Risk Factors for Salmonella Detection in Commercial Layer Flocks in Spain
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
- Farm level: location of the farm (municipality);
- Flock level: size of the flock (number of birds) and housing type;
- Sampling level: date of sampling (day), reason for sampling (see below), sampler (CA or FBOp), number of samples analyzed independently and age of the flock at sampling;
- Sample level: sample type (i.e., specimen) and laboratory results from the sample (isolation of Salmonella and, when positive, if a target serotype—Enteritidis or Typhimurium—was identified).
2.2. Data Preparation
2.3. Data Analysis
3. Results
3.1. Descriptive Results
3.2. Salmonella spp. Detection
3.3. Model Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- EFSA; ECDC. The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, 324. [Google Scholar] [CrossRef]
- Havelaar, A.H.; Ivarsson, S.; Lofdahl, M.; Nauta, M.J. Estimating the true incidence of campylobacteriosis and salmonellosis in the European Union, 2009. Epidemiol. Infect. 2013, 141, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Guillier, L.; Thebault, A.; Fravalo, P.; Mughini-Gras, L.; da Silva, N.J.; David, J.; Kooh, P.; Cadavez, V.; Gonzales-Barron, U. Risk factors for sporadic salmonellosis: A systematic review and meta-analysis. Microb. Risk Anal. 2021, 17, 11. [Google Scholar] [CrossRef]
- Pijnacker, R.; Dallman, T.J.; Tijsma, A.S.L.; Hawkins, G.; Larkin, L.; Kotila, S.M.; Amore, G.; Amato, E.; Suzuki, P.M.; Denayer, S.; et al. An international outbreak of Salmonella enterica serotype Enteritidis linked to eggs from Poland: A microbiological and epidemiological study. Lancet Infect. Dis. 2019, 19, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Pires, S.M.; Vieira, A.R.; Hald, T.; Cole, D. Source attribution of human salmonellosis: An overview of methods and estimates. Foodborne Pathog. Dis. 2014, 11, 667–676. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific opinion on the Salmonella control in poultry flocks and its public health impact. EFSA J. 2019, 17, 155. [Google Scholar]
- Schulz, J.; Van Hoorebeke, S.; Hald, B.; Hartung, J.; Van Immerseel, F.; Radtke, I.; Kabell, S.; Dewulf, J. The dynamics of Salmonella occurrence in commercial laying hen flocks throughout a laying period. Avian Pathol. 2011, 40, 243–248. [Google Scholar] [CrossRef]
- Carrique-Mas, J.J.; Davies, R.H. Salmonella Enteritidis in commercial layer flocks in Europe: Legislative background, on-farm sampling and main challenges. Braz. J. Poult. Sci. 2008, 10, 10. [Google Scholar] [CrossRef]
- Menanteau, P.; Kempf, F.; Trotereau, J.; Virlogeux-Payant, I.; Gitton, E.; Dalifard, J.; Gabriel, I.; Rychlik, I.; Velge, P. Role of systemic infection, cross contaminations and super-shedders in Salmonella carrier state in chicken. Environ. Microbiol. 2018, 20, 3246–3260. [Google Scholar] [CrossRef]
- Denagamage, T.; Jayarao, B.; Patterson, P.; Wallner-Pendleton, E.; Kariyawasam, S. Risk Factors Associated With Salmonella in Laying Hen Farms: Systematic Review of Observational Studies. Avian Dis. 2015, 59, 291–302. [Google Scholar] [CrossRef]
- Mahe, A.; Bougeard, S.; Huneau-Salaun, A.; Le Bouquin, S.; Petetin, I.; Rouxel, S.; Lalande, F.; Beloeil, P.A.; Rose, N. Bayesian estimation of flock-level sensitivity of detection of Salmonella spp., Enteritidis and Typhimurium according to the sampling procedure in French laying-hen houses. Prev. Vet. Med. 2008, 84, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Carrique-Mas, J.J.; Davies, R.H. Sampling and bacteriological detection of Salmonella in poultry and poultry premises: A review. Rev. Sci. Tech. 2008, 27, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Spiegelhalter, D.; Best, N.; Carlin, B.P.; Van der Linde, A. Bayesian measures of model complexity and fit. J. R. Stat. Soc. B 2002, 64, 583–639. [Google Scholar] [CrossRef]
- Lunn, D.; Spiegelhalter, D.; Thomas, A.; Best, N. The BUGS project: Evolution, critique and future directions. Stat. Med. 2009, 28, 3049–3067. [Google Scholar] [CrossRef]
- Sturtz, S.; Ligges, U.; Gelman, A. R2WinBUGS: A Package for Running WinBUGS from R. J. Stat. Softw. 2005, 12, 1–16. [Google Scholar] [CrossRef]
- R Core Team Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. 2020. Available online: http://www.R-project.org/ (accessed on 1 September 2023).
