The Yeast and Hypha Phases of Candida krusei Induce the Apoptosis of Bovine Mammary Epithelial Cells via Distinct Signaling Pathways
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of C. krusei Yeast Phase and Hypha Phase
2.2. Cell Culture
2.3. Infections of BMECs with C. krusei
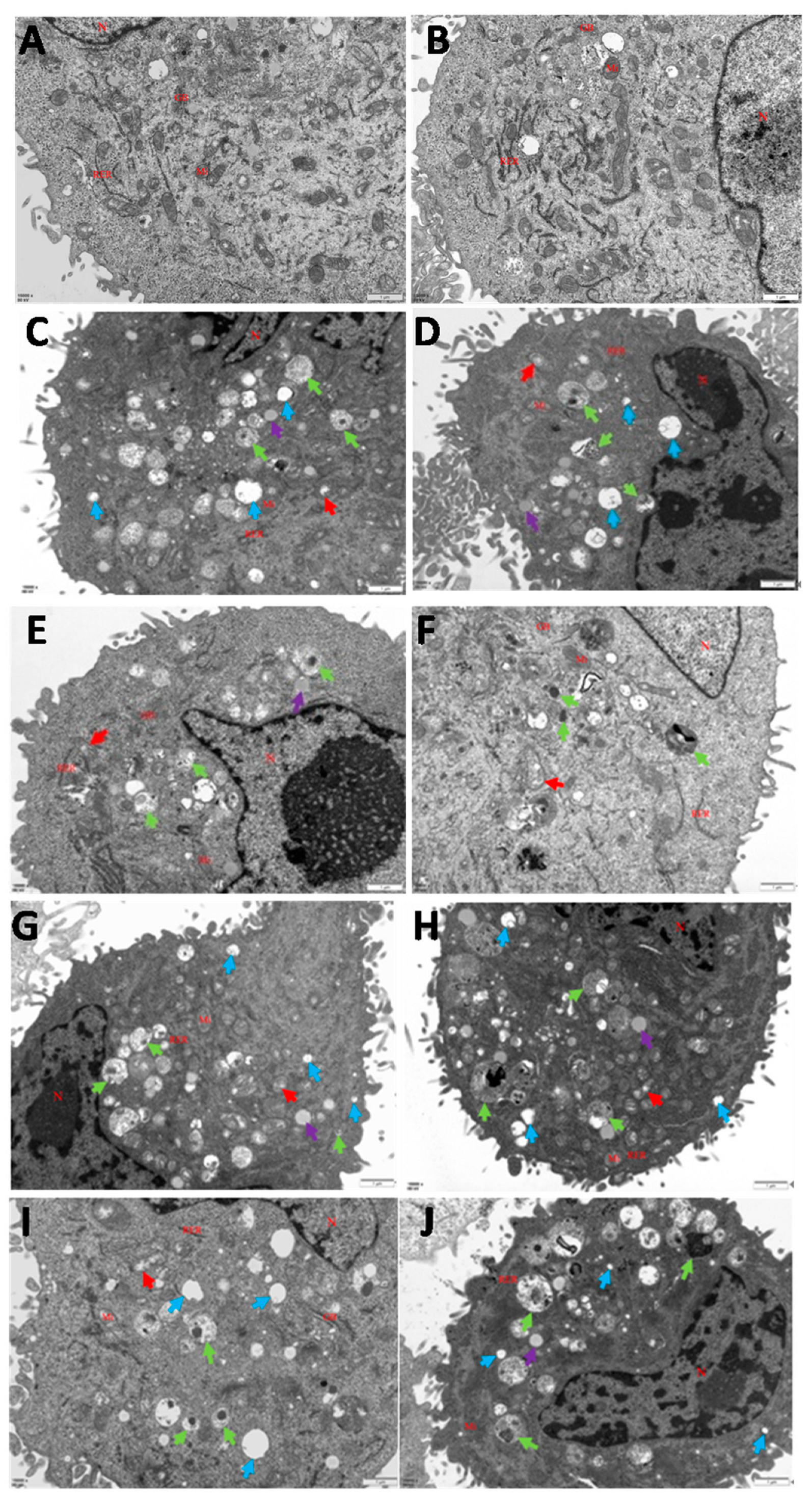
2.4. Transmission Electron Microscopy
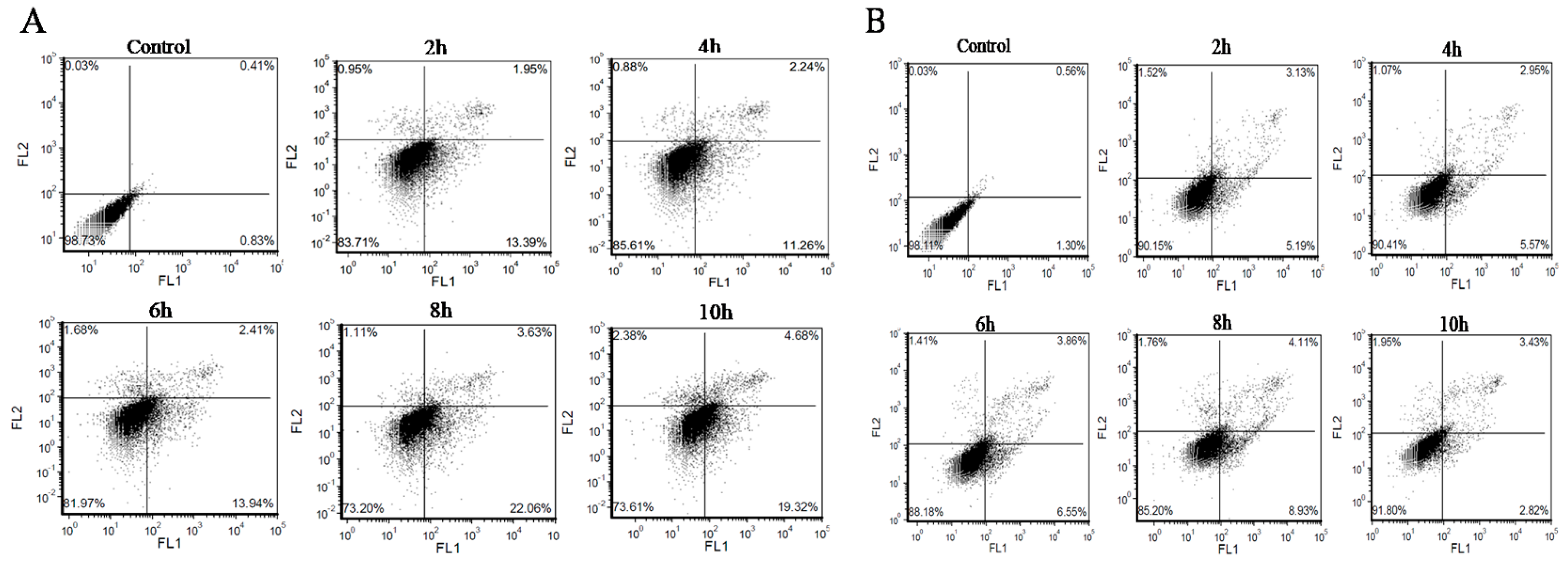
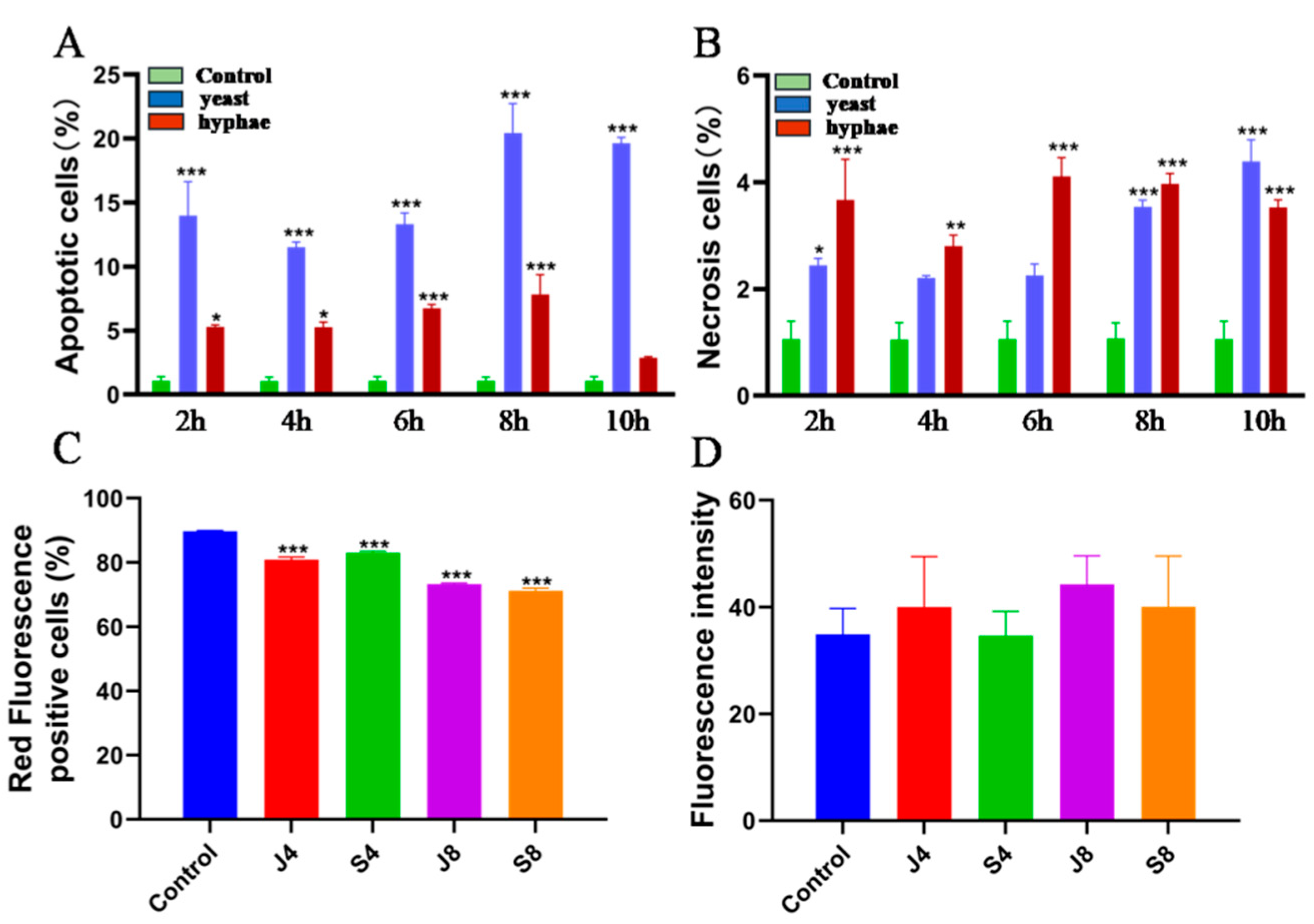
2.5. Analysis of Apoptosis/Necrosis with AV/PI Double Staining
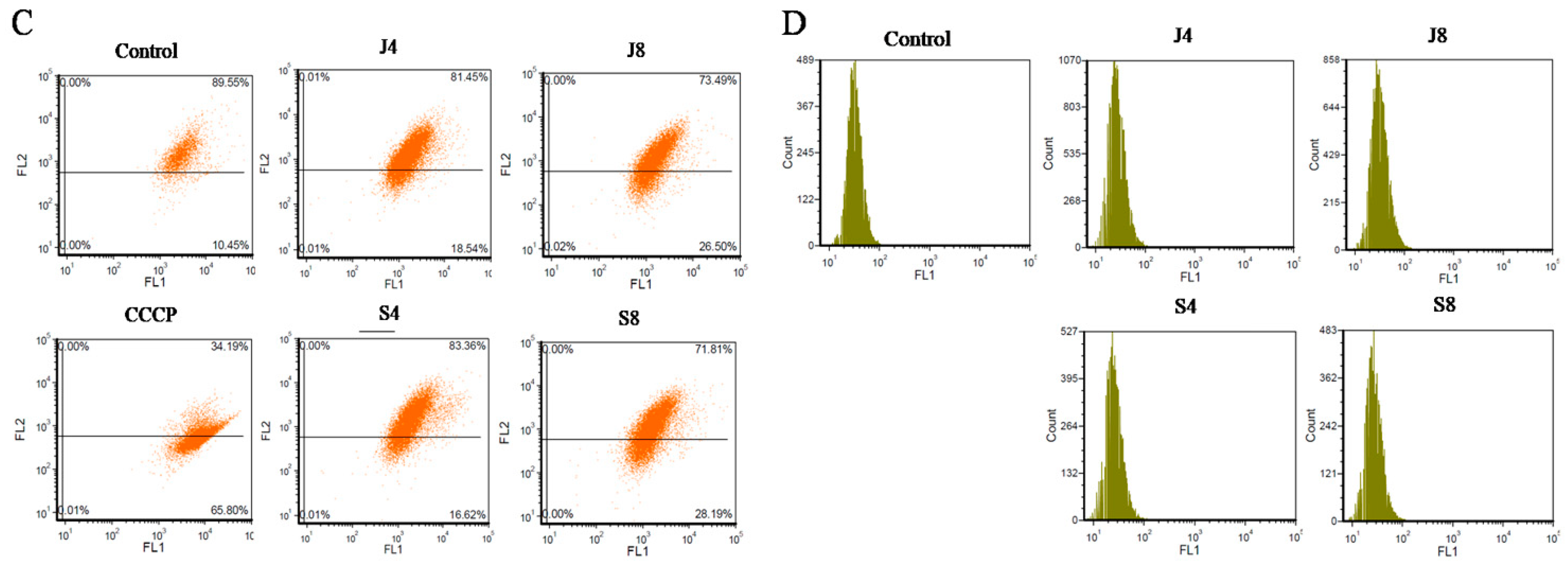
2.6. MMP Assay
2.7. Measurement of Intracellular ROS
2.8. TdT-Mediated dUTP Nick End Labeling (TUNEL) Assay
2.9. Western Blot Analysis
2.10. Treatment with Inhibitors
2.11. Statistical Analysis
3. Results
3.1. Infections of C. krusei Yeast Phase and Hypha Phase Induced Morphological Changes in Apoptosis in BMECs
3.2. BMECs Infected with C. krusei Exhibit an Apoptotic Phenotype
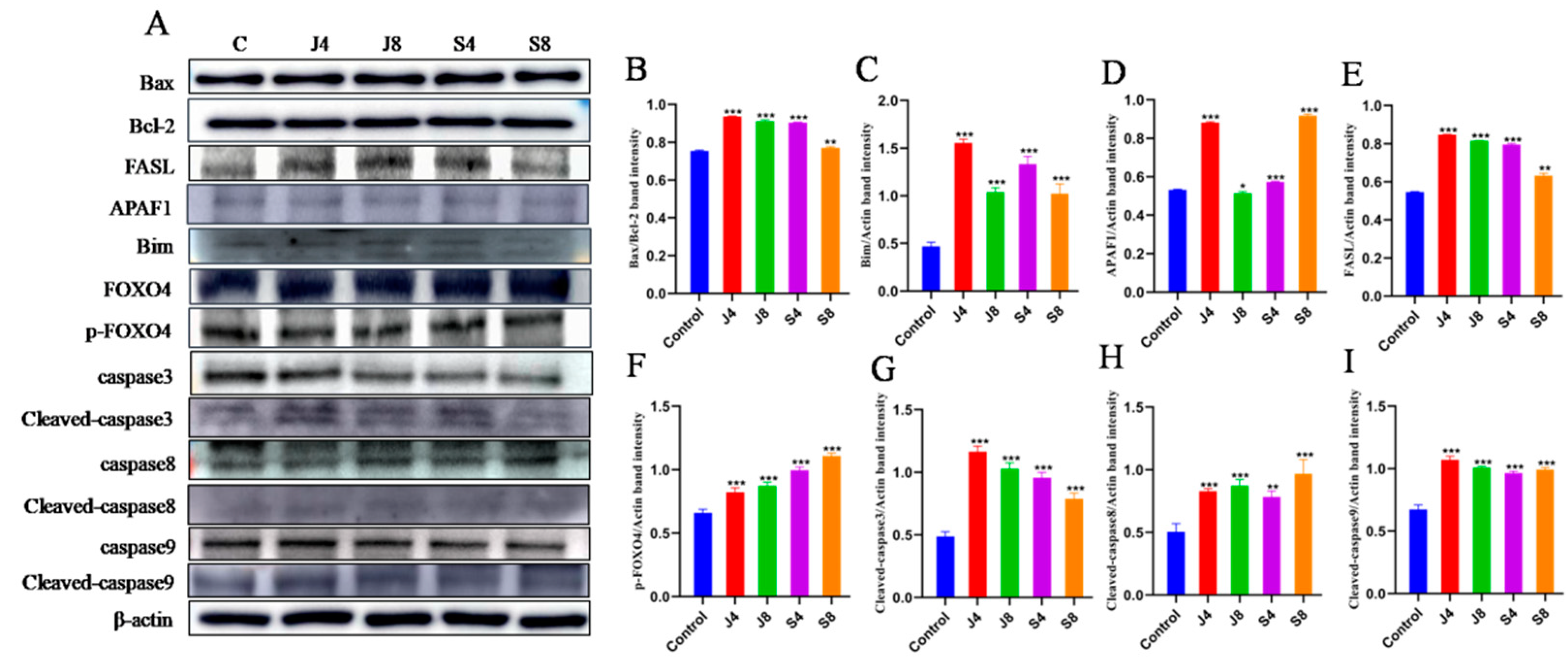
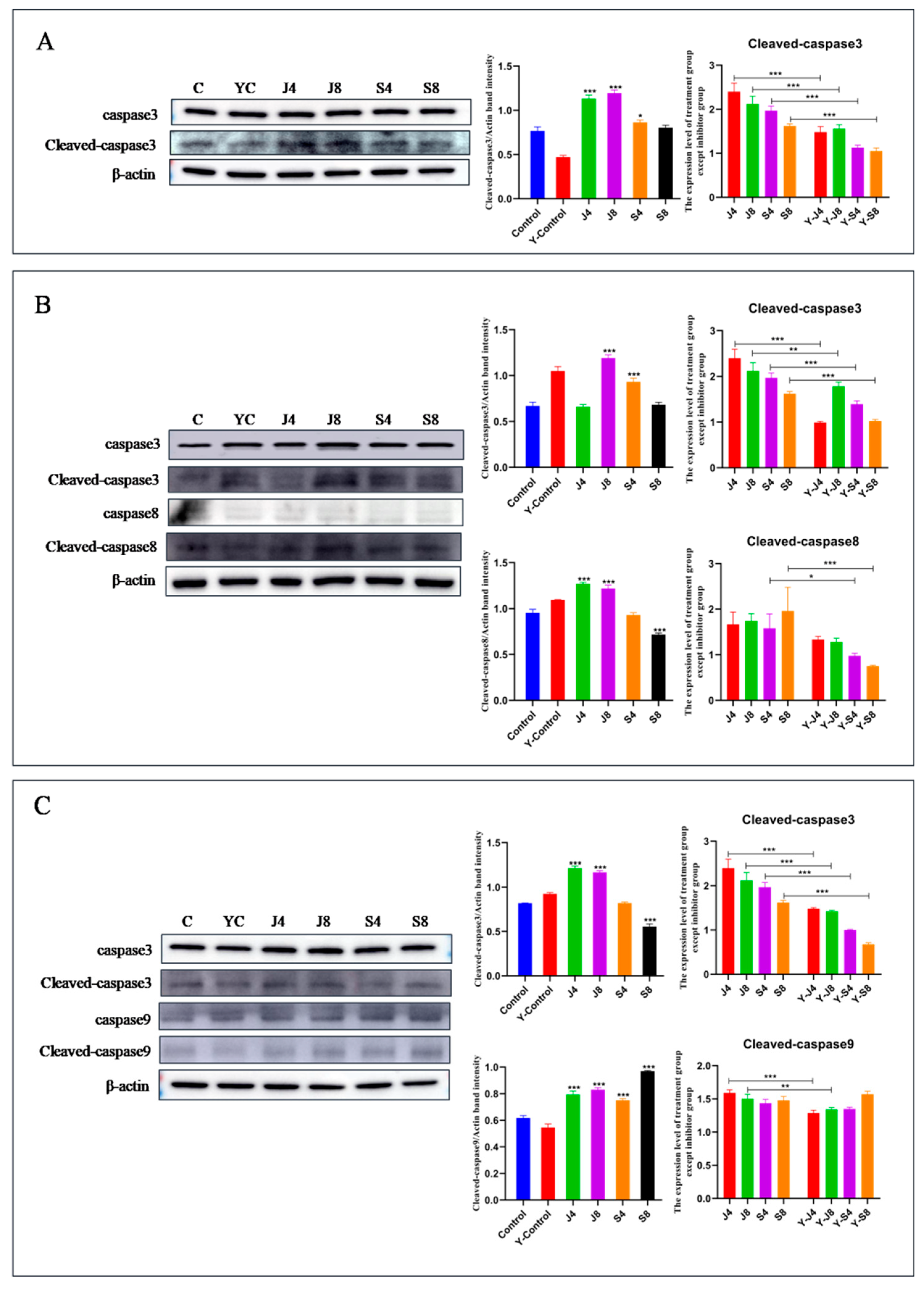
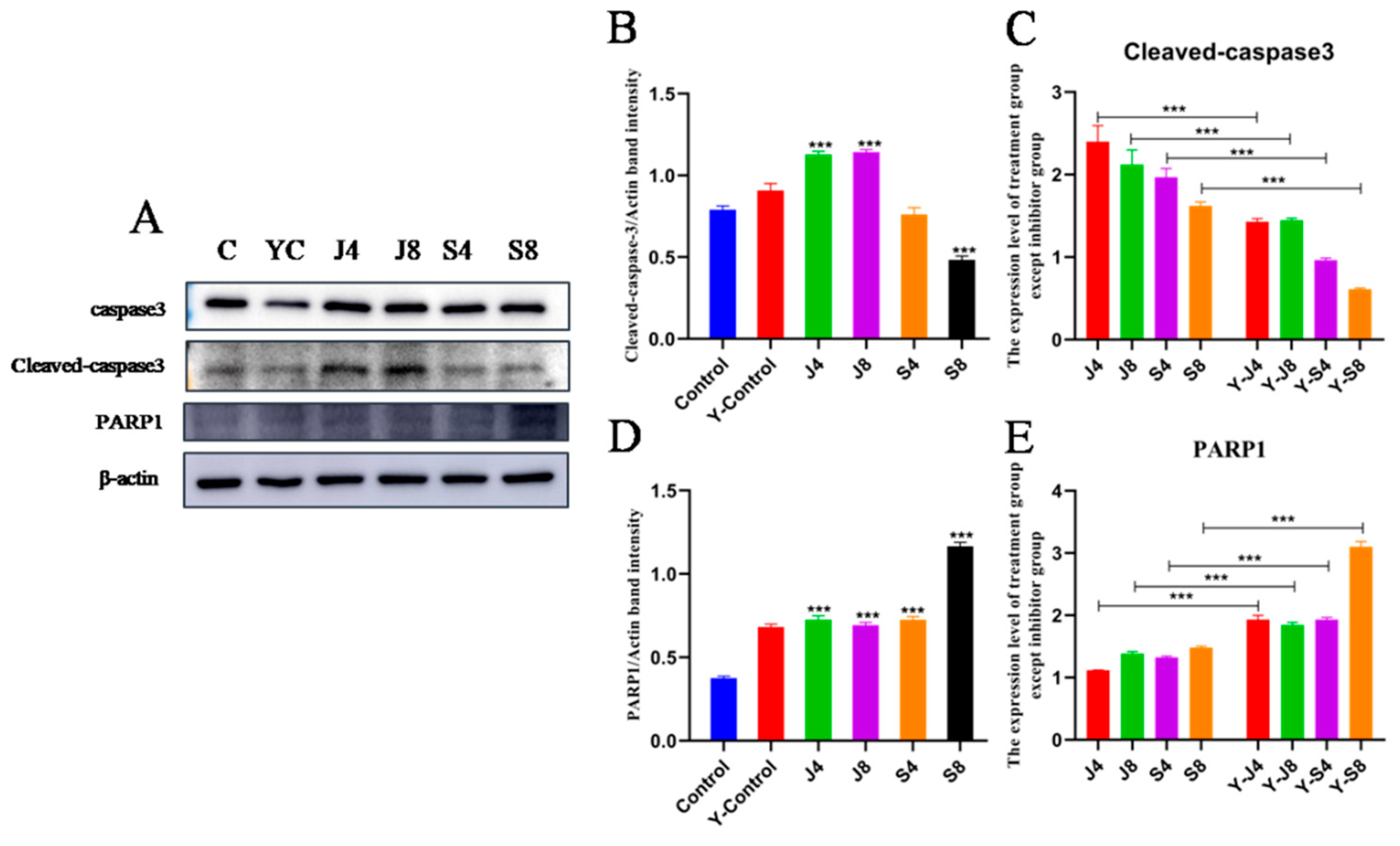
3.3. Distinct Apoptosis Signaling Pathways in BMECs Infected with the Yeast Phase and Hypha Phase of C. krusei
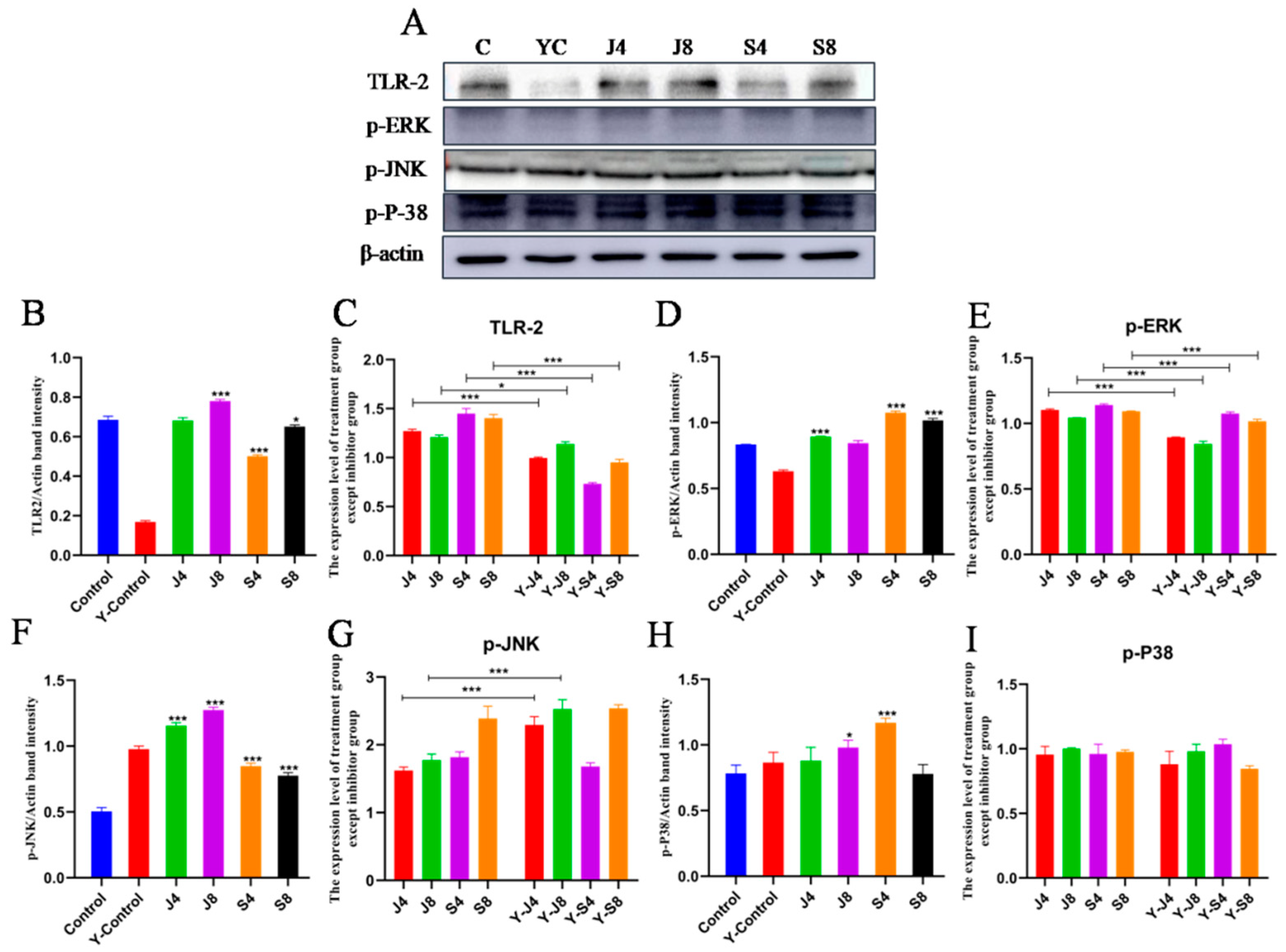
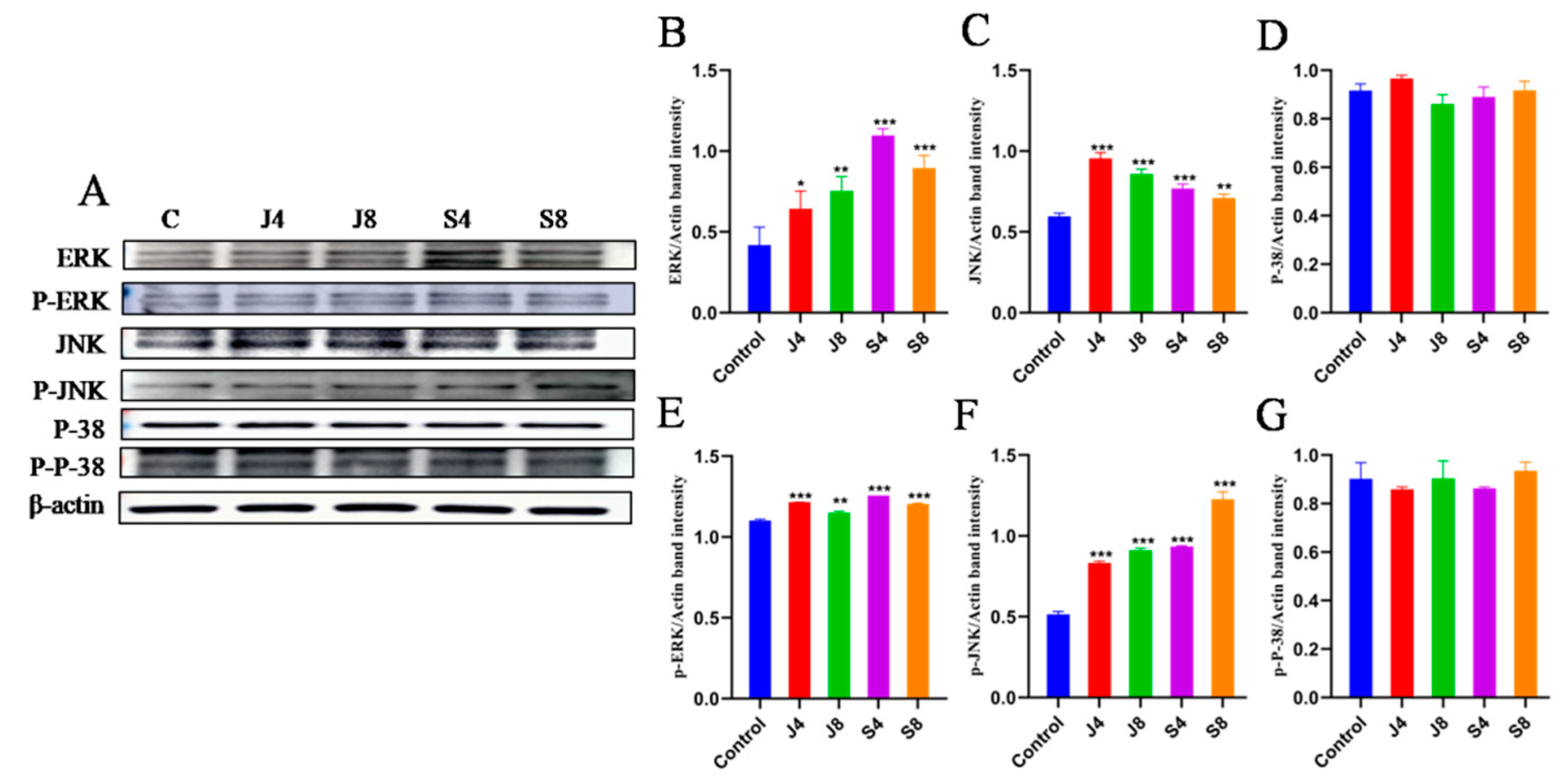
3.4. TLR/ERK Signaling Is Activated in BMECs in Response to C. krusei Infection
3.5. JNK and ERK Pathways Are Involved in BMEC Apoptosis in Response to C. krusei Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, J.S.S.; Bezawada, J.; Yan, S.; Tyagi, R.; Surampalli, R. Candida krusei: Biotechnological potentials and concerns about its safety. Can. J. Microbiol. 2012, 58, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Jamiu, A.T.; Albertyn, J.; Sebolai, O.M.; Pohl, C.H. Update on Candida krusei, a potential multidrug-resistant pathogen. Med. Mycol. 2020, 59, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gaviria, M.; Ramírez-Sotelo, U.; Mora-Montes, H.M. Non-albicans Candida Species: Immune Response, Evasion Mechanisms, and New Plant-Derived Alternative Therapies. J. Fungi 2023, 9, 11. [Google Scholar] [CrossRef]
