Simple Summary
In this study, we utilized the methods of spatiotemporal analysis to reveal and visualize the areas in the Russian Federation where an increased density of wild boar population might be related to the concentration of African swine fever (ASF) cases in wild boar. We demonstrated the areas (at the municipality level), where the elevated wild boar population density has continued to rise in recent years, that may be treated as high-risk areas, subject to the application of enhanced surveillance and population control measures.
Abstract
African swine fever (ASF) is an infectious disease that affects both domestic pigs (DPs) and wild boar (WB). The WB population plays an important role in the spread of ASF as the WB acts as a natural reservoir of the virus and transmits it to other susceptible wild and domestic pigs. Our study was aimed at revealing the areas with a high concentration of the WB population, and their potential relationships with the grouping of ASF cases in WB during the course of the ASF spread in the Russian Federation (2007–2022). We collected the annual data on WB numbers by municipalities within the regions of the most intensive ASF spread. We then conducted spatiotemporal analysis to identify clustering areas of ASF cases and compare them with the territories with a high density of WB population. We found that some of the territories with elevated ASF incidence in WB demonstrated spatial and temporal coincidence with the areas with a high WB population density. We also visualized the zones (“emerging hot spots”) with a statistically significant rise in the WB population density in recent years, which may be treated as areas of paramount importance for the application of surveillance measures and WB population control.
1. Introduction
African swine fever (ASF) is a contagious viral disease in domestic and wild pigs [,,]. It causes high fever, loss of appetite, weakness, and ultimately, death in infected animals. The ASF virus can survive for long periods of time in both pork products and the environment, making it difficult to eradicate the disease [,,]. There is currently no vaccine or cure for African swine fever, and the main method of prevention is through strict biosecurity measures and monitoring of wild boar and livestock pig populations [].
ASF is an ongoing problem for many countries due to the transboundary spread of the disease and the enormous economic damage that arises from the costs associated with restrictions on the trade and movement of pigs and pork products, as well as the hunting ban and the implementation of veterinary and other quarantine measures in the outbreak areas [].
The circulation of infection within the population of wild boar is a part of the African swine fever epidemic, promoting the formation of natural foci of the disease and its further spread to unaffected areas [,]. The presence of wild boar in the ecosystem is of great importance in the transmission of the African swine fever virus both among wild boar individuals and to populations of domestic pigs, which is recognized by many studies [,,].
For the regions of the Russian Federation, the circulation of African swine fever in the wild boar population is typical. Recently, such a mechanism of an epidemic disease has been clearly traced in the Far East [,].
In addition to the density of the wild boar population, factors such as the stability of the ASF virus in wild boar carcasses, the social structure of the population, geography, and many others may also affect the transmission process of the virus [,,]. The wild boar is a social animal and can therefore group together even in low-density regions, resulting in geographical areas with a higher local concentration of animals and creating conditions for the emergence of new African swine fever epidemics [,].
The emergence of ASF outbreaks in wild boar from 2007 to 2022 has been mainly recorded sporadically as isolated occurrences, rarely as sweeping short-duration epidemics. Ecological factors that contribute to the preservation of the ASF virus in the environment make it difficult to eradicate the disease in the wild boar population [,,]. Monitoring the wild boar population is an important component of surveillance and control when planning preventive measures []. The monitoring activity is used to timely detect disease cases and to apply effective measures to prevent the spread of infection to unaffected areas [,]. The density of the wild boar population varies dynamically as a result of active depopulation measures as well as boars’ migration. Thus, accounting for these changes as well as their visualization provide important information for decision-making in the field of animal disease control [,,]. It is necessary to accurately estimate the population density of wild boars in specific areas in order to determine the volume of removal of this species []. Modern information technologies, particularly geo-information systems (GIS) available in various areas of science, allow for the automatic data processing, display, and analysis of information in a spatial-temporal format.
The analysis of spatial-temporal patterns of disease occurrence in relation to the susceptible population density allows for determining and evaluating areas (or foci) where the active transmission of pathogens occurs, which potentially may contribute to the assessment of introduction and spread risks [,,]. In the Russian Federation, the methods of planned monitoring, accounting, and assessment of wild boar populations traditionally depend on the geographic location of protected areas and hunting grounds and represent a government system of observations and evaluations []. Due to the current epidemic situation of African swine fever in Russia, comprehensive studies of the spatial-temporal structure and dynamics of the wild boar population density at local and regional scales are especially relevant today. The counting, analysis, and visualization of the actual size of susceptible animal populations are important for making informed management decisions aimed at regulating the population density and to determine strategies for disease prevention and elimination.
Therefore, our study aimed to investigate and analyze the spatial-temporal patterns of African swine fever (ASF) cases depending on the density of the susceptible wild boar population by municipalities of affected regions in Russia, and to reveal and visualize hot spots of increased wild boar population density through 2007–2022, which may indicate the areas for the paramount application of monitoring and risk-reduction efforts.
2. Materials and Methods
2.1. Study Area
The study area was determined by the regions of the Russian Federation where (1) ASF outbreaks in wild boar were recorded within the study period of 2007–2022, and (2) annual data on wild boar population were available at the municipality level (Figure 1). The entire model region consisted of 39 subjects (first-level administrative divisions). It was divided into two zones, namely European (35 subjects) and Far Eastern (4 subjects). The model subjects consisted of 2440 s-level divisions (municipalities or districts).
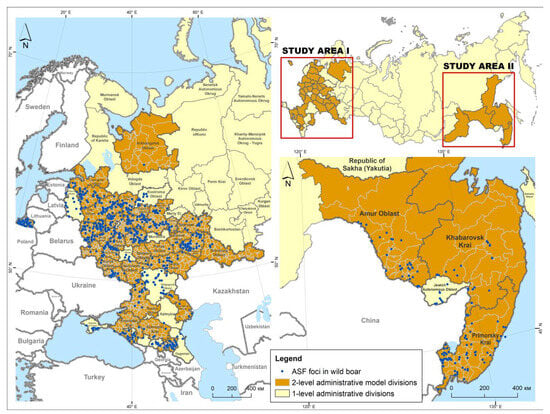
Figure 1.