- Brooks, S.P.; Gelman, A. General methods for monitoring convergence of iterative simulations. J. Comput. Graph. Stat. 1998, 7, 434–455. [Google Scholar]
- Gelman, A.; Rubin, D.B. Inference from iterative simulation using multiple sequences. Stat. Sci. 1992, 7, 457–511. [Google Scholar] [CrossRef]
- Messens, W.; Vivas-Alegre, L.; Bashir, S.; Amore, G.; Romero-Barrios, P.; Hugas, M. Estimating the public health impact of setting targets at the European level for the reduction of zoonotic Salmonella in certain poultry populations. Int. J. Environ. Res. Public Health 2013, 10, 4836–4850. [Google Scholar] [CrossRef]
- European Commission (Directorate-General for Health and Food Safety). Communication from the Commission on the European Citizen’s Initiative (Eci) “End the Cage Age”. 2021. Available online: https://ec.europa.eu/transparency/documents-register/detail?ref=C(2021)4747&lang=en (accessed on 6 December 2022).
- Burkholder, K.M.; Thompson, K.L.; Einstein, M.E.; Applegate, T.J.; Patterson, J.A. Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella enteritidis colonization in broilers. Poult. Sci. 2008, 87, 1734–1741. [Google Scholar] [CrossRef]
- Van Hoorebeke, S.; Van Immerseel, F.; Haesebrouck, F.; Ducatelle, R.; Dewulf, J. The influence of the housing system on Salmonella infections in laying hens: A review. Zoonoses Public Health 2011, 58, 304–311. [Google Scholar] [CrossRef]
- EFSA. Report of the task force on zoonoses data collection on the analysis of the baseline study on the prevalence of Salmonella in holdings of laying hen flocks of Gallus gallus. EFSA J. 2007, 97, 85. [Google Scholar]