- Domán, M.; Makrai, L.; Bányai, K. Molecular Phylogenetic Analysis of Candida krusei. Mycopathologia 2022, 187, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, X.; Luo, H.; Wang, Y.; Liu, X.; Zhou, X. Epidemiological investigation of non-albicans Candida species recovered from mycotic mastitis of cows in Yinchuan, Ningxia of China. BMC Vet. Res. 2018, 14, 251. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, M.; Erbas, G. Isolation and Identification of Candida spp. from Mastitis Cattle Milk and Determination of Antifungal Susceptibilities. Int. J. Vet. Sci. 2017, 6, 104–107. [Google Scholar]
- Şeker, E. Identification of Candida Species Isolated from Bovine Mastitic Milk and Their In Vitro Hemolytic Activity in Western Turkey. Mycopathologia 2010, 169, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Casia dos Santos, R.D.; Marin, J.M. Isolation of Candida spp. from mastitic bovine milk in Brazil. Mycopathologia 2005, 159, 251–253. [Google Scholar] [CrossRef]
- Sartori, L.; Santos, R.; Marin, J. Identification of Candida species isolated from cows suffering mastitis in four Brazilian states. Arq. Bras. Med. Vet. Zootec. 2014, 66, 1615–1617. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, M.; Vasishta, N.K.; Sharma, N.S. Mycotic Isolates from the Uterus of Endometritic Cows and Buffaloes in Himachal Pradesh; Indian Council of Agricultural Research: New Delhi, India, 2008. [Google Scholar]
- Chhabra, D.; Moghe, M.N.; Tanwani, S.K. Prevalence of mycotic mastitis in cows and buffaloes in Madhya Pradesh. Indian Vet. J. 1996, 73, 1–3. [Google Scholar]