The African swine fever foci in wild boar population in the model regions of the Russian Federation from 2007 to 2022.
2.2. Data Sources
Data on African Swine Fever outbreaks in the Russian Federation were obtained from the WOAH World Animal Health Information System (WAHIS) for the period from 2007 to 2022 []. In total, 608 foci of African swine fever in the population of wild boar in the European zone and 103 foci in the Far Eastern region were included in the analysis. In this study, we considered an “outbreak” as a notified occurrence of dead or infected wild boar(s) with laboratory-confirmed ASF, determined by geographic coordinates and the date of occurrence. A single dead or infected wild boar was referred to as a “case”.
Data on the wild boar population size in the studied municipalities of the model regions annually from 2007 to 2022 were obtained through requests from the regional administrations of the Ministries of Natural Resources and Ecology of the Russian Federation []. The data on the wild boar population size are estimated indicators that have been extrapolated to the entire territory of the district. One of the main methods used for monitoring the population of wild boars in the regions of the model territories is winter route counting, which is currently applied across Russia wherever there is stable snow cover. This method provides a general characteristic of the population in the area and is widely used in hunting farms throughout Russia.
2.3. Spatiotemporal Cluster Analysis of ASF Cases in the Wild Boar
The study included three stages:
- (a)
- Identifying spatial-temporal clusters of African swine fever incidence in wild boars in the model regions from 2007 to 2022, depending on animal density using a discrete Poisson model;
- (b)
- Identifying spatial-temporal clusters of increased wild boar population density in the model regions from 2007 to 2022 using a normal probability model;
- (c)
- Identifying “hot and cold spots”, i.e., geographic areas with increasing and decreasing trends in wild boar population density, using the Emerging Hot Spot Analysis (EHSA) method.
The search for spatial-temporal clusters was conducted using the Kulldorff spatial-temporal scan statistic [,]. The method scans the whole study area with a cylindrical scanning window of changing radius and height, where the height represents time. The number of cases (or associated attributes) within each candidate cluster are compared with the one expected under a null hypothesis. The maximum spatial and temporal search size for clusters were taken by default for 50% of the study area and time period, respectively. Windows with a significant excess of observed cases compared to expected ones are reported as spatiotemporal clusters. Municipalities of the 2nd order (districts) were used as spatial modeling units. The annual number of ASF cases in each district and the population density of wild boars were linked to the centroid of the corresponding district. Centroids were obtained using the Feature to Point tool in ArcGIS Pro (Version 2.9).
2.3.1. ASF Incidence Cluster Analysis with Poisson Model
A discrete spatial-temporal Poisson probability model was used to identify clusters of increased ASF incidence in the wild boar population. The null hypothesis for this model assumes that the expected number of disease cases within some areas is Poisson distributed depending on the population size (i.e., is proportional to the wild boar population size) at the same area [,,]. The p-value for all clusters was estimated using 999 Monte Carlo simulations. Clusters with p < 0.05 were considered significant [].
2.3.2. Wild Boar Density Cluster Analysis with Normal Probability Model
To identify distribution patterns of areas with high wild boar density, a spatial-temporal cluster analysis was performed using a normal probability model []. This model is used to analyze continuously distributed data, assuming that the theoretical (expected) distribution is normal. The model identifies clusters with a statistically significant (p < 0.05) excess of population density over the expected value [].
2.4. Emerging Hot Spot Analysis (EHSA) of Annual Wild Boar Population Density
Since the results obtained from SaTScan software may be relatively unstable, being strongly dependent on the selected size of the scanning window [], hot and cold spot analysis was also conducted to justify the areas with certain groupings of high/low animal density and trends in its temporal changes [].
To perform EHSA, the data on the wild boar population density were represented as a spatiotemporal structure referred to as a “cube,” where the spatial units were the analyzed municipalities with wild boar density data for the sufficient number of years (n = 1187 in the European zone and n = 82 in the Far Eastern zone), and the vertical dimension represented time with a yearly interval.
The primary objects of analysis are time-series data, known as “population density bins,” for each municipality over the entire study period. The EHSA method combines two statistical indicators: the Getis–Ord Gi* hot spot statistic test, which determines the location and intensity of spatial clusters of wild boar density (hot and cold spots), and the Mann–Kendall statistic to evaluate the temporal trends in hot and cold spots’ emergence. The statistical significance of hot and cold spots, as well as their trends, is determined based on the variance of the time-series values [,,].
The results of the hot and cold spot analysis were visualized based on z-scores and corresponding p-value significance criteria. Seventeen categories of hot and cold spots were identified depending on the temporal trends in their formation (Table S1): new, consecutive, intensifying, constant, decreasing, sporadic, fluctuating, and historical. For example, a “new” hot spot indicates the formation of a high-density wild boar cluster in recent years of the study period. A “constant” hot spot indicates a consistently observed high wild boar density in the area throughout the study years.
We compared clusters of increased wild boar population density with clusters of increased ASF incidence, based on the principle of spatial and temporal intersection. Additionally, the identified hot and cold spots were used to reveal the geographic territories with increasing and decreasing trends in the wild boar population density and areas with intensive hunting resource utilization.
2.5. Software
Spatiotemporal cluster analysis was conducted using SaTScan software version 9.7 []. Statistical data processing was performed using the MS Office Excel v.1 application package. Emerging Hot and Cold Spot Analysis (EHSA), spatial data processing, and mapping of results were carried out using ArcGIS Pro (Esri, Redlands, CA, USA).
