Identification of Candidate Genes for Economically Important Carcass Cutting in Commercial Pigs through GWAS
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Samples and Phenotype Data
2.3. SNP Genotyping and Quality Control
2.4. Population Structure and Single-Locus GWAS Analysis
2.5. Identification of Candidate Genes and Functional Analysis
3. Results
3.1. Phenotypic Variation and SNP Genotyping
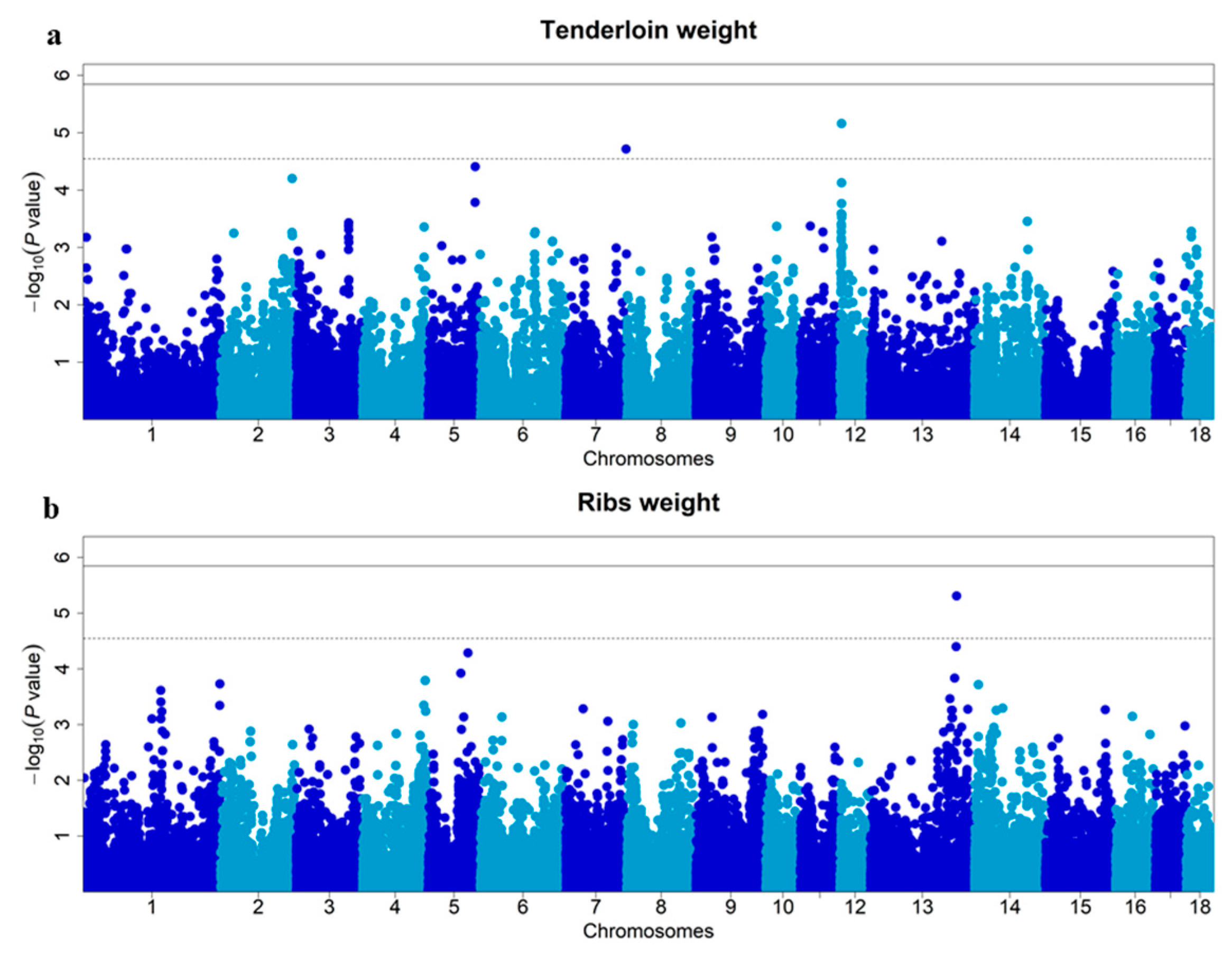
3.2. Single-Locus GWAS for TLNW and RIBW
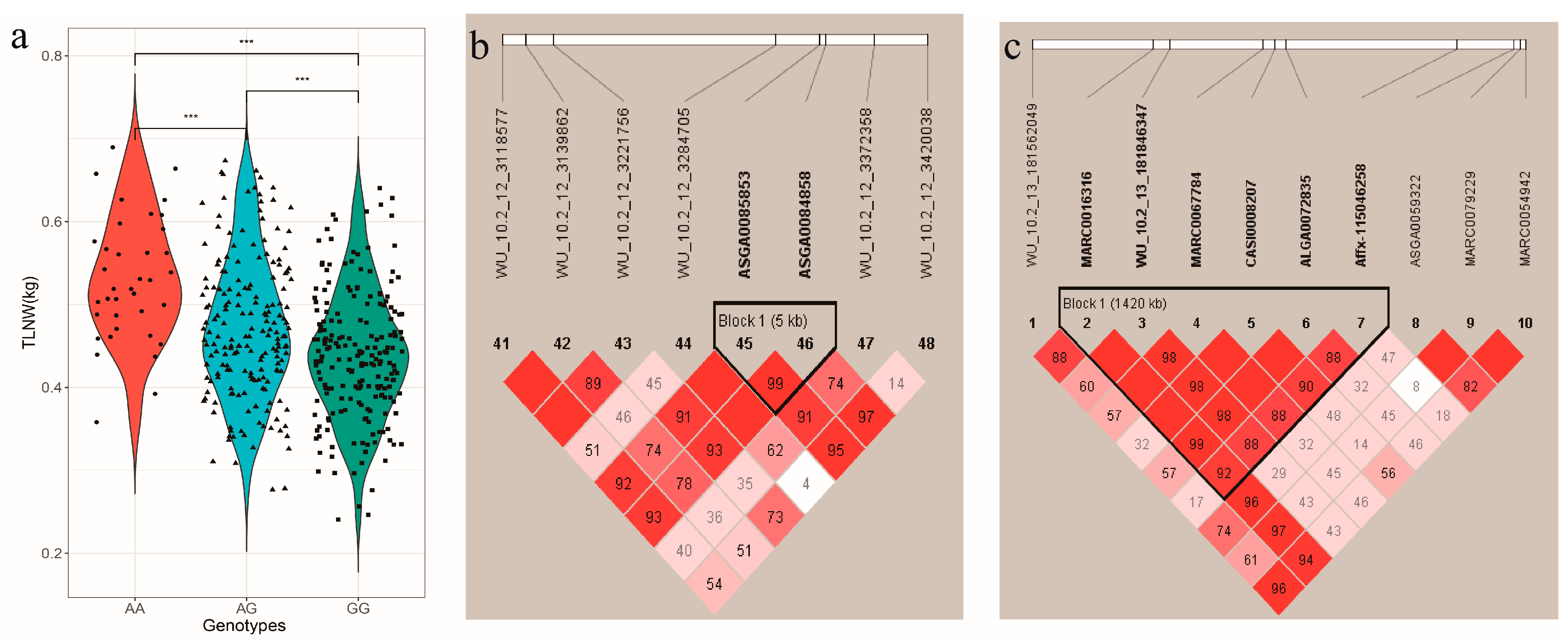
3.3. Effects of the QTL for TLNW and RIBW
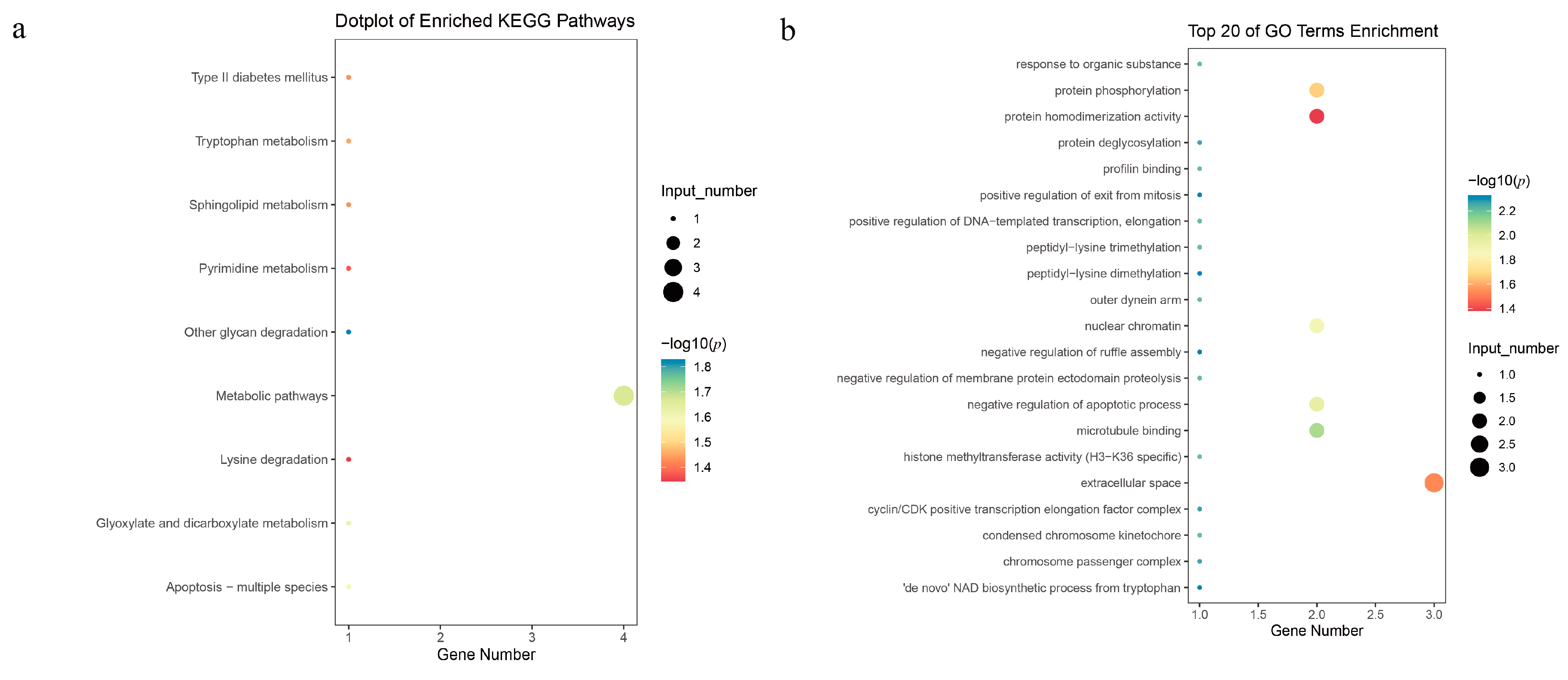
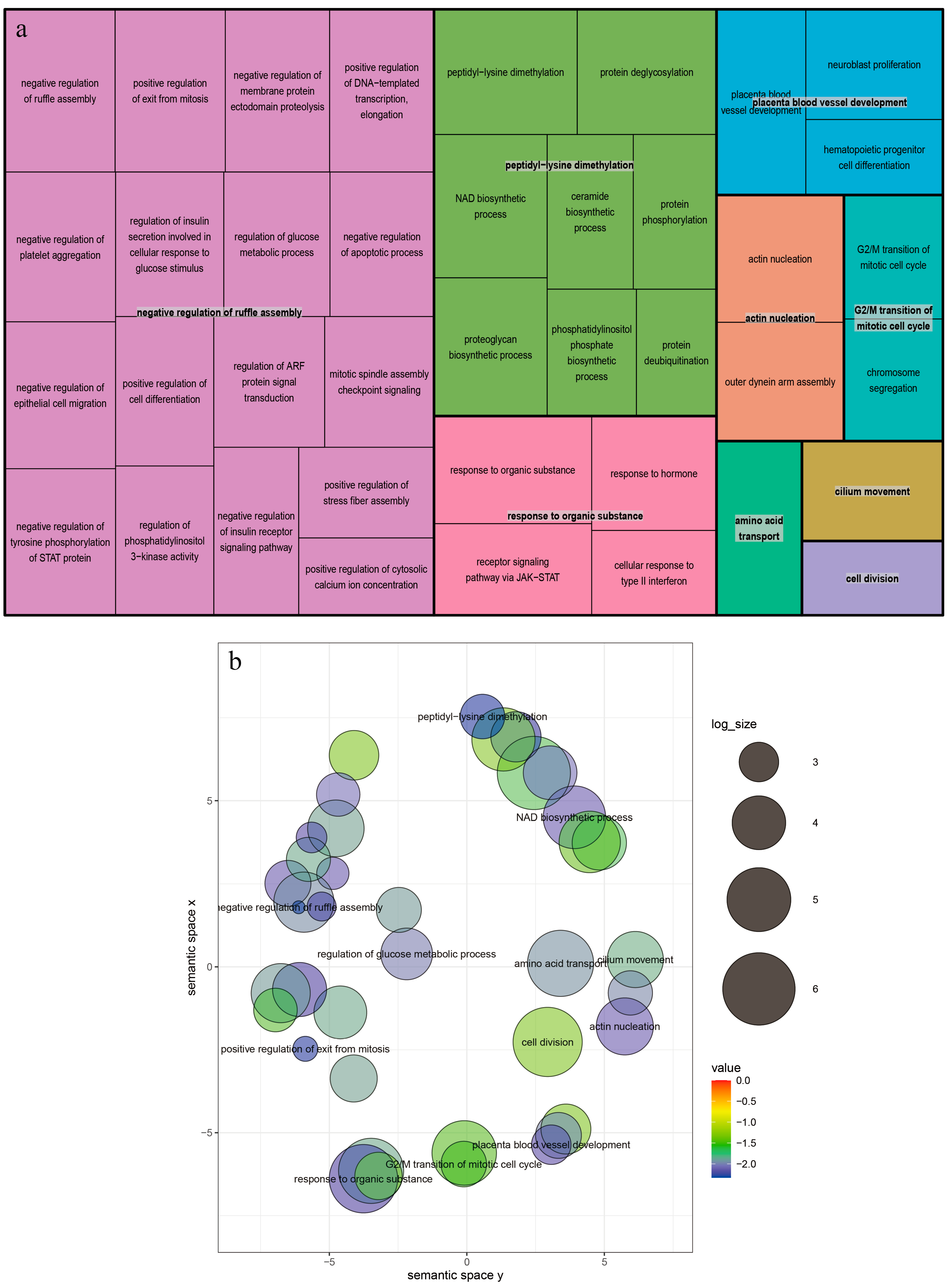
3.4. Candidate Genes Search and Functional Annotation
4. Discussion
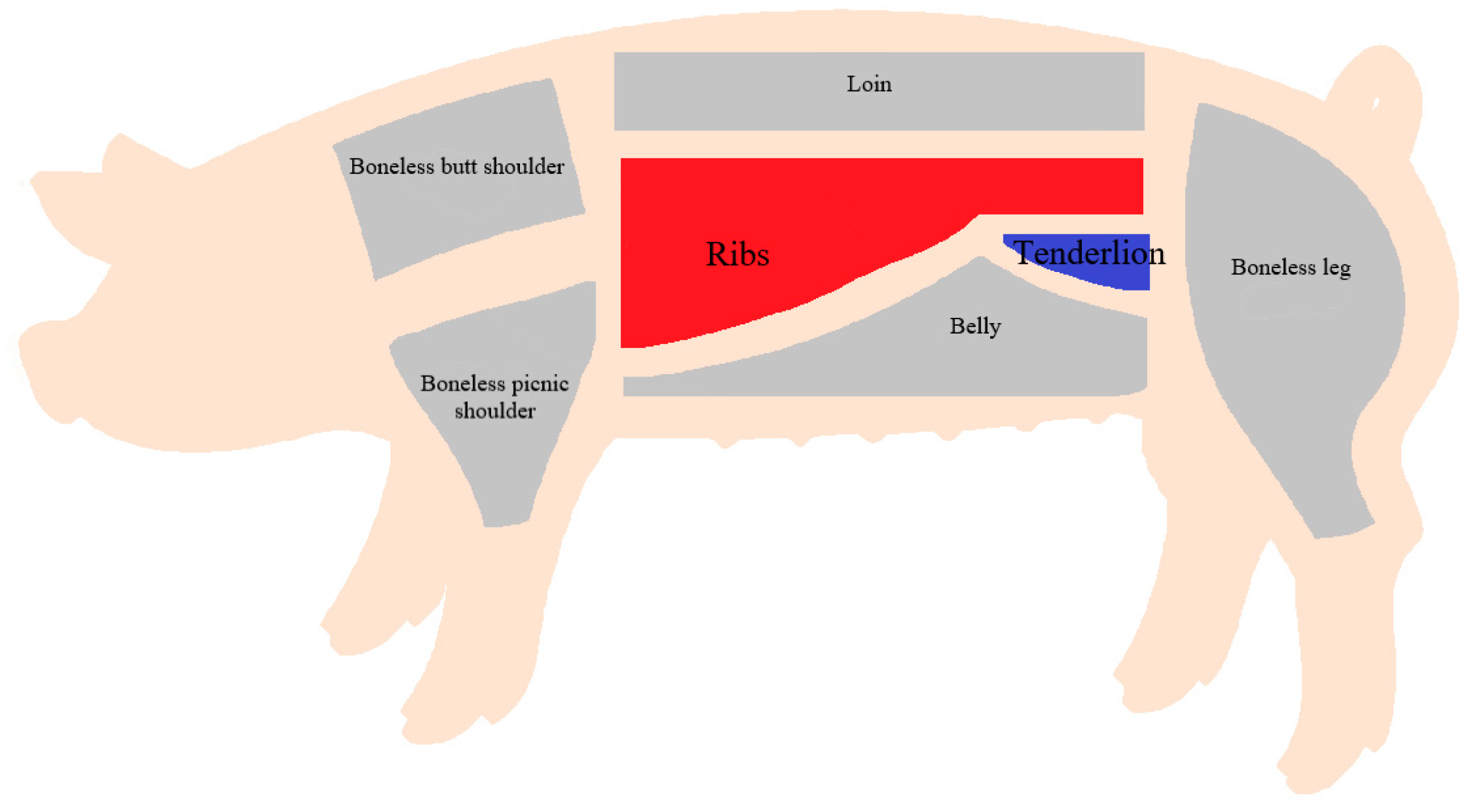
4.1. Fine Segmentation and Sale of Pig Carcasses
4.2. Genetic Loci and Candidate Genes for the TLNW Trait
4.3. Genetic Loci and Candidate Genes for the RIBW Trait
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Font, I.F.M.; Guerrero, L. Consumer preference, behavior and perception about meat and meat products: An overview. Meat Sci. 2014, 98, 361–371. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.P.; de Alencar Nääs, I.; Abe, J.M.; da Silva Cordeiro, A.F. Classification of piglet (Sus Scrofa) stress conditions using vocalization pattern and applying paraconsistent logic Eτ. Comput. Electron. Agric. 2019, 166, 105020. [Google Scholar] [CrossRef]
- Lim, M.S.M.; Grohn, Y.T. Comparison of China’s and the European Union’s Approaches to Antimicrobial Stewardship in the Pork Industry. Foodborne Pathog. Dis. 2021, 18, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhuang, Z.; Meng, F.; Ruan, D.; Xu, C.; Ma, F.; Peng, L.; Ding, R.; Cai, G.; Yang, M.; et al. Identification of candidate genes associated with carcass component weights in commercial crossbred pigs through a combined GWAS approach. J. Anim. Sci. 2023, 101, skad121. [Google Scholar] [CrossRef]
- Zhang, L.C.; Yue, J.W.; Pu, L.; Wang, L.G.; Liu, X.; Liang, J.; Yan, H.; Zhao, K.B.; Li, N.; Shi, H.B.; et al. Genome-wide study refines the quantitative trait locus for number of ribs in a Large White × Minzhu intercross pig population and reveals a new candidate gene. Mol. Genet. Genom. 2016, 291, 1885–1890. [Google Scholar] [CrossRef]