- Zhou, Y.; Ren, Y.; Fan, C.; Hong, S. Survey of mycotic mastitis in dairy cows from Heilongjiang Province, China. Trop. Anim. Health Prod. 2013, 45, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Krukowski, H.; Tietze, M.; Majewski, T.; Róański, P. Survey of yeast mastitis in dairy herds of small-type farms in the Lublin region, Poland. Mycopathologia 2001, 150, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Ksouri, S.; Djebir, S.; Hadef, Y.; Benakhla, A. Survey of Bovine Mycotic Mastitis in Different Mammary Gland Statuses in TwoNorth-Eastern Regions of Algeria. Mycopathologia 2015, 179, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Bourdichon, F.; Casaregola, S.; Farrokh, C.; Frisvad, J.C.; Gerds, M.L.; Hammes, W.P.; Harnett, J.; Huys, G.; Laulund, S.; Ouwehand, A.; et al. Food fermentations: Microorganisms with technological beneficial use. Int. J. Food Microbiol. 2012, 154, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, Y.H.; Wu, P.C.; Samaranayake, L.P.; Ho, P.L. The relative pathogenicity of Candida krusei and C. albicans in the ratoral mucosa. J. Med. Microbiol. 1998, 47, 1047–1057. [Google Scholar] [CrossRef]
- Dorko, E.; Zibrín, M.; Jenca, A.; Pilipcinec, E.; Danko, J.; Tkáčiková, Ĺ. The histopathological characterization of oral Candida leukoplakias. Folia Microbiol. 2001, 46, 447–451. [Google Scholar] [CrossRef]
- Wellnitz, O.; Bruckmaier, R.M. The innate immune response of the bovine mammary gland to bacterial infection. Vet. J. 2012, 192, 148–152. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, F.; Ma, W.; Li, X.; Zhou, X. DAD3 targets ACE2 to inhibit the MAPK and NF-κB signaling pathways and protect against LPS-induced inflammation in bovine mammary epithelial cells. Vet. Res. 2022, 53, 104. [Google Scholar] [CrossRef]
- Boya, P.; Mellén, M.A.; De la Rosa, E.J. How autophagyis related to programmed cell death during the development of the nervous system. Biochem. Soc. Trans. 2008, 36, 813–817. [Google Scholar] [CrossRef]