3. Results
3.1. Descriptive Analysis
The number of ASF outbreaks among the population of wild boars from 2007 to 2022 accounted for 42.5% of all ASF outbreaks in Russia during this period (2678). ASF outbreaks in the wild boar population demonstrate a pronounced seasonality, when peaks occur in November and December during the cold season and July and August during the summer season (Figure 2).
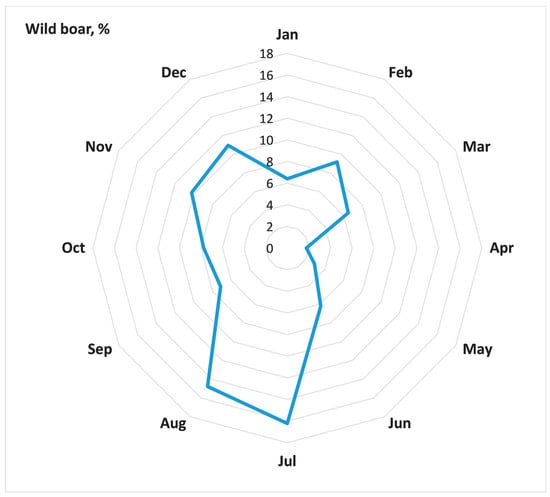
Figure 2.
Seasonality of ASF outbreaks in wild boar within model district of Russia, 2007–2022 (a share of outbreaks in a particular month in the total number of outbreaks).
3.2. Spatiotemporal Cluster Analysis of ASF Foci in Wild Boar Population Density
Analysis of spatial-temporal clusters of ASF incidence in populations of wild boar in the model regions of the Russian Federation from 2007 to 2022 using a Poisson probability model revealed 24 statistically significant clusters with significantly different durations of formation ranging from 1 year to 9 years (Figure 3, Table 1).
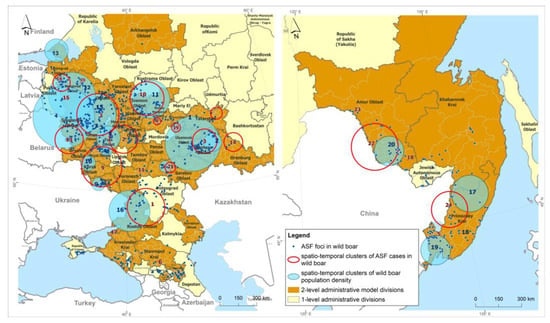
Figure 3.
Spatiotemporal clusters of ASF cases in wild boar, and spatiotemporal clusters of districts with high wild boar population density in the Russian Federation, 2007–2022.

Table 1.
Characteristics of spatiotemporal clusters of ASF incidence in the affected model districts of Russia, 2007–2022.
In total, 2 prolonged clusters (clusters #10 and #16), with a duration of more than 7 years, 10 short-term clusters, with durations of up to 1 year, and 12 intermediate clusters, with a duration of 2 to 6 years, were identified.
The cluster of African swine fever foci #16, which coincided in the period from 2011 to 2016 with density cluster #12, was prolonged (duration time amounted to 9 years), although its relative risk was not particularly high and amounted to 199.
Clusters #4, 5, and 6 had moderate geographic areas within the radius of one district and were short-lived (duration time from 1 to 3 years) but presented the highest risk of infection spread due to the high density of susceptible animal populations (ODE4 = 1523.59, ODE5 = 2013.61, ODE6 = 1947.59).
Areas of high risk for registered outbreaks of ASF can also be identified in clusters #11 and #17, where the ODE was 1915.12 and 286.29, respectively.
As a result of cluster analysis of the yearly wild boar population density data, 20 clusters were identified, including 4 significant clusters that combine areas of the Russian Far East (Figure 3, Table 2). Cluster number 19, with the highest LLR of 181.86, is located in Primorsky Krai and consists of 13 districts where the wild boar density remains high at 5.27 head/km2. The wild boar density in the areas within cluster number 17, located in the territories of Primorsky and Khabarovsk Krai, was lower at 4.27 head/km2.

Table 2.
Characteristics of spatiotemporal clusters of wild boar population density in the model districts of Russia, 2010–2022.
In the central zone of the Russian Federation, statistically significant clusters, numbers 3 and 7, with LLR values of 169.98 and 199.74, respectively, are locally located in two adjacent districts, Medynsky and Dzerzhinsky, in the Kaluga region, where the wild boar density had the highest values of 1.89 head/km2 and 2.02 head/km2, respectively (Table 2).
Cluster numbers 1, 5, and 9, located in the territories of 15 districts (Republic of Tatarstan, Saratov, and Belgorod regions), had wild boar densities of 0.88, 0.47, and 0.35 head/km2, respectively, exceeding the threshold wild boar density of 0.025 head/km2 recommended by the ASF elimination plan [] by 353%, 189%, and 140%, respectively.
The results of the spatial-temporal analysis using the normal probability model are presented in Table 2.
The #5 cluster for ASF cases, located in Primorsky Krai, had a temporal coincidence with cluster #19 in the animal population density, which demonstrated a high density of individuals in the areas forming the cluster—5.27 head/km2. This could be a potential risk factor for the concentration of ASF outbreaks in the region in 2020.
Cluster #10 for ASF cases among wild boars, encompassing territories in the Nizhny Novgorod, Vladimir, Ivanovo, and Kostroma regions, coincided in the time of formation with cluster #11 for population density (2016), identified by the normal probability method. The density of wild boars in that cluster was 0.21 head/km2.
Cluster #3 for ASF cases, located in the Samara region and with a temporal period in 2020, was the shortest major epidemic, resulting in over 60 ASF foci registered among wild boars and coincided with cluster #6 for population density, in which the animal density was 1.88 head/km2.
