Thoracolumbar Retrolaminar Block: Anatomical and Radiological Study of Injectate Pattern Distribution in Canine Cadavers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Retrolaminar Injections
2.3. Computed Tomography
2.4. Transverse Anatomical Dissection
2.5. Statistical Analysis
3. Results
3.1. Retrolaminar Injections
3.2. Computed Tomography
3.3. Transverse Anatomical Dissections
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Onishi, E.; Toda, N.; Kameyama, Y.; Yamauchi, M. Comparison of Clinical Efficacy and Anatomical Investigation between Retrolaminar Block and Erector Spinae Plane Block. Biomed. Res. Int. 2019, 2019, 2578396. [Google Scholar] [CrossRef] [PubMed]
- Skolnick, P. The Opioid Epidemic: Crisis and Solutions. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Chia, P.A.; Cannesson, M.; Bui, C.C.M. Opioid free anesthesia: Feasible? Curr. Opin. Anaesthesiol. 2020, 33, 512–517. [Google Scholar] [CrossRef] [PubMed]
- White, D.M.; Mair, A.R.; Martinez-Taboada, F. Opioid-free anaesthesia in three dogs. Open Vet. J. 2017, 7, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.J. Thoracic wall blocks: From paravertebral to retrolaminar to serratus to erector spinae and back again—A review of evidence. Best Pract. Res. Clin. Anaesthesiol. 2019, 33, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, G.; Oppitz, N.; Schone, S.; Richter-Heine, I.; Hohne, M.; Koltermann, C. Analgesia of the axilla using a paravertebral catheter in the lamina technique. Anaesthesist 2006, 55, 423–427. [Google Scholar] [CrossRef]
- Sabouri, A.S.; Crawford, L.; Bick, S.K.; Nozari, A.; Anderson, T.A. Is a Retrolaminar Approach to the Thoracic Paravertebral Space Possible?: A Human Cadaveric Study. Reg. Anesth. Pain Med. 2018, 43, 864–868. [Google Scholar] [CrossRef]
- Bouman, E.A.C.; Sieben, J.M.; Balthasar, A.J.R.; Joosten, E.A.; Gramke, H.F.; van Kleef, M.; Lataster, A. Boundaries of the thoracic paravertebral space: Potential risks and benefits of the thoracic paravertebral block from an anatomical perspective. Surg. Radiol. Anat. 2017, 39, 1117–1125. [Google Scholar] [CrossRef]
