Seasonal Dynamics and Physiological Age of Ixodid Ticks Collected from Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tick Sampling
2.2. Tick Identification and Morphometric Measurement
2.3. Physiological Age Calculation
2.4. Statistical Analyses
3. Results
3.1. Tick Seasonal Dynamics
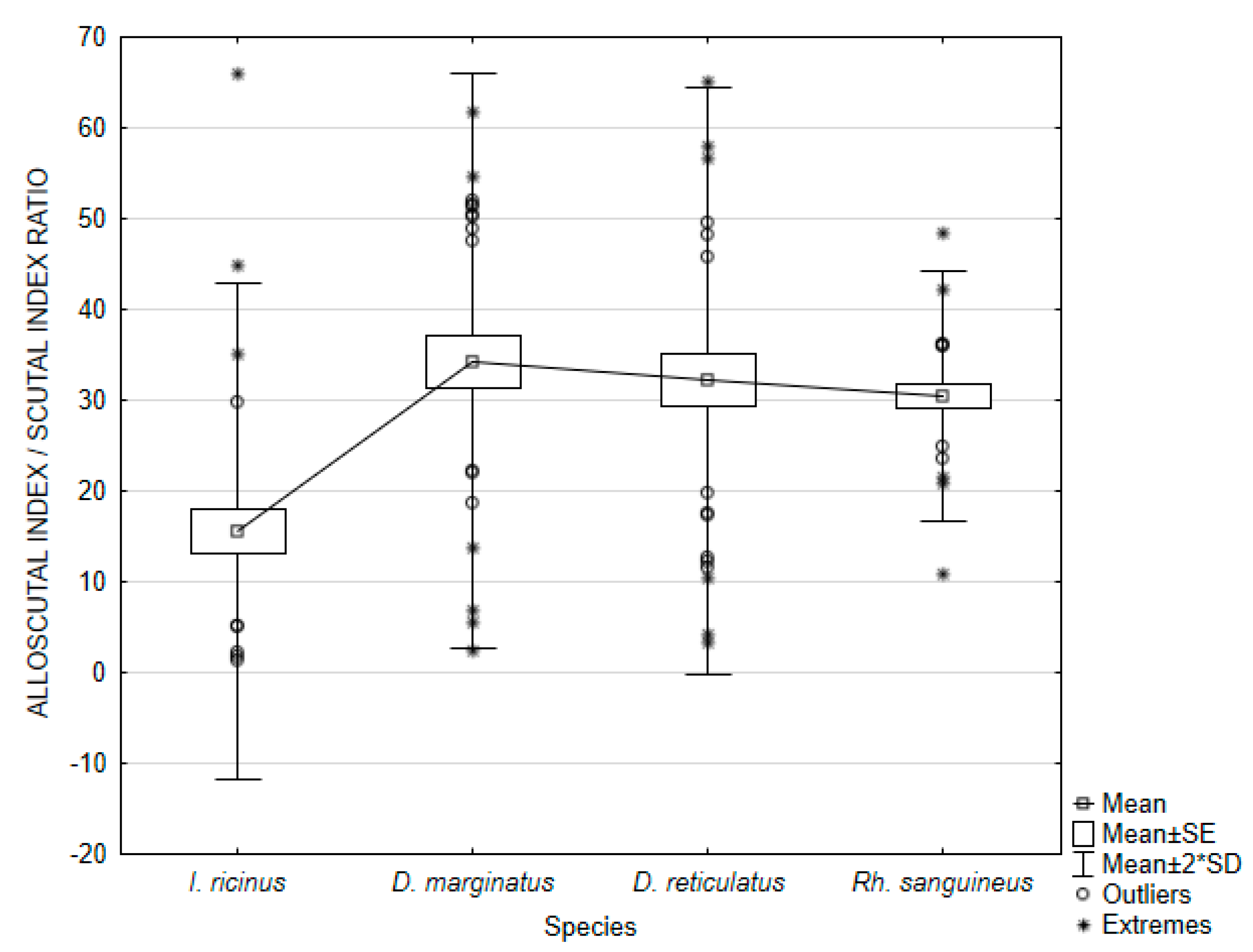
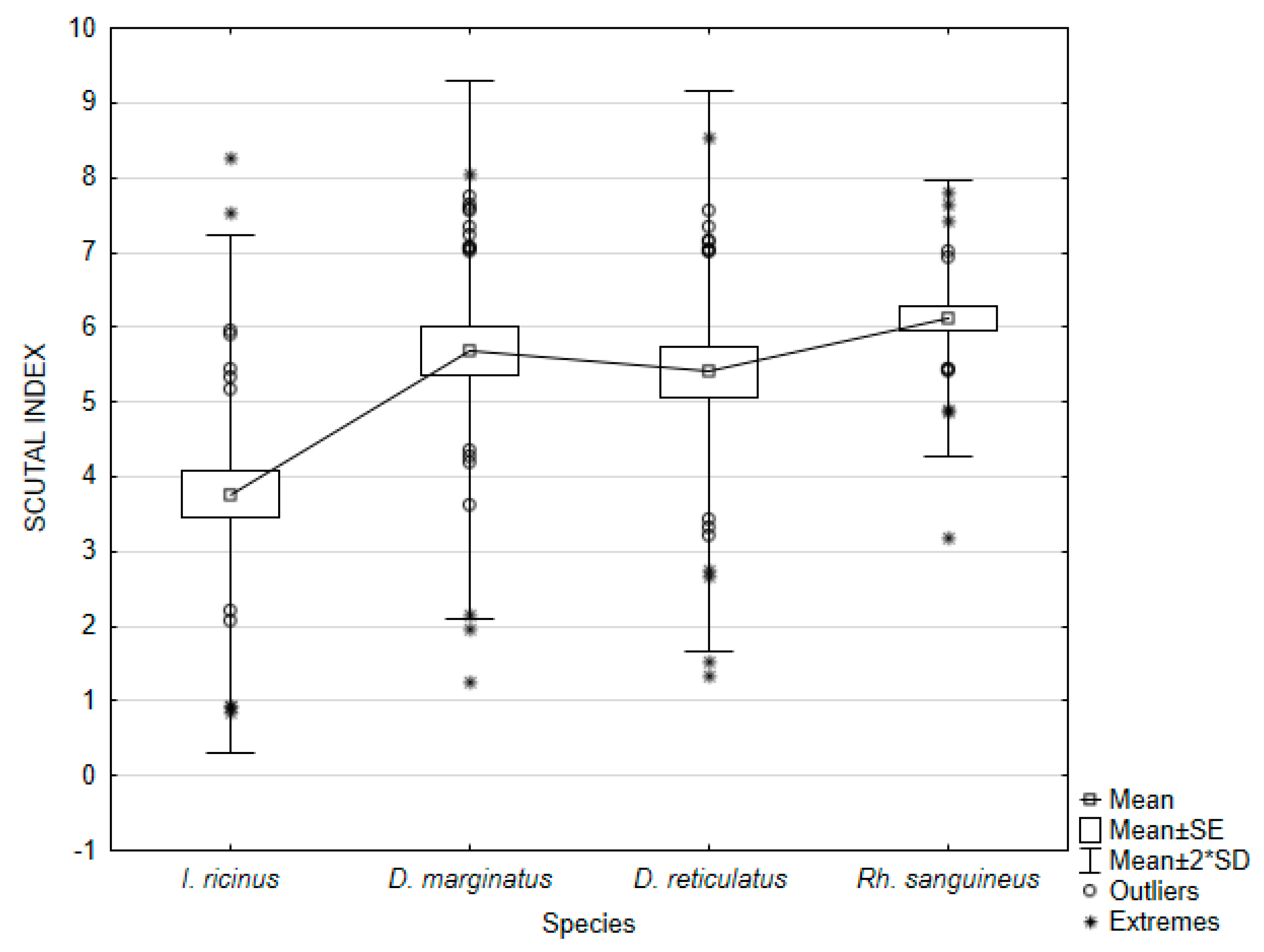
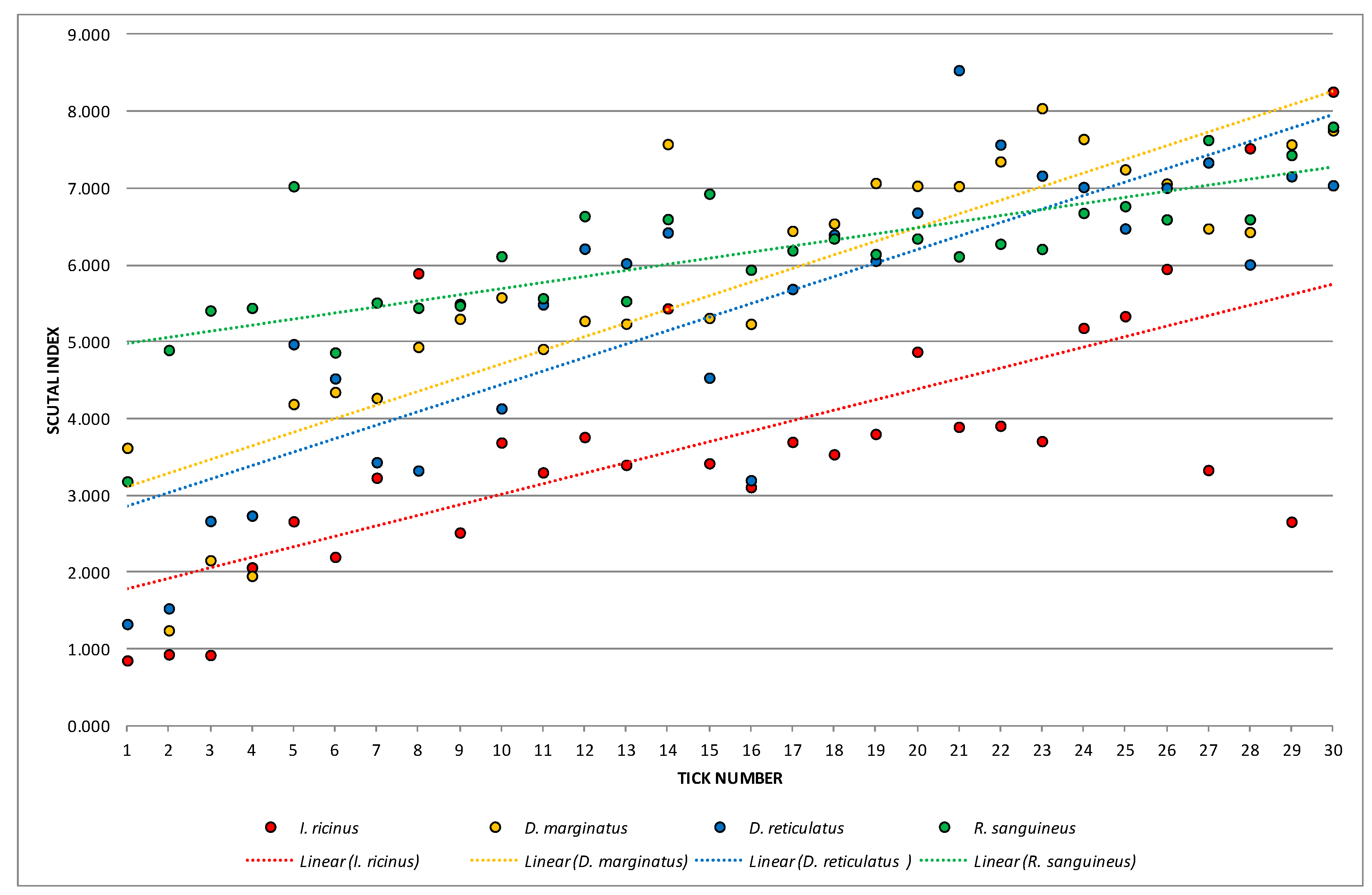
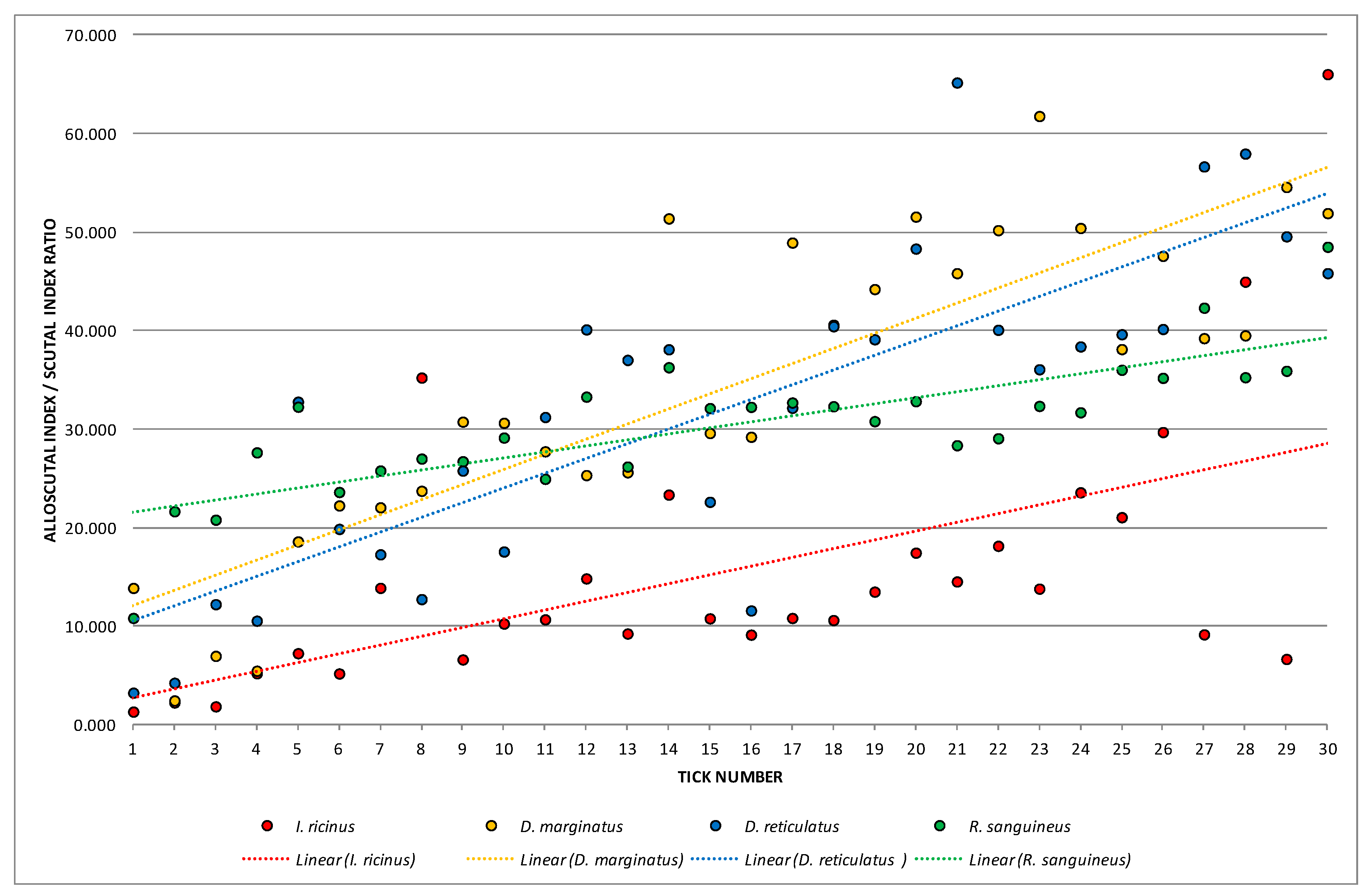
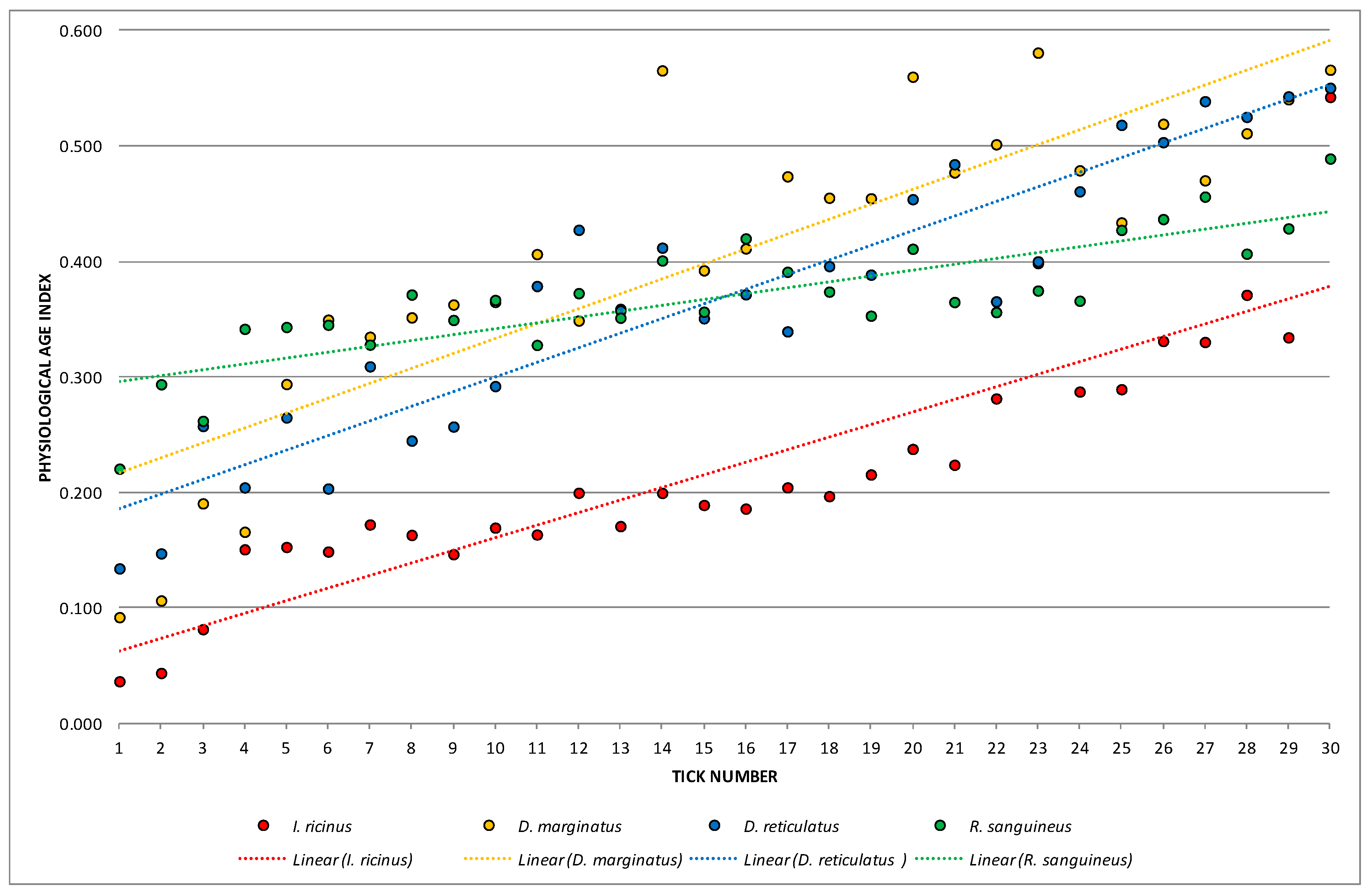
3.2. Female Physiological Age
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Morphometric Characteristic | Mean | Min. | Max. | Variance | Standard Deviation | Coefficient of Variation | Standard Error |
|---|---|---|---|---|---|---|---|
| capitulum base length | 211.78 | 172.11 | 282.54 | 984.383 | 31.375 | 14.815 | 5.728 |
| capitulum base width | 456.26 | 324.68 | 568.19 | 4023.402 | 63.430 | 13.902 | 11.580 |
| length between cornua | 450.54 | 370.83 | 522.58 | 1403.307 | 37.461 | 8.315 | 6.839 |
| scutum length | 1250.47 | 1132.63 | 1394.08 | 5167.820 | 71.888 | 5.749 | 13.125 |
| scutum width | 1294.79 | 968.93 | 1467.51 | 14,633.151 | 120.968 | 9.3433 | 22.086 |
| alloscutum length | 4675.89 | 1046.60 | 9678.88 | 4,245,321.253 | 2060.418 | 44.065 | 376.179 |
| alloscutum width | 3890.44 | 1561.61 | 8467.96 | 2,297,215.126 | 1515.657 | 38.958 | 276.720 |
| lateral width | 2009.09 | 519.41 | 4437.96 | 772,588.741 | 878.970 | 43.749 | 160.477 |
| body mass | 37.42 | 0.10 | 254.90 | 2616.720 | 51.154 | 136.714 | 9.339 |
| Morphometric Characteristic | Mean | Min. | Max. | Variance | Standard Deviation | Coefficient of Variation | Standard Error |
|---|---|---|---|---|---|---|---|
| capitulum base length | 254.05 | 183.23 | 409.91 | 3213.630 | 56.689 | 22.314 | 10.350 |
| capitulum base width | 562.92 | 467.37 | 737.23 | 3902.831 | 62.473 | 11.098 | 11.406 |
| length between cornua | 425.65 | 326.81 | 535.71 | 2785.842 | 52.781 | 12.400 | 9.636 |
| scutum length | 1523.76 | 1136.26 | 1764.04 | 18,145.371 | 134.705 | 8.840 | 24.594 |
| scutum width | 1339.64 | 946.61 | 1602.67 | 20,545.064 | 143.335 | 10.699 | 26.169 |
| alloscutum length | 8640.90 | 2104.05 | 12,756.17 | 7,799,865.211 | 2792.824 | 32.321 | 509.897 |
| alloscutum width | 6696.52 | 2510.08 | 9108.71 | 3,117,684.124 | 1765.696 | 26.367 | 322.371 |
| lateral width | 4121.02 | 649.44 | 6653.48 | 2,151,281.139 | 1466.725 | 35.591 | 267.786 |
| body mass | 199.43 | 2.60 | 543.40 | 20,494.022 | 143.157 | 71.783 | 26.137 |
| Morphometric Characteristic | Mean | Min. | Max. | Variance | Standard Deviation | Coefficient of Variation | Standard Error |
|---|---|---|---|---|---|---|---|
| capitulum base length | 285.77 | 174.54 | 376.92 | 1645.662 | 40.567 | 14.196 | 7.286 |