3.3. Emerging Hot Spot Analysis of Wild Boar Population Density
As a result of hot and cold spot analysis, typical areas were identified in the central European zone of the Russian Federation and in the southeastern part of the country with an increased/decreased concentration in the wild boar population density, and classified as sporadic, persistent, new, and fluctuating depending on the temporal patterns of clustering (Figure 4). Sporadic and persistent hot spots in the wild boar population concentration were identified in the northwest part of Russia, in the Leningrad region bordering Finland and the Baltic States. Territories in the central part of the Russian Federation, specifically in Vladimir, Tver, Nizhny Novgorod regions, the Republic of Chuvashia, and the western and southwestern parts such as Smolensk, Kaluga, Tula, Orel, and Kursk regions, are characterized by temporary increases in the animal population density and likely by the persistence of the African swine fever (ASF) virus in the environment fomites, including in the carcasses and remains of infected wild boars.
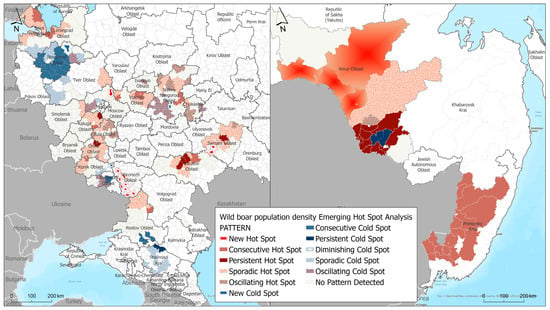
Figure 4.
Results of Emerging Hot Spot Analysis of wild boar population density in European part (left) and Far East (right) of the Russian Federation.
We also identified cold spot areas, where changes toward a decrease in the population density were observed during the analyzed period or no dynamics were observed. Cold spots were identified in the southern part of the Russian Federation—in the Stavropol Krai, Rostov, Tver, Novgorod, and southern parts of the Leningrad Oblasts. These areas likely experience constant or periodic decreases in the wild boar density population.
In the Far East part of the Russian Federation, where mainly hot spots were revealed, a persistent cold spot was identified, presenting an area where the population density remained at the same level or constantly decreased as a result of measures being taken to regulate the wild boar population. This cold spot is located in the eastern part of the Amur Oblast, in the Romnensky and Zavitinsky districts. Characteristics of the obtained hot and cold spots (geographic locations) are presented in the Supplementary Material (Table S1).
Territorial trends in the increasing wild boar population density were identified in the central European zone of the Russian Federation in subjects such as Vladimir, Moscow, Kaluga, Bryansk, Oryol, Kursk, and Nizhny Novgorod regions, the Mari El Republic, as well as in the southern and southeastern parts of Russia in the Rostov, Samara, Saratov, and Astrakhan regions. A trend in the increasing wild boar population density was also noted in the Leningrad region in the northwest of Russia (Figure 5).
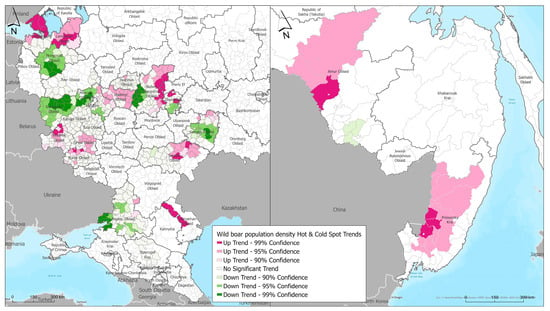
Figure 5.
Temporal trends in wild boar population density hot spots across in European part (left) and Far East (right) of the Russian Federation.
In the Far Eastern region of Russia, a trend in the increasing wild boar population density was noted in Amur Oblast and Primorsky Krai.
Territories with a decreasing trend in the wild boar population density have been identified in the central part of the European part of the Russian Federation in the Novgorod and Smolensk regions, the western part of the Nizhny Novgorod, Moscow, and Rostov regions, and the central part of the Samara region. Such a change in the population density is likely linked to measures being taken to regulate the number of wild boar.
4. Discussion
Animal infectious diseases cause enormous damage to the food security of many countries, resulting in significant financial losses due to the mass culling of animals [,]. As a reliable vaccine against ASF has not yet been developed, the main methods of combating ASF involve implementing strict biosecurity measures in the management of pigs and wild boar [,,]. This includes compartmentalization on pig farms based on zoosanitary status, and preventive measures aimed at regulating the population density of susceptible animals [].
There is no standard strategy for controlling ASF epidemics that could be applied to all ASF affected areas due to the different geographic and regional characteristics of populations of wild boars [,,]. Measures to prevent the introduction of the ASF virus from an affected region to unaffected region are a preferable option for standard prevention measures [,].
The spread of ASF in the wild boar population in the affected districts in the Russian Federation has distinct characteristics observed in the dynamics of epidemics [,]. The incidence of ASF among the wild boar population exhibits a seasonality of outbreak registrations []. The new foci of ASF were recorded during both winter peaks in November and December, and summer peaks in July and August. It is possible that the winter peak in ASF incidence is due to the biological characteristics of the animals, such as the mating season and herd re-groupings. The summer peak is likely caused by human activities, such as frequent visits to forests and hunting activities. An indirect contact between contaminated pork products or fomites left in the field can be a source of infection in the wild boar population in proximity to domestic pig farms, which presumably may happen mainly in the periods of high economic activity in the population, i.e., in the summer season.
Spatial and spatiotemporal methods for identifying areas of concentration (clusters) of disease outbreaks have an important role in modern epidemiological research, as well as in the practice of public health and preventive veterinary medicine. Their use enables the identification of potential etiological and pathogenic causes of epidemics and determines the key ways to address the disease eradication.
Therefore, in our study, we aimed to demonstrate the necessity of using cluster analysis methods to identify areas with a concentration of ASF foci in wild boars depending on the localization of areas with a high density of populations. We conducted a comparative analysis of geographic regions forming these clusters based on the animal population density and identified high-risk areas where the density of the wild boar population remains elevated.
Spatial-temporal analysis of ASF foci in the wild boar population using a Poisson probability model that took into account the density of the wild boar population revealed clusters with a high risk of detecting new foci of the disease in the central part of the European Zone of the Russian Federation.
