; Chien, Y.H.; Wang, Y.J. Antioxidant defence toammonia stress of characins (Hyphessobrycon eques Steindachner) fed diets supplemented with carotenoids. Aquac. Nutr. 2011, 17, 258–266. [Google Scholar] [CrossRef]
- Huang, J.-N.; Wen, B.; Li, X.-X.; Xu, L.; Gao, J.-Z.; Chen, Z.-Z. Astaxanthin mitigates oxidative stress caused by microplastics at the expense of reduced skin pigmentation in discus fish. Sci. Total Environ. 2023, 874, 162494. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Guo, Y.-C.; Huai, M.-Y.; Li, L.; Man, C.; Pelletier, W.; Wei, H.-L.; Yao, R.; Niu, J. Comparison of the Retention Rates of Synthetic and Natural Astaxanthin in Feeds and Their Effects on Pigmentation, Growth, and Health in Rainbow Trout (Oncorhynchus mykiss). Antioxidants 2022, 11, 2473. [Google Scholar] [CrossRef] [PubMed]
- Ulloa-Rodríguez, P.; Figueroa, E.; Díaz, R.; Lee-Estevez, M.; Short, S.; Farías, J.G. Mitochondria in teleost spermatozoa. Mitochondrion 2017, 34, 49–55. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; Yeste, M.; Salas-Huetos, A. The Relationship between Sperm Oxidative Stress Alterations and IVF/ICSI Outcomes: A Systematic Review from Nonhuman Mammals. Biology 2020, 9, 178. [Google Scholar] [CrossRef]
- Takalani, N.B.; Monageng, E.M.; Mohlala, K.; Monsees, T.K.; Henkel, R.; Opuwari, C.S. Role of oxidative stress in male infertility. Reprod. Fertil. 2023, 4, e230024. [Google Scholar] [CrossRef]
- Mao, L.; Jia, W.; Zhang, L.; Zhang, Y.; Zhu, L.; Sial, M.U.; Jiang, H. Embryonic development and oxidative stress effects in the larvae and adult fish livers of zebrafish (Danio rerio) exposed to the strobilurin fungicides, kresoxim-methyl and pyraclostrobin. Sci. Total Environ. 2020, 729, 139031. [Google Scholar] [CrossRef]
- Lopes, R.A.; Neves, K.B.; Tostes, R.C.; Montezano, A.C.; Touyz, R.M. Downregulation of Nuclear Factor Erythroid 2-Related Factor and Associated Antioxidant Genes Contributes to Redox-Sensitive Vascular Dysfunction in Hypertension. Hypertension 2015, 66, 1240–1250. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Q.; Yuan, Y.; Zhang, Z.; Jiang, B.; Yang, S.; Jian, J. Silencing of Nrf2 in Litopenaeus vannamei, decreased the antioxidant capacity, and increased apoptosis and autophagy. Fish Shellfish Immunol. 2022, 122, 257–267. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, P.; Liu, S.; Bian, Y.; Xu, Y.; Zhang, Q.; Wang, H.; Pi, J. Is Nuclear Factor Erythroid 2-Related Factor 2 a Target for the Intervention of Cytokine Storms? Antioxidants 2023, 12, 172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Davies, K.J.; Forman, H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Quan, Y.N.; Huang, Z.Q.; Wang, H.H.; Wu, L.F. Monitoring oxidative stress, immune response, Nrf2/NF-κB signaling molecules of Rhynchocypris lagowski living in BFT system and exposed to waterborne ammonia. Ecotoxicol. Environ. Saf. 2020, 205, 111161. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Ding, Y.; Li, X.; Dong, X.; Mai, K.; Ai, Q. Nrf2 pathway in vegetable oil-induced inflammation of large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2022, 127, 778–787. [Google Scholar] [CrossRef]
- Zaynab, M.; Al-Yahyai, R.; Ameen, A.; Sharif, Y.; Ali, L.; Fatima, M.; Khan, K.A.; Li, S. Health and environmental effects of heavy metals. J. King Saud Univ. Sci. 2021, 34, 101653. [Google Scholar] [CrossRef]
- Subaramaniyam, U.; Allimuthu, R.S.; Vappu, S.; Ramalingam, D.; Balan, R.; Paital, B.; Panda, N.; Rath, P.K.; Ramalingam, N.; Sahoo, D.K. Effects of microplastics, pesticides and nano-materials on fish health, oxidative stress and antioxidant defense mechanism. Front. Physiol. 2023, 14, 1217666. [Google Scholar] [CrossRef]