- Namata, H.; Meroc, E.; Aerts, M.; Faes, C.; Abrahantes, J.C.; Imberechts, H.; Mintiens, K. Salmonella in Belgian laying hens: An identification of risk factors. Prev. Vet. Med. 2008, 83, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Snow, L.C.; Davies, R.H.; Christiansen, K.H.; Carrique-Mas, J.J.; Cook, A.J.; Evans, S.J. Investigation of risk factors for Salmonella on commercial egg-laying farms in Great Britain, 2004–2005. Vet. Rec. 2010, 166, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Van Hoorebeke, S.; Van Immerseel, F.; Schulz, J.; Hartung, J.; Harisberger, M.; Barco, L.; Ricci, A.; Theodoropoulos, G.; Xylouri, E.; De Vylder, J.; et al. Determination of the within and between flock prevalence and identification of risk factors for Salmonella infections in laying hen flocks housed in conventional and alternative systems. Prev. Vet. Med. 2010, 94, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.; Breslin, M. Observations on Salmonella contamination of eggs from infected commercial laying flocks where vaccination for Salmonella enterica serovar Enteritidis had been used. Avian. Pathol. 2004, 33, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Gast, R.K.; Guraya, R.; Jones, D.R.; Anderson, K.E.; Karcher, D.M. Frequency and Duration of Fecal Shedding of Salmonella Enteritidis by Experimentally Infected Laying Hens Housed in Enriched Colony Cages at Different Stocking Densities. Front. Vet. Sci. 2017, 4, 47. [Google Scholar] [CrossRef]
- Gast, R.K.; Guraya, R.; Jones, D.R.; Guard, J.; Anderson, K.E.; Karcher, D.M. Frequency and Duration of Fecal Shedding of Salmonella Serovars Heidelberg and Typhimurium by Experimentally Infected Laying Hens Housed in Enriched Colony Cages at Different Stocking Densities. Avian. Dis. 2017, 61, 366–371. [Google Scholar] [CrossRef]
- Holt, P.S. Centennial Review: A revisiting of hen welfare and egg safety consequences of mandatory outdoor access for organic egg production. Poult. Sci. 2021, 100, 101436. [Google Scholar] [CrossRef]