The ASF incidence clusters in the wild boar population had different temporal durations, with some epidemics characterized by the formation of short-term clusters with a time duration up to 1 year, as well as prolonged clusters lasting up to 9 years. The trend toward the formation of short-duration clusters with a high risk of new foci is likely due to the prolonged epidemic situation among wild boars and the expansion of ASF disease to previously unaffected territories of the Russian Federation.
Geographically, ASF hot spots characterized by high relative risk values were localized in subjects in the central part of European Russia. The spatial-temporal spread of ASF was characterized by the movement of cluster territories from Cluster #6 in 2010 toward the north to Cluster #17 in 2012, and then to Clusters #11 (2021) and #4 (2015). In 2022, a high-risk cluster #5 was identified in Primorsky Krai in the Far East region, which may be associated with the prohibition of wild boar depopulation in the bordering territories of China with Russia.
The distinguishing epidemiological feature of the identified clusters was the duration of their existence, with some having a short period of 1 or 2 years, while others were long-lasting for 7 years or more (clusters #6 and #17). This can be explained by the fact that outbreaks of African swine fever (ASF) in wild boar populations in different regions of Russia have had varying durations, ranging from widespread epidemics in a short period, such as in the Samara region in 2020, where more than 60 foci of ASF in carcasses of infected wild boars were registered [], to sporadic outbreaks that are predominantly found in the most affected regions of the Russian Federation [].
Therefore, there is a view among international scientists that even at a very low population density of wild boars, there is a window of uncertainty when African swine fever (ASF) is still present among animals, but practically undetected by standard diagnostic methods, which complicates any further disease management, including possible infection-elimination strategies [,,].
As a result of a spatial-temporal analysis of geographic areas where foci of African swine fever (ASF) were registered from 2007 to 2022, with a predisposing factor likely being the increased population density of wild boar, several clusters were identified in the northwestern, central, and southeastern regions of the Russian Federation. The territorial and temporal coincidence of these high-density clusters may be explained by the continued concentration of animals in the ASF foci areas, despite the ongoing disease control measures implemented in the affected regions of Russia, which involve the annual population reduction in wild boars [].
Based on the estimated density of wild boar populations and through the analysis of emerging hotspots (EHSA), the geographic scope of regions affected by African swine fever (ASF) in the Russian Federation with high densities of wild boars has been determined. These areas have a tendency to experience increases or decreases in animal population density, making them important for conducting targeted measures aimed at regulating boar populations.
As a result of the EHSA analysis, an increasing spatiotemporal trend was observed, which can be attributed to the existing high density of animal populations in the territories of the northwestern and southeastern parts of Russia (with an average density of over 0.85 boars per square kilometer within the trend subjects). The density of wild boars in the subjects of the European zone has been found to be 0.025 animals per square kilometer of hunting grounds, based on the plan for the eradication of African swine fever in the subjects of the Russian Federation.
Areas of risk can be considered territories with a tendency toward the clustering of areas of concentration of animals where this trend is maintained and expressed as an increasing trend in the population density of wild boar. An increasing trend in the population density of wild boar has been observed in the following subjects of the European part of Russia: Leningrad, Vladimir, Kaluga, Bryansk, Oryol, Kursk regions, the north part of the Nizhny Novgorod region, Chuvash Republic, the center of the Saratov region, the northeastern part of the Samara and Moscow regions. In the Far East, areas with high densities of wild boar, such as the Amur region and Primorsky Krai, provide an opportunity to assess areas at risk of ASF occurrence and to some extent represent an example of the transboundary spread of ASF among the population of wild boar.
Currently, depopulation is carried out annually in all problematic regions of Russia, which has an irrational basis due to the high cost of implementing the measures. We have studied some clustering methods for identifying areas with a certain risk of ASF epidemics to more effectively plan measures to regulate the population density of wild boar in the subjects of the Russian Federation.
It should be noted that the data on wild boar population size and density used in this study can be affected by a bias related to imperfect counting methods. Normally, wild boar counts in Russia are obtained via “winter route counting”. However, when this method is used in individual hunting farms and nature reserves (i.e., smaller areas), it often underestimates the population size of wild boar. This is because the counting is conducted in the second half of winter when the movement of wild boar on the ground is disrupted by heavy snow, and their transitional activity is drastically reduced. The obtained assessment data are often extrapolated to the remaining area of the district, which can lead to serious errors.
5. Conclusions
The lack of an effective vaccine against ASF currently determines the key points in the strategy for combating and eliminating the infection, which remains a global problem for many countries in the world. Due to the ability of the ASF virus to maintain its virulent properties in the environment for a long period, problem areas are formed in the subjects of the Russian Federation, with the potential for the further spread of the infection. The situation is aggravated by the presence of local areas with a high density of wild boar, which can be risk zones for the spread of ASF. The location and duration of clusters of ASF outbreaks among wild boar in the problematic regions of Russia provide an idea of areas with the most extended epidemics and the presence of a high potential for further persistence of the ASF virus in the wild.
The clustering methods used in this study, which investigate hot spots—areas of high wild boar population density—can be used to determine high-risk territories for the purpose of enhancing active and passive monitoring in certain geographical areas and applying biosecurity measures during hunting, as well as other risk-reduction measures. Knowledge of the identified trends and patterns of ASF spread in the wild boar population enables the improvement of implemented measures within the territorial boundaries of local epidemics. The use of spatial-temporal analysis methods of ASF foci and wild boar population density using clustering tools presents a risk-oriented approach for identifying priority zones (risk territories) and assessing the effectiveness of prevention measures.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani13193081/s1, Table S1: Patterns of emerging hot spot analysis results.