| capitulum base width | 576.780 | 452.79 | 714.83 | 3041.439 | 55.149 | 9.562 | 9.905 |
| length between cornua | 479.56 | 326.55 | 630.41 | 3159.693 | 56.211 | 11.721 | 10.096 |
| scutum length | 1553.69 | 1283.13 | 1767.55 | 14,693.701 | 121.218 | 7.802 | 21.771 |
| scutum width | 1346.76 | 1015.76 | 1694.81 | 23,473.073 | 153.209 | 11.376 | 27.517 |
| alloscutum length | 8222.30 | 2108.31 | 12,198.59 | 7,734,051.021 | 2781.016 | 33.823 | 499.485 |
| alloscutum width | 6545.19 | 2780.21 | 11,482.18 | 3,522,816.365 | 1876.917 | 28.676 | 337.104 |
| lateral width | 4063.22 | 1356.41 | 7885.06 | 2,953,108.074 | 1718.461 | 42.293 | 308.645 |
| body mass | 154.10 | 7.80 | 373.70 | 14,924.442 | 122.166 | 79.277 | 21.942 |
| Morphometric Characteristic | Mean | Min. | Max. | Variance | Standard Deviation | Coefficient of Variation | Standard Error |
|---|---|---|---|---|---|---|---|
| capitulum base length | 246.67 | 180.68 | 301.37 | 977.779 | 31.269 | 12.677 | 5.709 |
| capitulum base width | 748.97 | 688.12 | 832.63 | 1848.157 | 42.990 | 5.739 | 7.849 |
| length between cornua | 386.31 | 313.80 | 451.17 | 1117.167 | 33.424 | 8.652 | 6.102 |
| scutum length | 1367.98 | 1198.11 | 1527.27 | 6756.647 | 82.199 | 6.009 | 15.007 |
| scutum width | 1368.80 | 1208.08 | 1530.67 | 9121.892 | 95.509 | 6.978 | 17.437 |
| alloscutum length | 8353.50 | 4862.39 | 11,391.66 | 1,601,128.141 | 1265.357 | 15.148 | 231.021 |
| alloscutum width | 5956.59 | 4283.75 | 7267.46 | 365,927.403 | 604.919 | 10.155 | 110.443 |
| lateral width | 3816.96 | 2294.94 | 5021.98 | 447,542.625 | 668.986 | 17.527 | 122.140 |
| body mass | 134.14 | 37.00 | 241.30 | 2568.976 | 50.685 | 37.784 | 9.254 |

References
- Nicholson, W.L.; Allen, K.E.; McQuiston, J.H.; Breitschwerdt, E.B.; Little, S.E. The increasing recognition of rickettsial pathogens in dogs and people. Trends Parasitol. 2010, 26, 205–212. [Google Scholar] [CrossRef]
- De la Fuente, J.; Villar, M.; Cabezas-Cruz, A.; Estrada-Peña, A.; Ayllón, N.; Alberdi, P. Tick–Host–Pathogen Interactions: Conflict and Cooperation. PLoS Pathog. 2016, 12, e1005488. [Google Scholar] [CrossRef]
- Namina, A.; Capligina, V.; Selezņova, M.; Krumins, R.; Aleinikova, D.; Kivrane, A.; Akopjana, S.; Lazovska, M.; Berzina, I.; Ranka, R. Tick-borne pathogens in ticks collected from dogs, Latvia, 2011–2016. BMC Vet. Res. 2019, 15, 398. [Google Scholar] [CrossRef]
- Kiewra, D.; Lonc, E. Epidemiological consequences of host specificity of ticks (Ixodida). Ann. Parasitol. 2012, 58, 181–187. [Google Scholar] [PubMed]
- Zanet, S.; Battisti, E.; Pepe, P.; Ciuca, L.; Colombo, L.; Trisciuoglio, A.; Ferroglio, E.; Cringoli, G.; Rinaldi, L.; Maurelli, M.P. Tick-borne pathogens in Ixodidae ticks collected from privately-owned dogs in Italy: A country-wide molecular survey. BMC Vet. Res. 2020, 16, 46. [Google Scholar] [CrossRef] [PubMed]