- Jones, D.R.; Guard, J.; Gast, R.K.; Buhr, R.J.; Fedorka-Cray, P.J.; Abdo, Z.; Plumblee, J.R.; Bourassa, D.V.; Cox, N.A.; Rigsby, L.L.; et al. Influence of commercial laying hen housing systems on the incidence and identification of Salmonella and Campylobacter. Poult. Sci. 2016, 95, 1116–1124. [Google Scholar] [CrossRef]
- Van Hoorebeke, S.; Van Immerseel, F.; De Vylder, J.; Ducatelle, R.; Haesebrouck, F.; Pasmans, F.; de Kruif, A.; Dewulf, J. The age of production system and previous Salmonella infections on-farm are risk factors for low-level Salmonella infections in laying hen flocks. Poult. Sci. 2010, 89, 1315–1319. [Google Scholar] [CrossRef]
- EFSA. Opinion of the Scientific Panel on Animal Health and Welfare (AHAW) on a request from the Commission related to the welfare aspects of various systems of keeping laying hens. EFSA J. 2005, 197, 23. [Google Scholar]
- Huneau-Salaun, A.; Marianne, C.; Sophie, L.B.; Francoise, L.; Isabelle, P.; Sandra, R.; Virginie, M.; Philippe, F.; Nicolas, R. Risk factors for Salmonella enterica subsp. enterica contamination in 519 French laying hen flocks at the end of the laying period. Prev. Vet. Med. 2009, 89, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Wales, A.; Breslin, M.; Carter, B.; Sayers, R.; Davies, R. A longitudinal study of environmental Salmonella contamination in caged and free-range layer flocks. Avian Pathol. 2007, 36, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Garber, L.; Smeltzer, M.; Fedorka-Cray, P.; Ladely, S.; Ferris, K. Salmonella enterica serotype enteritidis in table egg layer house environments and in mice in U.S. layer houses and associated risk factors. Avian Dis. 2003, 47, 134–142. [Google Scholar] [CrossRef]
- Gole, V.C.; Woodhouse, R.; Caraguel, C.; Moyle, T.; Rault, J.L.; Sexton, M.; Chousalkar, K. Dynamics of Salmonella Shedding and Welfare of Hens in Free-Range Egg Production Systems. Appl. Environ. Microbiol. 2017, 83, e03313-16. [Google Scholar] [CrossRef]
