Effects of Dietary Supplementation of Spirulina platensis on the Immune System, Intestinal Bacterial Microbiome and Skin Traits of Mink
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
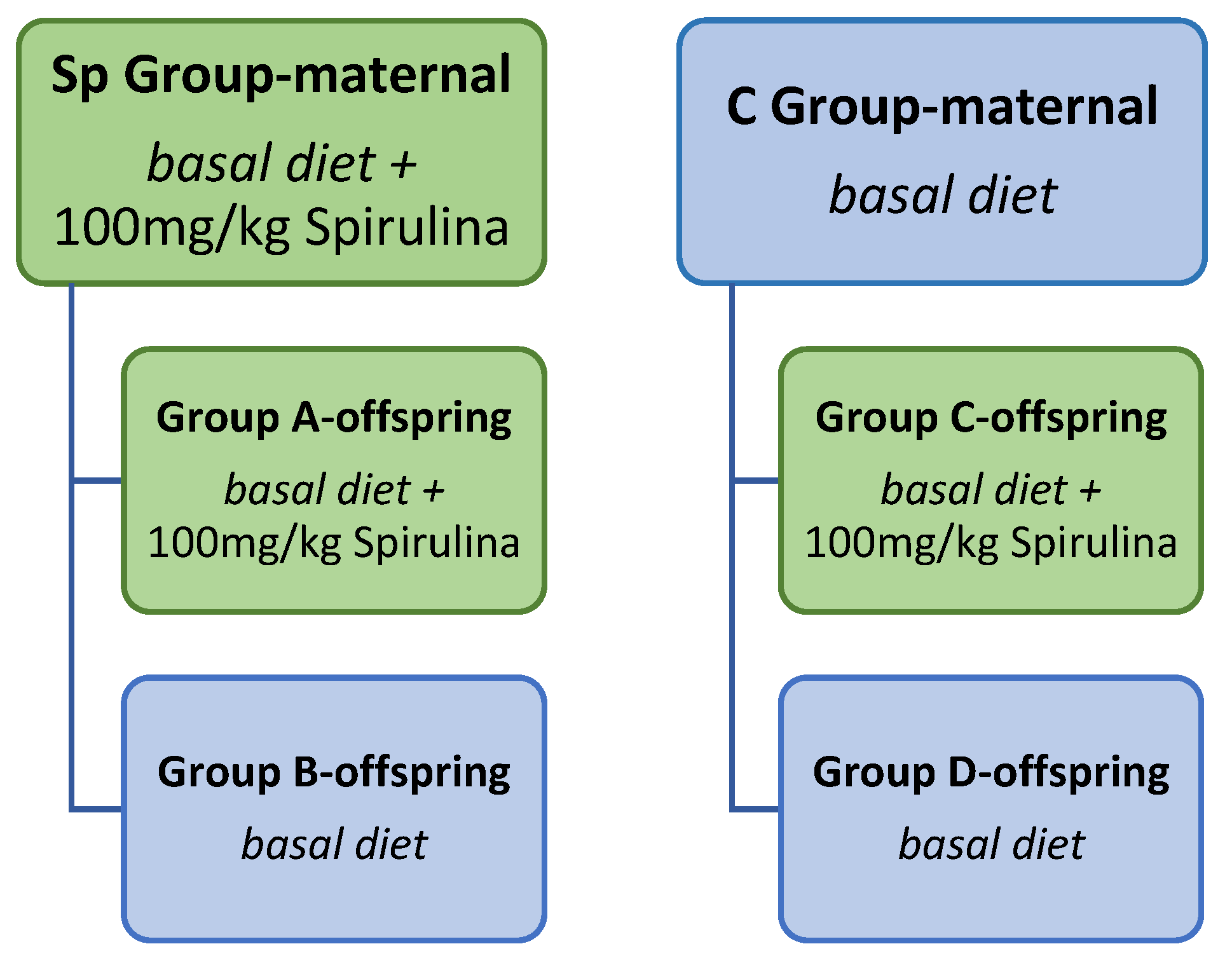
2.2. Animal Design, Diet and Sample Collection
2.3. Body Weight
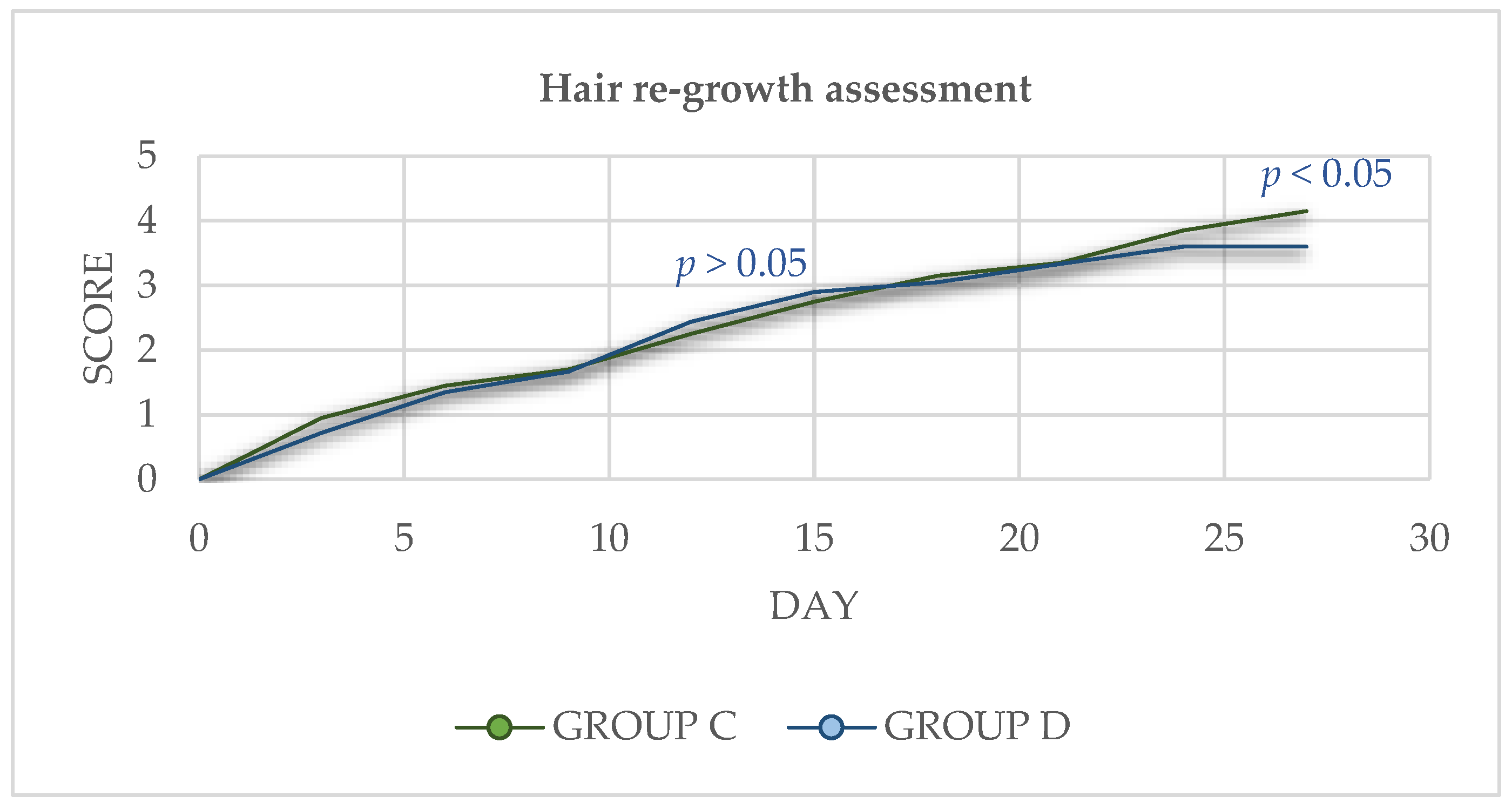
2.4. Hair Re-Growth Assessment
2.5. Grading
2.6. Euthanasia and Necropsy
2.7. Fur Quality Scoring
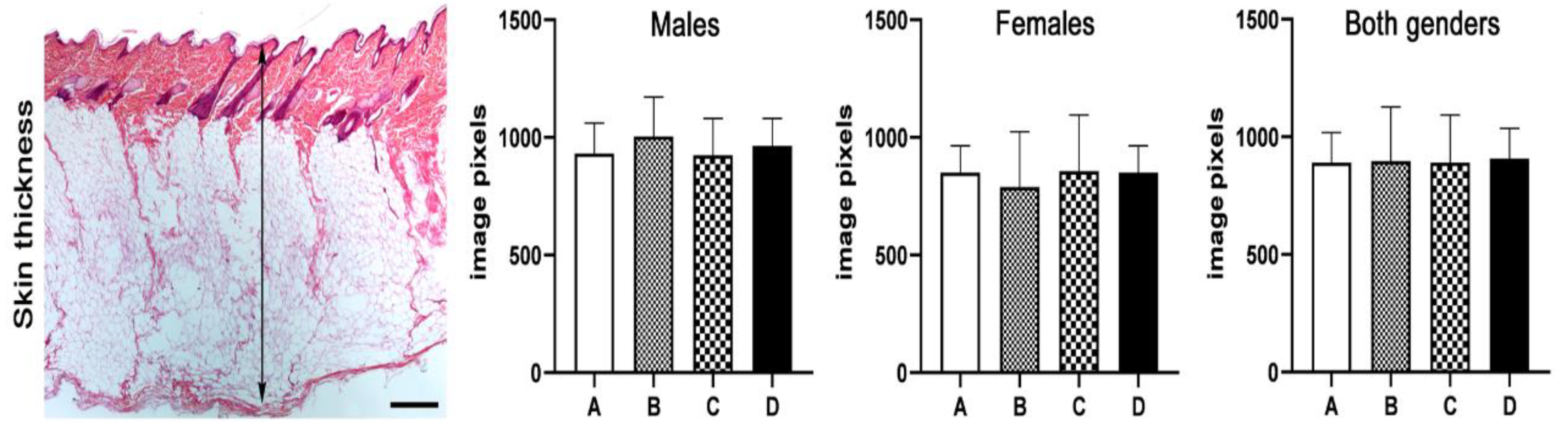
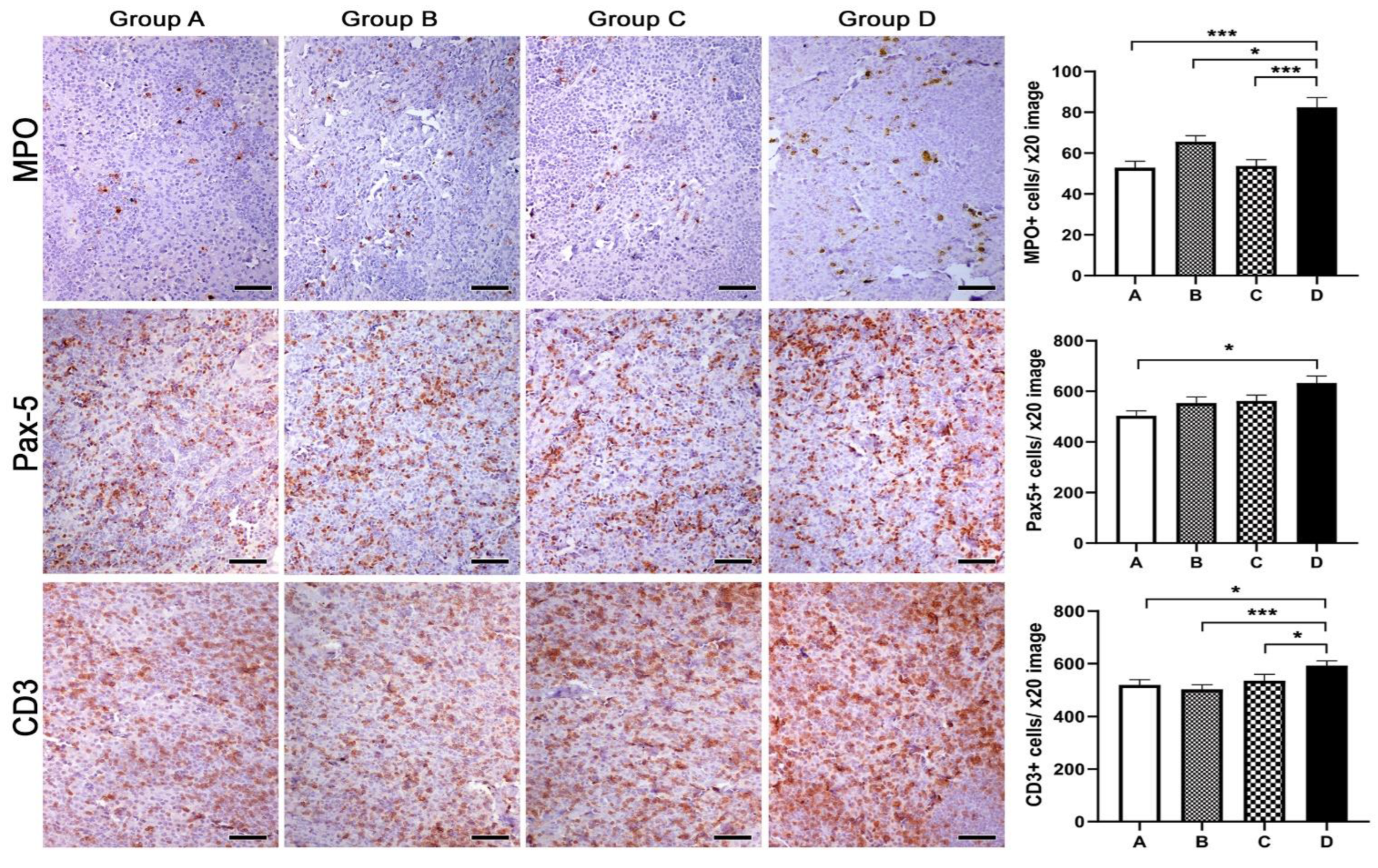
2.8. Histopathology, Immunohistochemistry and Morphometry
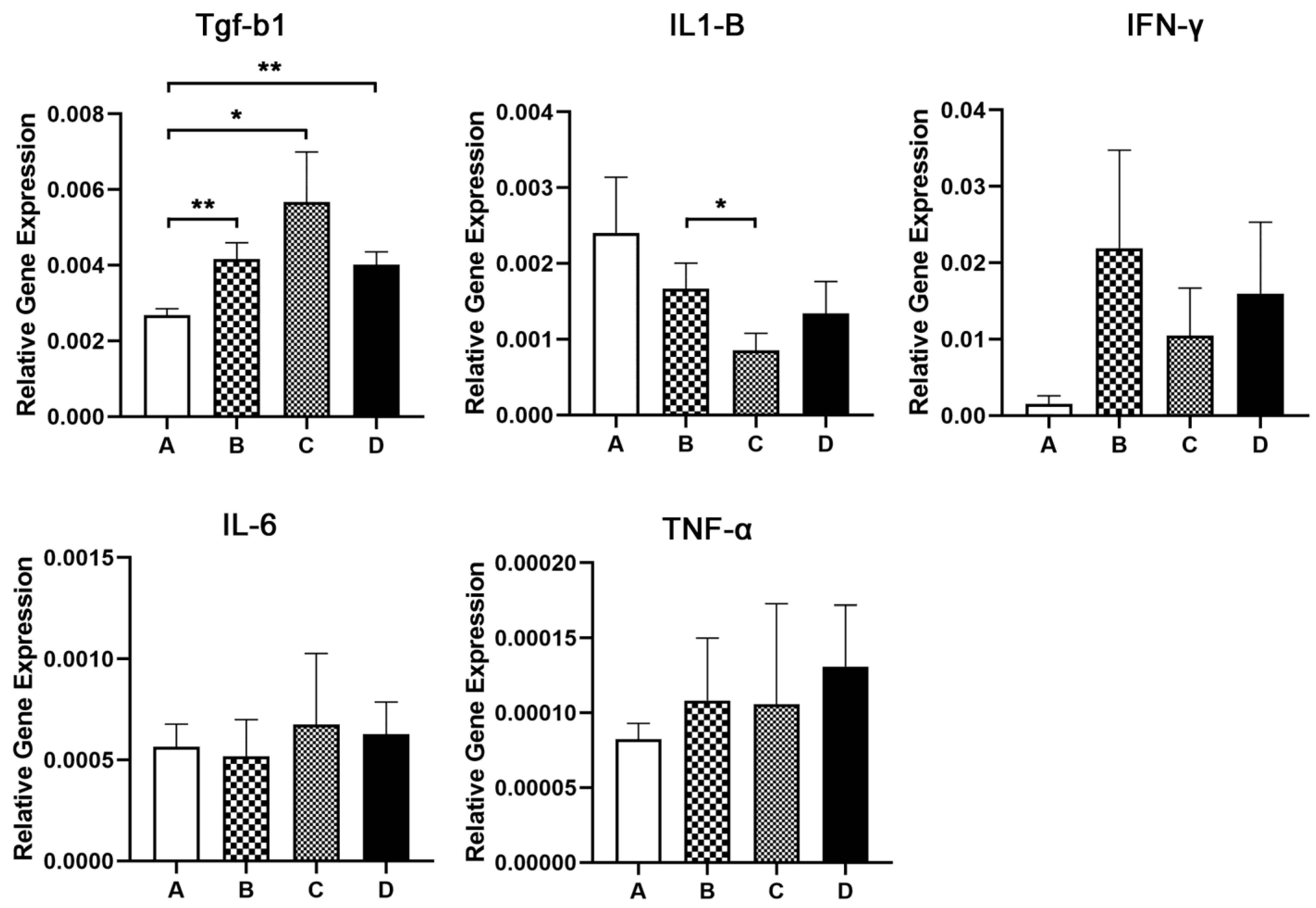
2.9. Quantitative Gene Expression Analysis
2.10. Stool Bacterial Flora Microbiome Analysis
2.10.1. Library Construction and Sequencing
2.10.2. Bioinformatics
2.11. Statistical Analyses
3. Results
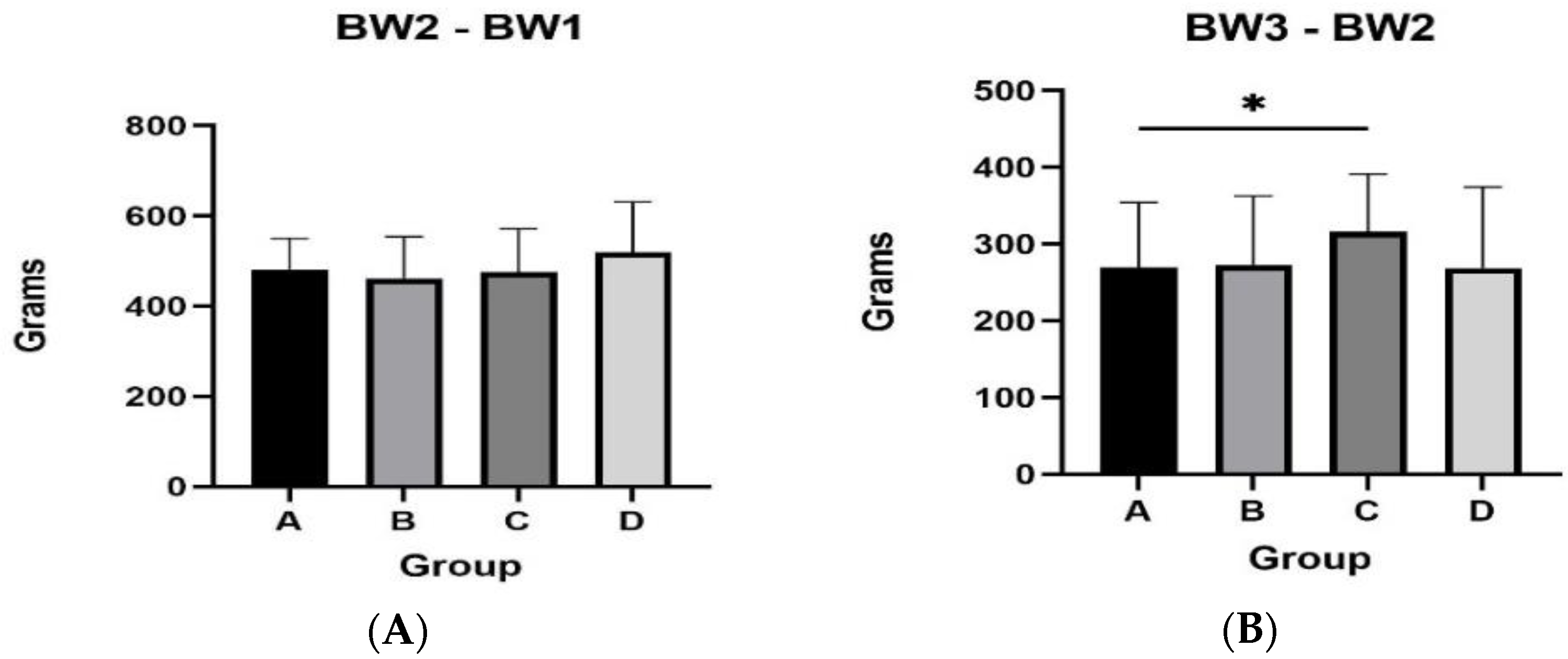
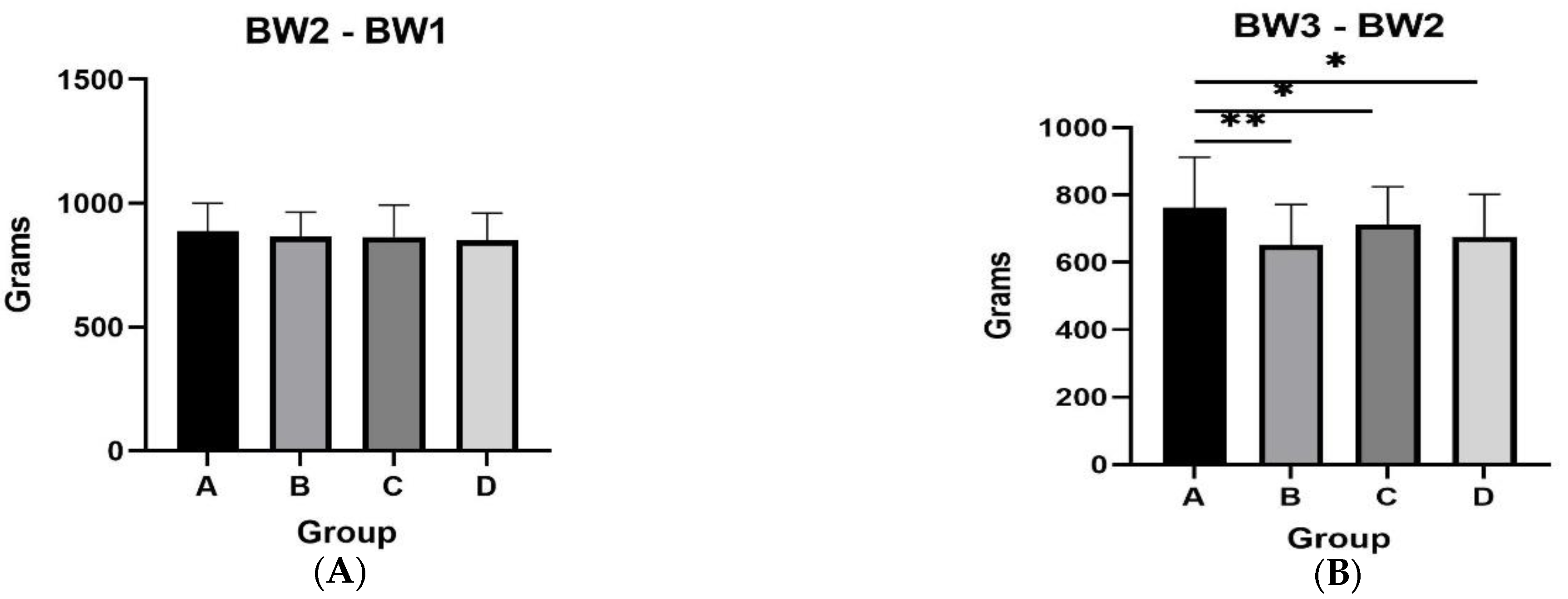
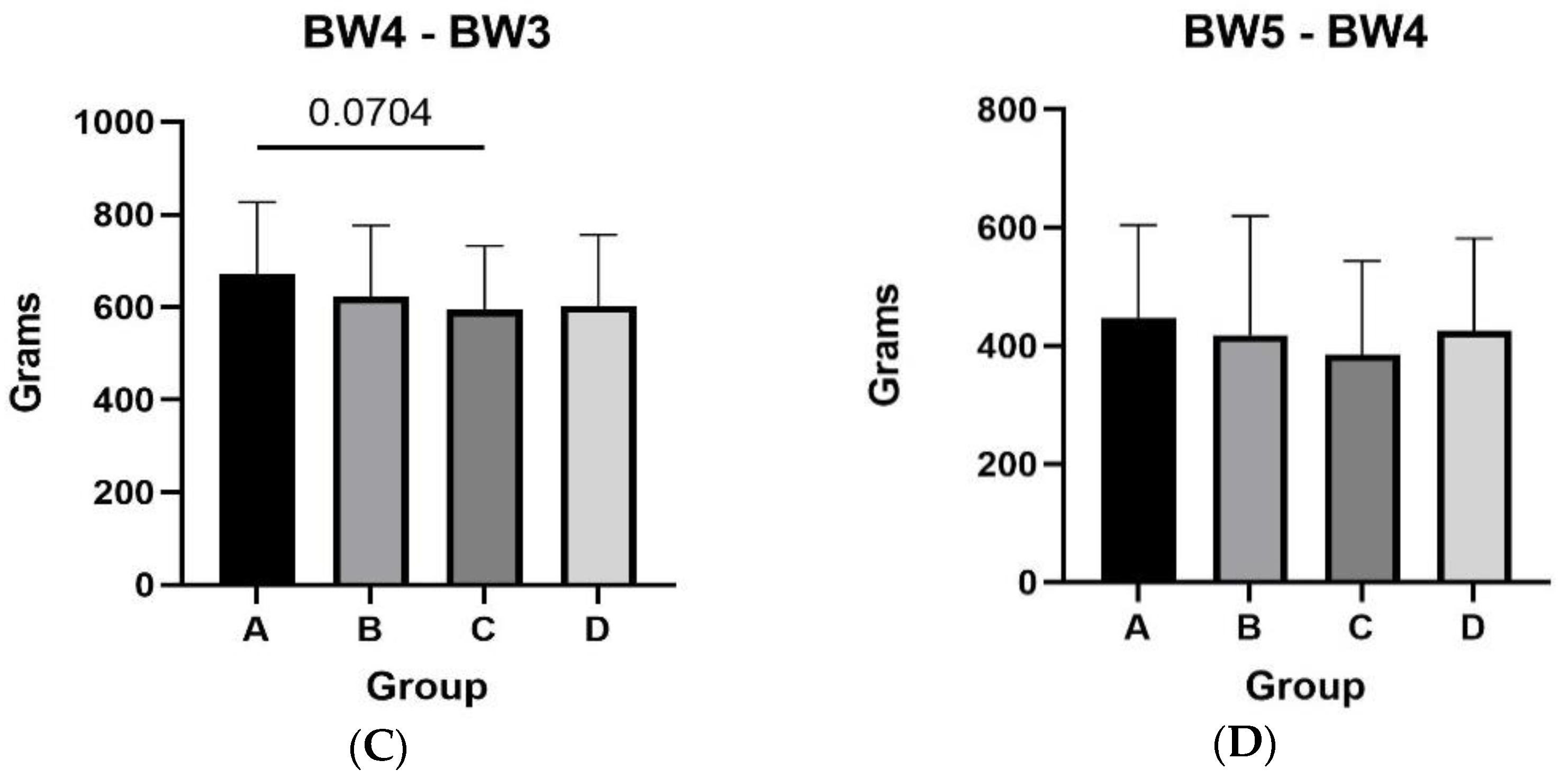
3.1. Body Weight
3.2. Skins
3.3. Hair Re-Growth Assessment
3.4. Histopathology, Immunohistochemistry and Morphometry
3.5. Gut Bacterial Microbiome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andresen, L.; Hammer, A.S.; Clausen, T.; Lassén, M.; Matthiesen, C.F.; Tauson, A.-H.; Bahl, M.I. Identification of the bacterial composition of the gut microtioba in danish farmed minks. NJF Semin. 2015, 485, 61–66. [Google Scholar]
- Rasmussen, H.E.; Martínez, I.; Lee, J.Y.; Walter, J. Alteration of the gastrointestinal microbiota of mice by edible blue-green algae. J. Appl. Microbiol. 2009, 107, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Sun, W.; Wang, Z.; Zhang, T.; Fan, Y.; Gu, H.; Li, G. Mink (Mustela vison) Gut Microbial Communities from Northeast China and Its Internal Relationship with Gender and Food Additives. Curr. Microbiol. 2017, 74, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, Y.; Pakpour, S.; Wang, S.; Pan, Z.; Liu, J.; Wei, Q.; She, J.; Cang, H.; Zhang, R.X. Dose Effects of Orally Administered Spirulina Suspension on Colonic Microbiota in Healthy Mice. Front. Cell. Infect. Microbiol. 2019, 9, 243. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Arck, P.; Handjiski, B.; Hagen, E.; Pincus, M.; Bruenahl, C.; Bienenstock, J.; Paus, R. Is there a “gut-brain-skin axis”? Exp. Dermatol. 2010, 19, 401–405. [Google Scholar] [CrossRef]
- Shanahan, F. Brain-gut axis and mucosal immunity: A perspective on mucosal psychoneuroimmunology. Semin. Gastrointest. Dis. 1999, 10, 8–13. [Google Scholar]
- Romijn, J.A.; Corssmit, E.P.; Havekes, L.M.; Pijl, H. Gut-brain axis. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 518–521. [Google Scholar] [CrossRef]
- Maurice, C.F.; Haiser, H.J.; Turnbaugh, P.J. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell 2013, 152, 39–50. [Google Scholar] [CrossRef]
