Identification, Typing, and Drug Resistance Analysis of Escherichia coli in Two Different Types of Broiler Farms in Hebei Province
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
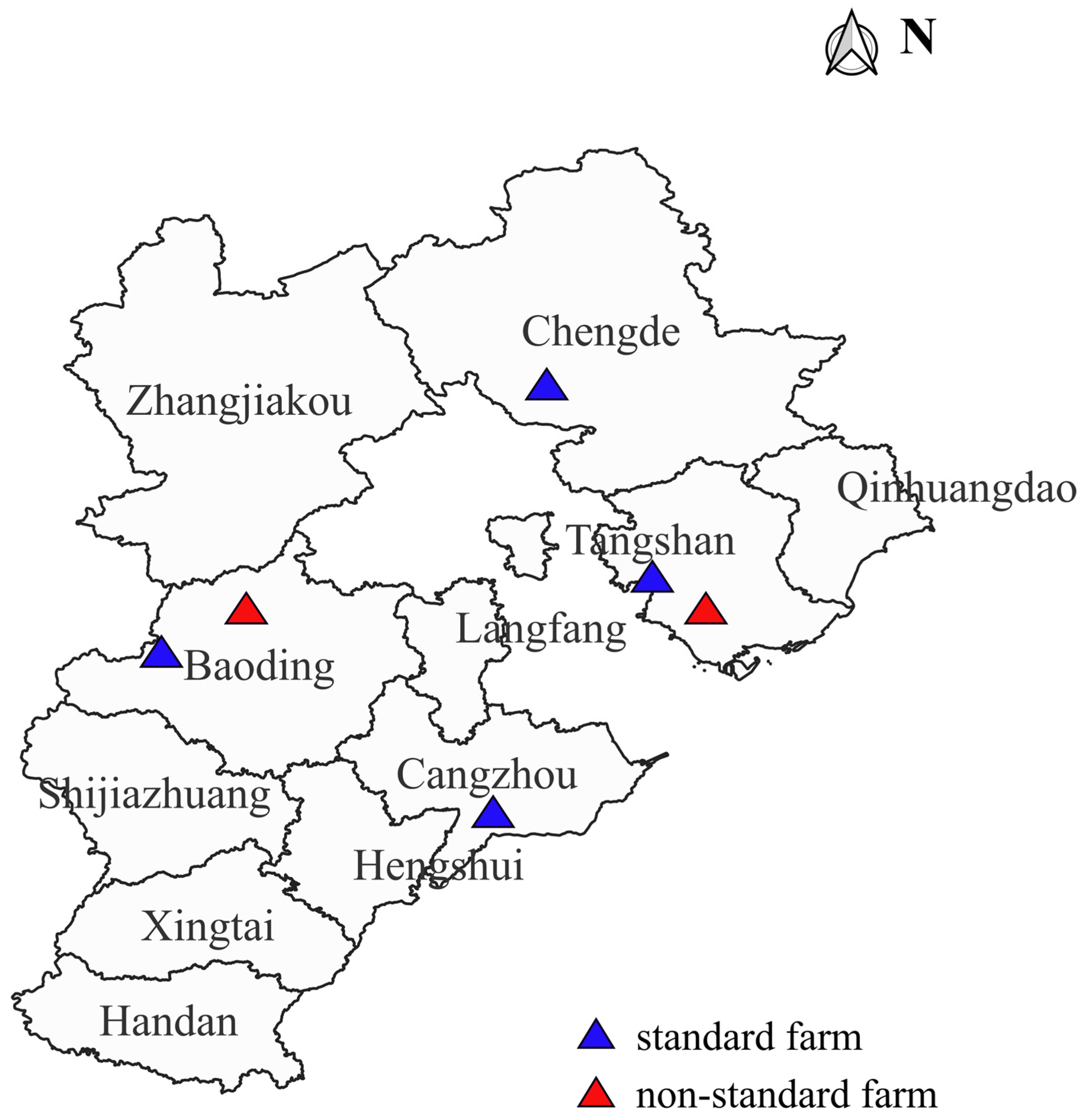
2.1. Sample Source
2.2. Main Reagents
2.3. Isolation and Identification of E. coli
2.4. Drug Susceptibility Testing
2.5. Detection of Resistance Genes
2.6. Multilocus Sequence Typing
2.7. Statistical Analysis
3. Results
3.1. Isolation and Identification
3.2. Results of Drug Resistance Phenotype Detection
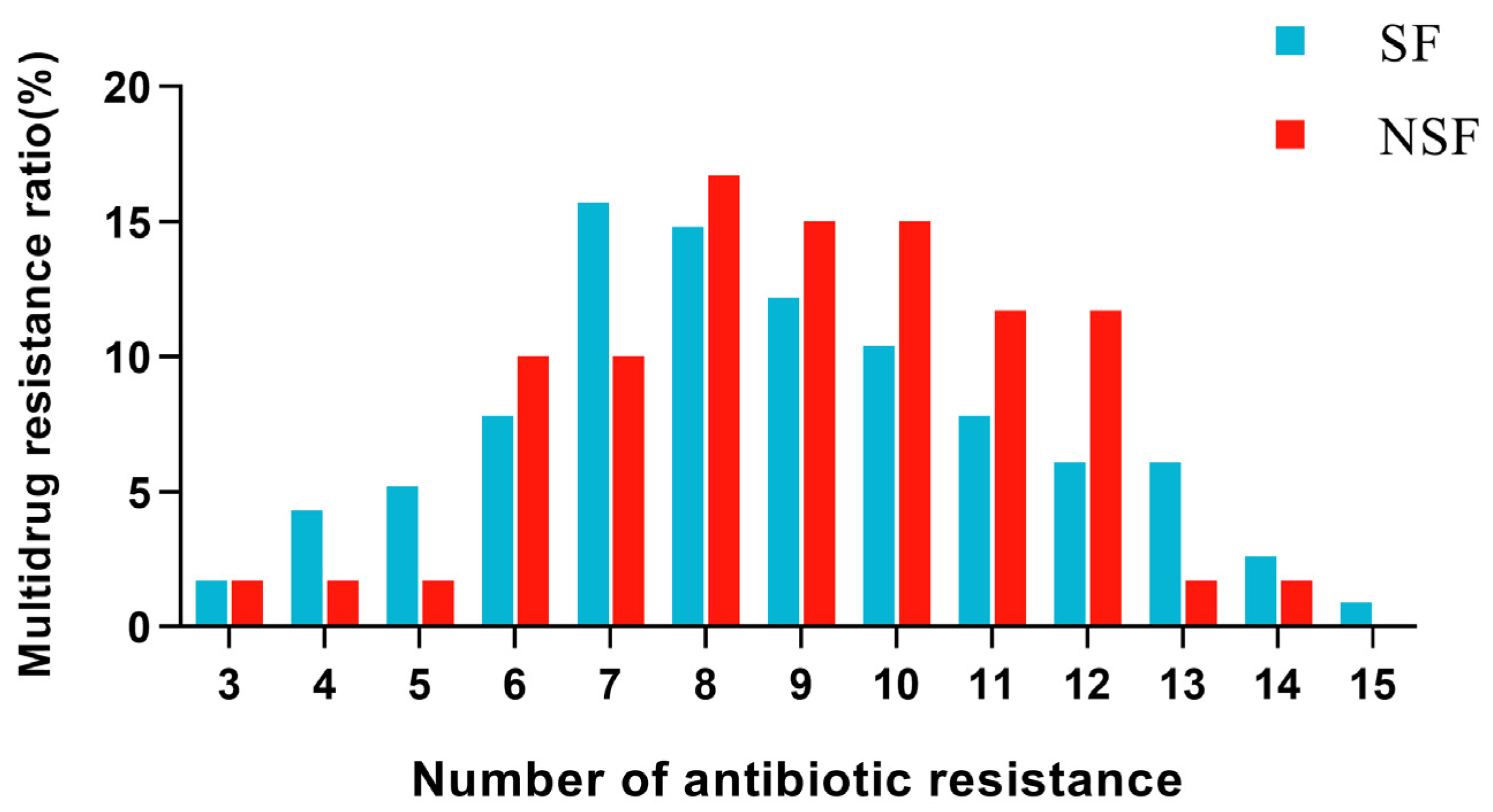
3.3. Results of Multidrug Resistance of Isolated Strains
3.4. Resistance Gene Detection Results
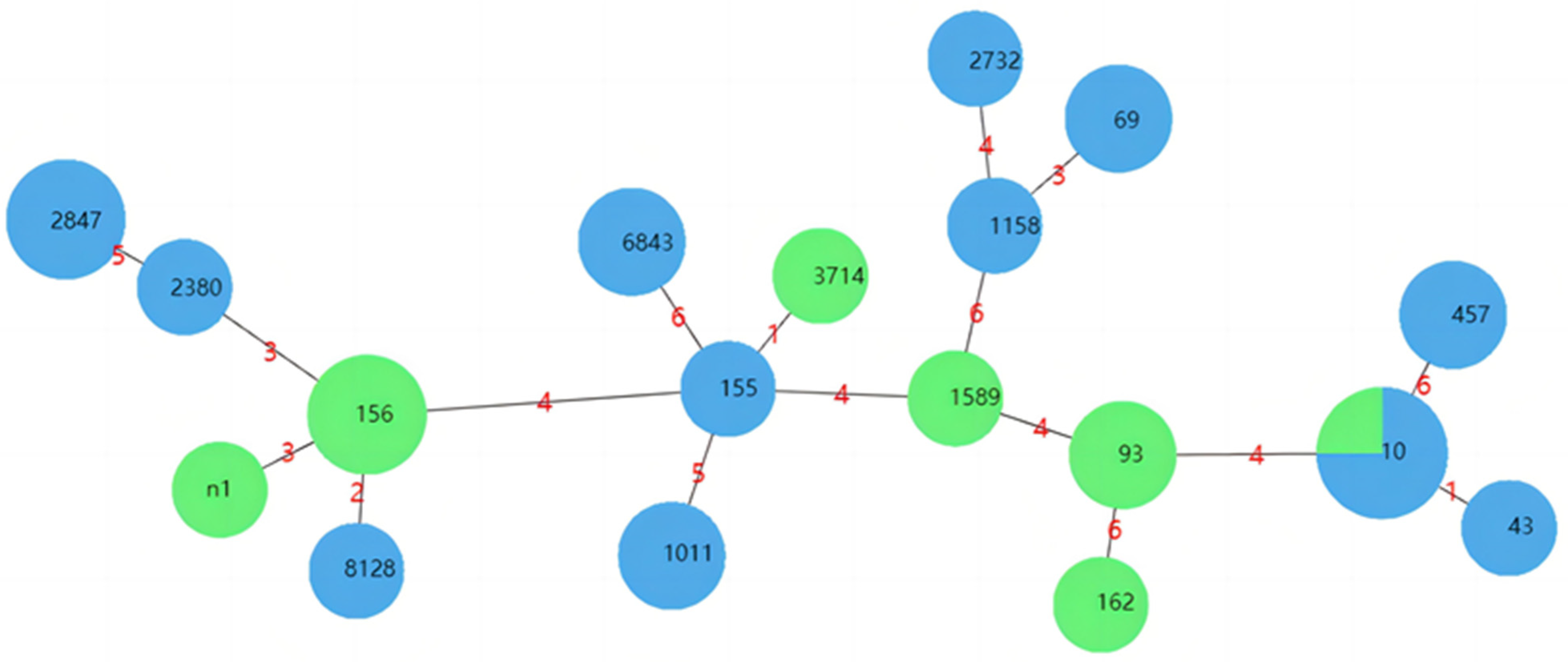
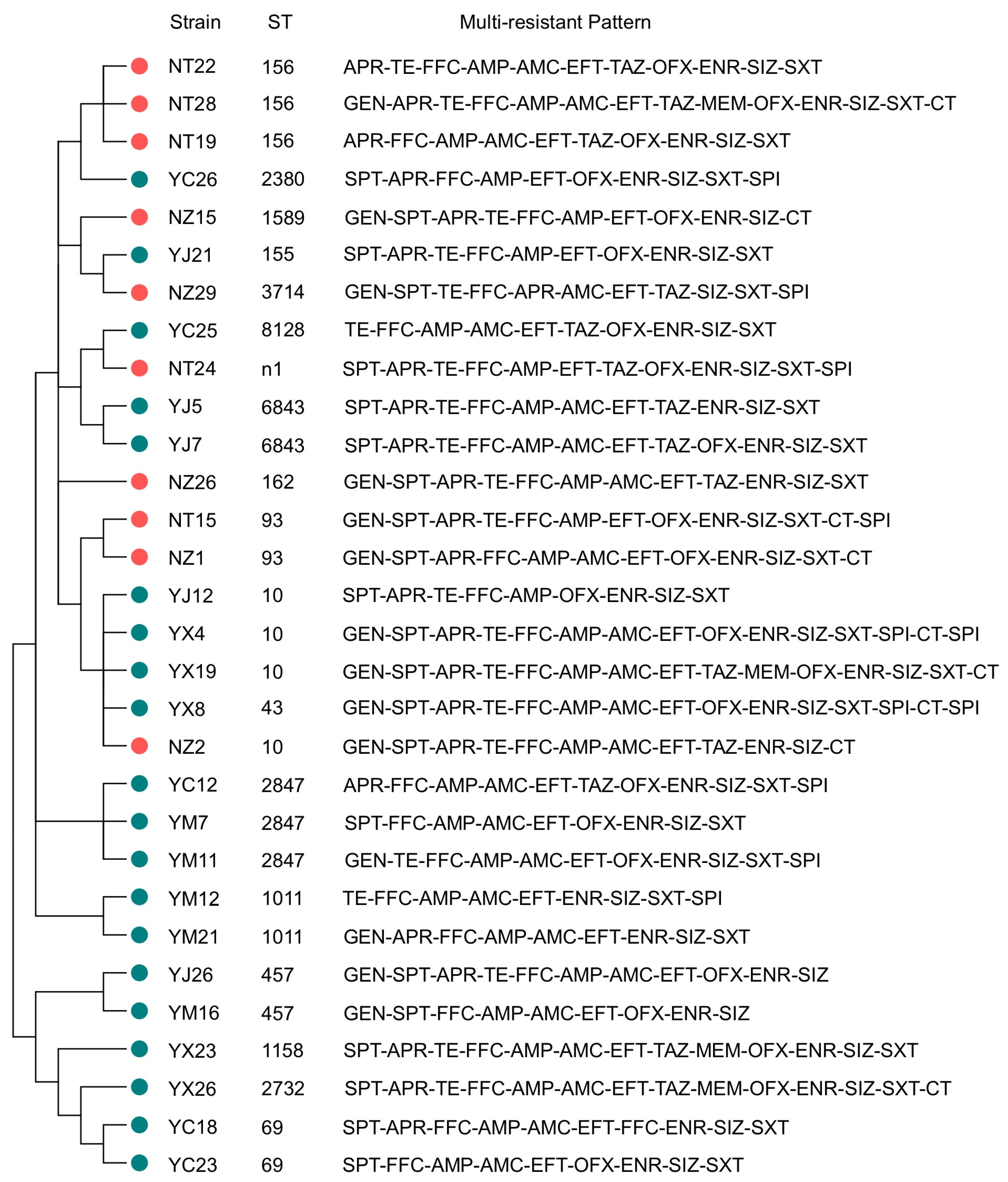
3.5. MLST Typing Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hopkins, K.L.; Davies, R.H.; Threlfall, E.J. Mechanisms of Quinolone Resistance in Escherichia coli and Salmonella: Recent Developments. Int. J. Antimicrob. Agents 2005, 25, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Castrignanò, E.; Kannan, A.M.; Proctor, K.; Petrie, B.; Hodgen, S.; Feil, E.J.; Lewis, S.E.; Lopardo, L.; Camacho-Muñoz, D.; Rice, J.; et al. (Fluoro)quinolones and quinolone resistance genes in the aquatic environment: A river catchment perspective. Water Res. 2020, 182, 116015. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, R.; Chen, B.; Zhang, T.; Hu, L.; Zou, S. Characterization of Airborne Antibiotic Resistance Genes from Typical Bioaerosol Emission Sources in the Urban Environment Using Metagenomic Approach. Chemosphere 2018, 213, 463–471. [Google Scholar] [CrossRef]
- Costa, D.; Poeta, P.; Sáenz, Y.; Coelho, A.C.; Matos, M.; Vinué, L.; Rodrigues, J.; Torres, C. Prevalence of Antimicrobial Resistance and Resistance Genes in Faecal Escherichia coli Isolates Recovered from Healthy Pets. Vet. Microbiol. 2008, 127, 97–105. [Google Scholar] [CrossRef]