- Powell, M.R.; Crim, S.M.; Hoekstra, R.M.; Williams, M.S.; Gu, W. Temporal patterns in principal Salmonella serotypes in the USA; 1996–2014. Epidemiol. Infect. 2018, 146, 437–441. [Google Scholar] [CrossRef]
- Arnold, M.E.; Martelli, F.; McLaren, I.; Davies, R.H. Estimation of the sensitivity of environmental sampling for detection of Salmonella in commercial layer flocks post-introduction of national control programmes. Epidemiol. Infect. 2014, 142, 1061–1069. [Google Scholar] [CrossRef]
- Jackson, B.R.; Griffin, P.M.; Cole, D.; Walsh, K.A.; Chai, S.J. Outbreak-associated Salmonella enterica serotypes and food Commodities, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 1239–1244. [Google Scholar] [CrossRef]
- Machado Junior, P.C.; Chung, C.; Hagerman, A. Modeling Salmonella Spread in Broiler Production: Identifying Determinants and Control Strategies. Front. Vet. Sci. 2020, 7, 564. [Google Scholar] [CrossRef]
- Le Bouquin, S.; Allain, V.; Rouxel, S.; Petetin, I.; Picherot, M.; Michel, V.; Chemaly, M. Prevalence and risk factors for Salmonella spp. contamination in French broiler-chicken flocks at the end of the rearing period. Prev. Vet. Med. 2010, 97, 245–251. [Google Scholar] [CrossRef]
- Namata, H.; Welby, S.; Aerts, M.; Faes, C.; Abrahantes, J.C.; Imberechts, H.; Vermeersch, K.; Hooyberghs, J.; Meroc, E.; Mintiens, K. Identification of risk factors for the prevalence and persistence of Salmonella in Belgian broiler chicken flocks. Prev. Vet. Med. 2009, 90, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Volkova, V.V.; Bailey, R.H.; Rybolt, M.L.; Dazo-Galarneau, K.; Hubbard, S.A.; Magee, D.; Byrd, J.A.; Wills, R.W. Inter-relationships of Salmonella status of flock and grow-out environment at sequential segments in broiler production and processing. Zoonoses Public Health 2010, 57, 463–475. [Google Scholar] [CrossRef] [PubMed]