- Cani, P.D.; Everard, A. Talking microbes: When gut bacteria interact with diet and host organs. Mol. Nutr. Food Res. 2016, 60, 58–66. [Google Scholar] [CrossRef]
- Marsland, B.J. Regulating inflammation with microbial metabolites. Nat. Med. 2016, 22, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Erdman, S.E.; Poutahidis, T. Probiotic “glow of health”: It’s more than skin deep. Benef. Microbes 2014, 5, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The gut microbiome as a major regulator of the gut-skin axis. Front. Microbiol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Levkovich, T.; Poutahidis, T.; Smillie, C.; Varian, B.J.; Ibrahim, Y.M.; Lakritz, J.R.; Alm, E.J.; Erdman, S.E. Probiotic Bacteria Induce a “Glow of Health”. PLoS ONE 2013, 8, e53867. [Google Scholar] [CrossRef] [PubMed]
- Bahl, M.I.; Hammer, A.S.; Clausen, T.; Jakobsen, A.; Skov, S.; Andresen, L. The gastrointestinal tract of farmed mink (Neovison vison) maintains a diverse mucosa-associated microbiota following a 3-day fasting period. Microbiologyopen 2017, 6, e00434. [Google Scholar] [CrossRef]
- Williams, C.; Elnif, J.; Buddington, R.K. The Gastrointestinal Bacteria of Mink (Mustela vison L): Influence of Age and Diet. Acta Vet. Scand. 1998, 39, 473–482. [Google Scholar] [CrossRef]
- Compo, N.R.; Gomez, D.E.; Tapscott, B.; Weese, J.S.; Turner, P.V. Fecal bacterial microbiota of Canadian commercial mink (Neovison vison): Yearly, life stage, and seasonal comparisons. PLoS ONE 2018, 13, e0208136. [Google Scholar] [CrossRef]
- Tjernsbekk, M.T.; Tauson, A.H.; Ahlstrøm, Ø. Ileal, colonic and total tract nutrient digestibility in dogs (Canis familiaris) compared with total tract digestibility in mink (Neovison vison). Arch. Anim. Nutr. 2014, 68, 245–261. [Google Scholar] [CrossRef]
- Vulfson, L.; Pedersen, K.; Chriel, M.; Andersen, T.H.; Dietz, H.H. Assessment of the aerobic faecal microflora in mink (Mustela vison Schreiber) with emphasis on Escherichia coli and Staphylococcus intermedius. Vet. Microbiol. 2003, 93, 235–245. [Google Scholar] [CrossRef]
- Gugołek, A.; Juśkiewicz, J.; Strychalski, J.; Konstantynowicz, M.; Zwoliński, C. Nutrient digestibility and colonic fermentation processes in species of the families Mustelidae and Canidae fed the same diet. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2015, 323, 637–644. [Google Scholar] [CrossRef]
- Birch, J.M.; Ullman, K.; Struve, T.; Agger, J.F.; Hammer, A.S.; Leijon, M.; Jensen, H.E. Investigation of the viral and bacterial microbiota in intestinal samples from mink (Neovison vison) with pre-weaning diarrhea syndrome using next generation sequencing. PLoS ONE 2018, 13, e0205890. [Google Scholar] [CrossRef] [PubMed]