- Serra, R.M.; Jimenez, C.P.; Monticelli, P.; Plested, M.; Viscasillas, J. Assessment of an ultrasound-guided technique for catheterization of the caudal thoracic paravertebral space in dog cadavers. Open Vet. J. 2019, 9, 230–237. [Google Scholar] [CrossRef]
- Aamir, F.; Cronin, M.; Lee, P.; Iohom, G.; Shorten, G. A sono-anatomical and cadaveric study of ultrasound-guided retrolaminar block. Med. Ultrason. 2021, 23, 418–423. [Google Scholar] [CrossRef]
- Damjanovska, M.; Stopar Pintaric, T.; Cvetko, E.; Vlassakov, K. The ultrasound-guided retrolaminar block: Volume-dependent injectate distribution. J. Pain Res. 2018, 11, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Choi, Y.J.; Kwon, H.J.; O, J.; Cho, T.H.; Kim, S.H. Comparison of injectate spread and nerve involvement between retrolaminar and erector spinae plane blocks in the thoracic region: A cadaveric study. Anaesthesia 2018, 73, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Juttner, T.; Werdehausen, R.; Hermanns, H.; Monaca, E.; Danzeisen, O.; Pannen, B.H.; Janni, W.; Winterhalter, M. The paravertebral lamina technique: A new regional anesthesia approach for breast surgery. J. Clin. Anesth. 2011, 23, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Voscopoulos, C.; Palaniappan, D.; Zeballos, J.; Ko, H.; Janfaza, D.; Vlassakov, K. The ultrasound-guided retrolaminar block. Can. J. Anaesth. 2013, 60, 888–895. [Google Scholar] [CrossRef]
- Murouchi, T.; Yamakage, M. Retrolaminar block: Analgesic efficacy and safety evaluation. J. Anesth. 2016, 30, 1003–1007. [Google Scholar] [CrossRef]
- Onishi, E.; Murakami, M.; Nishino, R.; Ohba, R.; Yamauchi, M. Analgesic Effect of Double-Level Retrolaminar Paravertebral Block for Breast Cancer Surgery in the Early Postoperative Period: A Placebo-Controlled, Randomized Clinical Trial. Tohoku J. Exp. Med. 2018, 245, 179–185. [Google Scholar] [CrossRef]
- Kamel, A.A.F.; Elhossieny, K.M.; Hegab, A.S.; Salem, D.A.E. Ultrasound-guided Retrolaminar Block Versus Thoracic Epidural Analgesia for Pain Control Following Laparoscopic Cholecystectomy. Pain Physician 2022, 25, E795–E803. [Google Scholar]
