Evaluation of Antiviral Activity of Ivermectin against Infectious Bovine Rhinotracheitis Virus in Rabbit Model
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Culture
2.2. Virus and Growth
2.3. Effect of IVM on Viral Binding and Entry
2.4. IVM Viral Inhibition Assay
2.5. Effect of IVM on Cytokine Production by Infected Cells
2.6. Animal Experiment
2.7. Clinical Sign Observation and Scoring
2.8. Neutralization Antibody Test
2.9. Evaluation of Lung Lesions
2.10. Primer Design
2.11. Statistical Analysis
3. Results
3.1. Effects of IVM’s Inhibition of IBRV Replication in Cultured Cells
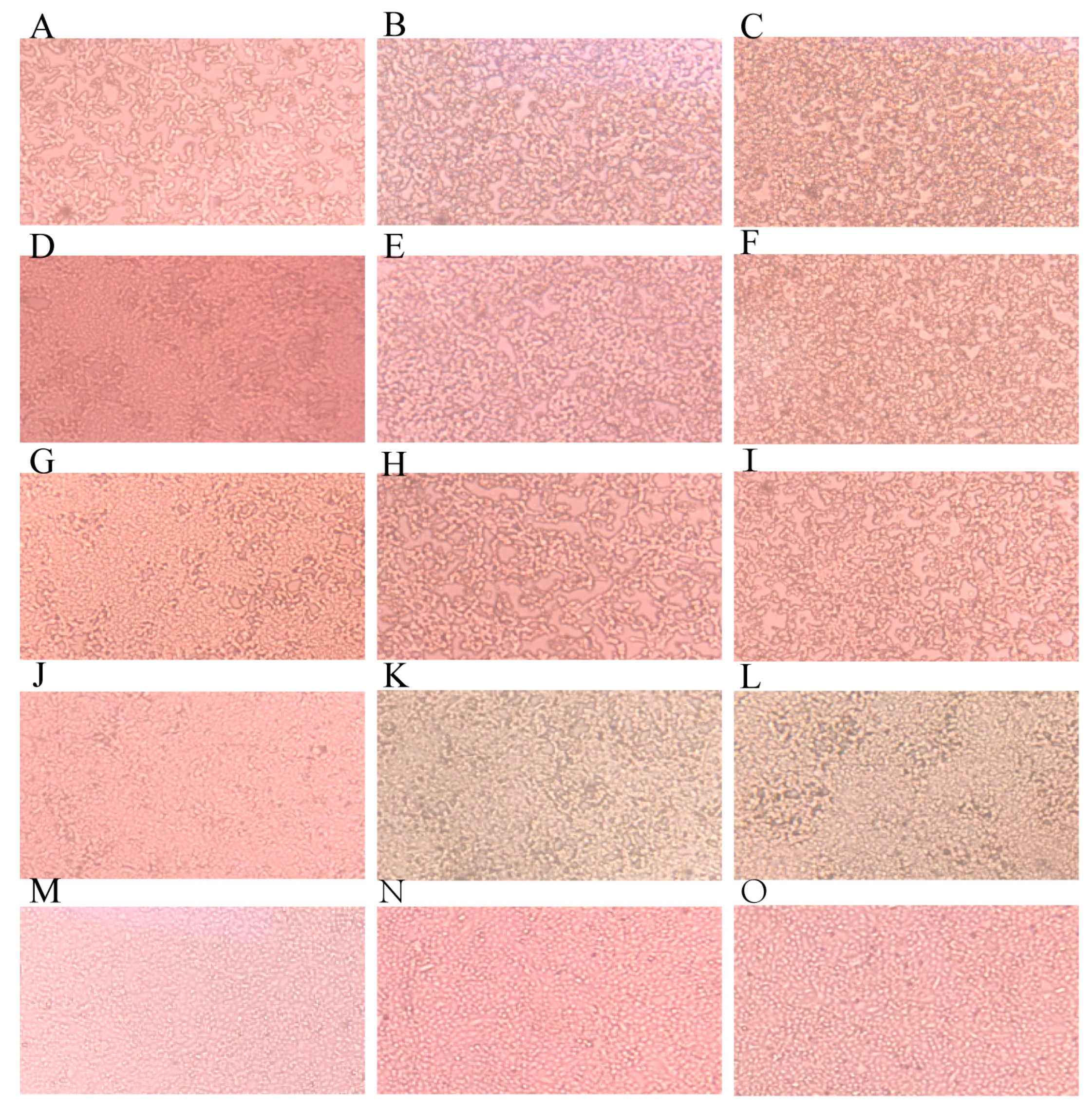
3.1.1. IVM Delayed CPE Appearance
3.1.2. IVM Did Not Affect Viral Binding and Entry
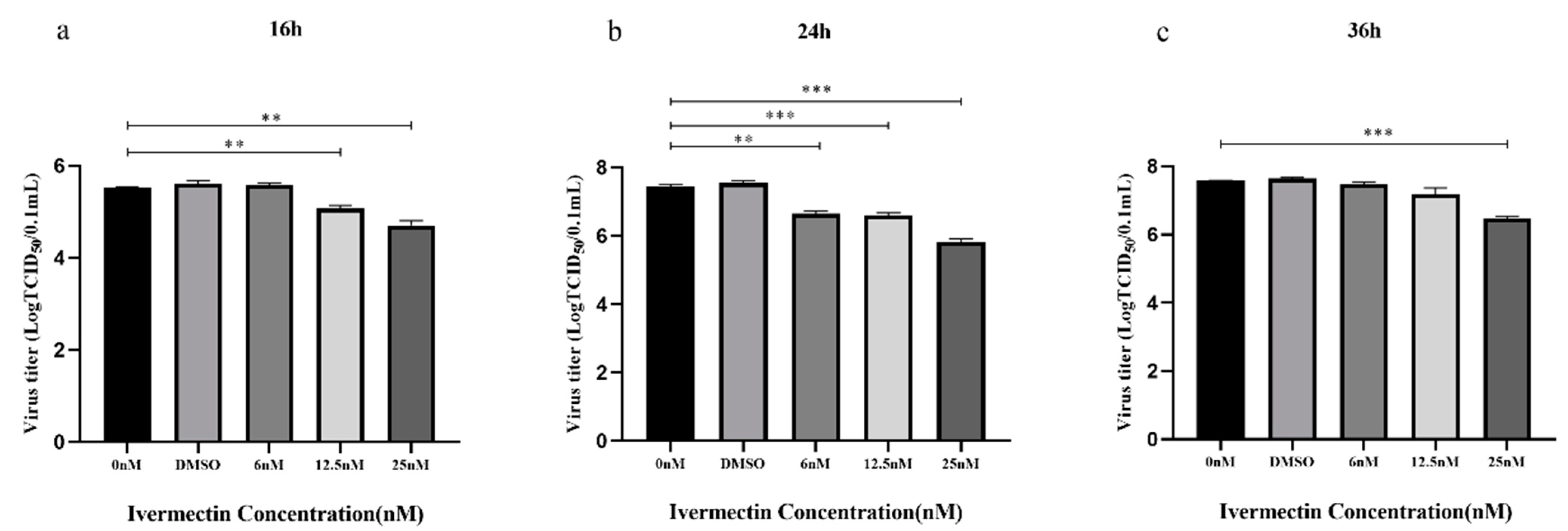
3.1.3. IVM Inhibited Viral Replication
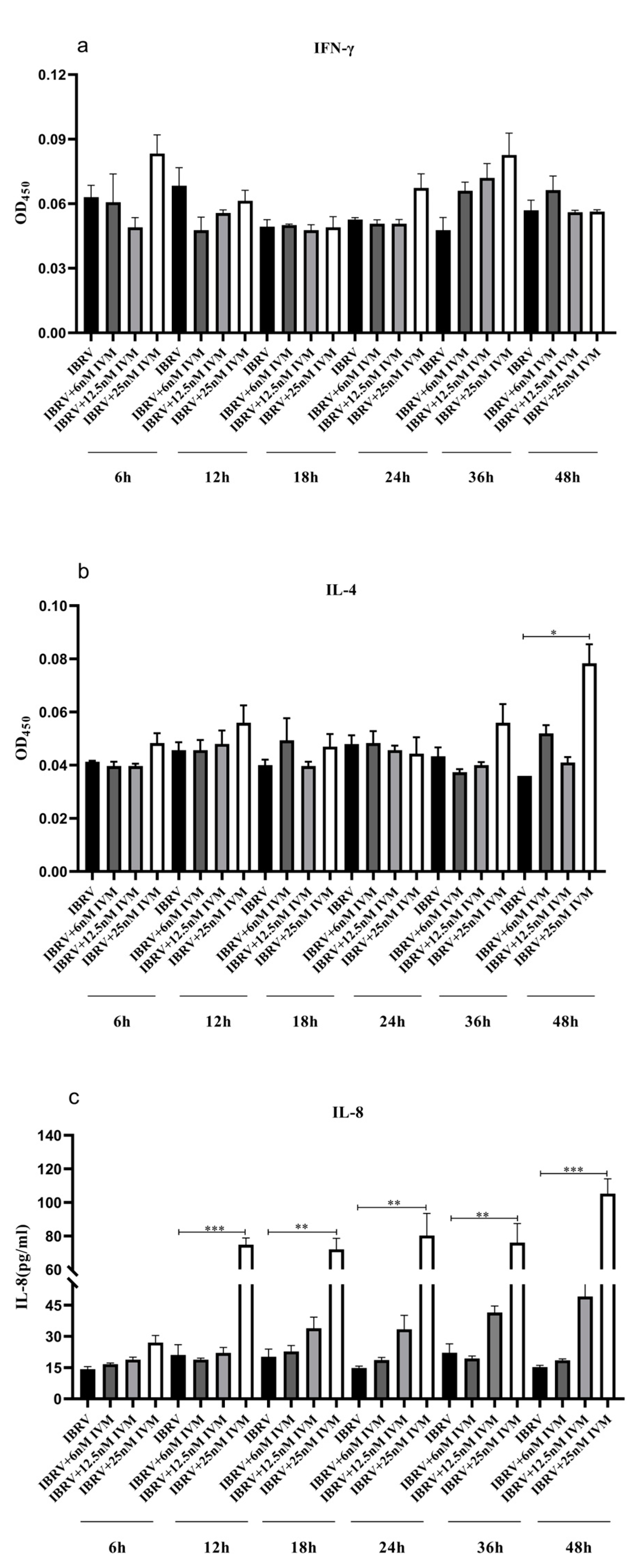
3.1.4. IVM’s Effects on Cytokine Production in IBRV Infected Cells
3.2. IVM’s Effects on IBRV Infection in Rabbits
3.2.1. Temperature Change of the Experimental Rabbits
3.2.2. Clinical Signs of Infected Rabbits
3.2.3. Nasal Virus Shedding
3.2.4. Neutralization Antibody Titers
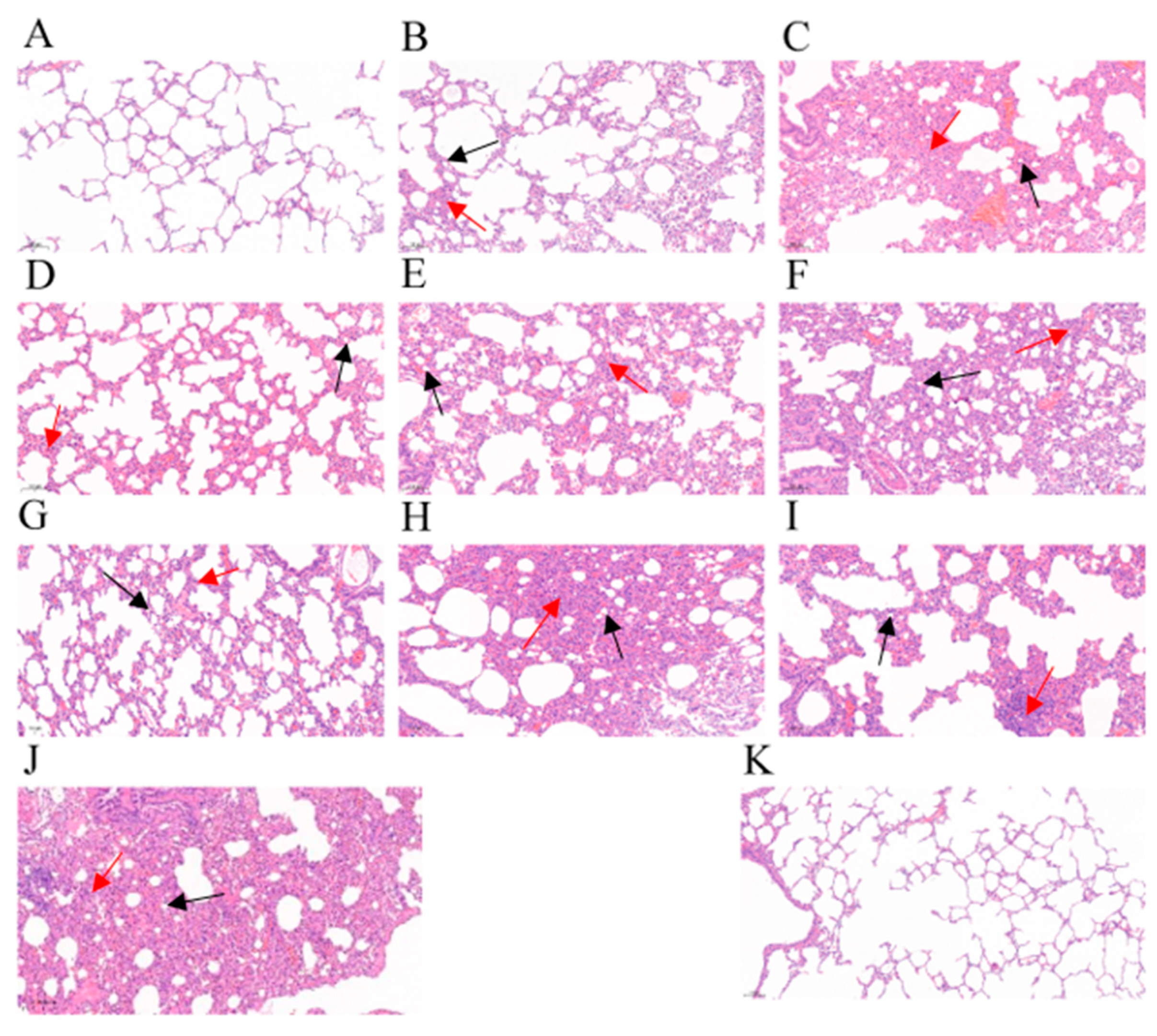
3.2.5. Gross Lesions and Histopathological Change of Lung Tissues
3.2.6. Detection of the Virus in Tissues Detected with qPCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wyler, R.; Engels, M.; Schwyzer, M. Infectious Bovine Rhinotracheitis/Vulvovaginitis (BHV1). In Herpesvirus Diseases of Cattle, Horses, and Pigs; Springer: Boston, MA, USA, 1989. [Google Scholar]