- Jiang, N.; Liu, C.; Lan, T.; Zhang, Q.; Cao, Y.; Pu, G.; Niu, P.; Zhang, Z.; Li, Q.; Zhou, J.; et al. Polymorphism of VRTN Gene g.20311_20312ins291 Was Associated with the Number of Ribs, Carcass Diagonal Length and Cannon Bone Circumference in Suhuai Pigs. Animals 2020, 10, 484. [Google Scholar] [CrossRef]
- Kim, G.W.; Kim, H.Y. Physicochemical Quality Properties of Loin and Tenderloin Ham from Sows. Food Sci. Anim. Resour. 2020, 40, 474–483. [Google Scholar] [CrossRef]
- Ortiz, A.; Díaz-Caro, C.; Tejerina, D.; Escribano, M.; Crespo, E.; Gaspar, P. Consumption of fresh Iberian pork: Two-stage cluster for the identification of segments of consumers according to their habits and lifestyles. Meat Sci. 2021, 173, 108373. [Google Scholar] [CrossRef]
- Mikawa, S.; Hayashi, T.; Nii, M.; Shimanuki, S.; Morozumi, T.; Awata, T. Two quantitative trait loci on Sus scrofa chromosomes 1 and 7 affecting the number of vertebrae. J. Anim. Sci. 2005, 83, 2247–2254. [Google Scholar] [CrossRef]
- Uemoto, Y.; Nagamine, Y.; Kobayashi, E.; Sato, S.; Tayama, T.; Suda, Y.; Shibata, T.; Suzuki, K. Quantitative trait loci analysis on Sus scrofa chromosome 7 for meat production, meat quality, and carcass traits within a Duroc purebred population. J. Anim. Sci. 2008, 86, 2833–2839. [Google Scholar] [CrossRef]
- Ren, D.R.; Ren, J.; Ruan, G.F.; Guo, Y.M.; Wu, L.H.; Yang, G.C.; Zhou, L.H.; Li, L.; Zhang, Z.Y.; Huang, L.S. Mapping and fine mapping of quantitative trait loci for the number of vertebrae in a White Duroc × Chinese Erhualian intercross resource population. Anim. Genet. 2012, 43, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Mikawa, S.; Sato, S.; Nii, M.; Morozumi, T.; Yoshioka, G.; Imaeda, N.; Yamaguchi, T.; Hayashi, T.; Awata, T. Identification of a second gene associated with variation in vertebral number in domestic pigs. BMC Genet. 2011, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Qin, J.; Yao, T.; Tang, X.; Cui, D.; Chen, L.; Rao, L.; Xiao, S.; Zhang, Z.; Huang, L. Genetic dissection of 26 meat cut, meat quality and carcass traits in four pig populations. Genet. Sel. Evol. 2023, 55, 43. [Google Scholar] [CrossRef] [PubMed]