- Seol, A.; Tsui, B.C.H. Retrolaminar Continuous Nerve Block Catheter for Multiple Rib Fractures: A Case Report. A A Pract. 2022, 16, e01614. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, A.; Sinha, C.; Kumar, A.; Kumari, A. Continuous retrolaminar block in percutaneous nephrolithotomy surgery. Saudi J. Anaesth. 2023, 17, 132–133. [Google Scholar] [CrossRef]
- Nobukuni, K.; Hatta, M.; Nakagaki, T.; Yoshino, J.; Obuchi, T.; Fujimura, N. Retrolaminar versus epidural block for postoperative analgesia after minor video-assisted thoracic surgery: A retrospective, matched, non-inferiority study. J. Thorac. Dis. 2021, 13, 2758–2767. [Google Scholar] [CrossRef]
- Alseoudy, M.M.; Abdelbaser, I. Ultrasound-guided retrolaminar block versus ilioinguinal nerve block for postoperative analgesia in children undergoing inguinal herniotomy: A randomized controlled trial. J. Clin. Anesth. 2021, 74, 110421. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xu, X.; Zhu, Y.; Liu, X.; Zhao, F.; Liang, G.; Zhu, Z. Safety and Efficacy of Ultrasound-Guided Retrolaminar Block of Multiple Injections in Retroperitoneal Laparoscopic Nephrectomy: A Prospective Randomized Controlled Study. J. Pain Res. 2021, 14, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Peker, K. The Efficacy of Ultrasound-guided Retrolaminar Block on Postoperative Analgesia for Lumbar İnstrumentation Surgery. J. Coll. Physicians Surg. Pak. 2020, 30, 1355–1356. [Google Scholar] [CrossRef] [PubMed]
- Pentsou, K.; Huuskonen, V. Thoracolumbar retrolaminar block in seven dogs undergoing spinal surgery. Ir. Vet. J. 2022, 75, 17. [Google Scholar] [CrossRef]
- Freeman, L.; Becvarova, I.; Cave, N.; MacKay, C.; Nguyen, P.; Rama, B.; Takashima, G.; Tiffin, R.; Tsjimoto, H.; van Beukelen, P. WSAVA Nutritional Assessment Guidelines. J. Small Anim. Pract. 2011, 52, 385–396. [Google Scholar] [CrossRef]
- Ferreira, T.H.; St James, M.; Schroeder, C.A.; Hershberger-Braker, K.L.; Teixeira, L.B.C.; Schroeder, K.M. Description of an ultrasound-guided erector spinae plane block and the spread of dye in dog cadavers. Vet. Anaesth. Analg. 2019, 46, 516–522. [Google Scholar] [CrossRef]