- Kornuta, C.A.; Bidart, J.E.; Soria, I.; Gammella, M.; Quattrocchi, V.; Pappalardo, J.S.; Salmaso, S.; Torchilin, V.P.; Valenzuela, F.C.; Hecker, Y.P. MAN alpha 1-2MAN decorated liposomes enhance the immunogenicity induced by a DNA vaccine against BoHV-1. Transbound. Emerg. Dis. 2021, 68, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.I.; Wei, H.; Weiss, M.; Pannhorst, K.; Paulsen, D.B. A triple gene mutant of BoHV-1 administered intranasally is significantly more efficacious than a BoHV-1 glycoprotein E-deleted virus against a virulent BoHV-1 challenge. Vaccine 2014, 32, 4909–4915. [Google Scholar] [CrossRef] [PubMed]
- White, D.W.; Beard, R.S.; Barton, E.S. Immune modulation during latent herpesvirus infection. Immunol. Rev. 2015, 245, 189–208. [Google Scholar]
- Wiebe, M.; Alexer, J.; Wang, J.; Jones, C. Bovine herpesvims 1 productive infection stimulates inflammasome formation and caspase 1 activity. Virus Res. Int. J. Mol. Cell. Virol. 2014, 185, 72–76. [Google Scholar]
- Caka, B.; Cala, B.; Jeba, B.; Is, A.; Vq, A.; Mg, A.; Fcvb, G.; Acmb, D.; Sf, E.; Bc, F. A plasmid encoding the extracellular domain of CD40 ligand and Montanide GEL01 as adjuvants enhance the immunogenicity and the protection induced by a DNA vaccine against BoHV-1—ScienceDirect. Vaccine 2021, 39, 1007–1017. [Google Scholar]
- Lv, C.; Liu, W.; Wang, B.; Dang, R.; Qiu, L.; Ren, J.; Yan, C.; Yang, Z.; Wang, X. Ivermectin inhibits DNA polymerase UL42 of pseudorabies virus entrance into the nucleus and proliferation of the virus in vitro and vivo. Antivir. Res. 2018, 159, 55–62. [Google Scholar]
- Raza, S.; Shahin, F.; Zhai, W.; Li, H.; Guo, A. Ivermectin Inhibits Bovine Herpesvirus 1 DNA Polymerase Nuclear Import and Interferes With Viral Replication. Microorganisms 2020, 8, 409. [Google Scholar] [CrossRef]