- Lugli, E.; Troiano, L.; Ferraresi, R.; Roat, E.; Prada, N.; Nasi, M.; Pinti, M.; Cooper, E.L.; Cossarizza, A. Characterization of cells with different mitochondrial membrane potential during apoptosis. Cytom. Part A 2005, 68, 28–35. [Google Scholar] [CrossRef]
- Jia, F.; Ma, W.; Zhang, X.; Wang, D.; Zhou, X. Matrine and baicalin inhibit apoptosis induced by Panton-Valentine leukocidin of Staphylococcus aureus in bovine mammary epithelial cells. J. Dairy Sci. 2020, 103, 2731–2742. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, F.B.; Cunha, P.; Jensen, K.; Glass, E.J.; Foucras, G.; Robert-Granié, C.; Rupp, R.; Rainard, P. Differential response of bovine mammary epithelial cells to Staphylococcus aureus or Escherichia coli agonists of the innate immune system. Vet. Res. 2013, 44, 40. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yao, M.; Li, Y.; Yang, Z.; Feng, X. Inhibition of Lipopolysaccharide(LPS)-induced inflammatory responses by selenium in bovine mammary epithelial cells in primary culture. Inflammation 2015, 38, 152–158. [Google Scholar] [CrossRef]
- Ibata-Ombetta, S.; Idziorek, T.; Trinel, P.-A.; Poulain, D.; Jouault, T. Candida albicans phospholipomannan promotes survival ofphagocytosed yeasts through modulation of bad phosphorylation and macrophage apoptosis. J. Biol. Chem. 2003, 278, 13086–13093. [Google Scholar] [CrossRef]
- Meng, M.; Huo, R.; Wang, Y.; Ma, N.; Shi, X.; Shen, X.; Chang, G. Lentinan inhibits oxidative stress and alleviates LPS-induced inflammation and apoptosis of BMECs by activating the Nrf2 signaling pathway. Int. J. Biol. Macromol. 2022, 222 Pt B, 2375–2391. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Ali, T.; Alkasir, R.; Yin, J.; Liu, G.; Han, B. Staphylococcal enterotoxin H induced apoptosis of bovine mammary epithelial cells in vitro. Toxins 2014, 6, 3552–3567. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Erbaş, G.; Parin, U.; Kirkan, Ş.; Savaşan, S.; Özavci, V.; Yüksel, H.T. Identification of Candida strains with nested PCR in bovine mastitis and determination of antifungal susceptibilities. Turk. J. Vet. Anim. Sci. 2017, 41, 757–763. [Google Scholar] [CrossRef]