Author Contributions
Conceptualization, O.I.Z. and F.I.K.; methodology, O.I.Z. and F.I.K.; software, O.I.Z.; validation, F.I.K.; formal analysis, O.I.Z.; investigation, O.A.B.; resources, E.A.L., N.A.G. and I.V.R.; data curation, A.A.B. and I.V.Y.; writing—original draft preparation, O.I.Z.; writing—review and editing, O.I.Z. and F.I.K.; visualization, O.I.Z.; supervision, I.V.Y.; project administration, A.A.B.; funding acquisition, A.A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was performed with the support of the Federal Research Center for Virology and Microbiology, budgetary program number FGNM-2021-0004. This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dixon, L.K.; Sun, H.; Roberts, H. African Swine Fever. Antivir. Res. 2019, 165, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, W.; Qiu, Z.; Li, Y.; Fan, J.; Wu, K.; Li, X.; Zhao, M.; Ding, H.; Fan, S.; et al. African Swine Fever Virus: A Review. Life 2022, 12, 1255. [Google Scholar] [CrossRef] [PubMed]
- Blome, S.; Franzke, K.; Beer, M. African Swine Fever—A Review of Current Knowledge. Virus Res. 2020, 287, 198099. [Google Scholar] [CrossRef] [PubMed]
- Gervasi, V.; Guberti, V. African Swine Fever Endemic Persistence in Wild Boar Populations: Key Mechanisms Explored through Modelling. Transbound. Emerg. Dis. 2021, 68, 2812–2825. [Google Scholar] [CrossRef]
- Carlson, J.; Fischer, M.; Zani, L.; Eschbaumer, M.; Fuchs, W.; Mettenleiter, T.; Beer, M.; Blome, S. Stability of African Swine Fever Virus in Soil and Options to Mitigate the Potential Transmission Risk. Pathogens 2020, 9, 977. [Google Scholar] [CrossRef]
- Niederwerder, M.C.; Khanal, P.; Foland, T.; Constance, L.A.; Stoian, A.M.M.; Deavours, A.; Haase, K.; Cino-Ozuna, A.G. Stability of African Swine Fever Virus in Feed during Environmental Storage. Transbound. Emerg. Dis. 2022, 69, 3216–3224. [Google Scholar] [CrossRef]
- Guberti, V.; Khomenko, S.; Masiulis, M.; Kerba, S. African Swine Fever in Wild Boar Ecology and Biosecurity; Food and Agriculture Organization: Rome, Italy, 2022; ISBN 978-92-5-136565-6. [Google Scholar]
- Vergne, T.; Korennoy, F.; Combelles, L.; Gogin, A.; Pfeiffer, D.U. Modelling African Swine Fever Presence and Reported Abundance in the Russian Federation Using National Surveillance Data from 2007 to 2014. Spat. Spatiotemporal. Epidemiol. 2016, 19, 70–77. [Google Scholar] [CrossRef]
- Depner, K.; Gortazar, C.; Guberti, V.; Masiulis, M.; More, S.; Oļševskis, E.; Thulke, H.; Viltrop, A.; Woźniakowski, G.; Cortiñas Abrahantes, J.; et al. Epidemiological Analyses of African Swine Fever in the Baltic States and Poland. EFSA J. 2017, 15, e05068. [Google Scholar] [CrossRef]
- More, S.; Miranda, M.A.; Bicout, D.; Bøtner, A.; Butterworth, A.; Calistri, P.; Edwards, S.; Garin-Bastuji, B.; Good, M.; Michel, V.; et al. African Swine Fever in Wild Boar. EFSA J. 2018, 16, e05344. [Google Scholar] [CrossRef]
- Cadenas-Fernández, E.; Ito, S.; Aguilar-Vega, C.; Sánchez-Vizcaíno, J.M.; Bosch, J. The Role of the Wild Boar Spreading African Swine Fever Virus in Asia: Another Underestimated Problem. Front. Vet. Sci. 2022, 9, 844209. [Google Scholar] [CrossRef]
- Sauter-Louis, C.; Conraths, F.J.; Probst, C.; Blohm, U.; Schulz, K.; Sehl, J.; Fischer, M.; Forth, J.H.; Zani, L.; Depner, K.; et al. African Swine Fever in Wild Boar in Europe—A Review. Viruses 2021, 13, 1717. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, O.I.; Titov, I.A.; Gogin, A.E.; Sevskikh, T.A.; Korennoy, F.I.; Kolbasov, D.V.; Abrahamyan, L.; Blokhin, A.A. African Swine Fever in the Russian Far East (2019–2020): Spatio-Temporal Analysis and Implications for Wild Ungulates. Front. Vet. Sci. 2021, 8, 882. [Google Scholar] [CrossRef] [PubMed]
- Andrey, B.; Nadezhda, T.; Olga, B.; Timofey, S.; Andrey, G.; Zoran, D.; Olga, Z. Spatio-Temporal Analysis of the Spread of ASF in the Russian Federation in 2017–2019. Acta Vet.-Beogr. 2020, 70, 194–206. [Google Scholar] [CrossRef]
- Pepin, K.M.; Golnar, A.; Podgórski, T. Social Structure Defines Spatial Transmission of African Swine Fever in Wild Boar. J. R. Soc. Interface 2021, 18, 20200761. [Google Scholar] [CrossRef] [PubMed]
- Pepin, K.M.; Golnar, A.J.; Abdo, Z.; Podgórski, T. Ecological Drivers of African Swine Fever Virus Persistence in Wild Boar Populations: Insight for Control. Ecol. Evol. 2020, 10, 2846–2859. [Google Scholar] [CrossRef]
- Zani, L.; Masiulis, M.; Bušauskas, P.; Dietze, K.; Pridotkas, G.; Globig, A.; Blome, S.; Mettenleiter, T.; Depner, K.; Karvelienė, B. African Swine Fever Virus Survival in Buried Wild Boar Carcasses. Transbound. Emerg. Dis. 2020, 67, 2086–2092. [Google Scholar] [CrossRef]
- Guberti, V.; Khomenko, S.; Masiulis, M.; Kerba, S. GF-TADs Handbook on ASF in Wild Boar and Biosecurity during Hunting-Version Standing Group of Experts on African Swine Fever in Europe under the GF-TADs Umbrella Handbook on African Swine Fever in Wild Boar and Biosecurity during Hunting; Food and Agriculture Organization: Rome, Italy, 2018. [Google Scholar]
- Tiwari, S.; Dhakal, T.; Kim, T.S.; Lee, D.H.; Jang, G.S.; Oh, Y. Climate Change Influences the Spread of African Swine Fever Virus. Vet. Sci. 2022, 9, 606. [Google Scholar] [CrossRef]