- Van Laere, A.S.; Nguyen, M.; Braunschweig, M.; Nezer, C.; Collette, C.; Moreau, L.; Archibald, A.L.; Haley, C.S.; Buys, N.; Tally, M.; et al. A regulatory mutation in IGF2 causes a major QTL effect on muscle growth in the pig. Nature 2003, 425, 832–836. [Google Scholar] [CrossRef]
- Oczkowicz, M.; Tyra, M.; Walinowicz, K.; Rózycki, M.; Rejduch, B. Known mutation (A3072G) in intron 3 of the IGF2 gene is associated with growth and carcass composition in Polish pig breeds. J. Appl. Genet. 2009, 50, 257–259. [Google Scholar] [CrossRef]
- Burgos, C.; Galve, A.; Moreno, C.; Altarriba, J.; Reina, R.; García, C.; López-Buesa, P. The effects of two alleles of IGF2 on fat content in pig carcasses and pork. Meat Sci. 2012, 90, 309–313. [Google Scholar] [CrossRef]
- Daetwyler, H.D.; Capitan, A.; Pausch, H.; Stothard, P.; van Binsbergen, R.; Brøndum, R.F.; Liao, X.; Djari, A.; Rodriguez, S.C.; Grohs, C.; et al. Whole-genome sequencing of 234 bulls facilitates mapping of monogenic and complex traits in cattle. Nat. Genet. 2014, 46, 858–865. [Google Scholar] [CrossRef]
- Xu, S.S.; Gao, L.; Xie, X.L.; Ren, Y.L.; Shen, Z.Q.; Wang, F.; Shen, M.; Eyϸórsdóttir, E.; Hallsson, J.H.; Kiseleva, T.; et al. Genome-Wide Association Analyses Highlight the Potential for Different Genetic Mechanisms for Litter Size Among Sheep Breeds. Front. Genet. 2018, 9, 118. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, L.Y.; Xu, Z.C.; Kramer, L.M.; Yu, J.Q.; Zhang, X.Y.; Na, W.; Yang, L.L.; Cao, Z.P.; Luan, P.; et al. Haplotype-based genome-wide association studies for carcass and growth traits in chicken. Poult. Sci. 2020, 99, 2349–2361. [Google Scholar] [CrossRef]
- Fachal, L.; Dunning, A.M. From candidate gene studies to GWAS and post-GWAS analyses in breast cancer. Curr. Opin. Genet. Dev. 2015, 30, 32–41. [Google Scholar] [CrossRef]