- Monticelli, P.; Fitzgerald, E.; Viscasillas, J. A sonographic investigation for the development of ultrasound-guided paravertebral brachial plexus block in dogs: Cadaveric study. Vet. Anaesth. Analg. 2018, 45, 195–202. [Google Scholar] [CrossRef]
- McFadzean, W.J.M.; Macfarlane, P.; Granger, N.; Murrell, J.C. Influence of peri-incisional epaxial muscle infiltration with bupivacaine pre- or post-surgery on opioid administration in dogs undergoing thoraco-lumbar hemilaminectomy. Vet. J. 2021, 270, 105622. [Google Scholar] [CrossRef]
- Horowitz, F.B. Evaluation of Epidural Morphine and Incisional Bupivacaine for Analgesia Following Hemilaminectomy in the Dog. Master’s Thesis, University of Virginia-Maryland, Virginia, MD, USA, 2009. [Google Scholar]
- Macias, C.; McKee, W.M.; May, C.; Innes, J.F. Thoracolumbar disc disease in large dogs: A study of 99 cases. J. Small Anim. Pract. 2002, 43, 439–446. [Google Scholar] [CrossRef]
- Taniguchi, M.; Kasaba, T.; Takasaki, M. Epidural anesthesia enhances sympathetic nerve activity in the unanesthetized segments in cats. Anesth. Analg. 1997, 84, 391–397. [Google Scholar] [CrossRef]
- Veering, B.T.; Cousins, M.J. Cardiovascular and pulmonary effects of epidural anaesthesia. Anaesth. Intensive Care 2000, 28, 620–635. [Google Scholar] [CrossRef] [PubMed]
- Hotvedt, R.; Platou, E.S.; Refsum, H. Electrophysiological effects of thoracic epidural analgesia in the dog heart in situ. Cardiovasc. Res. 1983, 17, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, G.; Zhu, Y.; Liu, X.; Xu, S.; He, M.; Chen, S.; An, K.; Liang, G.; Zhu, Z. Effectiveness of Ultrasound-Guided Retrolaminar Block and Erector Spinae Plane Block in Retroperitoneal Laparoscopic Surgery: A Randomized Controlled Trial. J. Pain. Res. 2022, 15, 815–826. [Google Scholar] [CrossRef]
- Bray, J.P.; Burbidge, H.M. The canine intervertebral disk: Part one: Structure and function. J. Am. Anim. Hosp. Assoc. 1998, 34, 55–63. [Google Scholar] [CrossRef]
- Aghighi, S.A.; Tipold, A.; Piechotta, M.; Lewczuk, P.; Kastner, S.B. Assessment of the effects of adjunctive gabapentin on postoperative pain after intervertebral disc surgery in dogs. Vet. Anaesth. Analg. 2012, 39, 636–646. [Google Scholar] [CrossRef]