- Kim, J.-H.; Yu, Y.-B.; Choi, J.-H. Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and neurotoxicity in fish exposed to microplastics: A review. J. Hazard. Mater. 2021, 413, 125423. [Google Scholar] [CrossRef]
- Taslima, K.; Al-Emran, M.; Rahman, M.S.; Hasan, J.; Ferdous, Z.; Rohani, M.F.; Shahjahan, M. Impacts of heavy metals on early development, growth and reproduction of fish—A review. Toxicol. Rep. 2022, 9, 858–868. [Google Scholar] [CrossRef]
- Madeira, D.; Narciso, L.; Cabral, H.; Vinagre, C.; Diniz, M. Influence of temperature in thermal and oxidative stress responses in estuarine fish. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 166, 237–243. [Google Scholar] [CrossRef]
- Welker, A.F.; Moreira, D.C.; Campos, É.G.; Hermes-Lima, M. Role of redox metabolism for adaptation of aquatic animals to drastic changes in oxygen availability. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 165, 384–404. [Google Scholar]
- Mitra, A.; Abdel-Gawad, F.K.; Bassem, S.; Barua, P.; Assisi, L.; Parisi, C.; Temraz, T.A.; Vangone, R.; Kajbaf, K.; Kumar, V.; et al. Climate Change and Reproductive Biocomplexity in Fishes: Innovative Management Approaches towards Sustainability of Fisheries and Aquaculture. Water 2023, 15, 725. [Google Scholar] [CrossRef]
- Sawecki, J.; Miros, E.; Border, S.E.; Dijkstra, P.D. Reproduction and maternal care increase oxidative stress in a mouth-brooding cichlid fish. Behav. Ecol. 2019, 30, 1662–1671. [Google Scholar] [CrossRef]
- Bacou, E.; Walk, C.; Rider, S.; Litta, G.; Perez-Calvo, E. Dietary Oxidative Distress: A Review of Nutritional Challenges as Models for Poultry, Swine and Fish. Antioxidants 2021, 10, 525. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Kiani, A.; Santhiravel, S.; Holman, B.W.B.; Lauridsen, C.; Dunshea, F.R. The Importance of Dietary Antioxidants on Oxidative Stress, Meat and Milk Production, and Their Preservative Aspects in Farm Animals: Antioxidant Action, Animal Health, and Product Quality—Invited Review. Animals 2022, 12, 3279. [Google Scholar] [CrossRef]
- Wu, L.; Xu, W.; Li, H.; Dong, B.; Geng, H.; Jin, J.; Han, D.; Liu, H.; Zhu, X.; Yang, Y.; et al. Vitamin C Attenuates Oxidative Stress, Inflammation, and Apoptosis Induced by Acute Hypoxia through the Nrf2/Keap1 Signaling Pathway in Gibel Carp (Carassius gibelio). Antioxidants 2022, 11, 935. [Google Scholar] [CrossRef]
- Qiang, J.; Tao, Y.-F.; Lu, S.-Q.; Ma, J.-L.; He, J.; Xu, P. Role of Astaxanthin as a Stimulator of Ovarian Development in Nile Tilapia (Oreochromis niloticus) and Its Potential Regulatory Mechanism: Ameliorating Oxidative Stress and Apoptosis. Aquac. Nutr. 2022, 2022, 1245151. [Google Scholar] [CrossRef]
- Ahmadi, M.R.; Bazyar, A.A.; Safi, S.; Ytrestøyl, T.; Bjerkeng, B. Effects of dietary astaxanthin supplementation on repro-ductive characteristics of rainbow trout (Oncorhynchus mykiss). J. Appl. Ichthyol. 2006, 22, 388–394. [Google Scholar] [CrossRef]
- Tizkar, B.; Kazemi, R.; Alipour, A.; Seidavi, A.; Naseralavi, G.; Ponce-Palafox, J. Effects of dietary supplementation with astaxanthin and β-carotene on the semen quality of goldfish (Carassius auratus). Theriogenology 2015, 84, 1111–1117. [Google Scholar] [CrossRef]
- Dattilo, M.; D’amato, G.; Caroppo, E.; Ménézo, Y. Improvement of gamete quality by stimulating and feeding the endogenous antioxidant system: Mechanisms, clinical results, insights on gene-environment interactions and the role of diet. J. Assist. Reprod. Genet. 2016, 33, 1633–1648. [Google Scholar] [CrossRef]