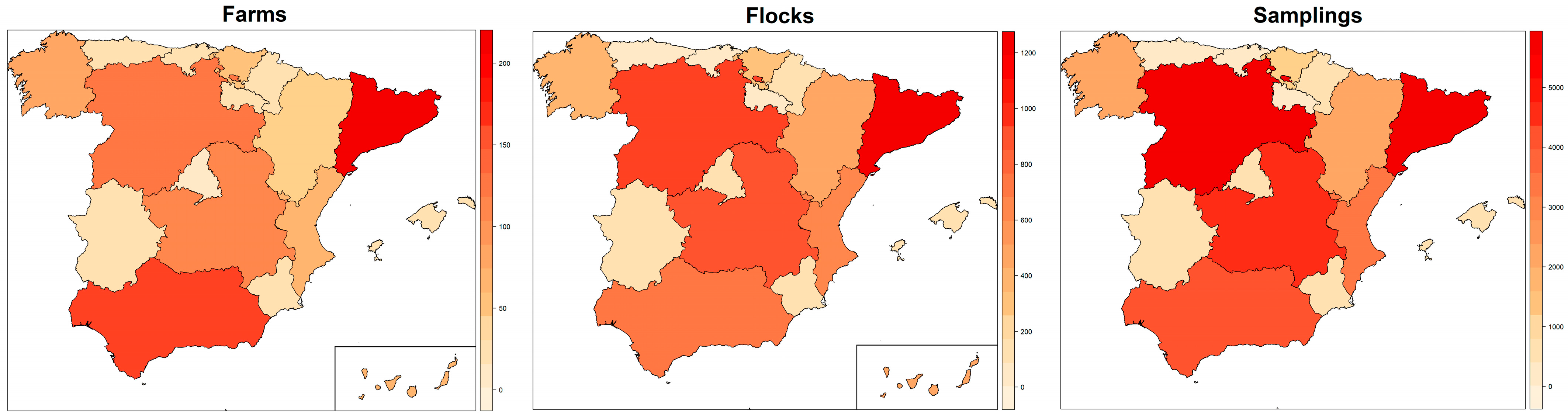
| Level | Variable | Category | N (%) | Salmonella spp. Positive (%) | Target Serovar Positive (%) |
|---|---|---|---|---|---|
| Farm (n = 1153) | Region | Region 0 | 206 (17.9) | 84 (40.8) | 16 (7.8) |
| Region 1 | 173 (15.0) | 86 (49.7) | 28 (16.2) | ||
| Region 2 | 53 (4.6) | 24 (45.3) | 6 (11.3) | ||
| Region 3 | 112 (9.7) | 54 (48.2) | 7 (6.3) | ||
| Region 4 | 124 (10.8) | 28 (22.6) | 4 (3.2) | ||
| Region 5 | 75 (6.5) | 45 (60.0) | 14 (18.7) | ||
| Region 6 | 97 (8.4) | 14 (14.4) | 3 (3.1) | ||
| Region 7 | 84 (7.3) | 33 (39.3) | 12 (9.1) | ||
| Region 8 | 164 (14.2) | 56 (34.1) | 15 (9.1) | ||
| Region 9 | 65 (5.6) | 13 (20.0) | 3 (4.6) | ||
| Flock (n = 7216) | Size | Q1 (<2500) | 1810 (25.1) | 170 (9.4) | 36 (2.0) |
| Q2 (2500–10,000) | 1817 (25.2) | 172 (9.5) | 35 (1.9) | ||
| Q3 (10,000–32,055) | 1784 (24.7) | 291 (16.3) | 36 (2.0) | ||
| Q4 (>32,055) | 1805 (25.0) | 205 (11.4) | 16 (0.9) | ||
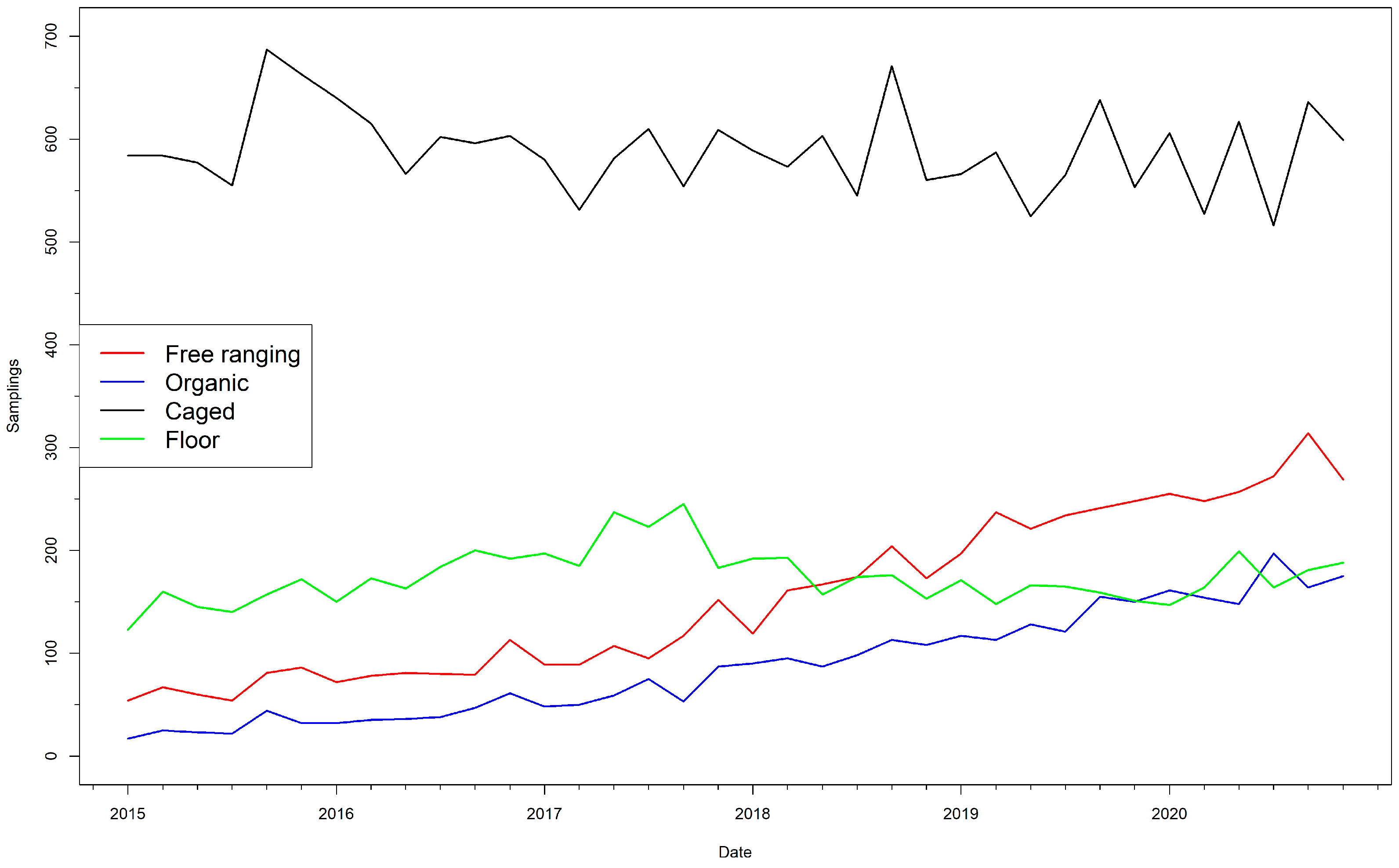
| Housing type | Caged | 3833 (53.1) | 586 (15.3) | 62 (1.6) | |
| Floor | 1465 (20.3) | 86 (5.9) | 19 (1.3) | ||
| Free-ranging | 1188 (16.5) | 86 (7.2) | 29 (2.4) | ||
| Organic | 730 (10.1) | 80 (11.0) | 13 (1.8) | ||
| Sampling (n = 36,193) | Sampler/reason sampling | FBOp—periodical | 28,481 (78.7) | 730 (2.6) | 23 (0.08) |
| FBOp—env | 4313 (11.9) | 45 (1.0) | 6 (0.14) | ||
| CA—periodical | 3399 (9.4) | 430 (12.7) | 103 (3.0) | ||
| Year | 2015 | 5112 (14.1) | 148 (2.9) | 8 (0.16) | |
| 2016 | 5436 (15.0) | 198 (3.6) | 23 (0.42) | ||
| 2017 | 5756 (15.9) | 199 (3.5) | 21 (0.36) | ||
| 2018 | 6175 (17.1) | 215 (3.5) | 26 (0.42) | ||
| 2019 | 6556 (18.1) | 224 (3.4) | 29 (0.44) | ||
| 2020 | 7158 (19.8) | 221 (3.1) | 25 (0.35) | ||
| Bimester | Jan–Feb | 5796 (16.0) | 148 (2.6) | 11 (0.19) | |