- Földvári, G.; Farkas, R. Ixodid tick species attaching to dogs in Hungary. Vet. Parasitol. 2005, 129, 125–131. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Roura, X.; Sainz, A.; Miró, G.; Solano-Gallego, L. Species of ticks and carried pathogens in owned dogs in Spain: Results of a one-year national survey. Ticks Tick Borne Dis. 2017, 8, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.; Helps, C.; Tasker, S.; Newbury, H.; Wall, R. Ticks infesting domestic dogs in the UK: A large-scale surveillance programme. Parasit. Vectors 2016, 9, 391. [Google Scholar] [CrossRef]
- Schreiber, C.; Krücken, J.; Beck, S.; Maaz, D.; Pachnicke, S.; Krieger, K.; Gross, M.; Kohn, B.; von Samson-Himmelstjerna, G. Pathogens in ticks collected from dogs in Berlin/Brandenburg, Germany. Parasit. Vectors 2014, 7, 535. [Google Scholar] [CrossRef]
- Claerebout, E.; Losson, B.; Cochez, C.; Casaert, S.; Dalemans, A.; De Cat, A.; Madder, M.; Saegerman, C.; Heyman, P.; Lempereur, L. Ticks and associated pathogens collected from dogs and cats in Belgium. Parasit. Vectors 2013, 6, 183. [Google Scholar] [CrossRef]
- Maurelli, M.P.; Pepe, P.; Colombo, L.; Armstrong, R.; Battisti, E.; Morgoglione, M.E.; Counturis, D.; Rinaldi, L.; Cringoli, G.; Ferroglio, E.; et al. A national survey of Ixodidae ticks on privately owned dogs in Italy. Parasit. Vectors 2018, 11, 420. [Google Scholar] [CrossRef] [PubMed]
- Latrofa, M.S.; Angelou, A.; Giannelli, A.; Annoscia, G.; Ravagnan, S.; Dantas-Torres, F.; Capelli, G.; Halos, L.; Beugnet, F.; Papadopoulos, E.; et al. Ticks and associated pathogens in dogs from Greece. Parasit. Vectors 2017, 10, 301. [Google Scholar] [CrossRef]
- Duscher, G.G.; Feiler, A.; Leschnik, M.; Joachim, A. Seasonal and spatial distribution of ixodid tick species feeding on naturally infested dogs from Eastern Austria and the influence of acaricides/repellents on these parameters. Parasit. Vectors 2013, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Földvári, G.; Márialigeti, M.; Solymosi, N.; Lukacs, Z.; Majoros, G.; Kósa, J.P.; Farkas, R. Hard Ticks Infesting Dogs in Hungary and their Infection with Babesia and Borrelia Species. Parasitol. Res. 2007, 101, 25–34. [Google Scholar] [CrossRef]
- Jameson, L.J.; Medlock, J.M. Tick surveillance in Great Britain. Vector Borne Zoonotic Dis. 2011, 11, 403–412. [Google Scholar] [CrossRef]
- Nijhof, A.M.; Bodaan, C.; Postigo, M.; Nieuwenhuijs, H.; Opsteegh, M.; Franssen, L.; Jebbink, F.; Jongejan, F. Ticks and associated pathogens collected from domestic animals in the Netherlands. Vector Borne Zoonotic Dis. 2007, 7, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.D.; Ballantyne, R.; Morgan, E.R.; Wall, R. Prevalence, distribution and risk associated with tick infestation of dogs in Great Britain. Med. Vet. Entomol. 2011, 25, 377–384. [Google Scholar] [CrossRef]