- Gronczewska, J.; Niedźwiecka, N.; Grzyb, K.; Skorkowski, E.F. Bioenergetics of fish spermatozoa with focus on some herring (Clupea harengus) enzymes. Fish Physiol. Biochem. 2019, 45, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Verakunpiriya, V.; Mushiake, K.; Kawano, K.; Watanabe, T. Supplemental effect of astaxanthin in broodstock diets on the quality of yellowtail eggs. Fish. Sci. 1997, 63, 816–823. [Google Scholar] [CrossRef]
- Hansen, J.; Puvanendran, V.; Bangera, R. Broodstock diet with water and astaxanthin improve condition and egg output of brood fish and larval survival in Atlantic cod, Gadus morhua L. Aquac. Res. 2016, 47, 819–829. [Google Scholar] [CrossRef]
- Craik, J.C.A. Egg quality and egg pigment content in salmonid fishes. Aquaculture 1985, 47, 61–88. [Google Scholar] [CrossRef]
- Wu, M.; Xu, H.; Shen, Y.; Qiu, W.; Yang, M. Oxidative stress in zebrafish embryos induced by short-term exposure to bisphenol A, nonylphenol, and their mixture. Environ. Toxicol. Chem. 2011, 30, 2335–2341. [Google Scholar] [CrossRef] [PubMed]
- Mugoni, V.; Camporeale, A.; Santoro, M.M. Analysis of oxidative stress in zebrafish embryos. J. Vis. Exp. 2014, 89, 51328. [Google Scholar]
- Sowmya, R.; Sachindra, N.M. Enhancement of non-specific immune responses in common carp, Cyprinus carpio, by dietary carotenoids obtained from shrimp exoskeleton. Aquac. Res. 2013, 46, 1562–1572. [Google Scholar] [CrossRef]
- Hue, N.T.N.; Lam, H.S.; Ngoc, D.T.H.; Tram, D.T.T.; Sang, H.M.; An, D.T.; Van Than, D.; Tai, N.T.T.; Dang, D.H.; An, H.T. Effect of dietary astaxanthin on reproductive performance, egg quality and larvae of clowfish Amphiprion ocellaris (Cuvier, 1830). Vietnam J. Mar. Sci. Technol. 2021, 20, 163–172. [Google Scholar] [CrossRef]
- Vasbinder, K.; Ainsworth, C. Early life history growth in fish reflects consumption-mortality tradeoffs. Fish. Res. 2020, 227, 105–538. [Google Scholar]
- Xie, S.; Yin, P.; Tian, L.; Yu, Y.; Liu, Y.; Niu, J. Dietary Supplementation of Astaxanthin Improved the Growth Performance, Antioxidant Ability and Immune Response of Juvenile Largemouth Bass (Micropterus salmoides) Fed High-Fat Diet. Mar. Drugs 2020, 18, 642. [Google Scholar] [CrossRef] [PubMed]
- Ytrestøyl, T.; Afanasyev, S.; Ruyter, B.; Hatlen, B.; Østbye, T.-K.; Krasnov, A. Transcriptome and functional responses to absence of astaxanthin in Atlantic salmon fed low marine diets. Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 39, 100841. [Google Scholar] [CrossRef]
- Xu, W.; Liu, Y.; Huang, W.; Yao, C.; Yin, Z.; Mai, K.; Ai, Q. Effects of dietary supplementation of astaxanthin (Ast) on growth performance, activities of digestive enzymes, antioxidant capacity and lipid metabolism of large yellow croaker (Larimichthys crocea) larvae. Aquac. Res. 2022, 53, 4605–4615. [Google Scholar] [CrossRef]
- Rashidian, G.; Rainis, S.; Prokić, M.D.; Faggio, C. Effects of different levels of carotenoids and light sources on swordtail fish (Xiphophorus helleri) growth, survival rate and reproductive parameters. Nat. Prod. Res. 2021, 35, 3675–3686. [Google Scholar] [CrossRef] [PubMed]
- Tizkar, B.; Hossainzadeh, H.; Zoghi, A.; Zahmatkesh, A.; Monsef, H. The effects of dietary astaxanthin on the physiological, growth and survival of gold fish larvae (Carassius auratus). Fish Farm. Dev. J. 2019, 13, 93–117. [Google Scholar]
- Zakariaee, H.; Sudagar, M.; Mazandarani, M.; Hoseini, S.A. The effect of Astaxanthin on sexual maturing and fecundity and survival larval of fighter fish (Betta splendens). J. Anim. Environ. 2015, 7, 227–234. [Google Scholar]
- Hernández, F.J.G. Improvements in Larval Culture Techniques of Sea Bream (Sparus aurata) in the First Feeding Phase. Bachelor’s Thesis, University of Las Palmas de Gran Canaria, Las Palmas de Gran Canaria, Spain, 2017. [Google Scholar]