- Benafif, S.; Kote-Jarai, Z.; Eeles, R.A. A Review of Prostate Cancer Genome-Wide Association Studies (GWAS). Cancer Epidemiol. Biomark. Prev. 2018, 27, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.B.; Ernst, C.W.; Raney, N.E.; Doumit, M.E.; Hoge, M.D.; Bates, R.O. Quantitative trait locus mapping in an F2 Duroc x Pietrain resource population: II. Carcass and meat quality traits. J. Anim. Sci. 2008, 86, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Ovilo, C.; Fernández, A.; Noguera, J.L.; Barragán, C.; Letón, R.; Rodríguez, C.; Mercadé, A.; Alves, E.; Folch, J.M.; Varona, L.; et al. Fine mapping of porcine chromosome 6 QTL and LEPR effects on body composition in multiple generations of an Iberian by Landrace intercross. Genet. Res. 2005, 85, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Grover, A.; Sharma, P.C. Development and use of molecular markers: Past and present. Crit. Rev. Biotechnol. 2016, 36, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Yang, M.; Wang, X.; Quan, J.; Zhuang, Z.; Zhou, S.; Li, S.; Xu, Z.; Zheng, E.; Cai, G.; et al. Genetic Architecture of Feeding Behavior and Feed Efficiency in a Duroc Pig Population. Front. Genet. 2018, 9, 220. [Google Scholar] [CrossRef]
- Zhou, S.; Ding, R.; Meng, F.; Wang, X.; Zhuang, Z.; Quan, J.; Geng, Q.; Wu, J.; Zheng, E.; Wu, Z.; et al. A meta-analysis of genome-wide association studies for average daily gain and lean meat percentage in two Duroc pig populations. BMC Genom. 2021, 22, 12. [Google Scholar] [CrossRef]
- Zhuang, Z.; Ding, R.; Peng, L.; Wu, J.; Ye, Y.; Zhou, S.; Wang, X.; Quan, J.; Zheng, E.; Cai, G.; et al. Genome-wide association analyses identify known and novel loci for teat number in Duroc pigs using single-locus and multi-locus models. BMC Genom. 2020, 21, 344. [Google Scholar] [CrossRef]
- Ruan, D.; Zhuang, Z.; Ding, R.; Qiu, Y.; Zhou, S.; Wu, J.; Xu, C.; Hong, L.; Huang, S.; Zheng, E.; et al. Weighted Single-Step GWAS Identified Candidate Genes Associated with Growth Traits in a Duroc Pig Population. Genes. 2021, 12, 117. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef]
- Yang, J.; Lee, S.H.; Goddard, M.E.; Visscher, P.M. GCTA: A tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 2011, 88, 76–82. [Google Scholar] [CrossRef]