- Drehmann, M.; Springer, A.; Lindau, A.; Fachet, K.; Mai, S.; Thoma, D.; Schneider, C.R.; Chitimia-Dobler, L.; Bröker, M.; Dobler, G.; et al. The Spatial Distribution of Dermacentor Ticks (Ixodidae) in Germany-Evidence of a Continuing Spread of Dermacentor reticulatus. Front Vet. Sci. 2020, 7, 578220. [Google Scholar] [CrossRef]
- Uspensky, I. Physiological age of ixodid ticks: Aspects of its determination and application. J. Med. Entomol. 1995, 32, 751–764. [Google Scholar] [CrossRef]
- Weiss, B.L.; Kaufman, W.R. Two feeding-induced proteins from the male gonad trigger engorgement of the female tick Amblyomma hebraeum. Proc. Natl. Acad. Sci. USA 2004, 101, 5874–5879. [Google Scholar] [CrossRef]
- Šimo, L.; Kazimirova, M.; Richardson, J.; Bonnet, S.I. The Essential Role of Tick Salivary Glands and Saliva in Tick Feeding and Pathogen Transmission. Front. Cell. Infect. Microbiol. 2017, 7, 281. [Google Scholar] [CrossRef] [PubMed]
- Suppan, J.; Engel, B.; Marchetti-Deschmann, M.; Nürnberger, S. Tick attachment cement-reviewing the mysteries of a biological skin plug system. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1056–1076. [Google Scholar] [CrossRef] [PubMed]
- Flynn, P.C.; Kaufman, W.R. Female ixodid ticks grow endocuticle during the rapid phase of engorgement. Exp. Appl. Acarol. 2011, 53, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Bartosik, K.; Buczek, A. Determination of the parameters of the parasitic stage in Ixodes ricinus females. Ann. Agric. Environ. Med. 2013, 20, 441–446. [Google Scholar]
- Yeh, M.T.; Bak, J.M.; Hu, R.; Nicholson, M.C.; Kelly, C.; Mather, T.N. Determining the duration of Ixodes scapularis (Acari: Ixodidae) attachment to tick-bite victims. J. Med. Entomol. 1995, 32, 853–858. [Google Scholar] [CrossRef]
- Meiners, T.; Hammer, B.; Göbel, U.B.; Kahl, O. Determining the tick scutal index allows assessment of tick feeding duration and estimation of infection risk with Borrelia burgdorferi sensu lato in a person bitten by an Ixodes ricinus nymph. Int. J. Med. Microbiol. 2006, 296, 103–107. [Google Scholar] [CrossRef]
- Uspensky, I.; Kovalevskii, Y.V.; Korenberg, E.I. Physiological age of field-collected female taiga ticks, Ixodes persulcatus (Acari: Ixodidae), and their infection with Borrelia burgdorferi sensu lato. Exp. Appl. Acarol. 2006, 38, 201–209. [Google Scholar] [CrossRef]
- Chaka, G.; Madder, M.; Speybroeck, N.; Tempia, S.; Tona, K.; Berkvens, D.L. Determination of the physiological age of Rhipicephalus appendiculatus (Acari: Ixodidae). Syst. Appl. Acarol. 2001, 10, 1–16. [Google Scholar] [CrossRef][Green Version]