- Sorandra-Rotta, C. Effect of the Pigmentation of Rotifers brachionus sp. on the Larviculture of the Clownfish Amphiprion clarkia. Master’s Thesis, Federal University of Santa Catarina, Florianópolis, Brazil, 2014. [Google Scholar]
- Tizkar, B.; Soudagar, M.; Bahmani, M.; Hosseini, S.A.; Chamani, M. The effects of dietary supplementation of astaxanthin and β-caroten on the reproductive performance and egg quality of female goldfish (Carassius auratus). Casp. J. Environ. Sci. 2013, 11, 217–231. [Google Scholar]
- Sawanboonchun, J.; Roy, W.J.; Robertson, D.A.; Bell, J.G. The impact of dietary supplementation with astaxanthin on egg quality in Atlantic cod broodstock (Gadus morhua L.). Aquaculture 2008, 283, 97–101. [Google Scholar] [CrossRef]
- Vassallo-Agius, R.; Watanabe, T.; Imaizumi, H.; Yamazaki, T.; Satoh, S.; Kiron, V. Effects of dry pellets containing astaxanthin and squid meal on the spawning performance of striped jack Pseudocaranx dentex. Fish. Sci. 2001, 67, 667–674. [Google Scholar] [CrossRef]
- Elbahnaswy, S.; Elshopakey, G.E. Recent progress in practical applications of a potential carotenoid astaxanthin in aquaculture industry: A review. Fish Physiol. Biochem. 2023, 1–30. [Google Scholar] [CrossRef]
- Tuan Harith, Z.; Mohd Sukri, S.; Remlee, N.F.S.; Mohd Sabir, F.N.; Zakaria, N.N.A. Effects of dietary astaxanthin enrichment on enhancing the colour and growth of red tilapia, Oreochromis sp. Aquac. Fish. 2022, 9, 52–56. [Google Scholar] [CrossRef]
- Jia, R.; Wang, L.; Hou, Y.; Feng, W.; Li, B.; Zhu, J. Effects of Stocking Density on the Growth Performance, Physiological Parameters, Redox Status and Lipid Metabolism of Micropterus salmoides in Integrated Rice-Fish Farming Systems. Antioxidants 2022, 11, 1215. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Dietary astaxanthin augments disease resistance of Asian seabass, Lates calcarifer (Bloch, 1790), against Vibrio alginolyticus infection. Fish Shellfish Immunol. 2021, 114, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Storebakken, T.; No, H.K. Pigmentation of rainbow trout. Aquaculture 1992, 100, 209–914. [Google Scholar] [CrossRef]
- Hamrang Omshi, A.; Bahri, A.; Khara, H.; Mohammadizadeh, F. The effects of lucantin red, yellow and astaxanthin on growth, hematological, immunological parameters and coloration in the Tiger Oscar (Astronotus ocellatus Agassiz,1831). IJFS 2019, 18, 798–811. [Google Scholar]
- Aracati, M.F.; Rodrigues, L.F.; de Oliveira, S.L.; Rodrigues, R.A.; Conde, G.; Cavalcanti, E.N.F.; Borba, H.; Charlie-Silva, I.; Fernandes, D.C.; Eto, S.F.; et al. Astaxanthin improves the shelf-life of tilapia fillets stored under refrigeration. J. Sci. Food Agric. 2022, 102, 4287–4295. [Google Scholar] [CrossRef] [PubMed]
- Torrissen, O.J. Pigmentation of salmonids: Interactions of astaxanthin and canthaxanthin on pigment deposition in rainbow trout. Aquaculture 1989, 79, 363–374. [Google Scholar] [CrossRef]
- Paibulkichakul, C.; Piyatiratitivorakul, S.; Sorgeloos, P.; Menasveta, P. Improved maturation of pondreared, black tiger shrimp Penaeus monodon using fish oil and astaxanthin feed supplements. Aquaculture 2008, 282, 83–89. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Astaxanthin as feed supplement in aquatic animals. Rev. Aquac. 2018, 10, 738–773. [Google Scholar] [CrossRef]
- Harmon, V.L.; Cysewski, G.R. Astaxanthin: Feed Supplement Enhances Pigmentation, Health. Global Aquaculture Advocate. 2007. Available online: https://www.globalseafood.org/advocate/astaxanthin-feed-supplement-enhances-pigmentation-health/?headlessPrint=AAAAAPIA9c8r7gs82o (accessed on 17 September 2023).