- Ungur, A.; Cazan, C.D.; Panait, L.C.; Coroian, M.; Cătoi, C. What Is the Real Influence of Climatic and Environmental Factors in the Outbreaks of African Swine Fever? Animals 2022, 12, 781. [Google Scholar] [CrossRef]
- Korennoy, F.I.; Gulenkin, V.M.; Malone, J.B.; Mores, C.N.; Dudnikov, S.A.; Stevenson, M.A. Spatio-Temporal Modeling of the African Swine Fever Epidemic in the Russian Federation, 2007–2012. Spat. Spatiotemporal. Epidemiol. 2014, 11, 135–141. [Google Scholar] [CrossRef]
- Ryser-Degiorgis, M.P. Wildlife Health Investigations: Needs, Challenges and Recommendations. BMC Vet. Res. 2013, 9, 223. [Google Scholar] [CrossRef]
- Woodford, M.H. Veterinary Aspects of Ecological Monitoring: The Natural History of Emerging Infectious Diseases of Humans, Domestic Animals and Wildlife. Trop. Anim. Health Prod. 2009, 41, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Ryser-Degiorgis, M.P.; Pewsner, M.; Angst, C. Joining the Dots—Understanding the Complex Interplay between the Values We Place on Wildlife, Biodiversity Conservation, Human and Animal Health: A Review. Schweiz. Arch. Tierheilkd. 2015, 157, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Kay, S.L.; Fischer, J.W.; Monaghan, A.J.; Beasley, J.C.; Boughton, R.; Campbell, T.A.; Cooper, S.M.; Ditchkoff, S.S.; Hartley, S.B.; Kilgo, J.C.; et al. Quantifying Drivers of Wild Pig Movement across Multiple Spatial and Temporal Scales. Mov. Ecol. 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Cross, P.C.; Caillaud, D.; Heisey, D.M. Underestimating the Effects of Spatial Heterogeneity Due to Individual Movement and Spatial Scale: Infectious Disease as an Example. Landsc. Ecol. 2013, 28, 247–257. [Google Scholar] [CrossRef]
- Meng, X.J.; Lindsay, D.S. Wild Boars as Sources for Infectious Diseases in Livestock and Humans. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2697–2707. [Google Scholar] [CrossRef] [PubMed]
- Consortium, E.; Keuling, O.; Sange, M.; Acevedo, P.; Podgorski, T.; Smith, G.; Scandura, M.; Apollonio, M.; Ferroglio, E.; Vicente, J. Guidance on Estimation of Wild Boar Population Abundance and Density: Methods, Challenges, Possibilities. EFSA Support. Publ. 2018, 15, 1449E. [Google Scholar] [CrossRef]
- Oganesyan, A.S.; Shibayev, M.A.; Baskakova, H..Y.; Korennoy, F.I.; Karaulov, A.K. African Swine Fever Epidemic in 2007–2017 Part 1 Common Trends for ASF in the Russian Federationand in Eurasia. Vet. Sci. Today 2018, 2, 18–25. [Google Scholar] [CrossRef]
- Malkhazova, C.; Korennoy, F.; Petrova, O.; Gulenkin, V.; Karaulov, A. Spatio-temporal analysis of the local african swine fever spread in the russian federation in 2007–2015 African. Mosc. Univ. Bull. Ser. 5 Geogr. 2017, 22, 33–42. [Google Scholar]
- Glazunova, A.A.; Korennoy, F.I.; Sevskikh, T.A.; Lunina, D.A. Risk Factors of African Swine Fever in Domestic Pigs of the Samara Region, Russian Federation. Front. Vet. Sci. 2021, 8, 723375. [Google Scholar] [CrossRef]
- Goverment of Natural Resources and Environment of the Russian Federation. Available online: https://www.mnr.gov.ru/en/ (accessed on 22 June 2023).
- WAHIS. Available online: https://wahis.woah.org/#/event-management (accessed on 22 June 2023).
- Kulldorff, M.; Heffernan, R.; Hartman, J.; Assunção, R.; Mostashari, F. A Space–Time Permutation Scan Statistic for Disease Outbreak Detection. PLoS Med. 2005, 2, e59. [Google Scholar] [CrossRef]
- Punyapornwithaya, V.; Seesupa, S.; Phuykhamsingha, S.; Arjkumpa, O.; Sansamur, C.; Jarassaeng, C. Spatio-Temporal Patterns of Lumpy Skin Disease Outbreaks in Dairy Farms in Northeastern Thailand. Front. Vet. Sci. 2022, 9, 957306. [Google Scholar] [CrossRef]
- Kulldorff, M. A Spatial Scan Statistic. Commun. Stat. Theory Methods 2007, 26, 1481–1496. [Google Scholar] [CrossRef]
- Amoako-Sakyi, D.; Obiri-Yeboah, D.; Ofosu, A.; Kusi, K.A.; Osei, K.; Adade, R.; Aniakwaa-Bonsu, E.; Quansah, R.; Arko-Mensah, J.; Amoah, B.Y.; et al. Preponderance of Vaccine-Preventable Diseases Hotspots in Northern Ghana: A Spatial and Space-Time Clustering Analysis from 2010 to 2014. BMC Public Health 2022, 22, 1899. [Google Scholar] [CrossRef]
- Kulldorff, M. Prospective Time Periodic Geographical Disease Surveillance Using a Scan Statistic. J. R. Stat. Soc. Ser. A Stat. Soc. 2001, 164, 61–72. [Google Scholar] [CrossRef]
- Kulldorff, M.; Huang, L.; Konty, K. A Scan Statistic for Continuous Data Based on the Normal Probability Model. Int. J. Health Geogr. 2009, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Kulldorff, M. Software for the Spatial and Space-Time Scan Statistics; v9.6; SaTScanTM Inc.: Edinburgh, UK, 2018. [Google Scholar]
- Chen, J.; Roth, R.E.; Naito, A.T.; Lengerich, E.J.; MacEachren, A.M. Geovisual Analytics to Enhance Spatial Scan Statistic Interpretation: An Analysis of U.S. Cervical Cancer Mortality. Int. J. Health Geogr. 2008, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- How Emerging Hot Spot Analysis Works—ArcGIS Pro. Documentation. Available online: https://pro.arcgis.com/en/pro-app/latest/tool-reference/space-time-pattern-mining/learnmoreemerging.htm (accessed on 22 June 2023).