- Plata, G.; Baxter, N.T.; Susanti, D.; Volland-Munson, A.; Gangaiah, D.; Nagireddy, A.; Mane, S.P.; Balakuntla, J.; Hawkins, T.B.; Kumar Mahajan, A. Growth Promotion and Antibiotic Induced Metabolic Shifts in the Chicken Gut Microbiome. Commun. Biol. 2022, 5, 293. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.S.; Rama Rao, S.V.; Hegde, N.; Williams, N.J.; Chatterjee, R.N.; Raju, M.; Reddy, G.N.; Kumar, V.; Phani Kumar, P.S.; Mallick, S.; et al. Effects of Dietary Antimicrobial Growth Promoters on Performance Parameters and Abundance and Diversity of Broiler Chicken Gut Microbiome and Selection of Antibiotic Resistance Genes. Front. Microbiol. 2022, 13, 905050. [Google Scholar] [CrossRef]
- Chokshi, A.; Sifri, Z.; Cennimo, D.; Horng, H. Global Contributors to Antibiotic Resistance. J. Glob. Infect. Dis. 2019, 11, 36–42. [Google Scholar] [CrossRef]
- Qiao, M.; Ying, G.G.; Singer, A.C.; Zhu, Y.G. Review of Antibiotic Resistance in China and Its Environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef]
- Tang, B.; Wang, J.; Zheng, X.; Chang, J.; Ma, J.; Wang, J.; Ji, X.; Yang, H.; Ding, B. Antimicrobial Resistance Surveillance of Escherichia coli from Chickens in the Qinghai Plateau of China. Front. Microbiol. 2022, 13, 885132. [Google Scholar] [CrossRef]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial Resistance: Prevalence, Economic Burden, Mechanisms of Resistance and Strategies to Overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef]
- Zhou, W.; Lin, R.; Zhou, Z.; Ma, J.; Lin, H.; Zheng, X.; Wang, J.; Wu, J.; Dong, Y.; Jiang, H.; et al. Antimicrobial Resistance and Genomic Characterization of Escherichia coli from Pigs and Chickens in Zhejiang, China. Front. Microbiol. 2022, 13, 1018682. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [PubMed]
- Sabino, Y.N.V.; Santana, M.F.; Oyama, L.B.; Santos, F.G.; Moreira, A.J.S.; Huws, S.A.; Mantovani, H.C. Characterization of Antibiotic Resistance Genes in the Species of the Rumen Microbiota. Nat. Commun. 2019, 10, 5252. [Google Scholar] [CrossRef] [PubMed]
- Opatowski, L.; Opatowski, M.; Vong, S.; Temime, L. A One-Health Quantitative Model to Assess the Risk of Antibiotic Resistance Acquisition in Asian Populations: Impact of Exposure Through Food, Water, Livestock and Humans. Risk Anal. 2021, 41, 1427–1446. [Google Scholar] [CrossRef]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef]