- Pool, J.R.; Petronglo, J.R.; Falco, R.C.; Daniels, T.J. Energy Usage of Known-Age Blacklegged Ticks (Acari: Ixodidae): What Is the Best Method for Determining Physiological Age? J. Med. Entomol. 2017, 54, 949–956. [Google Scholar] [CrossRef]
- Gray, J.; Stanek, G.; Kundi, M.; Kocianova, E. Dimensions of engorging Ixodes ricinus as a measure of feeding duration. Int. J. Med. Microbiol. 2005, 295, 567–572. [Google Scholar] [CrossRef]
- Walker, A.R.; Bouattour, A.; Camicas, J.L.; Estrada-Peña, A.; Horak, I.G.; Latif, A.A.; Pegram, R.G.; Preston, P.M. Ticks of Domestic Animals in Africa: A Guide to Identification of Species; Bioscience Reports: Edinburgh, UK, 2003; pp. 1–221. [Google Scholar]
- Hornok, S.; Kováts, D.; Horvath, G.D.; Kontschán, J.; Farkas, R. Checklist of the hard tick (Acari: Ixodidae) fauna of Hungary with emphasis on host-associations and the emergence of Rhipicephalus sanguineus. Exp. Appl. Acarol. 2020, 80, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Chitimia-Dobler, L. Spatial distribution of Dermacentor reticulatus in Romania. Vet. Parasitol. 2015, 214, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Ciuca, L.; Martinescu, G.; Miron, L.D.; Roman, C.; Acatrinei, D.; Cringoli, G.; Rinaldi, L.; Maurelli, M.P. Occurrence of Babesia Species and Co-Infection with Hepatozoon canis in Symptomatic Dogs and in Their Ticks in Eastern Romania. Pathogens 2021, 10, 1339. [Google Scholar] [CrossRef] [PubMed]
- Stanilov, I.; Blazhev, A.; Miteva, L. Anaplasma and Ehrlichia Species in Ixodidae Ticks Collected from Two Regions of Bulgaria. Microorganisms 2023, 11, 594. [Google Scholar] [CrossRef]
- Krčmar, S.; Klobučar, A.; Vucelja, M.; Boljfetić, M.; Kučinić, M.; Madić, J.; Cvek, M.; Bruvo Mađarić, B. DNA barcoding of hard ticks (Ixodidae), notes on distribution of vector species and new faunal record for Croatia. Ticks Tick Borne Dis. 2022, 13, 101920. [Google Scholar] [CrossRef]
- Geurden, T.; Becskei, C.; Six, R.H.; Maeder, S.; Latrofa, M.S.; Otranto, D.; Farkas, R. Detection of tick-borne pathogens in ticks from dogs and cats in different European countries. Ticks Tick Borne Dis. 2018, 9, 1431–1436. [Google Scholar] [CrossRef]
- Dörr, B.; Gothe, R. Cold-hardiness of Dermacentor marginatus (Acari: Ixodidae). Exp. Appl. Acarol. 2001, 25, 151–169. [Google Scholar] [CrossRef]
- Król, N.; Kiewra, D.; Szymanowski, M.; Lonc, E. The role of domestic dogs and cats in the zoonotic cycles of ticks and pathogens. Preliminary studies in the Wrocław Agglomeration (SW Poland). Vet. Parasitol. 2015, 214, 208–212. [Google Scholar] [CrossRef]
- Földvári, G.; Široký, P.; Szekeres, S.; Majoros, G.; Sprong, H. Dermacentor reticulatus: A vector on the rise. Parasit. Vectors 2016, 9, 314. [Google Scholar] [CrossRef]