- Gargouri, M.; Soussi, A.; Akrouti, A.; Magné, C.; El Feki, A. Potential protective effects of the edible alga arthrospira platensis against lead-induced oxidative stress, anemia, kidney injury, and histopathological changes in adult rats. Appl. Physiol. Nutr. Metab. 2019, 44, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Chen, S.; Wu, Y.; Ma, Y.; Qiao, H.; Fan, J.; Wu, H. Dietary supplementation with microalgae enhances the zebrafish growth performance by modulating immune status and gut microbiota. Appl. Microbiol. Biotechnol. 2022, 106, 773–788. [Google Scholar] [CrossRef]
- Afkhami-Ardakani, M.; Hasanzadeh, S.; Shahrooz, R.; Delirezh, N.; Malekinejad, H. Antioxidant effects of Spirulina platensis (Arthrospira platensis) on cyclophosphamide-induced testicular injury in rats. Vet. Res. Forum 2018, 9, 35–41. [Google Scholar] [PubMed]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.T.; Jin, P.; Zhao, H.; Liu, X.; Sheng, Q. Effects of oral administration of Spirulina platensis and probiotics on serum immunity indexes, colonic immune factors, fecal odor, and fecal flora in mice. Anim. Sci. J. 2021, 92, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Satyaraj, E.; Reynolds, A.; Engler, R.; Labuda, J.; Sun, P. Supplementation of Diets With Spirulina Influences Immune and Gut Function in Dogs. Front. Nutr. 2021, 8, 667072. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Su, L.; Zhang, L.; Zeng, J.; Chen, Q.; Deng, R.; Wang, Z.; Kuang, W.; Jin, X.; Gui, S.; et al. Spirulina platensis aqueous extracts ameliorate colonic mucosal damage and modulate gut microbiota disorder in mice with ulcerative colitis by inhibiting inflammation and oxidative stress. J. Zhejiang Univ. Sci. B 2022, 23, 481–501. [Google Scholar] [CrossRef]
- Liu, P.; Choi, J.W.; Lee, M.K.; Choi, Y.H.; Nam, T.J. Spirulina protein promotes skin wound repair in a mouse model of full-thickness dermal excisional wound. Int. J. Mol. Med. 2020, 46, 351–359. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Author Correction: Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2 (Nature Biotechnology, (2019), 37, 8, (852-857), 10.1038/s41587-019-0209-9). Nat. Biotechnol. 2019, 37, 1091. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Phyloseq: A bioconductor package for handling and analysis of high-throughput phylogenetic sequence data. Pac. Symp. Biocomput. 2012, 2012, 235–246. [Google Scholar] [CrossRef]
- R Development Core Team R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Volume 35, ISBN 9780387981406. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Ibrahim, J.G.; Love, M.I. Heavy-Tailed prior distributions for sequence count data: Removing the noise and preserving large differences. Bioinformatics 2019, 35, 2084–2092. [Google Scholar] [CrossRef]
- Bonos, E.; Kasapidou, E.; Kargopoulos, A.; Karampampas, A.; Christaki, E.; Florou-Paneri, P.; Nikolakakis, I. Spirulina as a functional ingredient in broiler chicken diets. S. Afr. J. Anim. Sci. 2016, 46, 94. [Google Scholar] [CrossRef]