- Mendelson, M.; Matsoso, M.P. The World Health Organization Global Action Plan for Antimicrobial Resistance. S. Afr. Med. J. 2015, 105, 325. [Google Scholar] [CrossRef]
- Ying, G.G.; He, L.Y.; Ying, A.J.; Zhang, Q.Q.; Liu, Y.S.; Zhao, J.L. China Must Reduce Its Antibiotic Use. Environ. Sci. Technol. 2017, 51, 1072–1073. [Google Scholar] [CrossRef]
- Zhou, Z.; Alikhan, N.F.; Mohamed, K.; Fan, Y.; Achtman, M. The Enterobase User’s Guide, with Case Studies on Salmonella Transmissions, Yersinia Pestis Phylogeny, and Escherichia Core Genomic Diversity. Genome Res. 2020, 30, 138–152. [Google Scholar] [CrossRef]
- Wirth, T.; Falush, D.; Lan, R.; Colles, F.; Mensa, P.; Wieler, L.H.; Karch, H.; Reeves, P.R.; Maiden, M.C.; Ochman, H.; et al. Sex and Virulence in Escherichia coli: An Evolutionary Perspective. Mol. Microbiol. 2006, 60, 1136–1151. [Google Scholar] [CrossRef]
- O’Brien, T.F. Emergence, Spread, and Environmental Effect of Antimicrobial Resistance: How Use of an Antimicrobial Anywhere Can Increase Resistance to Any Antimicrobial Anywhere Else. Clin. Infect. Dis. 2002, 34 (Suppl. S3), S78–S84. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Zhang, Q.; Liu, N.; Wang, J.; Li, Y.; Huang, X.; Liu, J.; Wang, J.; Qu, Z.; Qi, K. Changes in Antibiotic Resistance of Escherichia coli During the Broiler Feeding Cycle. Poult. Sci. 2020, 99, 6983–6989. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, Z.; Zhang, Y.; Yuan, X.; Hu, M.; Liu, Y. Prevalence and Molecular Characteristics of Avian-Origin Mcr-1-Harboring Escherichia coli in Shandong Province, China. Front. Microbiol. 2020, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Afayibo, D.J.A.; Zhu, H.; Zhang, B.; Yao, L.; Abdelgawad, H.A.; Tian, M.; Qi, J.; Liu, Y.; Wang, S. Isolation, Molecular Characterization, and Antibiotic Resistance of Avian Pathogenic Escherichia coli in Eastern China. Vet. Sci. 2022, 9, 319. [Google Scholar] [CrossRef]