- Raheel, I.A.E.R.; Hassan, W.H.; Salam, H.S.H.; Abed, A.H.; Salem, S.S.R. Recovery rate of fungal pathogens isolated from cases o fbovine and ovine mycotic mastitis. J. Vet. Med Res. 2023. [Google Scholar] [CrossRef]
- Wu, H.; Downs, D.; Ghosh, K.; Ghosh, A.K.; Staib, P.; Monod, M.; Tang, J. Candida albicans secreted aspartic proteases 4–6 induce apoptosis of epithelial cells by a novel Trojan horse mechanism. FASEB J. 2013, 27, 2132–2144. [Google Scholar] [CrossRef]
- Sauter, A.; McDuffie, Y.; Boehm, H.; Martinez, A.; Spatz, J.P.; Appel, S. Surface-mediated priming during in vitro generation of monocyte-derived dendritic cells. Scand. J. Immunol. 2015, 81, 56–65. [Google Scholar] [CrossRef]
- Quintin, J.; Saeed, S.; Martens, J.H.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.-J.; Wijmenga, C.; et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 2012, 12, 223–232. [Google Scholar] [CrossRef]
- Bie, X.; Zhang, S.; Luo, X.; Qi, R.-Q. Candida albicans cell wall mannoprotein synergizes with lipopolysaccharide to affect RAW264.7 proliferation, phagocytosis and apoptosis. Microb. Pathog. 2019, 131, 98–105. [Google Scholar] [CrossRef]
- Nguyen, T.N.Y.; Padungros, P.; Wongsrisupphakul, P.; Sa-Ard-Iam, N.; Mahanonda, R.; Matangkasombut, O.; Choo, M.K.; Ritprajak, P. Cell wall mannan of Candida krusei mediates dendritic cell apoptosis and orchestrates Th17 polarizationviaTLR-2/MyD88-dependent pathway. Sci. Rep. 2018, 8, 17123. [Google Scholar] [CrossRef]
- Naglik, J.R.; Moyes, D.L.; Wächtler, B.; Hube, B. Candida albicans interactions with epithelial cells and mucosal immunity. Microbes Infect. 2011, 13, 963–976. [Google Scholar] [CrossRef]