- Lamont, L.A. Multimodal pain management in veterinary medicine: The physiologic basis of pharmacologic therapies. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 1173–1186. [Google Scholar] [CrossRef] [PubMed]
- Pascal, M.; Allison, A.; Kaartinen, J. Opioid-sparing effect of a medetomidine constant rate infusion during thoraco-lumbar hemilaminectomy in dogs administered a ketamine infusion. Vet. Anaesth. Analg. 2020, 47, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Schmierer, P.A.; Tunsmeyer, J.; Tipold, A.; Hartnack-Wilhelm, S.; Lesczuk, P.; Kastner, S.B.R. Randomized controlled trial of pregabalin for analgesia after surgical treatment of intervertebral disc disease in dogs. Vet. Surg. 2020, 49, 905–913. [Google Scholar] [CrossRef]
- Epstein, M.; Rodan, I.; Griffenhagen, G.; Kadrlik, J.; Petty, M.; Robertson, S.; Simpson, W. 2015 AAHA/AAFP Pain Management Guidelines for Dogs and Cats. J. Am. Anim. Hosp. Assoc. 2015, 51, 67–84. [Google Scholar] [CrossRef]
- Muir, W.; Woolf, C.J. Mechanisms of pain and their therapeutic implications. J. Am. Vet. Med. Assoc. 2001, 219, 1346–1356. [Google Scholar] [CrossRef]
- Gaynor, J.S.; Dunlop, C.I.; Wagner, A.E.; Wertz, E.M.; Golden, A.E.; Demme, W.C. Complications and mortality associated with anesthesia in dogs and cats. J. Am. Anim. Hosp. Assoc. 1999, 35, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Bruniges, N.; Rioja, E. Intraoperative anaesthetic complications in dogs undergoing general anaesthesia for thoracolumbar hemilaminectomy: A retrospective analysis. Vet. Anaesth. Analg. 2019, 46, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, N.D.; Levine, J.M.; Olby, N.J.; Stein, V.M. Intervertebral disk degeneration in dogs: Consequences, diagnosis, treatment, and future directions. J. Vet. Intern. Med. 2013, 27, 1318–1333. [Google Scholar] [CrossRef]
- Olby, N. The Pathogenesis and Treatment of Acute Spinal Cord Injuries in Dogs. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 791–807. [Google Scholar] [CrossRef]