- Osman, K.M.; Kappell, A.D.; Elhadidy, M.; ElMougy, F.; El-Ghany, W.A.A.; Orabi, A.; Mubarak, A.S.; Dawoud, T.M.; Hemeg, H.A.; Moussa, I.M.I.; et al. Poultry Hatcheries as Potential Reservoirs for Antimicrobial-Resistant Escherichia coli: A Risk to Public Health and Food Safety. Sci. Rep. 2018, 8, 5859. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, W.; Wu, J.; Liu, X.; Lin, J.; Ji, X.; Lin, H.; Wang, J.; Jiang, H.; Zhou, Q.; et al. Large-Scale Studies on Antimicrobial Resistance and Molecular Characterization of Escherichia coli from Food Animals in Developed Areas of Eastern China. Microbiol. Spectr. 2022, 10, e0201522. [Google Scholar] [CrossRef]
- Tan, M.F.; Li, H.Q.; Yang, Q.; Zhang, F.F.; Tan, J.; Zeng, Y.B.; Wei, Q.P.; Huang, J.N.; Wu, C.C.; Li, N.; et al. Prevalence and Antimicrobial Resistance Profile of Bacterial Pathogens Isolated from Poultry in Jiangxi Province, China from 2020 to 2022. Poult. Sci. 2023, 102, 102830. [Google Scholar] [CrossRef]
- De Jong, A.; El Garch, F.; Hocquet, D.; Prenger-Berninghoff, E.; Dewulf, J.; Migura-Garcia, L.; Perrin-Guyomard, A.; Veldman, K.T.; Janosi, S.; Skarzynska, M.; et al. European-Wide Antimicrobial Resistance Monitoring in Commensal Escherichia coli Isolated from Healthy Food Animals between 2004 and 2018. J. Antimicrob. Chemother. 2022, 77, 3301–3311. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V.; Pereira, J.E.; Maltez, L.; Igrejas, G.; Valentão, P.; Falco, V.; Poeta, P. Antimicrobial Resistance and Clonal Lineages of Escherichia coli from Food-Producing Animals. Antibiotics 2023, 12, 1061. [Google Scholar] [CrossRef]
- Liao, M.; Wu, J.; Li, Y.; Lu, X.; Tan, H.; Chen, S.; Huang, W.; Lian, X.; Sun, J.; Liao, X.; et al. Prevalence and Persistence of Ceftiofur-Resistant Escherichia coli in a Chicken Layer Breeding Program. Animals 2022, 13, 90. [Google Scholar] [CrossRef]
- Sodagari, H.R.; Varga, C. Evaluating Antimicrobial Resistance Trends in Commensal Escherichia coli Isolated from Cecal Samples of Swine at Slaughter in the United States, 2013–2019. Microorganisms 2023, 11, 1033. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Husna, A.; Elshabrawy, H.A.; Alam, J.; Runa, N.Y.; Badruzzaman, A.T.M.; Banu, N.A.; Al Mamun, M.; Paul, B.; Das, S.; et al. Isolation and Molecular Characterization of Multidrug-Resistant Escherichia coli from Chicken Meat. Sci. Rep. 2020, 10, 21999. [Google Scholar] [CrossRef]