- Zhou, X.; Stephens, M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 2012, 44, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Price, A.L.; Patterson, N.J.; Plenge, R.M.; Weinblatt, M.E.; Shadick, N.A.; Reich, D. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 2006, 38, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef]
- Smedley, D.; Haider, S.; Ballester, B.; Holland, R.; London, D.; Thorisson, G.; Kasprzyk, A. BioMart—Biological queries made easy. BMC Genom. 2009, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]
- Supek, F.; Bošnjak, M.; Škunca, N.; Šmuc, T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE 2011, 6, e21800. [Google Scholar] [CrossRef]
- Zhang, Z.; Kryvokhyzha, D.; Orsucci, M.; Glémin, S.; Milesi, P.; Lascoux, M. How broad is the selfing syndrome? Insights from convergent evolution of gene expression across species and tissues in the Capsella genus. New Phytol. 2022, 236, 2344–2357. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.; Ding, R.; Wang, X.; Yang, M.; Yang, Y.; Zheng, E.; Gu, T.; Cai, G.; Wu, Z.; Liu, D.; et al. Genome-wide association study reveals genetic loci and candidate genes for average daily gain in Duroc pigs. Asian-Australas. J. Anim. Sci. 2018, 31, 480–488. [Google Scholar] [CrossRef]
- Verbeke, W.; Pérez-Cueto, F.J.; Grunert, K.G. To eat or not to eat pork, how frequently and how varied? Insights from the quantitative Q-PorkChains consumer survey in four European countries. Meat Sci. 2011, 88, 619–626. [Google Scholar] [CrossRef]
- Grunert, K.; Wognum, N.; Trienekens, J.; Wever, M.; Olsen, N.V.; Scholderer, J. Consumer demand and quality assurance: Segmentation basis and implications for chain governance in the pork sector. J. Chain. Netw. Sci. 2011, 11, 89–97. [Google Scholar] [CrossRef]
- Choe, J.-H.; Yang, H.-S.; Lee, S.-H.; Go, G.-W. Characteristics of pork belly consumption in South Korea and their health implication. J. Anim. Sci. Technol. 2015, 57, 22. [Google Scholar] [CrossRef] [PubMed]