- Park, E.H.; White, G.A.; Tieber, L.M. Mechanisms of injury and emergency care of acute spinal cord injury in dogs and cats. J Vet. Emerg. Crit. Care 2012, 22, 160–178. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.H.; Teixeira, L.B.C.; Schroeder, C.A.; de Miguel Garcia, C.; Schroeder, K.M. Description of an ultrasound-guided thoracic paravertebral block technique and the spread of dye in dog cadavers. Vet. Anaesth. Analg. 2018, 45, 811–819. [Google Scholar] [CrossRef]
- Portela, D.A.; Campoy, L.; Otero, P.E.; Martin-Flores, M.; Gleed, R.D. Ultrasound-guided thoracic paravertebral injection in dogs: A cadaveric study. Vet. Anaesth. Analg. 2017, 44, 636–645. [Google Scholar] [CrossRef]
- Portela, D.A.; Romano, M.; Zamora, G.A.; Garcia-Pereira, F.; Pablo, L.S.; Gatson, B.J.; Johnson, A.N.; Otero, P.E. The effect of erector spinae plane block on perioperative analgesic consumption and complications in dogs undergoing hemilaminectomy surgery: A retrospective cohort study. Vet. Anaesth. Analg. 2021, 48, 116–124. [Google Scholar] [CrossRef]
- Zannin, D.; Isaka, L.J.; Pereira, R.H.; Mencalha, R. Opioid-free total intravenous anesthesia with bilateral ultrasound-guided erector spinae plane block for perioperative pain control in a dog undergoing dorsal hemilaminectomy. Vet. Anaesth. Analg. 2020, 47, 728–731. [Google Scholar] [CrossRef]
- Portela, D.A.; Castro, D.; Romano, M.; Gallastegui, A.; Garcia-Pereira, F.; Otero, P.E. Ultrasound-guided erector spinae plane block in canine cadavers: Relevant anatomy and injectate distribution. Vet. Anaesth. Analg. 2020, 47, 229–237. [Google Scholar] [CrossRef]
- Klop, A.C.; Vester, M.E.M.; Colman, K.L.; Ruijter, J.M.; Van Rijn, R.R.; Oostra, R.J. The effect of repeated freeze-thaw cycles on human muscle tissue visualized by postmortem computed tomography (PMCT). Clin. Anat. 2017, 30, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Heng, H.G.; Selvarajah, G.T.; Lim, H.T.; Ong, J.S.; Lim, J.; Ooi, J.T. Serial postmortem thoracic radiographic findings in canine cadavers. Forensic Sci. Int. 2009, 188, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Lamon, A.M.; Habib, A.S. Managing anesthesia for cesarean section in obese patients: Current perspectives. Local. Reg. Anesth. 2016, 9, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Portela, D.A.; Otero, P.E.; Sclocco, M.; Romano, M.; Briganti, A.; Breghi, G. Anatomical and radiological study of the thoracic paravertebral space in dogs: Iohexol distribution pattern and use of the nerve stimulator. Vet. Anaesth. Analg. 2012, 39, 398–408. [Google Scholar] [CrossRef] [PubMed]