- Liu, C.; Wang, P.; Dai, Y.; Liu, Y.; Song, Y.; Yu, L.; Feng, C.; Liu, M.; Xie, Z.; Shang, Y.; et al. Longitudinal Monitoring of Multidrug Resistance in Escherichia coli on Broiler Chicken Fattening Farms in Shandong, China. Poult. Sci. 2021, 100, 100887. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yu, L.; Zhang, Y.; Dai, Y.; Wang, P.; Feng, C.; Liu, M.; Sun, S.; Xie, Z.; Wang, F. Prevalence and Characteristics of Multidrug-Resistant Mcr-1-Positive Escherichia coli Isolates from Broiler Chickens in Tai’an, China. Poult. Sci. 2020, 99, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, Q.; Mao, X.; Li, L.; Dou, J.; He, Q.; Shao, H.; Luo, Q. Prevalence of Extended-Spectrum β-Lactamase-Resistant Genes in Escherichia coli Isolates from Central China during 2016–2019. Animals 2022, 12, 3191. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, K.; Zhang, Y.; Xia, L.; Zhao, L.; Guo, C.; Liu, X.; Qin, L.; Hao, Z. High Prevalence and Diversity Characteristics of Bla(Ndm), Mcr, and Bla(Esbls) Harboring Multidrug-Resistant Escherichia coli from Chicken, Pig, and Cattle in China. Front. Cell. Infect. Microbiol. 2021, 11, 755545. [Google Scholar] [CrossRef]
- Nhung, N.T.; Chansiripornchai, N.; Carrique-Mas, J.J. Antimicrobial Resistance in Bacterial Poultry Pathogens: A Review. Front. Vet. Sci. 2017, 4, 126. [Google Scholar] [CrossRef]
- Awad, A.; Arafat, N.; Elhadidy, M. Genetic Elements Associated with Antimicrobial Resistance among Avian Pathogenic Escherichia coli. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 59. [Google Scholar] [CrossRef]
- Li, P.; Zhu, T.; Zhou, D.; Lu, W.; Liu, H.; Sun, Z.; Ying, J.; Lu, J.; Lin, X.; Li, K.; et al. Analysis of Resistance to Florfenicol and the Related Mechanism of Dissemination in Different Animal-Derived Bacteria. Front. Cell. Infect. Microbiol. 2020, 10, 369. [Google Scholar] [CrossRef]
- Aworh, M.K.; Kwaga, J.K.P.; Hendriksen, R.S.; Okolocha, E.C.; Thakur, S. Genetic Relatedness of Multidrug Resistant Escherichia coli Isolated from Humans, Chickens and Poultry Environments. Antimicrob. Resist. Infect. Control 2021, 10, 58. [Google Scholar] [CrossRef]