- Lopes, J.P.; Lionakis, M.S. Pathogenesis and virulence of Candida albicans. Virulence 2022, 13, 89–121. [Google Scholar] [CrossRef]
- Kumamoto, C.A.; Vinces, M.D. Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence. Cell. Microbiol. 2005, 7, 1546–1554. [Google Scholar] [CrossRef]
- Filler, S.G.; Sheppard, D.C. Fungal invasion of normally non-phagocytic host cells. PLoS Pathog. 2006, 2, e129. [Google Scholar] [CrossRef]
- Wächtler, B.; Wilson, D.; Haedicke, K.; Dalle, F.; Hube, B. From attachment to damage: Defined genes of Candida albicans mediate adhesion, invasion and damage during interaction with oral epithelial cells. PLoS ONE 2011, 6, e17046. [Google Scholar] [CrossRef]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida albicans Secreted Aspartyl Proteinases in Virulence and Pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 400–428. [Google Scholar] [CrossRef]
- Yan, L.; Yang, C.; Tang, J. Disruption of the intestinal mucosal barrier in Candida albicans infections. Microbiol. Res. 2013, 168, 389–395. [Google Scholar] [CrossRef]
- Jiménez-López, C.; Lorenz, M.C. Fungal immune evasion in a model host–pathogen interaction: Candida albicans versus macrophages. PLoS Pathog. 2013, 9, e1003741. [Google Scholar] [CrossRef]
- Uwamahoro, N.; Verma-Gaur, J.; Shen, H.-H.; Qu, Y.; Lewis, R.; Lu, J.; Bambery, K.; Masters, S.L.; Vince, J.E.; Naderer, T.; et al. The Pathogen Candida albicans Hijacks Pyroptosis for Escape from Macrophages. MBio 2014, 5, e000013-14. [Google Scholar] [CrossRef]
- Lorenz, M.C.; Bender, J.A.; Fink, G.R. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot. Cell 2004, 3, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Wartenberg, A.; Linde, J.; Martin, R.; Schreiner, M.; Horn, F.; Jacobsen, I.D.; Jenull, S.; Wolf, T.; Kuchler, K.; Guthke, R.; et al. Microevolution of Candida albicans in macrophages restores filamentation in a nonfilamentous mutant. PLoS Genet. 