- BASF (Badische Anilin- und Sodafabrik). Carotenoids. In BASF Technical Handbook Animal Nutrition; BASF SE, Animal Nutrition: Ludwigshafen, Germany, 2014; pp. 48–70. [Google Scholar]
- EFSA (European Food Safety Authority). Scientific Opinion on the safety and efficacy of synthetic astaxanthin as feed additive for salmon and trout, other fish, ornamental fish, crustaceans and ornamental birds. EFSA J. 2014, 12, 3724. [Google Scholar]
- Christiansen, R.; Lie, O.; Torrissen, O.J. Effect of astaxanthin and vitamin A on growth and survival during first feeding of Atlantic salmon, Salmo salar L. Aquac. Res. 1994, 25, 903–914. [Google Scholar] [CrossRef]
- Nadarajah, S.; Flaaten, O. Global aquaculture growth and institutional quality. Mar. Policy 2017, 84, 142–151. [Google Scholar] [CrossRef]
- Henriksson, P.J.G.; Troell, M.; Banks, L.K.; Belton, B.; Beveridge, M.C.M.; Klinger, D.H.; Pelletier, N.; Phillips, M.J.; Tran, N. Interventions for improving the productivity and environmental performance of global aquaculture for future food security. One Earth 2021, 4, 1220–1232. [Google Scholar] [CrossRef]
- Lu, Q.; Li, H.; Zou, Y.; Liu, H.; Yang, L. Astaxanthin as a microalgal metabolite for aquaculture: A review on the synthetic mechanisms, production techniques, and practical application. Algal Res. 2021, 54, 102178. [Google Scholar]
- Torrissen, O.J. Pigmentation of salmonids: Factors affecting carotenoid deposition in rainbow trout (Salmo gairdneri). Aquaculture 1985, 46, 133–142. [Google Scholar] [CrossRef]
- Page, G.I. Physiological and Biochemical Factors Affecting Carotenoid Utilization in Salmonid Fish. Ph.D. Thesis, University of Plymouth, Plymouth, UK, 2001. [Google Scholar]
- Natnan, M.E.; Low, C.-F.; Chong, C.-M.; Bunawan, H.; Baharum, S.N. Integration of Omics Tools for Understanding the Fish Immune Response due to Microbial Challenge. Front. Mar. Sci. 2021, 8, 668771. [Google Scholar] [CrossRef]
- Long, J.A. The ‘omics’ revolution: Use of genomic, transcriptomic, proteomic and metabolomic tools to predict male reproductive traits that impact fertility in livestock and poultry. Anim. Reprod. Sci. 2020, 220, 106354. [Google Scholar] [CrossRef]
- De León, L.F.; Silva, B.; Avilés-Rodríguez, K.J.; Buitrago-Rosas, D. Harnessing the omics revolution to address the global biodiversity crisis. Curr. Opin. Biotechnol. 2023, 80, 102901. [Google Scholar] [CrossRef]
- Ye, H.; Lin, Q.; Luo, H. Applications of transcriptomics and proteomics in understanding fish immunity. Fish Shellfish Immunol. 2018, 77, 319–327. [Google Scholar] [CrossRef]
- Pereiro, P. Transcriptome and Genome Analyses Applied to Aquaculture Research. Biology 2022, 11, 1312. [Google Scholar] [CrossRef]