- Benameur, Q.; Gervasi, T.; Dahloum, L.; Rechidi-Sidhoum, N.; Boutaiba Benklaouz, M.; Yakubu, A. Multidrug-Resistant Escherichia coli Isolated from Cleaned and Disinfected Poultry Houses Prior to Day-Old Chick Placement. J. Environ. Qual. 2023, 52, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Feil, E.J.; Enright, M.C. Analyses of Clonality and the Evolution of Bacterial Pathogens. Curr. Opin. Microbiol. 2004, 7, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, J.; Li, X.; Ma, L.; Cao, X.; Hu, W.; Zhao, L.; Jing, W.; Lan, X.; Li, Y.; et al. Genetic Diversity, Antimicrobial Resistance and Extended-Spectrum Β-Lactamase Type of Escherichia coli Isolates from Chicken, Dog, Pig and Yak in Gansu and Qinghai Provinces, China. J. Glob. Antimicrob. Resist. 2020, 22, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhang, W.; Luo, L.; Wang, H.; Shao, H.; Zhang, T.; Luo, Q. Genetic Diversity and Multidrug Resistance of Phylogenic Groups B2 and D in Inpec and Expec Isolated from Chickens in Central China. BMC Microbiol. 2022, 22, 60. [Google Scholar] [CrossRef] [PubMed]
- Danzeisen, J.L.; Wannemuehler, Y.; Nolan, L.K.; Johnson, T.J. Comparison of Multilocus Sequence Analysis and Virulence Genotyping of Escherichia coli from Live Birds, Retail Poultry Meat, and Human Extraintestinal Infection. Avian Dis. 2013, 57, 104–108. [Google Scholar] [CrossRef]
| Drug Type | Drug Name | Abbreviation | SF (%) | NSF (%) |
|---|---|---|---|---|
| Aminoglycosides | Gentamicin | GEN | 26.1 A | 56.7 B |
| Spectinomycin | SPT | 40.0 A | 71.7 B | |
| Apramycin | APR | 69.6 | 75.0 | |
| Tetracyclines | Tetracycline | TE | 45.2 | 53.3 |
| Chloramphenicol | Florfenicol | FFC | 86.1 | 93.3 |
| β-lactams | Ampicillin | AMP | 93.9 | 98.3 |
| Amoxicillin/clavulanic acid | AMC | 58.3 | 45.0 | |
| Ceftiofur | EFT | 82.6 a | 96.7 b | |
| Ceftazidime | TAZ | 16.5 | 16.7 | |
| Meropenem | MEM | 7.0 | 1.7 | |
| Quinolones | Ofloxacin | FOX | 31.3 | 30.0 |
| Enrofloxacin | ENR | 75.7 | 76.7 | |
| Sulfonamides | Sulfisoxazole | SIZ | 79.1 | 71.7 |