- Mirzaie, S.; Zirak-Khattab, F.; Hosseini, S.A.; Donyaei-Darian, H. Effects of dietary Spirulina on antioxidant status, lipid profile, immune response and performance characteristics of broiler chickens reared under high ambient temperature. Asian Australas. J. Anim. Sci. 2018, 31, 556–563. [Google Scholar] [CrossRef]
- Iatrou, A.M.; Papadopoulos, G.A.; Giannenas, I.; Lymberopoulos, A.; Fortomaris, P. Effects of Dietary Inclusion of Spirulina platensis on the Reproductive Performance of Female Mink. Vet. Sci. 2022, 9, 428. [Google Scholar] [CrossRef]
- Toyomizu, M.; Sato, K.; Taroda, H.; Kato, T.; Akiba, Y. Effects of dietary Spirulina on meat colour in muscle of broiler chickens. Br. Poult. Sci. 2001, 42, 197–202. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Meineri, G. Effects of diets with increasing levels of Spirulina platensis on the performance and apparent digestibility in growing rabbits. Livest. Sci. 2008, 118, 173–177. [Google Scholar] [CrossRef]
- Kaoud, H.A. Effect of spirulina platensis as a dietary supplement on broiler performance in comparison with prebiotics. Sci. J. Appl. Res. 2012, 2, 46–51. [Google Scholar]
- Hassanein, H.A.M.; Arafa, M.M.; Warda, M.A.A. Effect of Using Spirulina Platensis and Chlorella Vulgaris As Feed Additives on Growing Rabbit Performance. Egypt. J. Rabbit Sci. 2014, 24, 413–431. [Google Scholar] [CrossRef]
- Furbeyre, H.; Van Milgen, J.; Mener, T.; Gloaguen, M.; Labussiere, E. Effects of oral supplementation with Spirulina and Chlorella on growth and digestive health in piglets around weaning. Animal 2018, 12, 2264–2273. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, S.M.; Khosravi-Darani, K.; Mozafari, M.R. Nutritional and Medical Applications of Spirulina Microalgae. Mini Rev. Med. Chem. 2013, 13, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.T.; Takemoto, J.Y.; Chang, P.E.; AlFindee, M.N.; Lin, Y.Y. Effects of Mesobiliverdin IXα-Enriched Microalgae Feed on Gut Health and Microbiota of Broilers. Front. Vet. Sci. 2021, 7, 586813. [Google Scholar] [CrossRef]
- Bhowmik, D.; Dubey, J.; Mehra, S. Probiotic Efficiency of Spirulina platensis—Stimulating Growth of Lactic Acid Bacteria. World J. Dairy Food Sci. 2009, 4, 160–163. [Google Scholar]
- Chen, H.; Zeng, F.; Li, S.; Liu, Y.; Gong, S.; Lv, X.; Zhang, J.; Liu, B. Spirulina active substance mediated gut microbes improve lipid metabolism in high-fat diet fed rats. J. Funct. Foods 2019, 59, 215–222. [Google Scholar] [CrossRef]
- Yu, T.; Wang, Y.; Chen, X.; Xiong, W.; Tang, Y.; Lin, L. Spirulina platensis alleviates chronic inflammation with modulation of gut microbiota and intestinal permeability in rats fed a high-fat diet. J. Cell. Mol. Med. 2020, 24, 8603–8613. [Google Scholar] [CrossRef]