- Cunze, S.; Glock, G.; Kochmann, J.; Klimpel, S. Ticks on the move-climate change-induced range shifts of three tick species in Europe: Current and future habitat suitability for Ixodes ricinus in comparison with Dermacentor reticulatus and Dermacentor marginatus. Parasitol. Res. 2022, 121, 2241–2252. [Google Scholar] [CrossRef]
- Alkishe, A.; Cobos, M.E.; Osorio-Olvera, L.; Peterson, A.T. Ecological niche and potential geographic distributions of Dermacentor marginatus and Dermacentor reticulatus (Acari: Ixodidae) under current and future climate conditions. Web Ecol. 2022, 22, 33–45. [Google Scholar] [CrossRef]
- Milutinović, M.; Radulović, Ž. Ecological notes on ticks, Acari: Ixodidae, in Serbia, central regions. Acta Vet. 2002, 52, 49–58. [Google Scholar]
- Medlock, J.M.; Hansford, K.M.; Bormane, A.; Derdakova, M.; Estrada-Peña, A.; George, J.C.; Golovljova, I.; Jaenson, T.G.; Jensen, J.K.; Jensen, P.M.; et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit. Vectors 2013, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit. Vectors 2010, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Savić-Jevđenić, S.; Grgić, Ž.; Vidić, B.; Petrović, A. Lyme disease: The great imitator. Biotechnol. Anim. Husb. 2007, 23, 215–221. [Google Scholar] [CrossRef]
- Bona, M.; Blaňárová, L.; Stanko, M.; Mošanský, L.; Čepčeková, E.; Víchová, B. Impact of climate factors on the seasonal activity of ticks and temporal dynamics of tick-borne pathogens in an area with a large tick species diversity in Slovakia, Central Europe. Biologia 2022, 77, 1619–1631. [Google Scholar] [CrossRef]
- Ivanović, I.; Stošić, M.Ž.; Sabljić, E.R.; Kišek, T.C.; Špik, V.C.; Popović, A.; Savić, S. Ecology and prevalence of Borrelia burgdorferi s.l. in Ixodes ricinus (Acari: Ixodidae) ticks. Acta Vet. Hung. 2022, 70, 15–23. [Google Scholar] [CrossRef]
- Vucelja, M.; Krčmar, S.; Habuš, J.; Perko, V.M.; Boljfetić, M.; Bjedov, L.; Margaletić, J. Altitudinal Distribution, Seasonal Dynamics and Borrelia burgdorferi Sensu Lato Infections in Hard Ticks (Acari: Ixodidae) in Different Forest Communities in Inland Croatia. Sustainability 2023, 15, 4862. [Google Scholar] [CrossRef]
- Schulz, M.; Mahling, M.; Pfister, K. Abundance and seasonal activity of questing Ixodes ricinus ticks in their natural habitats in southern Germany in 2011. J. Vector Ecol. 2014, 39, 56–65. [Google Scholar] [CrossRef]
- Hornok, S.; Farkas, R. Influence of biotope on the distribution and peak activity of questing ixodid ticks in Hungary. Med. Vet. Entomol. 2009, 23, 41–46. [Google Scholar] [CrossRef]