- Ranade, A.V.; Hegde, P.S.; Bhat, M.A.; Rai, P.; Vinodini, N.A.; Aravind, A.; Prasad, T.S.K.; Damodara Gowda, K.M. Astaxanthin and DHA supplementation ame-liorates the proteomic profile of perinatal undernutrition-induced adipose tissue dysfunction in adult life. Sci. Rep. 2023, 13, 12312. [Google Scholar]
- Yang, B.-T.; Wen, B.; Ji, Y.; Wang, Q.; Zhang, H.-R.; Zhang, Y.; Gao, J.-Z.; Chen, Z.-Z. Comparative metabolomics analysis of pigmentary and structural coloration in discus fish (Symphysodon haraldi). J. Proteom. 2020, 233, 104085. [Google Scholar] [CrossRef]
- Nakano, T.; Wiegertjes, G. Properties of Carotenoids in Fish Fitness: A Review. Mar. Drugs 2020, 18, 568. [Google Scholar] [CrossRef] [PubMed]
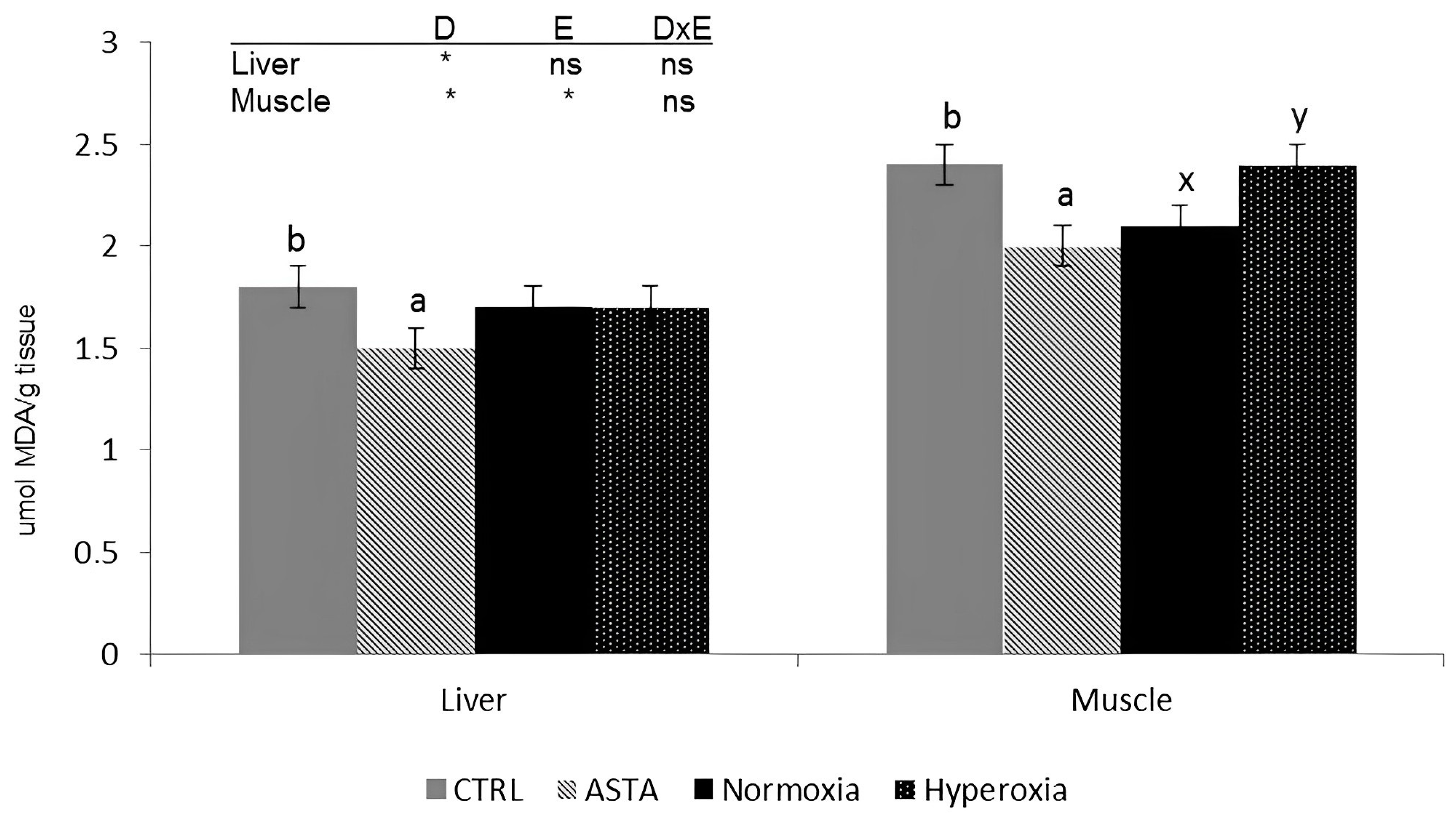
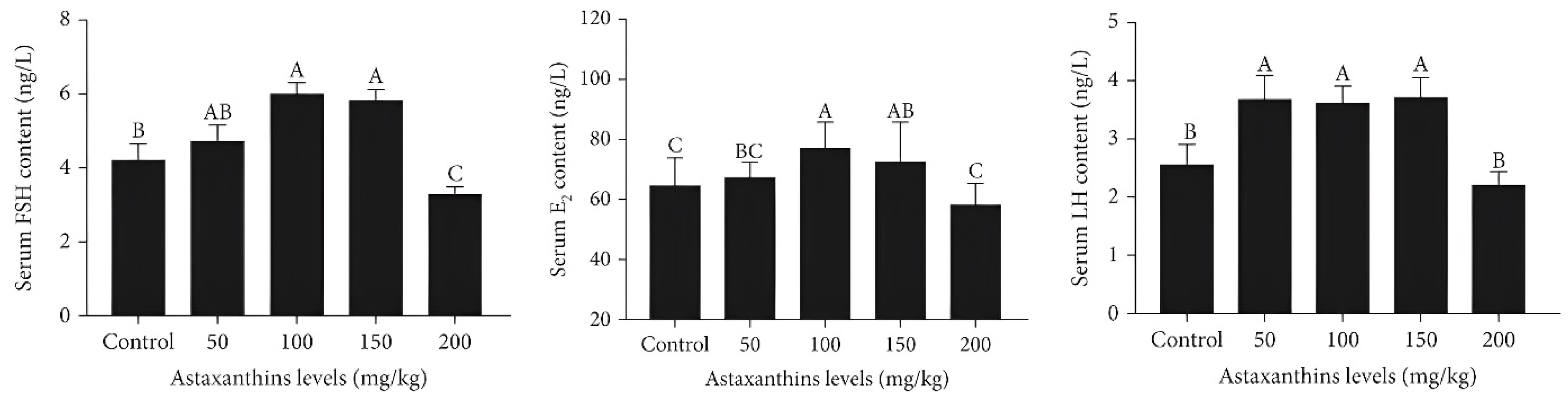
| Stressor | Permissible Limit | Effects on Fish | Reference |
|---|---|---|---|
| Oxygen depletion | Dissolved oxygen levels should not fall below 5 mg/L for most freshwater fish. | Low dissolved oxygen can lead to fish suffocation and reduced growth. | [66] |
| Temperature fluctuations | Diurnal fluctuations in water temperature should not exceed a certain threshold, which is species-specific. | Rapid temperature changes can stress fish and impact their metabolism. | [67] |
| Pollutants (heavy metals) | Varies by metal and species. In general, allowable concentrations are low (micrograms per liter or lower). | Heavy metals like lead, mercury, and cadmium can accumulate in fish tissues and harm health. | [68] |
| Pesticides and herbicides | Varies by chemical and species. Generally, very low concentrations are allowed (parts per billion). | These chemicals can disrupt fish physiology and impair reproduction. | [69] |
| Ammonia | Total ammonia nitrogen levels should be below 0.02 mg/L for freshwater fish. | High ammonia can damage fish gills and cause respiratory distress. | [70] |
| pH | Optimal pH ranges from 6.5 to 9.0, depending on the fish species. | Extreme pH levels can stress fish, affecting ion balance and survival. | [71] |
| Salinity | Varies widely by fish species. Some tolerate freshwater, while others require high salinity. | Salinity outside a fish’s tolerance range can cause osmotic stress. | [72] |
| UV radiation | Exposure should be limited, especially in shallow, clear waters. | Prolonged UV exposure can damage fish skin and eyes. | [73] |
| Microorganisms (pathogens) | The presence of pathogens like bacteria, viruses, and parasites should be minimized. | Infections can weaken fish and lead to disease outbreaks. | [74] |
| Toxic algal blooms | Concentrations of harmful algae should be monitored and controlled. | Toxins produced by algae can harm fish and other aquatic organisms. | [75] |
| T-AOC (U/mg Protein) | SOD (U/mg Protein) | GSH (μmol/g Protein) | |
|---|---|---|---|
| Diet 1 | 0.11 ± 0.01 a | 240.87 ± 5.76 a | 82.44 ± 4.87 a |