- Formiga, F.R.; Leblanc, R.; Rebouas, J.D.S.; Farias, L.P.; Oliveira, R.N.D.; Pena, L. Ivermectin: An Award-Winning Drug with Expected Antiviral Activity against COVID-19; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Schmith, V.D.; Zhou, J.J.; Lohmer, L.R.L. The Approved Dose of Ivermectin Alone is not the Ideal Dose for the Treatment of COVID-19. Clin. Pharmacol. Ther. 2020, 108, 762–765. [Google Scholar] [CrossRef]
- Lanusse, C.; Lifschitz, A.; Virkel, G.; Alvarez, L.; SÁnchez, S.; Sutra, J.F.; Galtier, P.; Alvinerie, M. Comparative plasma disposition kinetics of ivermectin, moxidectin and doramectin in cattle. J. Vet. Pharmacol. Ther. 1997, 20, 91–99. [Google Scholar] [CrossRef]
- Valera, A.; Pidone, C.; Massone, A.; Quiroga, M.; Riganti, J.; Corva, S.; Galosi, C. A simple method of infecting rabbits with Bovine herpesvirus 1 and 5. J. Virol. Methods 2008, 150, 77–79. [Google Scholar] [CrossRef]
- Jairo, R.S.; Jaime, J.; Vera, V. An inactivated vaccine from a field strain of bovine herpesvirus-1 (BoHV-1) has high antigenic mass and induces strong efficacy in a rabbit model. Virol. Sin. 2013, 28, 36–42. [Google Scholar]
- Ferrer, M.F.; Del Médico Zajac, M.P.; Zanetti, F.A.; Valera, A.R.; Zabal, O.; Calamante, G. Recombinant MVA expressing secreted glycoprotein D of BoHV-1 induces systemic and mucosal immunity in animal models. Viral Immunol. 2011, 24, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Valera, A.R.; Fuentealba, N.A.; Zanuzzi, C.N.; Corva, S.G.; Pecoraro, M.R.; Barbeito, C.G.; Galosi, C.M. Systemic infection induced by intranasal inoculation of Bovine herpesvirus 1.1 in pregnant and non-pregnant rabbits. Res. Vet. Ence 2013, 95, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Butchi, N.B.; Jones, C.; Perez, S.; Doster, A.; Chowdhury, S.I. Envelope protein Us9 is required for the anterograde transport of bovine herpesvirus type 1 from trigeminal ganglia to nose and eye upon reactivation. J. Neurovirology 2007, 13, 384. [Google Scholar] [CrossRef] [PubMed]
- Heidary, F.; Gharebaghi, R. Ivermectin: A systematic review from antiviral effects to COVID-19 complementary regimen. J. Antibiot. 2020, 73, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Deng, M. Investigation of Gene Deleted Vaccine of Infectious Bovine Rhinotracheitis Virus and Its Application. Ph.D. Thesis, Hua Zhong Agriculture University, Wuhan, China, 2015. [Google Scholar]
- Naeem, Z.; Raza, S.; Afzal, S.; Sheikh, A.A.; Altaf, I. Antiviral potential of ivermectin against foot-and-mouth disease virus, serotype O, A and Asia-1. Microb. Pathog. 2021, 155, 104914. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, C. Ivermectin inhibits porcine reproductive and respiratory syndrome virus in cultured porcine alveolar macrophages. Arch. Virol. 2016, 161, 257–268. [Google Scholar]
- Yesilbag, K.; Toker, E.B.; Ates, O. Ivermectin also inhibits the replication of bovine respiratory viruses (BRSV, BPIV-3, BoHV-1, BCoV and BVDV) in vitro. Virus Res. 2021, 297, 198384. [Google Scholar] [CrossRef]
- Wagstaff, K.M.; Sivakumaran, H.; Heaton, S.M.; Harrich, D.; Jans, D.A. Ivermectin is a specific inhibitor of importin alpha/beta-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem. J. 2012, 443, 851–856. [Google Scholar] [CrossRef]
- Barber, S.; Bowles, V.; Lespine, A.; Alvinerie, M. The comparative serum disposition kinetics of subcutaneous administration of doramectin, ivermectin and moxidectin in the Australian Merino sheep. J. Vet. Pharmacol. Ther. 2010, 26, 343–348. [Google Scholar] [CrossRef]