- Bahl, M.I.; Honoré, A.L.; Skønager, S.T.; Honoré, O.L.; Clausen, T.; Andresen, L.; Hammer, A.S. The microbiota of farmed mink (Neovison vison) follows a successional development and is affected by early life antibiotic exposure. Sci. Rep. 2020, 10, 20434. [Google Scholar] [CrossRef]
- Biström, M.; Moisander-Jylhä, A.M.; Heinikainen, S.; Pelkola, K.; Raunio-Saarnisto, M. Isolation of Clostridium limosum from an outbreak of metritis in farmed mink. Acta Vet. Scand. 2016, 58, 49. [Google Scholar] [CrossRef] [PubMed]
- Hammer, A.S.; Andresen, L.; Aalbæk, B.; Damborg, P.; Weiss, V.; Christiansen, M.L.; Selsing, S.; Bahl, M.I. Abortion and mortality in farm mink (Neovison vison) associated with feed-born Clostridium limosum. Vet. Microbiol. 2017, 203, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Priscila, V.; De Medeiros, B.; Leite, E.; Souza, D.; Mariano, T.; De Albuquerque, R.; Francisca, C.; Lima, S.; Sivieri, K.; Colombo, T.; et al. Freshwater microalgae biomasses exert a prebiotic effect on human colonic microbiota. Algal Res. 2021, 60, 102547. [Google Scholar] [CrossRef]
- Hua, P.; Yu, Z.; Xiong, Y.; Liu, B.; Zhao, L. Regulatory efficacy of spirulina platensis protease hydrolyzate on lipid metabolism and gut microbiota in high-fat diet-fed rats. Int. J. Mol. Sci. 2018, 19, 4023. [Google Scholar] [CrossRef]
- Li, T.T.; Tong, A.J.; Liu, Y.Y.; Huang, Z.R.; Wan, X.Z.; Pan, Y.Y.; Jia, R.B.; Liu, B.; Chen, X.H.; Zhao, C. Polyunsaturated fatty acids from microalgae Spirulina platensis modulates lipid metabolism disorders and gut microbiota in high-fat diet rats. Food Chem. Toxicol. 2019, 131, 110558. [Google Scholar] [CrossRef]
- Li, T.T.; Liu, Y.Y.; Wan, X.Z.; Huang, Z.R.; Liu, B.; Zhao, C. Regulatory efficacy of the polyunsaturated fatty acids from microalgae Spirulina platensis on lipid metabolism and gut microbiota in high-fat diet rats. Int. J. Mol. Sci. 2018, 19, 3075. [Google Scholar] [CrossRef]
- Gerritsen, J.; Hornung, B.; Ritari, J.; Paulin, L.; Rijkers, G.T.; Schaap, P.J.; de Vos, W.M.; Smidt, H. A comparative and functional genomics analysis of the genus Romboutsia provides insight into adaptation to an intestinal lifestyle. bioRxiv 2019, rxiv:845511. [Google Scholar] [CrossRef]
- Gill, A.; Austin, J.W. The Ecology of Bacterial Agents of Foodborne Illness; Springer: Cham, Switzerland, 2018; ISBN 9783319923734. [Google Scholar]
- Bai, X.; Liu, S.; Zhao, J.; Cheng, Y.; Zhang, H.; Hu, B.; Zhang, L.; Shi, Q.; Zhang, Z.; Wu, T.; et al. Epidemiology and molecular characterization of the antimicrobial resistance of pseudomonas aeruginosa in Chinese mink infected by hemorrhagic pneumonia. Can. J. Vet. Res. 2019, 83, 122–132. [Google Scholar]
- Krogdahl, Å.; Jaramillo-Torres, A.; Ahlstrøm, Ø.; Chikwati, E.; Aasen, I.M.; Kortner, T.M. Protein value and health aspects of the seaweeds Saccharina latissima and Palmaria palmata evaluated with mink as model for monogastric animals. Anim. Feed Sci. Technol. 2021, 276, 114902. [Google Scholar] [CrossRef]