| Diet 2 | 0.15 ± 0.01 b | 214.24 ± 5.71 b | 118.52 ± 8.93 b |
| Fish Species | Astaxanthin Supplementation Levels in Feed | Form | Challenge 2 | Astaxanthin Effects on Oxidative Status | Astaxanthin Effects on Growth | Reference |
|---|---|---|---|---|---|---|
| Rainbow trout (Oncorhynchus mykiss) | 0.5, 2.0 g/kg | Synthetic | Yes | Reduced MDA and peroxide values and upregulation of the expression of antioxidant-relevant genes in fish fillet | Improved final weight and FCR | [97] |
| Yellow catfish (Pelteobagrus fulvidraco) | 0.08 g/kg | Synthetic | Yes | Higher levels of catalase activity and reduced MDA in the liver | Improved final weight and specific growth rate | [107] |
| Rainbow trout (Oncorhynchus mykiss) | 0.5, 2.0, 5.0 g/kg | Synthetic | Yes | Reduced MDA and improved T-AOC in blood serum, upregulation of the expression of antioxidant-relevant genes in the liver | Improved final weight and specific growth rate | [23] |
| Characin (Hyphessobrycon eques Stein-dachner) | 0.01, 0.02, 0.04 g/kg | Synthetic | Yes | Improved antioxidant capacity as measured by total antioxidant status and superoxide dismutase activity | No effect | [108] |
| Rainbow trout (Oncorhynchus mykiss) | 0.1 g/kg | Synthetic | Yes | Decrease in TBARS in muscle and liver cells; increased glutathione reductase activity; improved ratio of GSH to GSSG | Numerical improvement in final weight | [96] |
| Discus fish (Symphysodon aequifasciatus) | 0.2 g/kg | Synthetic | Yes | Improved antioxidant defense status | No effect | [109] |
| Coral trout (Plectropomus leopardus) | 0.05, 0.1, 0.2 g/kg | Natural | No | Elevated levels of catalase, superoxide dismutase, and glutathione peroxidase activities increased T-AOC in the serum and liver | No effect | [5] |
| Golden pompano (Trachinotus ovatus) | 0.2 g/kg | Synthetic | No | Elevated hepatic T-AOC and augmented levels of GSH to GSSG | Improved weight gain, specific growth rate and FCR | [98] |
| Rainbow trout (Oncorhynchus mykiss) | 0.1 g/kg | Synthetic and natural | No | IncreasedNrf2/HO-1 signaling and antioxidant enzyme activity | Improved final body weight and FCR | [110] |
| Fish Species | Astaxanthin Supplementation Levels in Feed | Form | Astaxanthin Effects on Reproduction and Larval Survival Criteria | Reference |
|---|---|---|---|---|
| Clownfish (Amphiprion ocellaris) | 0.05, 0.1, 0.15, 0.2 | Synthetic | Improved hatching rate of eggs, reduced malformed rate, and increased survival rate of larvae in 3 days post-hatch | [144] |
| Swordtail fish (Xiphophorus helleri) | 0.05, 0.1 and 0.2 | Synthetic | Improved reproductive parameters | [149] |
| Nile tilapia (Oreochromis niloticus) | 0.05, 0.1, 0.15 and 0.2 g/kg | Natural | Improved gonad development, higher levels of serum E2, FSH, and LH, reduced apoptosis, and fewer instances of follicular atresia | [133] |