- Gokbulut, C.; Biligili, A.; Kart, A.; Turgut, C. Plasma dispositions of ivermectin, doramectin and moxidectin following subcutaneous administration in rabbits. Lab. Anim. 2010, 44, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Arévalo, A.; Pagotto, R.; Pórfido, J.; Daghero, H.; Crispo, M. Ivermectin Reduces Coronavirus Infection In Vivo: A Mouse Experimental Model; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 2020. [Google Scholar]
- Uematsu, T.; Takano, T.; Matsui, H.; Kobayashi, N.; Ōmura, S.; Hanaki, H. Prophylactic administration of ivermectin attenuates SARS-CoV-2 induced disease in a Syrian Hamster Model. J. Antibiot. 2023, 76, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Padhy, B.M.; Mohanty, R.R.; Das, S.; Meher, B.R. Therapeutic potential of ivermectin as add on treatment in COVID 19: A systematic review and meta-analysis: Ivermectin in COVID-19: A meta-analysis. J. Pharm. Pharm. Sci. 2020, 23, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.; Doster, A.; Sur, J.-H.; Jones, C. Analysis of bovine trigeminal ganglia following infection with bovine herpesvirus 1. Vet. Microbiol. 2002, 86, 139–155. [Google Scholar]
- Winkler, M.; Doster, A.; Jones, C. Persistence and Reactivation of Bovine Herpesvirus 1 in the Tonsils of Latently Infected Calves. J. Virol. 2000, 74, 5337–5346. [Google Scholar] [CrossRef]

| Groups | N | Inoculums | IVM Doses (mg/kg) | Days of IVM Administered Post Viral Challenge (DPC) |
|---|---|---|---|---|
| 1 | 3 | IBRV HB06, 107.0 TCID50/mL, 2 mL | 0.6 | 0 |
| 2 | 3 | 0.4 | 0 | |