- Juárez, M.; Clemente, I.; Polvillo, O.; Molina, A. Meat quality of tenderloin from Iberian pigs as affected by breed strain and crossbreeding. Meat Sci. 2009, 81, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Takawale, A.; Basu, R.; Patel, V.; Lee, J.; Kandalam, V.; Wang, X.; Oudit, G.Y.; Kassiri, Z. Differential role of TIMP2 and TIMP3 in cardiac hypertrophy, fibrosis, and diastolic dysfunction. Cardiovasc. Res. 2014, 103, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Fontes, M.; Costa, T.C.; Lopes, M.M.; Souza, R.O.; Carneiro, L.S.; Paulino, P.V.R.; Chizzotti, M.L.; Silva, F.F.; Serão, N.V.L.; Duarte, M.S. Intramuscular collagen characteristics and expression of related genes in skeletal muscle of cull cows receiving a high-energy diet. Meat Sci. 2021, 177, 108495. [Google Scholar] [CrossRef]
- Chen, Y.; Gondro, C.; Quinn, K.; Herd, R.M.; Parnell, P.F.; Vanselow, B. Global gene expression profiling reveals genes expressed differentially in cattle with high and low residual feed intake. Anim. Genet. 2011, 42, 475–490. [Google Scholar] [CrossRef]
- Taye, M.; Kim, J.; Yoon, S.H.; Lee, W.; Hanotte, O.; Dessie, T.; Kemp, S.; Mwai, O.A.; Caetano-Anolles, K.; Cho, S.; et al. Whole genome scan reveals the genetic signature of African Ankole cattle breed and potential for higher quality beef. BMC Genet. 2017, 18, 11. [Google Scholar] [CrossRef]
- Paganini, C.; Monti, L.; Costantini, R.; Besio, R.; Lecci, S.; Biggiogera, M.; Tian, K.; Schwartz, J.M.; Huber, C.; Cormier-Daire, V.; et al. Calcium activated nucleotidase 1 (CANT1) is critical for glycosaminoglycan biosynthesis in cartilage and endochondral ossification. Matrix Biol. 2019, 81, 70–90. [Google Scholar] [CrossRef]
- Oegema, R.; McGillivray, G.; Leventer, R.; Le Moing, A.G.; Bahi-Buisson, N.; Barnicoat, A.; Mandelstam, S.; Francis, D.; Francis, F.; Mancini, G.M.S.; et al. EML1-associated brain overgrowth syndrome with ribbon-like heterotopia. Am. J. Med. Genet. C Semin. Med. Genet. 2019, 181, 627–637. [Google Scholar] [CrossRef]
- Collin, G.B.; Won, J.; Krebs, M.P.; Hicks, W.J.; Charette, J.R.; Naggert, J.K.; Nishina, P.M. Disruption in murine Eml1 perturbs retinal lamination during early development. Sci. Rep. 2020, 10, 5647. [Google Scholar] [CrossRef]