| Goldfish (Carassius auratus) | 0.05, 0.1, 0.15 g/kg | Synthetic | Improved osmolality, motility, spermatocrit value, sperm concentration, and fertilization rate | [135] |
| Rainbow trout (Oncorhynchus mykiss) | 0.07, 12.5, 33.3, 65.1 or 92.9 mg/kg | Synthetic | Improved fertilization rates, the proportion of eggs exhibiting eye pigmentation and those that hatched, as well as the reduced mortality rate of developed embryos | [134] |
| Yellowtail (Seriola quinqueradiata) | 0.02, 0.03, 0.04 g/kg | Synthetic | Improved fertilization rate, egg quality, hatching rate, and the count of normally developing larvae | [138] |
| Atlantic cod (Gadus morhua L.) | 0.1 g/kg | Synthetic | Lower egg incubation mortality and higher larval growth and survival | [139] |
| Gold fish (Carassius auratus) | 0.05, 0.1, 0.15 g/kg | Synthetic | Higher number of hatched eggs, larvae produced, and survivability | [150] |
| Fighter fish (Betta splendens) | 0.05, 0.1, 0.15 g/kg | Synthetic | Higher hatchability and a higher survival rate of larvae | [151] |
| Sea bream (Sparus aurata) | “Enriched in astaxanthin” | Natural | Higher survival rate of larvae | [152] |
| Clownfish (Amphiprion clarkia) | “Enriched in astaxanthin” | Natural | Improved development and survival of larvae | [153] |
| Goldfish (Carassius auratus) | 0.05, 0.1, 0.15 g/kg | Synthetic | Higher diameter and number of eggs per gram of fertilized eggs result in higher egg survival rates in the incubation period | [154] |
| Atlantic Cod (Gadus morhua L.) | 0.074 g/kg | Synthetic | A higher number of eggs per batch spawned and improved numbers of fertilized eggs per kg of female | [155] |
| Striped Jack (Pseudocaranx dentex) | 0.01 g/kg | Synthetic | Improved overall spawning performance | [156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shastak, Y.; Pelletier, W. Captivating Colors, Crucial Roles: Astaxanthin’s Antioxidant Impact on Fish Oxidative Stress and Reproductive Performance. Animals 2023, 13, 3357. https://doi.org/10.3390/ani13213357
Shastak Y, Pelletier W. Captivating Colors, Crucial Roles: Astaxanthin’s Antioxidant Impact on Fish Oxidative Stress and Reproductive Performance. Animals. 2023; 13(21):3357. https://doi.org/10.3390/ani13213357
Chicago/Turabian StyleShastak, Yauheni, and Wolf Pelletier. 2023. "Captivating Colors, Crucial Roles: Astaxanthin’s Antioxidant Impact on Fish Oxidative Stress and Reproductive Performance" Animals 13, no. 21: 3357. https://doi.org/10.3390/ani13213357
APA StyleShastak, Y., & Pelletier, W. (2023). Captivating Colors, Crucial Roles: Astaxanthin’s Antioxidant Impact on Fish Oxidative Stress and Reproductive Performance. Animals, 13(21), 3357. https://doi.org/10.3390/ani13213357