| 3 | 3 | 0.2 | 0 | |
| 4 | 3 | 0.6 | 3 | |
| 5 | 3 | 0.4 | 3 | |
| 6 | 3 | 0.2 | 3 | |
| 7 | 3 | 0.6 | 7 | |
| 8 | 3 | 0.4 | 7 | |
| 9 | 3 | 0.2 | 7 | |
| 10 | 3 | / | / | |
| 11 | 3 | DMEM, 2 mL | / | / |
| Observation Item | Scoring Criteria |
|---|---|
| Nose and eye discharge | None 0, mild discharge 1, moderate discharge with mild mucopurulent discharge 2, moderate mucopurulent discharge 3, severe mucopurulent discharge 4 |
| Mental disorder | None 0, mild 1, moderate 2, severe 3 |
| Nasal mucosa | Normal 0, congestion 1, ulcer 2 |
| Temperature | Increase of 0.5 °C, 1.0 °C, 1.5 °C, and 2.0 °C or more corresponds to 1, 2, 3, 4, respectively |
| Primers | Primer Sequences (5′-3′) |
|---|---|
| gB-F | TGTGGACCTAAACCTCACGGT |
| gB-R | GTAGTCGAGCAGACCCGTGTC |
| gB-Probe | [FAM]-AGGACCGCGAGTTCTTGCCGC-[TAMRA] |
| IVM Doses (mg/kg) | IBRV Challenge | IVM Administration Time (DPC) | Total | ||
|---|---|---|---|---|---|
| 0 | 3 | 7 | |||
| 0.6 | + | 8/33 | 13/33 | 11/33 | 32/99 ** |
| 0.4 | + | 11/33 | 8/33 | 17/33 | 36/99 * |
| 0.2 | + | 11/33 | 10/33 | 17/33 | 38/99 * |
| Total | + | 30/99 *** | 31/99 ** | 45/99 * | / |
| 0 | + | / | 23/33 | ||
| IVM Doses (mg/kg) | IBRV Challenge | Summed Scores at Different IVM Administration Times | Total Scores | ||
|---|---|---|---|---|---|
| 0 DPC | 3 DPC | 7 DPC | |||
| 0.6 | + | 14 ** | 43 | 26 | 83 |
| 0.4 | + | 26 | 24 ** | 39 | 89 |
| 0.2 | + | 36 | 24 * | 37 | 97 |
| Total | + | 76 | 91 | 102 | / |
| 0 | + | / | 62 | ||
| 0 | - | / | 0 | ||
| IVM Doses (mg/kg) | IBRV Challenge | IVM Administration Time (Positive/Total Samples) | Total Proportions | ||
|---|---|---|---|---|---|
| 0 DPC | 3 DPC | 7 DPC | |||