- Affar, E.B.; Gay, F.; Shi, Y.; Liu, H.; Huarte, M.; Wu, S.; Collins, T.; Li, E.; Shi, Y. Essential dosage-dependent functions of the transcription factor yin yang 1 in late embryonic development and cell cycle progression. Mol. Cell. Biol. 2006, 26, 3565–3581. [Google Scholar] [CrossRef]
- Wirth, B. An update of the mutation spectrum of the survival motor neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA). Hum. Mutat. 2000, 15, 228–237. [Google Scholar] [CrossRef]
- Poirier, A.; Weetall, M.; Heinig, K.; Bucheli, F.; Schoenlein, K.; Alsenz, J.; Bassett, S.; Ullah, M.; Senn, C.; Ratni, H.; et al. Risdiplam distributes and increases SMN protein in both the central nervous system and peripheral organs. Pharmacol. Res. Perspect. 2018, 6, e00447. [Google Scholar] [CrossRef] [PubMed]
- Gogliotti, R.G.; Quinlan, K.A.; Barlow, C.B.; Heier, C.R.; Heckman, C.J.; Didonato, C.J. Motor neuron rescue in spinal muscular atrophy mice demonstrates that sensory-motor defects are a consequence, not a cause, of motor neuron dysfunction. J. Neurosci. 2012, 32, 3818–3829. [Google Scholar] [CrossRef]
- Bowerman, M.; Anderson, C.L.; Beauvais, A.; Boyl, P.P.; Witke, W.; Kothary, R. SMN, profilin IIa and plastin 3: A link between the deregulation of actin dynamics and SMA pathogenesis. Mol. Cell Neurosci. 2009, 42, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Park, G.H.; Maeno-Hikichi, Y.; Awano, T.; Landmesser, L.T.; Monani, U.R. Reduced survival of motor neuron (SMN) protein in motor neuronal progenitors functions cell autonomously to cause spinal muscular atrophy in model mice expressing the human centromeric (SMN2) gene. J. Neurosci. 2010, 30, 12005–12019. [Google Scholar] [CrossRef]
- Lorson, M.A.; Spate, L.D.; Prather, R.S.; Lorson, C.L. Identification and characterization of the porcine (Sus scrofa) survival motor neuron (SMN1) gene: An animal model for therapeutic studies. Dev. Dyn. 2008, 237, 2268–2278. [Google Scholar] [CrossRef]