- La Fata, G.; Weber, P.; Mohajeri, M.H. Probiotics and the Gut Immune System: Indirect Regulation. Probiotics Antimicrob. Proteins 2018, 10, 11–21. [Google Scholar] [CrossRef]
- Ruff, W.E.; Greiling, T.M.; Kriegel, M.A. Host–microbiota interactions in immune-mediated diseases. Nat. Rev. Microbiol. 2020, 18, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Ruff, W.E.; Kriegel, M.A. Autoimmune host-microbiota interactions at barrier sites and beyond. Trends Mol. Med. 2015, 21, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Dehner, C.; Fine, R.; Kriegel, M.A. The microbiome in systemic autoimmune disease: Mechanistic insights from recent studies. Curr. Opin. Rheumatol. 2019, 31, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Bhadouria, P.; Bisen, P. Nutritional and Therapeutic Potential of Spirulina. Curr. Pharm. Biotechnol. 2005, 6, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Dartsch, P.C. Antioxidant Potential of Selected Spirulina platensis Preparations. Phytother. Res. 2008, 22, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Seyidoglu, N.; Köseli, E.; Gurbanli, R.; Aydin, C. The preventive role of Spirulina platensis (Arthrospira platensis) in immune and oxidative insults in a stress-induced rat model. J. Vet. Res. 2021, 65, 193–200. [Google Scholar] [CrossRef]
- Mahapatro, M.; Erkert, L.; Becker, C. Cytokine-mediated crosstalk between immune cells and epithelial cells in the gut. Cells 2021, 10, 111. [Google Scholar] [CrossRef]
- Nasirian, F.; Dadkhah, M.; Moradi-Kor, N.; Obeidavi, Z. Effects of Spirulina platensis microalgae on antioxidant and anti-inflammatory factors in diabetic rats. Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 375–380. [Google Scholar] [CrossRef]
- Ashraf, R.; Shah, N.P. Immune System Stimulation by Probiotic Microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 938–956. [Google Scholar] [CrossRef]
- Wang, K.; Hu, C.; Tang, W.; Azad, M.A.K.; Zhu, Q.; He, Q.; Kong, X. The Enhancement of Intestinal Immunity in Offspring Piglets by Maternal Probiotic or Synbiotic Supplementation Is Associated With the Alteration of Gut Microbiota. Front. Nutr. 2021, 8, 1–13. [Google Scholar] [CrossRef]
- Boudreau, L.; Benkel, B.; Astatkie, T.; Rouvinen-Watt, K. Ideal body condition improves reproductive performance and influences genetic health in female mink. Anim. Reprod. Sci. 2014, 145, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Thirstrup, J.P.; Jensen, J.; Lund, M.S. Genetic parameters for fur quality graded on live animals and dried pelts of American mink (Neovison vison). J. Anim. Breed. Genet. 2017, 134, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.V.; Børsting, C.F. Effects of variations in dietary protein levels on hair growth and pelt quality in mink (Mustela vison). Can. J. Anim. Sci. 2000, 80, 633–642. [Google Scholar] [CrossRef][Green Version]