- Walker, A.R. Age structure of a population of Ixodes ricinus (Acari: Ixodidae) in relation to its seasonal questing. Bull. Entomol. Res. 2001, 91, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Hornok, S. Allochronic seasonal peak activities of Dermacentor and Haemaphysalis spp. under continental climate in Hungary. Vet. Parasitol. 2009, 163, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Bartosik, K.; Wiśniowski, L.; Buczek, A. Abundance and seasonal activity of adult Dermacentor reticulatus (Acari: Amblyommidae) in eastern Poland in relation to meteorological conditions and the photoperiod. Ann. Agric. Environ. Med. 2011, 18, 340–344. [Google Scholar] [PubMed]
- Kohn, M.; Krücken, J.; McKay-Demeler, J.; Pachnicke, S.; Krieger, K.; von Samson-Himmelstjerna, G. Dermacentor reticulatus in Berlin/Brandenburg (Germany): Activity patterns and associated pathogens. Ticks Tick Borne Dis. 2019, 10, 191–206. [Google Scholar] [CrossRef]
- Lorusso, V.; Dantas-Torres, F.; Lia, R.P.; Tarallo, V.D.; Mencke, N.; Capelli, G.; Otranto, D. Seasonal dynamics of the brown dog tick, Rhipicephalus sanguineus, on a confined dog population in Italy. Med. Vet. Entomol. 2010, 24, 309–315. [Google Scholar] [CrossRef]
- Militzer, N.; Bartel, A.; Clausen, P.H.; Hoffmann-Köhler, P.; Nijhof, A.M. Artificial Feeding of All Consecutive Life Stages of Ixodes ricinus. Vaccines 2021, 9, 385. [Google Scholar] [CrossRef]
- Valim, J.R.; Rangel, C.P.; Baêta, B.A.; Ribeiro, C.C.; Cordeiro, M.D.; Teixeira, R.C.; Cepeda, P.B.; Fonseca, A.H. Using plastic tips in artificial feeding of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) females. Rev. Bras. Parasitol. Vet. 2017, 26, 110–114. [Google Scholar] [CrossRef]
- Springer, A.; Jordan, D.; Glass, A.; Kahl, O.; Fingerle, V.; Girl, P.; Chitimia-Dobler, L.; Strube, C. Borrelia Infections in Ageing Ticks: Relationship with Morphometric Age Ratio in Field-Collected Ixodes ricinus Nymphs. Microorganisms 2022, 10, 166. [Google Scholar] [CrossRef]
| Species | Stage/Gender | Jan. | Feb. | Mar. | Apr. | May | June | July | Aug. | Sept. | Oct. | Nov. | Dec. | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ixodes ricinus | Females | 1 | 2 | 4 | 18 | 22 | 3 | 2 | - | 4 | 5 | 4 | 3 | 68 |
| Males | - | - | - | 3 | 9 | 1 | - | - | 1 | 2 | - | - | 16 | |
| Nymphs | - | - | - | 1 | 5 | - | - | - | - | - | - | - | 6 | |
| Total | 1 | 2 | 4 | 22 | 36 | 4 | 2 | 0 | 5 | 7 | 4 | 3 | 90 | |
| Dermacentor marginatus | Females | 5 | 6 | 23 | 5 | - | - | - | - | - | 1 | - | 1 | 41 |
| Males | - | - | 14 | 2 | - | - | - | - | - | - | - | - | 16 | |
| Nymphs | - | - | - | - | - | - | - | - | - | - | - | - | 0 | |
| Total | 5 | 6 | 37 | 7 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 57 | |
| Dermacentor reticulatus | Females | 2 | 8 | 45 | 30 | 20 | 1 | - | - | - | 2 | 38 | 16 | 132 |