2014, 10, e1004824. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Wei, C.F.; Jiang, H.M. [Intravenous infection of C. albicans induces apoptosis of cortical thymocytes in mice]. Hunan Yi Ke Da Xue Xue Bao 2000, 25, 113–116. [Google Scholar] [PubMed]
- Lv, Y.; Song, S.; Zhang, K.; Gao, H.; Ma, R. CHIP regulates AKT/FoxO/Bim signaling in MCF7 and MCF10A cells. PLoS ONE 2013, 8, e83312. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, D.; Worku, T.; Dad, R.; Rehman, Z.U.; Gong, X.; Zhang, S. Mechanism of pattern recognition receptors (PRRs) and host pathogen interplay in bovine mastitis. Microb. Pathog. 2018, 120, 64–70. [Google Scholar] [CrossRef]
- Kober, A.K.M.H.; Saha, S.; Islam, A.; Rajoka, M.S.R.; Fukuyama, K.; Aso, H.; Villena, J.; Kitazawa, H. Immunomodulatory Effects of Probiotics: A Novel Preventive Approach for the Control of Bovine Mastitis. Microorganisms 2022, 10, 2255. [Google Scholar] [CrossRef]
- Wellnitz, O.; Bruckmaier, R. Invitedreview: The role of the blood–milk barrier and its manipulation for the efficacy of themammary immune response and milk production. J. Dairy Sci. 2021, 104, 6376–6388. [Google Scholar] [CrossRef]
- Lim, K.H.; Staudt, L.M. Toll-like receptor signaling. Cold Spring Harb. Perspect. Biol. 2013, 5, a011247. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Tang, J. Candida albicans infection and intestinal immunity. Microbiol. Res. 2017, 198, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Prieto, D.; Carpena, N.; Maneu, V.; Gil, M.L.; Pla, J.; Gozalbo, D. TLR2 modulates gut colonization and dissemination of Candida albicans in a murine model. Microbes Infect. 2016, 18, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bai, J.; Wu, C.L.; Wu, Y.; Zhao, Y.; Song, W.M.; Luo, X.P. Candida glabrata induced infection of rat tracheal epithelial cells is mediated by TLR-2 induced activation of NF-κB. Microb. Pathog. 