- Felska-Błaszczyk, L.; Ławrów, N.; Lasota, B.; Żuk, K.; Pęzińska-Kijak, K.; Muszczyński, Z. Badania nad zastosowaniem probiotyku w żywieniu fermowej norki amerykańskiej (Neovison vison). J. Cent. Eur. Agric. 2016, 17, 652–660. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Poutahidis, T.; Kearney, S.M.; Levkovich, T.; Qi, P.; Varian, B.J.; Lakritz, J.R.; Ibrahim, Y.M.; Chatzigiagkos, A.; Alm, E.J.; Erdman, S.E. Microbial symbionts accelerate wound healing via the neuropeptide hormone oxytocin. PLoS ONE 2013, 8, e78898. [Google Scholar] [CrossRef]
- Liu, P.; Choi, J.W.; Lee, M.K.; Choi, Y.H.; Nam, T.J. Wound healing potential of spirulina protein on CCD-986sk cells. Mar. Drugs 2019, 17, 130. [Google Scholar] [CrossRef]
- Syarina, P.N.A.; Karthivashan, G.; Abas, F.; Arulselvan, P.; Fakurazi, S. Wound healing potential of spirulina platensis extracts on human dermal fibroblast cells. EXCLI J. 2015, 14, 385–393. [Google Scholar] [CrossRef]
- An, E.; Park, H.; Lee, A.R.C. Inhibition of fibrotic contraction by C-phycocyanin through modulation of connective tissue growth factor and α-smooth muscle actin expression. Tissue Eng. Regen. Med. 2016, 13, 388–395. [Google Scholar] [CrossRef]





| Primer | Sequence (5′-3′) | Positions 1 | Amplicon Size (bp) | Concentration (nM) | Annealing Temperature (°C) |
|---|---|---|---|---|---|
| Tgf-b1-F | GCTAGACCGCTTGCTTCAGG | 262–281 | 111 | 100 | 61 |
| Tgf-b1-R | TGGTCCTCCTTCTGTTCCCC | 353–372 | |||
| Il-1b-F | CCTGAACCCACCAGTGAAGT | 110–129 | 144 | 250 | 62 |
| Il-1b-R | TTGAAGCTGGATGCCCTCCT | 234–253 | |||
| Ifng-F | AGATGGTGGGCCTCTTTTCT | 138–157 | 155 | 200 | 61 |
| Ifng-R | CCTTGATGGTATCCATGCTCCT | 271–292 | |||
| Il6-F | ACCTAGAGGCCAACTATGAGGG | 383–404 | 128 | 100 | 61 |
| Il6-R | ATCTGTGGTAGGGTTGGGGG | 491–510 | |||
| Tnf-a-F | CCAGATGGCCTCCAACTAATCA | 11–32 | 110 | 400 | 61 |
| Tnf-a-R | CCCTCAGCTTCAGGGTTTGC | 101–120 | |||
| Gapdh-F | GGGTGTGAACCACGAGAAGT | 6–25 | 122 | 250 | 61 |
| Gapdh-R | GTCCCTCCACAATGCCGAAG | 108–127 |
| Group | |||||
|---|---|---|---|---|---|
| Grams | A (±SD 1) | Β (±SD 1) | C (±SD 1) | D (±SD 1) | p-Value |
| BW1 | 949.28 (112.39) | 898.80 (85.45) | 931.84 (84.99) | 882.44 (100.02) | 0.065 |
| BW2 | 1429.72 (149.40) | 1361.08 (139.95) | 1408.08 (152.69) | 1402.48 (150.65) | 0.077 |
| BW3 | 4699.36 (200.22) | 1634.00 (188.66) | 1724.24 (177.04) | 1671.48 (231.16) | 0.345 |
| BW4 | 1913.36 (246.11) | 1833.76 (210.72) | 1949.04 (190.19) | 1883.76 (284.79) | 0.383 |
| BW5 | 2008.00 (297.04) | 1934.16 (248.89) | 2044.32 (230.24) | 2027.92 (307.83) | 0.151 |
| Group | |||||
|---|---|---|---|---|---|
| A (±SD 1) | Β (±SD 1) | C (±SD 1) | D (±SD 1) | p-Value | |
| BW1 | 1175.68 (168.14) | 1176.32 (297.04) | 1224.16 (156.69) | 1116.36 (121.85) | 0.077 |
| BW2 | 2064.16 (183.34) | 2042.6 (123.53) | 2089.08 (239.71) | 1966.88 (187.06) | 0.749 |