| Males | 9 | 4 | 16 | 16 | 9 | - | - | - | - | - | 8 | 6 | 98 | |
| Nymphs | - | - | - | - | - | - | - | - | - | - | - | - | 0 | |
| Total | 11 | 12 | 61 | 46 | 29 | 1 | 0 | 0 | 0 | 2 | 46 | 22 | 230 | |
| Rhipicephalus sanguineus | Females | - | - | - | 338 | 30 | 6 | 9 | 2 | 1 | - | - | - | 386 |
| Males | - | - | - | 175 | 32 | 3 | 2 | 1 | 1 | - | - | - | 214 | |
| Nymphs | - | - | - | 62 | 14 | 1 | 2 | 1 | - | - | - | - | 80 | |
| Total | 0 | 0 | 0 | 575 | 76 | 10 | 13 | 4 | 2 | 0 | 0 | 0 | 680 |
| Species | Index/Ratio | Mean | Min | Max | Variance | Standard Deviation | Coefficient of Variation | Standard Error |
|---|---|---|---|---|---|---|---|---|
| Ixodes ricinus | SI | 3.76748 | 0.85284 | 8.25582 | 3.01485 | 1.73633 | 46.08731 | 0.317009 |
| ASR | 15.57469 | 1.32172 | 66.03437 | 187.24392 | 13.68371 | 87.85861 | 2.498292 | |
| PAI | 0.22066 | 0.03657 | 0.54246 | 0.01141 | 0.10679 | 48.39508 | 0.019496 | |
| Dermacentor marginatus | SI | 5.69296 | 1.24443 | 8.04042 | 3.25125 | 1.80312 | 31.67286 | 0.329204 |
| ASR | 34.34066 | 2.45581 | 61.77639 | 249.68034 | 15.80128 | 46.01332 | 2.884905 | |
| PAI | 0.40394 | 0.09207 | 0.58080 | 0.01745 | 0.13211 | 32.70520 | 0.024120 | |
| Dermacentor reticulatus | SI | 5.40410 | 1.32653 | 8.53510 | 3.50176 | 1.87130 | 34.62740 | 0.341651 |
| ASR | 32.22059 | 3.24604 | 65.18115 | 261.39583 | 16.16774 | 50.17828 | 2.951812 | |
| PAI | 0.36935 | 0.13426 | 0.55036 | 0.01427 | 0.11945 | 32.34060 | 0.021809 | |
| Rhipicephalus sanguineus | SI | 6.12184 | 3.18371 | 7.79968 | 0.85205 | 0.92306 | 15.07822 | 0.168528 |
| ASR | 30.46824 | 10.83415 | 48.50751 | 46.82442 | 6.84284 | 22.45892 | 1.249325 | |
| PAI | 0.36955 | 0.22061 | 0.48910 | 0.00299 | 0.05469 | 14.79807 | 0.009984 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrović, A.; Stanić, K.; Popović, A.; Ivanović, I.; Supić, D.; Marinković, D.; Bursić, V. Seasonal Dynamics and Physiological Age of Ixodid Ticks Collected from Dogs. Animals 2023, 13, 3026. https://doi.org/10.3390/ani13193026
Petrović A, Stanić K, Popović A, Ivanović I, Supić D, Marinković D, Bursić V. Seasonal Dynamics and Physiological Age of Ixodid Ticks Collected from Dogs. Animals. 2023; 13(19):3026. https://doi.org/10.3390/ani13193026
Chicago/Turabian StylePetrović, Aleksandra, Ksenija Stanić, Aleksandra Popović, Ivana Ivanović, Dejan Supić, Dušan Marinković, and Vojislava Bursić. 2023. "Seasonal Dynamics and Physiological Age of Ixodid Ticks Collected from Dogs" Animals 13, no. 19: 3026. https://doi.org/10.3390/ani13193026
APA StylePetrović, A., Stanić, K., Popović, A., Ivanović, I., Supić, D., Marinković, D., & Bursić, V. (2023). Seasonal Dynamics and Physiological Age of Ixodid Ticks Collected from Dogs. Animals, 13(19), 3026. https://doi.org/10.3390/ani13193026