- The Esri Guide to GIS Analysis, Volume 2: Spatial Measurements and Statistics, Second Edition by Andy Mitchell. Esri Press. Available online: https://www.esri.com/en-us/esri-press/browse/the-esri-guide-to-gis-analysis-volume-2-spatial-measurements-and-statistics-second-edition (accessed on 22 June 2023).
- Mann, H.B. Nonparametric Tests Against Trend. Econometrica 1945, 13, 245. [Google Scholar] [CrossRef]
- Carlson, W.; Lackman, C. Can Business Firms Have Too Much Leverage? M&M, RJR 1990, and the Crisis of 2008. Mod. Econ. 2016, 7, 194–203. [Google Scholar] [CrossRef][Green Version]
- Plan of Measures (“Road Map”) to Improve the System of Veterinary Security Russian Federation. Gov. Russ. Fed. React. Formen. 2022. Available online: https://www.garant.ru/products/ipo/prime/doc/405796833/?ysclid=ln27in86l9958335046 (accessed on 18 September 2023).
- Guinat, C.; Vergne, T.; Jurado-Diaz, C.; Sánchez-Vizcaíno, J.M.; Dixon, L.; Pfeiffer, D.U. Effectiveness and Practicality of Control Strategies for African Swine Fever: What Do We Really Know? Vet. Rec. 2017, 180, 97. [Google Scholar] [CrossRef] [PubMed]
- Nga, B.T.T.; Padungtod, P.; Depner, K.; Chuong, V.D.; Duy, D.T.; Anh, N.D.; Dietze, K. Implications of Partial Culling on African Swine Fever Control Effectiveness in Vietnam. Front. Vet. Sci. 2022, 9, 957918. [Google Scholar] [CrossRef]
- Gervasi, V.; Marcon, A.; Guberti, V. Estimating the Risk of Environmental Contamination by Forest Users in African Swine Fever Endemic Areas. Acta Vet. Scand. 2022, 64, 16. [Google Scholar] [CrossRef] [PubMed]
- Cerberus_vetrf. Available online: https://cerberus.vetrf.ru/cerberus/compartment/pub (accessed on 18 June 2020).
- Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Depner, K.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortazar Schmidt, C.; Herskin, M.; et al. ASF Exit Strategy: Providing Cumulative Evidence of the Absence of African Swine Fever Virus Circulation in Wild Boar Populations Using Standard Surveillance Measures. EFSA J. 2021, 19, 6419. [Google Scholar] [CrossRef]
- Dankwa, E.A.; Lambert, S.; Hayes, S.; Thompson, R.N.; Donnelly, C.A. Stochastic Modelling of African Swine Fever in Wild Boar and Domestic Pigs: Epidemic Forecasting and Comparison of Disease Management Strategies. Epidemics 2022, 40, 100622. [Google Scholar] [CrossRef] [PubMed]
- European Commission Directorate-General for Health and Food Safety; Directorate G-Crisis Management in Food, Animals and Plants U.G. Official Controls and Eradication of Diseases in Animals Brussels Strategic Approach to the Management of African Swine Fever for the EU; Health and Food Safety—European Commission: Dublin, Ireland, 2020. [Google Scholar]
- Rozstalnyy, A.; Plavs, B. Strategic Challenges to Global Control of African Swine Fever. In Proceedings of the 87th General Session, Paris, France, 26–31 May 2019; World Organisation for Animal Health: Paris, France, 2019; Volume 33, pp. 26–31. [Google Scholar]
- Gallardo, M.C.; Reoyo, A.d.l.T.; Fernández-Pinero, J.; Iglesias, I.; Muñoz, M.J.; Arias, M.L. African Swine Fever: A Global View of the Current Challenge. Porc. Health Manag. 2015, 1, 21. [Google Scholar] [CrossRef]
- Gogin, A.; Gerasimov, V.; Malogolovkin, A.; Kolbasov, D. African Swine Fever in the North Caucasus Region and the Russian Federation in Years 2007–2012. Virus Res. 2013, 173, 198–203. [Google Scholar] [CrossRef]
- Mathes, R.W.; Lall, R.; Levin-Rector, A.; Sell, J.; Paladini, M.; Konty, K.J.; Olson, D.; Weiss, D. Evaluating and Implementing Temporal, Spatial, and Spatio-Temporal Methods for Outbreak Detection in a Local Syndromic Surveillance System. PLoS ONE 2017, 12, e0184419. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, O.I.; Blokhin, A.A.; Burova, O.A.; Yashin, I.V.; Korennoy, F.I. Spatiotemporal Analysis of African Swine Fever Spread in Wild Boar Population in Russian Federation, 2007–2022. Vet. Sci. Today 2023, 12, 57–65. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).