| Mar–Apr | 5792 (16.0) | 151 (2.6) | 13 (0.22) | ||
| May–Jun | 5910 (16.3) | 159 (2.7) | 18 (0.30) | ||
| Jul–Aug | 5903 (16.3) | 170 (2.9) | 19 (0.32) | ||
| Sep–Oct | 6512 (18.0) | 305 (4.7) | 32 (0.49) | ||
| Nov–Dec | 6280 (17.4) | 272 (4.3) | 39 (0.62) | ||
| Age (weeks) | Q1 (<39) | 8912 (24.6) | 216 (2.4) | 21 (0.24) | |
| Q2 (39–61) | 8947 (24.7) | 238 (2.7) | 16 (0.18) | ||
| Q3 (61–83) | 9223 (25.5) | 309 (3.4) | 31 (0.34) | ||
| Q4 (>83) | 9111 (25.2) | 442 (4.9) | 64 (0.70) | ||
| Number of analyses | 1 | 32,645 (90.2) | 801 (2.5) | 40 (0.12) | |
| 2–10 | 3548 (9.8) | 404 (11.4) | 92 (2.6) | ||
| Sample type | Feces | 20,065 (55.4) | 898 (4.5) | 64 (0.32) | |
| Boot swabs | 11,720 (32.4) | 249 (2.1) | 59 (0.50) | ||
| Dust | 3542 (9.8) | 48 (1.4) | 7 (0.20) | ||
| Fabric swab | 866 (2.4) | 10 (1.2) | 2 (0.23) |
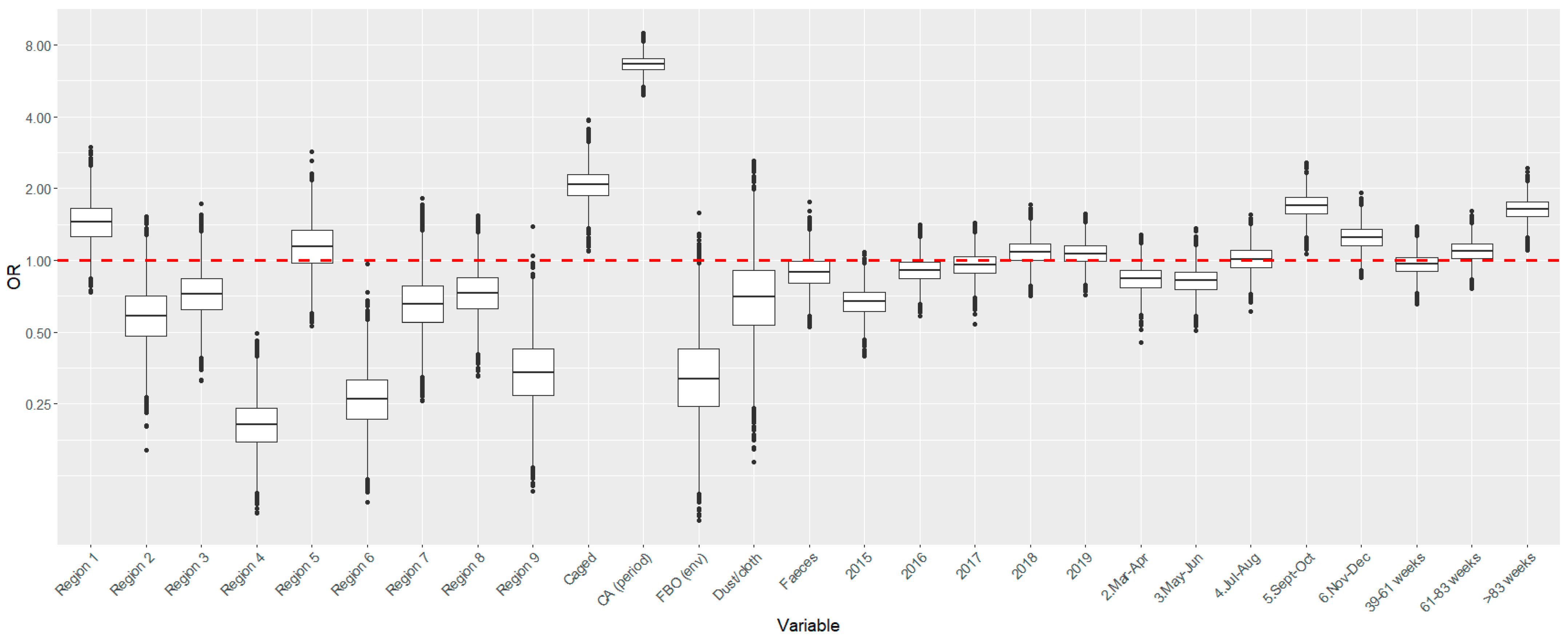
| Level (n) | Variable | Category | Median β, γ, δ | Rhat | Median OR (95% PPI) |
|---|---|---|---|---|---|
| Farm (n = 1153) | Region | Region 0 | Ref | ||
| Region 1 | 0.369 | 1.001 | 1.45 (0.97–2.13) | ||
| Region 2 | −0.539 | 1.001 | 0.58 (0.33–1.03) | ||
| Region 3 | −0.328 | 1.006 | 0.72 (0.45–1.1) | ||
| Region 4 | −1.590 | 1.004 | 0.2 (0.13–0.33) | ||
| Region 5 | 0.132 | 1.001 | 1.14 (0.71–1.81) | ||
| Region 6 | −1.340 | 1.002 | 0.26 (0.15–0.46) | ||
| Region 7 | −0.419 | 1.003 | 0.66 (0.38–1.14) | ||
| Region 8 | −0.317 | 1.003 | 0.73 (0.48–1.11) | ||
| Region 9 | −1.085 | 1.006 | 0.34 (0.18–0.63) | ||
| Flock (n = 7216) | Housing type | Others | Ref | ||
| Caged | 0.729 | 1.005 | 2.07 (1.52–2.85) | ||
| Sampling (n = 36,193) | Sampler/reason sampling | FBOp—periodical | Ref | ||
| FBOp—env | −1.142 | 1.001 | 0.32 (0.15–0.74) | ||
| CA—periodical | 1.893 | 1.001 | 6.64 (5.63–7.75) | ||
| Year | 2015 | −0.400 | 1.005 | 0.67 (0.52–0.87) | |
| 2016 | −0.097 | 1.003 | 0.91 (0.71–1.15) | ||