| 0.6 | + | 22/42 | 24/42 | 23/42 | 69/126 |
| 0.4 | + | 22/42 | 23/42 | 24/42 | 69/126 |
| 0.2 | + | 23/42 | 23/42 | 24/42 | 70/126 |
| Total | + | 67/126 | 70/126 | 71/126 | / |
| 0 | + | / | 29/42 | ||
| 0 | - | / | 0 | ||
| IVM Doses (mg/kg) | IBRV | IVM Administration Time | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 DPC | 3 DPC | 7 DPC | |||||||
| L | T | L | T | L | T | L | T | ||
| 0.6 | + | 1/3 | 2/3 | 1/3 | 3/3 | 2/3 | 1/3 | 4/9 | 6/9 |
| 0.4 | + | 1/3 | 2/3 | 2/3 | 1/3 | 2/3 | 3/3 | 5/9 | 7/9 |
| 0.2 | + | 2/3 | 2/3 | 3/3 | 1/3 | 3/3 | 3/3 | 8/9 | 6/9 |
| Total | + | 4/9 | 6/9 | 6/9 | 5/9 | 7/9 | 7/9 | ||
| 0 | + | / | 3/3 | 3/3 | |||||
| 0 | - | / | 0/3 | 0/3 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Chen, Y.; Chen, X.; Hu, C.; Chen, J.; Guo, A. Evaluation of Antiviral Activity of Ivermectin against Infectious Bovine Rhinotracheitis Virus in Rabbit Model. Animals 2023, 13, 3164. https://doi.org/10.3390/ani13203164
Wang C, Chen Y, Chen X, Hu C, Chen J, Guo A. Evaluation of Antiviral Activity of Ivermectin against Infectious Bovine Rhinotracheitis Virus in Rabbit Model. Animals. 2023; 13(20):3164. https://doi.org/10.3390/ani13203164
Chicago/Turabian StyleWang, Chen, Yingyu Chen, Xi Chen, Changmin Hu, Jianguo Chen, and Aizhen Guo. 2023. "Evaluation of Antiviral Activity of Ivermectin against Infectious Bovine Rhinotracheitis Virus in Rabbit Model" Animals 13, no. 20: 3164. https://doi.org/10.3390/ani13203164
APA StyleWang, C., Chen, Y., Chen, X., Hu, C., Chen, J., & Guo, A. (2023). Evaluation of Antiviral Activity of Ivermectin against Infectious Bovine Rhinotracheitis Virus in Rabbit Model. Animals, 13(20), 3164. https://doi.org/10.3390/ani13203164