2016, 91, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Schirbel, A.; Shouval, D.S.; Hebecker, B.; Hube, B.; Sturm, A.; Werner, L. Intestinal epithelial cells and T cells differentially recognize and respond to Candida albicans yeast and hypha. Eur. J. Immunol. 2018, 48, 1826–1837. [Google Scholar] [CrossRef]
- Moyes, D.L.; Runglall, M.; Murciano, C.; Shen, C.; Nayar, D.; Thavaraj, S.; Kohli, A.; Islam, A.; Mora-Montes, H.; Challacombe, S.J.; et al. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe 2010, 8, 225–235. [Google Scholar] [CrossRef]
- Moyes, D.L.; Murciano, C.; Runglall, M.; Islam, A.; Thavaraj, S.; Naglik, J.R. Candida albicans yeast and hyphae are discriminated by MAPK signaling in vaginal epithelial cells. PLoS ONE 2011, 6, e26580. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, Y.; Ding, T.; Liu, Y.; Zhou, X.; Du, J. The Yeast and Hypha Phases of Candida krusei Induce the Apoptosis of Bovine Mammary Epithelial Cells via Distinct Signaling Pathways. Animals 2023, 13, 3222. https://doi.org/10.3390/ani13203222
Miao Y, Ding T, Liu Y, Zhou X, Du J. The Yeast and Hypha Phases of Candida krusei Induce the Apoptosis of Bovine Mammary Epithelial Cells via Distinct Signaling Pathways. Animals. 2023; 13(20):3222. https://doi.org/10.3390/ani13203222
Chicago/Turabian StyleMiao, Yuhang, Tao Ding, Yang Liu, Xuezhang Zhou, and Jun Du. 2023. "The Yeast and Hypha Phases of Candida krusei Induce the Apoptosis of Bovine Mammary Epithelial Cells via Distinct Signaling Pathways" Animals 13, no. 20: 3222. https://doi.org/10.3390/ani13203222
APA StyleMiao, Y., Ding, T., Liu, Y., Zhou, X., & Du, J. (2023). The Yeast and Hypha Phases of Candida krusei Induce the Apoptosis of Bovine Mammary Epithelial Cells via Distinct Signaling Pathways. Animals, 13(20), 3222. https://doi.org/10.3390/ani13203222