| 2017 | −0.045 | 1.002 | 0.96 (0.75–1.21) | ||
| 2018 | 0.081 | 1.002 | 1.08 (0.87–1.36) | ||
| 2019 | 0.066 | 1.001 | 1.07 (0.86–1.33) | ||
| 2020 | Ref | ||||
| Bimester | Jan–Feb | Ref | |||
| Mar–Apr | −0.180 | 1.001 | 0.84 (0.65–1.07) | ||
| May–Jun | −0.195 | 1.002 | 0.82 (0.64–1.06) | ||
| Jul–Aug | 0.010 | 1.001 | 1.01 (0.79–1.29) | ||
| Sep–Oct | 0.531 | 1.002 | 1.70 (1.36–2.12) | ||
| Nov–Dec | 0.218 | 1.001 | 1.24 (0.99–1.56) | ||
| Age (weeks) | Q1 (<39) | Ref | |||
| Q2 (39–61) | −0.039 | 1.002 | 0.96 (0.78–1.18) | ||
| Q3 (61–83) | 0.090 | 1.001 | 1.09 (0.90–1.33) | ||
| Q4 (>83) | 0.492 | 1.002 | 1.63 (1.35–2.00) | ||
| Sample type | Boot swabs | Ref | |||
| Feces | −0.116 | 1.004 | 0.89 (0.65–1.23) | ||
| Dust/fabric swab | −0.350 | 1.001 | 0.7 (0.31–1.51) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samper-Cativiela, C.; Prieto, M.E.; Collado, S.; De Frutos, C.; Branscum, A.J.; Saez, J.L.; Alvarez, J. Risk Factors for Salmonella Detection in Commercial Layer Flocks in Spain. Animals 2023, 13, 3181. https://doi.org/10.3390/ani13203181
Samper-Cativiela C, Prieto ME, Collado S, De Frutos C, Branscum AJ, Saez JL, Alvarez J. Risk Factors for Salmonella Detection in Commercial Layer Flocks in Spain. Animals. 2023; 13(20):3181. https://doi.org/10.3390/ani13203181
Chicago/Turabian StyleSamper-Cativiela, Clara, Maria Esther Prieto, Soledad Collado, Cristina De Frutos, Adam J. Branscum, Jose Luis Saez, and Julio Alvarez. 2023. "Risk Factors for Salmonella Detection in Commercial Layer Flocks in Spain" Animals 13, no. 20: 3181. https://doi.org/10.3390/ani13203181
APA StyleSamper-Cativiela, C., Prieto, M. E., Collado, S., De Frutos, C., Branscum, A. J., Saez, J. L., & Alvarez, J. (2023). Risk Factors for Salmonella Detection in Commercial Layer Flocks in Spain. Animals, 13(20), 3181. https://doi.org/10.3390/ani13203181