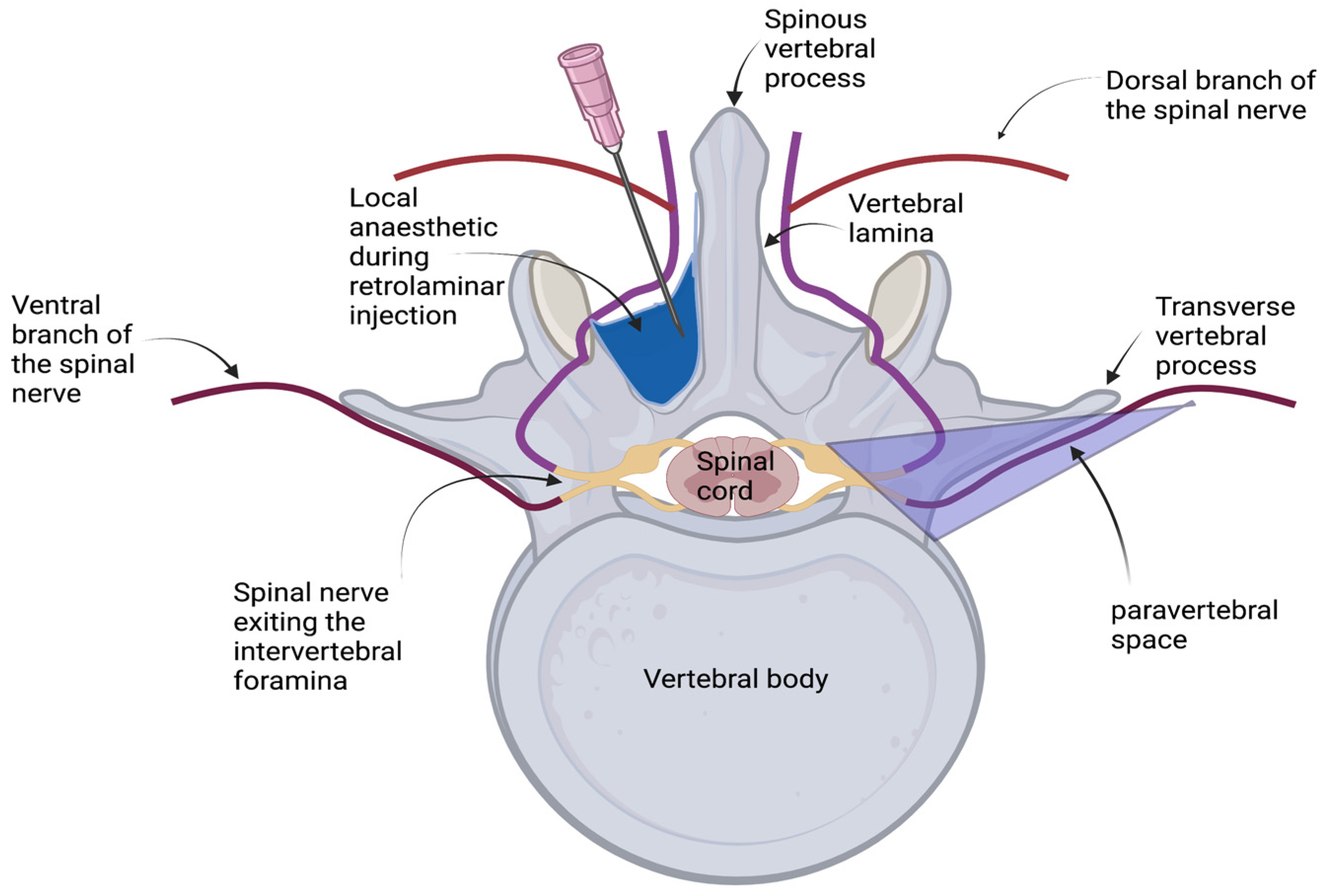


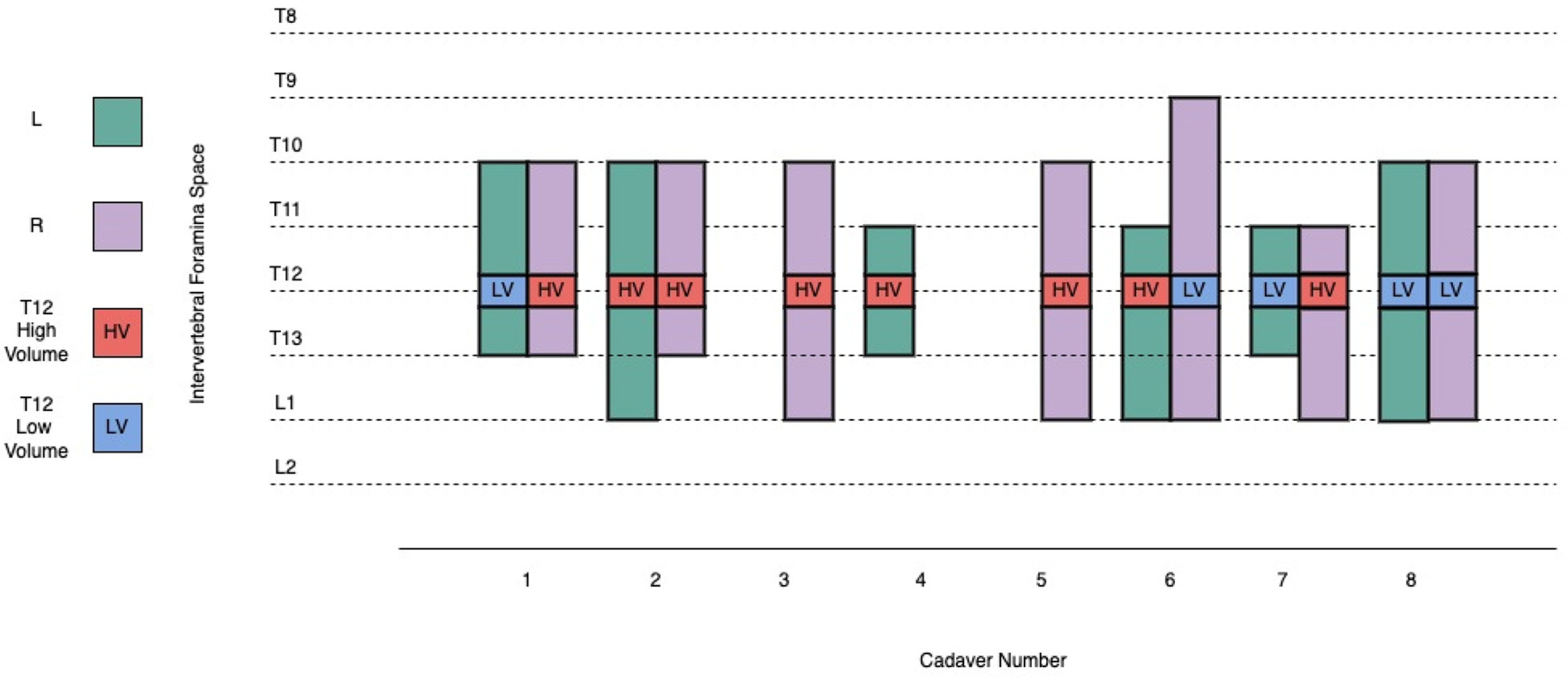
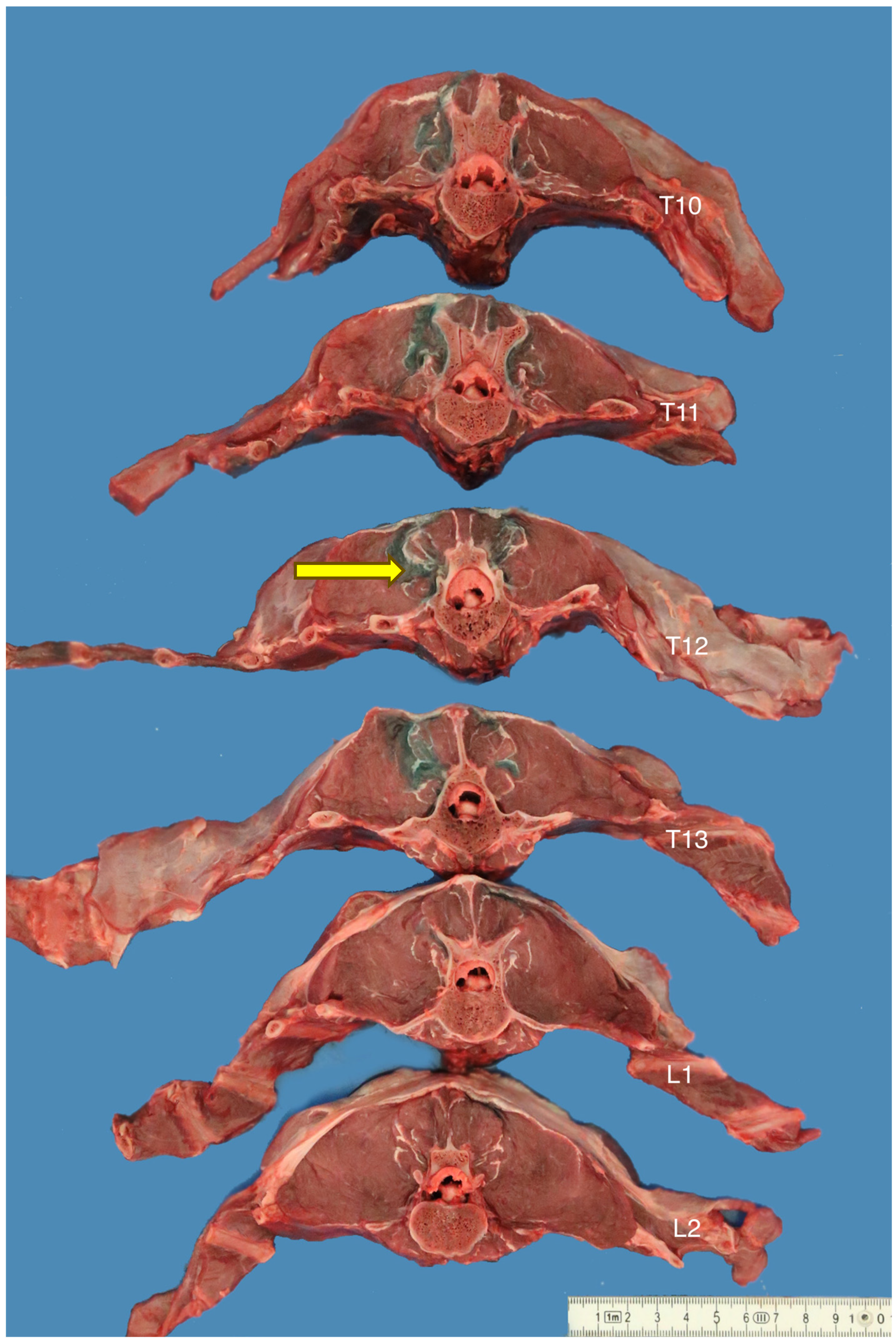
| Injection | Cadaver | Injection Volume (mL) | Retrolaminar Spread | Intervertebral Foramina Spread | Epidural Spread |
|---|---|---|---|---|---|
| 1 | 1 | 20 | T10–T13 | T10–T13 | T11–T13 |
| 2 | 1 | 10 | T10–T13 | T10–T13 | T11–T13 |
| 3 | 2 | 20 | T10–T13 | T10–T13 | L1 |
| 4 | 2 | 20 | T10–T13 | T10–L1 | L1 |
| 5 | 3 | 20 | T10–L1 | T10–L1 | T11–T13 |
| 6 | 4 | 20 | T11–T13 | T11–T13 | None |
| 7 | 5 | 20 | T10–T13 | T10–L1 | T11–T13 |
| 8 | 6 | 10 | T11–L1 | T9–L1 | T11–T12 |
| 9 | 6 | 20 | T11–L1 | T11–L1 | T11–T12 |
| 10 | 7 | 20 | T11–L1 | T11–L1 | T6–L1 |
| 11 | 7 | 10 | T11–T13 | T11–T13 | T6–L1 |
| 12 | 8 | 10 | T11–L1 | T10–L1 | T4–L4 |
| 13 | 8 | 10 | T11–T13 | T10–L1 | T4–L4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pentsou, J.; Vagias, M.; Davies, T.; Hoey, S.; Huuskonen, V. Thoracolumbar Retrolaminar Block: Anatomical and Radiological Study of Injectate Pattern Distribution in Canine Cadavers. Animals 2023, 13, 3008. https://doi.org/10.3390/ani13193008
Pentsou J, Vagias M, Davies T, Hoey S, Huuskonen V. Thoracolumbar Retrolaminar Block: Anatomical and Radiological Study of Injectate Pattern Distribution in Canine Cadavers. Animals. 2023; 13(19):3008. https://doi.org/10.3390/ani13193008
Chicago/Turabian StylePentsou, Julia, Michail Vagias, Thomas Davies, Séamus Hoey, and Vilhelmiina Huuskonen. 2023. "Thoracolumbar Retrolaminar Block: Anatomical and Radiological Study of Injectate Pattern Distribution in Canine Cadavers" Animals 13, no. 19: 3008. https://doi.org/10.3390/ani13193008
APA StylePentsou, J., Vagias, M., Davies, T., Hoey, S., & Huuskonen, V. (2023). Thoracolumbar Retrolaminar Block: Anatomical and Radiological Study of Injectate Pattern Distribution in Canine Cadavers. Animals, 13(19), 3008. https://doi.org/10.3390/ani13193008