- Schrank, B.; Götz, R.; Gunnersen, J.M.; Ure, J.M.; Toyka, K.V.; Smith, A.G.; Sendtner, M. Inactivation of the survival motor neuron gene, a candidate gene for human spinal muscular atrophy, leads to massive cell death in early mouse embryos. Proc. Natl. Acad. Sci. USA 1997, 94, 9920–9925. [Google Scholar] [CrossRef]
| Trait | N 3 | Mean (±SD)/kg 4 | Min/kg 5 | Max/kg 6 | C.V./% 7 | h2 (±SE) 8 |
|---|---|---|---|---|---|---|
| TLNW 1 | 431 | 0.46 ± 0.08 | 0.24 | 0.69 | 17.40 | 0.42 ± 0.11 |
| RIBW 2 | 408 | 4.51 ± 0.56 | 2.87 | 6.17 | 12.42 | 0.22 ± 0.09 |
| Trait | SNP | SSC 1 | Position (bp) | EPV 2 | MAF | p-Value | Distance 3 | Nearest Gene |
|---|---|---|---|---|---|---|---|---|
| TLNW | ASGA0085853 ALGA0112188 | 12 7 | 3,284,259 120,821,692 | 4.88% 3.90% | 0.306 0.325 | 6.88 × 10−6 1.92 × 10−5 | within within | TIMP2 EML1 |
| RIBW | Affx-115046258 | 13 | 172,454,121 | 5.19% | 0.268 | 4.88 × 10−6 | 150.8 kb | ENSSSCG00000029127 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, F.; Quan, J.; Ruan, D.; Qiu, Y.; Ding, R.; Xu, C.; Ye, Y.; Cai, G.; Liu, L.; Zhang, Z.; et al. Identification of Candidate Genes for Economically Important Carcass Cutting in Commercial Pigs through GWAS. Animals 2023, 13, 3243. https://doi.org/10.3390/ani13203243
Zhou F, Quan J, Ruan D, Qiu Y, Ding R, Xu C, Ye Y, Cai G, Liu L, Zhang Z, et al. Identification of Candidate Genes for Economically Important Carcass Cutting in Commercial Pigs through GWAS. Animals. 2023; 13(20):3243. https://doi.org/10.3390/ani13203243
Chicago/Turabian StyleZhou, Fuchen, Jianping Quan, Donglin Ruan, Yibin Qiu, Rongrong Ding, Cineng Xu, Yong Ye, Gengyuan Cai, Langqing Liu, Zebin Zhang, and et al. 2023. "Identification of Candidate Genes for Economically Important Carcass Cutting in Commercial Pigs through GWAS" Animals 13, no. 20: 3243. https://doi.org/10.3390/ani13203243
APA StyleZhou, F., Quan, J., Ruan, D., Qiu, Y., Ding, R., Xu, C., Ye, Y., Cai, G., Liu, L., Zhang, Z., Yang, J., Wu, Z., & Zheng, E. (2023). Identification of Candidate Genes for Economically Important Carcass Cutting in Commercial Pigs through GWAS. Animals, 13(20), 3243. https://doi.org/10.3390/ani13203243