| BW3 | 2826.76 (250.37) | 2694.24 (168.36) | 2802.32 (304.56) | 2643.08 (282.42) | 0.127 |
| BW4 | 3498.32 (357.05) | 3316.68 (333.23) | 3397.04 (376.61) | 3246.36 (392.64) | 0.133 |
| BW5 | 3945.84 (458.92) | 3735.28 (432.22) | 3782.44 (499.67) | 3671.84 (478.51) | 0.197 |
| Group | |||||
|---|---|---|---|---|---|
| Trait | A (±SD 1) | B (±SD 1) | C (±SD 1) | D (±SD 1) | p-Value |
| Skin size | 2.00 ± 0.89 | 1.67 ± 1.21 | 1.83 ± 1.17 | 1.50 ± 0.84 | 0.618 |
| Nap size | 1.83 ± 0.41 | 1.83 ± 0.41 | 1.50 ± 0.55 | 1.50 ± 0.55 | 0. 392 |
| Quality | 1.83 ± 0.75 | 1.66 ± 0.82 | 2.00 ± 0.63 | 1.83 ± 0.75 | 0.948 |
| Grading | 4.33 ± 0.52 | 4.17 ± 0.75 | 3.83 ± 0.75 | 4.33 ± 0.52 | 0.625 |
| Price | 25.83 ± 5.27 | 32.17 ± 5.23 | 28.83 ± 5.19 | 28.00 ± 6.20 | 0.279 |
| Group | |||||
|---|---|---|---|---|---|
| Trait | A (±SD 1) | B (±SD 1) | C (±SD 1) | D (±SD 1) | p-Value |
| Skin size | 2.83 ± 0.75 | 2.83 ± 0.41 | 2.5 ± 0.55 | 2.5 ± 0.83 | 0.504 |
| Nap size | 1.50 ± 0.55 | 1.33 ± 0.52 | 1.83 ± 0.41 | 1.5 ± 0.55 | 0.363 |
| Quality | 1.66 ± 0.52 | 2.83 ± 0.98 | 2.00 ± 0.89 | 2.17 ± 1.17 | 0.469 |
| Grading | 4.33 ± 0.82 | 4.33 ± 0.52 | 4.00 ± 0.89 | 4.5 ± 0.55 | 0.535 |
| Price | 50.17 ± 10.55 | 51.50 ± 3.08 | 51.83 ± 2.04 | 50.5 ± 6.63 | 0.965 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iatrou, A.M.; Michailidou, S.; Papadopoulos, G.A.; Afaloniati, H.; Lagou, M.K.; Kiritsi, M.; Argiriou, A.; Angelopoulou, K.; Poutahidis, T.; Fortomaris, P. Effects of Dietary Supplementation of Spirulina platensis on the Immune System, Intestinal Bacterial Microbiome and Skin Traits of Mink. Animals 2023, 13, 190. https://doi.org/10.3390/ani13020190
Iatrou AM, Michailidou S, Papadopoulos GA, Afaloniati H, Lagou MK, Kiritsi M, Argiriou A, Angelopoulou K, Poutahidis T, Fortomaris P. Effects of Dietary Supplementation of Spirulina platensis on the Immune System, Intestinal Bacterial Microbiome and Skin Traits of Mink. Animals. 2023; 13(2):190. https://doi.org/10.3390/ani13020190
Chicago/Turabian StyleIatrou, Anna Maria, Sofia Michailidou, Georgios A. Papadopoulos, Hara Afaloniati, Maria K. Lagou, Maria Kiritsi, Anagnostis Argiriou, Katerina Angelopoulou, Theofilos Poutahidis, and Paschalis Fortomaris. 2023. "Effects of Dietary Supplementation of Spirulina platensis on the Immune System, Intestinal Bacterial Microbiome and Skin Traits of Mink" Animals 13, no. 2: 190. https://doi.org/10.3390/ani13020190
APA StyleIatrou, A. M., Michailidou, S., Papadopoulos, G. A., Afaloniati, H., Lagou, M. K., Kiritsi, M., Argiriou, A., Angelopoulou, K., Poutahidis, T., & Fortomaris, P. (2023). Effects of Dietary Supplementation of Spirulina platensis on the Immune System, Intestinal Bacterial Microbiome and Skin Traits of Mink. Animals, 13(2), 190. https://doi.org/10.3390/ani13020190