| Cotrimoxazole | SXT | 73.9 a | 55.0 b | |
| Peptides | Colistin-E | CT | 22.6 | 28.3 |
| Quinoxalines | Mequindox | SPI | 22.6 | 13.3 |
| Drug Type | Gene | SF (%) | NSF (%) |
|---|---|---|---|
| Aminoglycosides | aadA | 78.3 | 88.3 |
| aph(3′)-Ⅱ | 100.0 | 100.0 | |
| aac2 | 13.0 A | 36.7 B | |
| aac4 | 70.4 | 75.0 | |
| β-lactams | CTX-M | 73.9 | 63.3 |
| SHV | 16.5 | 8.3 | |
| TEM | 97.4 | 96.7 | |
| OXA | 71.3 | 68.3 | |
| CMY-2 | 14.8 a | 1.7 b | |
| Tetracyclines | Tet(A) | 88.7 | 88.3 |
| Tet(B) | 55.7 A | 18.3 B | |
| Tet(M) | 10.4 | 8.3 | |
| Quinolones | qnrA | 75.7 a | 91.7 b |
| qnrB | 18.3 a | 8.3 b | |
| qnrs | 42.6 | 33.3 | |
| oqxA | 66.1 | 63.3 | |
| oqxB | 84.3 | 73.3 | |
| aac(6′)- Ib-cr | 10.4 | 11.7 | |
| gyrA | 100.0 | 100.0 | |
| gyrB | 100.0 | 100.0 | |
| parC | 100.0 | 100.0 | |
| Sulfonamides | sul-1 | 40.9 | 48.3 |
| sul-2 | 79.1 | 83.3 | |
| dfra | 7.0 | 3.3 | |
| Peptides | mcr-1 | 53.0 A | 28.3 B |
| Chloramphenicol | flor | 87.8 | 93.3 |
| ST Type | Number | Strain Number | Farm Type | Multidrug Resistance Profile |
|---|---|---|---|---|
| ST10 | 4 | YJ12 | SF | SPT-APR-TE-FFC-AMP-OFX-ENR-SIZ-SXT |
| NZ2 | NSF | GEN-SPT-APR-TE-FFC-AMP-AMC-EFT-TAZ-ENR-SIZ-CT | ||
| YX4 | SF | GEN-SPT-APR-TE-FFC-AMP-AMC-EFT-OFX-ENR-SIZ-SXT-SPI-CT-SPI | ||
| YX19 | SF | GEN-SPT-APR-TE-FFC-AMP-AMC-EFT-TAZ-MEM-OFX-ENR-SIZ-SXT-CT | ||
| ST43 | 1 | YX8 | SF | GEN-SPT-APR-TE-FFC-AMP-AMC-EFT-OFX-ENR-SIZ-SXT-SPI-CT-SPI |
| ST155 | 1 | YJ21 | SF | SPT-APR-TE-FFC-AMP-EFT-OFX-ENR-SIZ-SXT |
| ST156 | 3 | NT19 | NSF | APR-FFC-AMP-AMC-EFT-TAZ-OFX-ENR-SIZ-SXT |
| NT22 | NSF | APR-TE-FFC-AMP-AMC-EFT-TAZ-OFX-ENR-SIZ-SXT | ||
| NT28 | NSF | GEN-APR-TE-FFC-AMP-AMC-EFT-TAZ-MEM-OFX-ENR-SIZ-SXT-CT | ||
| ST93 | 2 | NZ1 | NSF | GEN-SPT-APR-FFC-AMP-AMC-EFT-OFX-ENR-SIZ-SXT-CT |
| NT15 | NSF | GEN-SPT-APR-TE-FFC-AMP-EFT-OFX-ENR-SIZ-SXT-CT-SPI | ||
| ST162 | 1 | NZ26 | NSF | GEN-SPT-APR-TE-FFC-AMP-AMC-EFT-TAZ-ENR-SIZ-SXT |
| ST69 | 2 | YC18 | SF | SPT-APR-FFC-AMP-AMC-EFT-FFC-ENR-SIZ-SXT |
| YC23 | SF | SPT-FFC-AMP-AMC-EFT-OFX-ENR-SIZ-SXT | ||
| ST6843 | 2 | YJ5 | SF | SPT-APR-TE-FFC-AMP-AMC-EFT-TAZ-ENR-SIZ-SXT |
| YJ7 | SF | SPT-APR-TE-FFC-AMP-AMC-EFT-TAZ-OFX-ENR-SIZ-SXT | ||
| ST457 | 2 | YJ26 | SF | GEN-SPT-APR-TE-FFC-AMP-AMC-EFT-OFX-ENR-SIZ |
| YM16 | SF | GEN-SPT-FFC-AMP-AMC-EFT-OFX-ENR-SIZ | ||
| ST1589 | 1 | NZ15 | NSF | GEN-SPT-APR-TE-FFC-AMP-EFT-OFX-ENR-SIZ-CT |
| ST1158 | 1 | YX23 | SF | SPT-APR-TE-FFC-AMP-AMC-EFT-TAZ-MEM-OFX-ENR-SIZ-SXT |
| ST2732 | 1 | YX26 | SF | SPT-APR-TE-FFC-AMP-AMC-EFT-TAZ-MEM-OFX-ENR-SIZ-SXT-CT |
| ST2847 | 3 | YM7 | SF | SPT-FFC-AMP-AMC-EFT-OFX-ENR-SIZ-SXT |
| YM11 | SF | GEN-TE-FFC-AMP-AMC-EFT-OFX-ENR-SIZ-SXT-SPI | ||
| YC12 | SF | APR-FFC-AMP-AMC-EFT-TAZ-OFX-ENR-SIZ-SXT-SPI | ||
| ST1011 | 2 | YM12 | SF | TE-FFC-AMP-AMC-EFT-ENR-SIZ-SXT-SPI |
| YM21 | SF | GEN-APR-FFC-AMP-AMC-EFT-ENR-SIZ-SXT | ||
| ST8128 | 1 | YC25 | SF | TE-FFC-AMP-AMC-EFT-TAZ-OFX-ENR-SIZ-SXT |
| ST2380 | 1 | YC26 | SF | SPT-APR-FFC-AMP-EFT-OFX-ENR-SIZ-SXT-SPI |
| ST3714 | 1 | NZ29 | NSF | GEN-SPT-TE-FFC-APR-AMC-EFT-TAZ-SIZ-SXT-SPI |
| n1 | 1 | NT24 | NSF | SPT-APR-TE-FFC-AMP-EFT-TAZ-OFX-ENR-SIZ-SXT-SPI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, C.; Cui, H.; Chen, L.; Zhang, H.; Zhang, C.; Liu, J. Identification, Typing, and Drug Resistance Analysis of Escherichia coli in Two Different Types of Broiler Farms in Hebei Province. Animals 2023, 13, 3194. https://doi.org/10.3390/ani13203194
Liang C, Cui H, Chen L, Zhang H, Zhang C, Liu J. Identification, Typing, and Drug Resistance Analysis of Escherichia coli in Two Different Types of Broiler Farms in Hebei Province. Animals. 2023; 13(20):3194. https://doi.org/10.3390/ani13203194
Chicago/Turabian StyleLiang, Chuncai, Huan Cui, Ligong Chen, Hailong Zhang, Cheng Zhang, and Juxiang Liu. 2023. "Identification, Typing, and Drug Resistance Analysis of Escherichia coli in Two Different Types of Broiler Farms in Hebei Province" Animals 13, no. 20: 3194. https://doi.org/10.3390/ani13203194
APA StyleLiang, C., Cui, H., Chen, L., Zhang, H., Zhang, C., & Liu, J. (2023). Identification, Typing, and Drug Resistance Analysis of Escherichia coli in Two Different Types of Broiler Farms in Hebei Province. Animals, 13(20), 3194. https://doi.org/10.3390/ani13203194





